Abstract
In this study, the effect of acidic extraction conditions (time of 30–90 min, temperature of 75–95 °C and pH of 1.5–3) on the yield and degree of esterification (DE) of citron peel pectin was investigated applying Box–Behnken design. The highest production yield of pectin (28.31 ± 0.11%) was achieved at extraction time of 90 min, temperature of 95 °C and pH of 1.5, as optimal extraction conditions, which was close to the predicted value (29.87%). Under optimum extraction conditions, the DE and the emulsifying activity were 51.33 and 46.2%, respectively. In addition, the emulsions were 93.9 and 93.5 stable at 4 °C, 93.7 and 93.1 at 23 °C after 1 and 30 days, respectively. The determination of flow behavior showed that the pectin solutions had a Newtonian behavior at low concentrations (< 1.0% w/v), while this behavior was changed to pseudoplastic with increasing concentration.
Keywords: Pectin, Citron, Optimization, Response surface methodology
Introduction
Pectin is an exclusive structural polysaccharide that exists in the cell walls and middle lamella of terrestrial plants [25]. Pectin includes a backbone of galacturonic acid residues which makes up at least 65% of total pectin [13]. The d-galacturonic acid includes carbonyl groups (COO) and these are partially esterified with methyl alcohol or acetic acid [13]. The degree of esterification (DE) is an effective property in gel production by pectin. Based on DE, pectin is classified into two categories: The high methyl ester pectin (HMP) with the DE percentage above 50% and the low methyl ester pectin (LMP) with the DE less than 50% [9].
Pectin are widely used in the food industry as a gelling, stabilizing, thickening and emulsifying agent [15, 31]. Furthermore, pectin is employed to diverse pharmaceutical activities such as wound healing, lipase inhibition, apoptosis induction of human cancer cell, immunostimulating, antimetastasis and lowering cholesterol [17, 19, 31].
Citrus fruits (family Rutaceae) are cultivated widely around the world. Some citrus fruits are oranges, mandarins, lemons, grapefruits and citrons (Citrus medica L.) [23]. The citron belongs to the citrus species with properties similar to lemon fruit, which was observed for the first time in the near East and the Mediterranean [29]. Nowadays, this fruit cultivated in the south of France, Southern Italy (Calabria), Greece, North Africa, Puerto Rico, China, Vietnam and Japan [11].
Citron is mainly used for the production of candied peel, flavoring of liquors and medical purposes [40]. The large volume of the fruit is its peel, which is included the plenty of bioactive compounds and polysaccharides, such as pectin [26]. So, this by-product can be a suitable for extraction of pectin.
Pectin is most commonly extracted by a hot diluted solution of the strong mineral acids [32]. Strong mineral acids such as hydrochloric, sulfuric, or nitric acid have low price and capable to generate pectin enriched in homogalacturonic blocks [43]. However, they have disadvantages include toxicity and damaging the environment and also requiring high cost for treating the wastes [42]. Therefore, the organic acids such as citric acids can used to extract the pectin [19]. In addition to, organic acids have a lower hydrolyzing capacity and are expected to cause less de-polymerization of pectin [20].
In acidic extraction, the diverse factors such as extraction period, extraction temperature and pH can be influenced on the yield and quality of extracted pectin [36]. Therefore, the optimization of extraction conditions is necessary to reach the maximum production.
Response Surface Methodology (RSM), which indicates the behavior of experimental data with objective of making the statistical previsions by a polynomial equation, is a complex of mathematical and statistical techniques [3]. Also, it is necessary to mention that this methodology widely employed for processes optimization. In these processes the output usually is affected by many input variables [2].
According to the above-mentioned, in the current study, our goals were to investigate the effects of the diverse parameters including extraction period, extraction temperature, pH and liquid/solid ratio on the acidic extraction yield and DE of citron peel pectin and also optimization these extraction conditions to reach the maximum yield.
Materials and methods
Materials
Citron peels were purchased from local grocery in Mashhad, Khorasan razavi, Iran. The peels were washed, cut into small pieces and then dried in 50 °C for 24 h by a hot air oven. In the next step, the dried peels were eroded and passed through a 40-mesh sieve to obtain powdered sample. The prepared powders were kept in sealable bag inside desiccator until use. Citric acid, sodium hydroxide, hydrochloric acid, phenolphthalein reagent, sulfuric acid, sodium tetraborate and sodium azide were procured from Merck Chemical Co. (Darmstadt, Germany).
Extraction of pectin from dried citron peel
Acidic extraction of pectin was carried out according to the methods described by Canteri-Schemin et al. [4] with some modification. The dried peel powder (liquid–solid ratio (LSR) of 30 (v/w), Fig. 1) was soaked into citric acid aqueous solution adjusted to the desirable pH values (1.5, 2.25, 3), and then stirred. The solution was extracted with three temperature of 75, 85, 95 °C for three times of 30, 60, 90 min. After the extraction process, the mixture was centrifuged (10,000 g, 20 min) to isolate impurities. Then, the same volume of ethanol (98%) was added to solution and stored at 7 °C for 12 h. After this period, pectin was separated from solution using centrifugation (10,000 g, 15 min) and dried in oven at 50 °C until constant weight was achieved. The yield of pectin (YP) was calculated from following equation [38]:
| 1 |
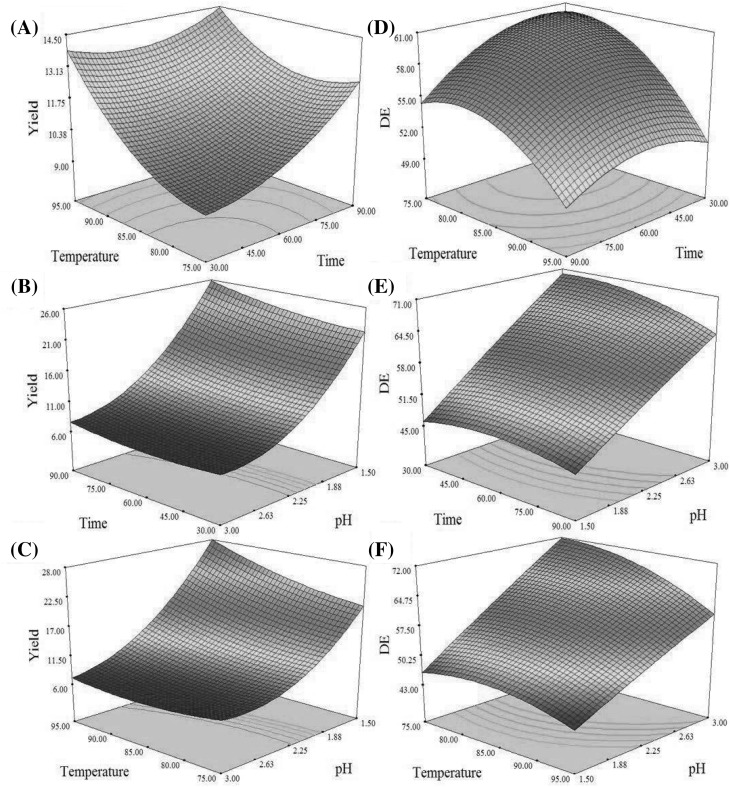
Fig. 1.
3-D response surface plots indicate the influence of time (min), temperature (°C) and pH on the extraction yield (%) and DE (%)
Determination of the degree of esterification
The degree of esterification was characterized by titration method, that explained by Santos et al. [38] with some modification. 20 mg pectin was soaked with 3 ml ethanol and dissolved in 20 ml of deionized water. The samples were stirred until the pectin dissolution completely. Thereafter, five drops of phenolphthalein reagent were added to solution and then with 0.1 N sodium hydroxide was titrated (V1). After that, 10 ml of 0.1 N sodium hydroxide was added to samples and shacked 15 min for hydrolysis. Subsequently, 10 ml of 0.1 N hydrochloric acid was added to solution and stirred until the pink color disappeared. The solution was titrated with 0.1 N sodium hydroxide until the reappearance of pink color (V2). Finally, the DE was determined according to the following equation:
| 2 |
Emulsifying properties
Generally, the ability to form an emulsion is one of the most important properties of some food hydrocolloids such as pectin and the emulsifying properties of this polysaccharide can be very important in using it in food products. It should also be noted that these properties are probably due to the presence of protein parts and acetyl groups in structure of this polysaccharide because the pectin itself is predominantly hydrophilic. In this part, emulsifying activity (EA) and emulsion stability (ES) of pectin emulsions were evaluated based on the procedure described by Hosseini et al. [17]. Generally, oil-in-water (O/W) emulsions were prepared by adding 5 ml sun flower oil to 5 ml pectin solutions (0.5% w/v) containing 0.02% sodium azide as a bacteriocide. Solutions were homogenized in the ultra-turax T-25 homogenizer (IKAT25 Digital Ultra-Turax, Staufen, Germany) at 10,000 g for 4 min, and then the emulsions were centrifuged for 5 min at 4000 g. The EA was determined as follows:
| 3 |
To investigate the emulsion stability (ES) as prepared above, the different tubes were kept at 4 and 23 °C for 1 and 30 days. The emulsion stability was characterized by following equation:
| 4 |
Determination of the viscosity
A rotational programmable viscometer (LVDV-II Pro, Brookfield Engineering Inc., Middleborough, MA, USA) by a LV spindle were employed to investigate of flow behavior of pectin solutions in diverse concentrations (0.1, 0.5, 1.0 and 2.0% w/v). Thus, the cylinder of the rotational viscometer was filled with 25 ml of sample and shear rate was adjusted from 1.22 to 79.50 s−1 within 5 s intervals.
Fourier transform infrared spectroscopy (FTIR)
FTIR is a structure spectroscopic technique that shows the bonding structure of atoms. This spectrum obtained from interaction of infrared radiation with matter. Analysis of FTIR spectrum was made to confirm presence of pectin in supernatant obtained from citron peel [36]. FTIR spectrum of citron peel pectin was registered by a device called Perkin Elmer FTIR spectrometer (Perkin Elmer Co., Waltham, MA, USA) applying the potassium bromide disk method with resolution of 4 cm−1. It should also be noted that the FTIR measurement was achieved over the range of 4000–450 cm−1.
Design of experiment
One factor at the time design was applied to investigate the most appropriate LSR. In this design, one factor variable and other factors are constant. For this purpose, different levels of LSR (20:1, 30:1, 40:1 and 50:1 v/w) were considered and other factors (time: 90 min, temp: 95 °C, pH: 1.5) considered to be constant. To optimize the effect of extraction conditions on the yield, a Box–Behnken response surface experimental design was applied. The levels of independent variables are shown in Table 1. It should also be noted that the all computation and graphics in this research were performed employing the statistical software Design Expert (version 8.0.0, Stat-Ease Inc., Minneapolis, MN, USA) and Excel (version 2010, Microsoft Corporation, Redmond, WA, USA).
Table 1.
Coded levels and actual values (in parentheses) of the variables in Box–Behnken design: extraction time (X1, min); temperature (X2, °C); pH (X3); pectin yield (PY, %); degree of esterification (DE, %)
| Run | Independent variables | Measured responses | Predicted responses | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | PY | DE | PY | DE | |
| 1 | − 1 (30) | − 1 (75) | 0 (2.25) | 9.20 | 61.23 | 9.09 | 60.15 |
| 2 | + 1 (90) | − 1 (75) | 0 (2.25) | 12.76 | 54.92 | 12.67 | 54.39 |
| 3 | − 1 (30) | + 1 (95) | 0 (2.25) | 13.75 | 50.05 | 13.83 | 50.58 |
| 4 | + 1 (90) | + 1 (95) | 0 (2.25) | 14.34 | 48.27 | 14.45 | 49.35 |
| 5 | − 1 (30) | 0 (85) | − 1 (1.50) | 22.26 | 45.83 | 22.14 | 45.93 |
| 6 | + 1 (90) | 0 (85) | − 1 (1.50) | 25.98 | 45.88 | 25.84 | 45.43 |
| 7 | − 1 (30) | 0 (85) | + 1 (3.00) | 7.03 | 69.48 | 7.17 | 69.92 |
| 8 | + 1 (90) | 0 (85) | + 1 (3.00) | 7.55 | 63.54 | 7.67 | 63.43 |
| 9 | 0 (60) | − 1 (75) | − 1 (1.50) | 20.41 | 45.11 | 20.64 | 46.08 |
| 10 | 0 (60) | + 1 (95) | − 1 (1.50) | 27.95 | 44.10 | 28.00 | 43.47 |
| 11 | 0 (60) | − 1 (75) | + 1 (3.00) | 8.20 | 71.15 | 8.16 | 71.78 |
| 12 | 0 (60) | + 1 (95) | + 1 (3.00) | 7.56 | 60.76 | 7.33 | 59.78 |
| 13 | 0 (60) | 0 (85) | 0 (2.25) | 11.02 | 58.57 | 10.62 | 58.54 |
| 14 | 0 (60) | 0 (85) | 0 (2.25) | 10.65 | 58.03 | 10.62 | 58.54 |
| 15 | 0 (60) | 0 (85) | 0 (2.25) | 10.20 | 59.01 | 10.62 | 58.54 |
Results and discussion
One factor at the time for evaluation of LSR effect on the yield of pectin
Liquid–solid ratio (LSR) is a extremely influential factor on the yield of pectin. One factor at the time was employed to evaluate the most appropriate LSR. As above-mentioned, the different levels of LSR (20:1, 30:1, 40:1, 50:1 v/w) were selected and other factors (time of 90 min, temperature of 95 °C and pH of 1.5) were considered to be constant. The results showed that the extraction yield increased by a increase in LSR up to 30:1 v/w, which this is probably due to increasing the content surface area between peel particles and solvent by increasing solvent volume and thereby, increase in extraction of pectin [34]. However, when the LSR continued to increase, the yield of pectin showed no significant change. Therefore, in the current study, in order to reduce alcohol consumption and therefore the cost and volume of work, the LSR of 30:1 v/w was selected for all experiments [37].
Model fitting and statistical analysis
In this paper, three factors, three levels Box–Behnken response surface design (BBD) was applied to evaluate and optimize the effect of process variables such as time (30–90 min), temperature (75–95 °C) and pH (1.5–3) on the extraction yield of pectin from citron peel. The predicted values and experimental results demonstrated in Table 1.
Second order polynomial equation includes linear, interactive and quadratic terms was employed to organize mathematical models to detect the optimum conditions and express the relationship between process variables and the responses [27, 35]. The second order equations related to the extraction yield and DE are given below:
| 5 |
| 6 |
where Xi is coded independent factor (X1 = time, X2 = temperature, X3 = pH).
The statistical meaningful of the models were investigated applying analysis of variance (ANOVA). ANOVA, with the aim of testing hypothesis on the parameters of the model, is a statistical method that subdivides the total variation in a collection of data into component parts associated with especial sources of variation (Table 2) [34]. As can be soon, the model p values of lower than 0.001 (significant) for both the yield and DE, and also lack of fit higher than 0.05 (insignificant) for these parameters demonstrated that the models were well adapted to the responses [16]. In the other hand, the determination coefficient of yield and DE (99.92 and 99.41%, respectively), adjusted determination coefficient (99.77 and 98.34%, respectively) and predicted determination coefficient (99.37% and 91.12%, respectively) are calculated to examine the adequacy of the models [1, 5]. The high values of R2, Adj-R2 values clearly demonstrated a very high degree of precision and good reliability of the conducted experiments [33].
Table 2.
The results of analysis of variance (ANOVA) for regression model of pectin yield and DE
| Source | Sum of squares | DF | Mean square | F value | p value |
|---|---|---|---|---|---|
| (A) Yield | |||||
| Regression | 671.395 | 9 | 74.599 | 674.69 | 0.000 |
| Linear | 578.820 | 3 | 192.940 | 1744.98 | 0.000 |
| Square | 71.081 | 3 | 23.694 | 214.29 | 0.000 |
| Interaction | 21.493 | 3 | 7.164 | 64.80 | 0.000 |
| Residual error | 0.553 | 5 | 0.111 | ||
| Lack-of-fit | 0.216 | 3 | 0.072 | 0.43 | 0.757 |
| Pure error | 0.337 | 2 | 0.169 | ||
| Total | 671.948 | 14 | |||
| R2 | 0.9992 | ||||
| Adj R2 | 0.9977 | ||||
| Pred R2 | 0.9937 | ||||
| (B) DE | |||||
| Regression | 1092.51 | 9 | 121.391 | 92.97 | 0.000 |
| Linear | 1013.16 | 3 | 337.719 | 258.66 | 0.000 |
| Square | 43.22 | 3 | 14.405 | 11.03 | 0.012 |
| Interaction | 36.14 | 3 | 12.048 | 9.23 | 0.018 |
| Residual error | 6.53 | 5 | 1.306 | ||
| Lack-of-fit | 6.03 | 3 | 2.009 | 8.02 | 0.113 |
| Pure error | 0.50 | 2 | 0.251 | ||
| Total | 1099.04 | 14 | |||
| R2 | 0.9941 | ||||
| Adj R2 | 0.9834 | ||||
| Pred R2 | 0.9112 | ||||
Optimization conditions and influence of process variables on the yield
Table 1, demonstrates that the yield of pectin was varied between 7.03 to 27.95%. The optimum conditions were calculated by solving Eq. (5), and the results showed that the highest yield (29.87%) were obtained under the extraction time of 90 min, temperature of 95 °C and pH of 1.5. The validation experiments were carried out in triplicate and indicated that the yield of pectin in optimum conditions was 28.31 ± 0.11%. As can be seen, the obtained yield was close to the predicted yield and showed that there is a high compatibility between measured and predicted values.
According to the experiments that were conducted at diverse pH levels (1.5–3) and the results were depicted in (Fig. 1B and C). The results clearly represent that the yield of pectin increased with decreasing pH value. The acidic solvent with low pH has the ability to hydrolysis of the insoluble pectin and converts to its soluble form, and thereby increasing the extraction yield of pectin from citron peel [10]. On the other hand, the low pH could diminish the molecular weight of pectin and so, increase its release from plant tissue without any degradation [12]. It should also be noted that at high pH levels, the yield of pectin considerably reduced thereby pectin accumulates that prevents its release [27]. This observation was also similar to the results published from apple pomace, sugar beet pulp, mango peel, and pomegranate peel by Canteri-Schemin et al. [4], Yapo et al. [43], Prakash Maran et al. [35] and Moorthy et al. [27] respectively.
Temperature is considered as one of the more crucial parameters affecting the amount of extraction yield of pectin. The results indicated that the yield of pectin was increased with increasing temperature (Fig. 1A and C). Increase in temperature can hasten the solubility and diffusivity of solvent into the plant tissue and increases the extraction yield of pectin [41]. This result agreed with pagan et al. [30] and Raji et al. [36], who extracted pectin from peach pomace and melon peel, respectively. Also, in the case of the effect of extraction time, it must be said that the yield of pectin was increased with increasing the time (Fig. 1A and B) because longer time provide more reaction time opportunity. The similar results were reported by Chen et al. [6] and Zheng et al. [44].
Influence of process variables on the DE
Table 1, indicated that DE under diverse extraction conditions was ranged from 44.10–69.48%. Also, the DE of pectin produced under optimal extraction conditions (Extraction time of 90 min, temperature of 95 °C and pH of 1.5) was 51.33%, which represented the citron peel pectin could be categorized as HMP. It should be said that this type of pectin (DE > 50%) is desirable for preparing high sugar products [4]. As Fig. 1(D), (E) and (F) depicted, under increased pH, decreased time and temperature of extraction, the DE was increased. The reason for this phenomenon is due to de-esterification of polygalacturonic chains, when use from harsh conditions such as very high temperature, long time and low pH [18, 28]. The similar results were observed by Raji et al. [36] for melon peel pectin and Tang et al. [39] for dragon fruit peel pectin.
Emulsifying features
The emulsifying features (emulsifying activity and emulsion stability) of pectin achieved under optimal extraction condition (Extraction time of 90 min, temperature of 95 °C and pH of 1.5) were assessed and the stability of emulsions was investigated during maintenance for 1 and 30 days at temperatures of 4 and 23 °C (Table 3). After centrifuging the emulsions, oil, emulsified layer and aqueous phase observed from top to bottom, respectively [24]. The emulsifying activity according to the Eq. 3 was 46.2%. This observation was similar to results obtained from sugar beet pulp (43–47%) and sour orange peel pectin (45%) by Yapo et al. [43] and Hosseini et al. [18], respectively. Also, the results illustrated that the emulsions were 93.9 and 93.5% stable at 4 °C, 93.7 and 93.1 at 23 °C after 1 and 30 days, respectively. These data express that the emulsions are more stable at low temperature (4 °C) that is similar to results published by Yapo et al. [43] and Cui et al. [8] for sugar beet pulp and pumpkin pectin, respectively.
Table 3.
Emulsifying features of oil/0.5% (w/v) pectin solutions
| Storage time | Emulsion activity (%) | Emulsion stability (%) | |||
|---|---|---|---|---|---|
| 1 day | 30 day | ||||
| Temperature (°C) | 23 | 4 | 23 | 4 | 23 |
| Pectina | 46.2 | 93.9 | 93.7 | 93.5 | 93.1 |
aCitron peel pectin extracted under optimal extraction conditions (temperature of 90 °C, extraction time of 180 min and LSR of 40 v/w)
Measuring pectin viscosity
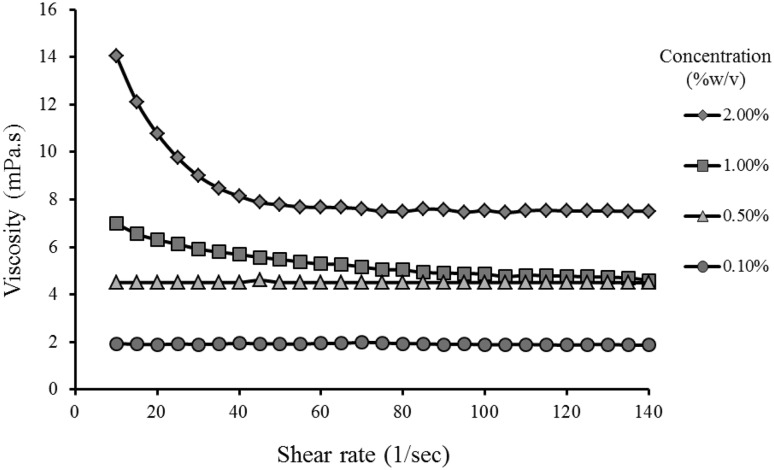
In this study, four solutions with various concentrations (0.1, 0.5, 1.0, and 2.0, % w/v) of citron peel pectin obtained under optimal extraction conditions (time: 90 min, temperature: 95 °C and pH: 1.5) were prepared to investigate the viscosity and flow behavior of pectin at room temperature (~ 25 °C). According to Fig. 2, the viscosity of pectin solutions was increased with increasing concentration. As can be seen, at low concentrations (< 1.0% w/v) of pectin solutions, all solutions exhibited Newtonian flow behavior. However, the pectin solution with high concentration (2.0% w/v) had a shear-thinning (pseudoplastic) behavior. These findings are in agreement with the results reported on Abelmoschus esclentus and sour orange peel pectin by Chen et al. [7] and Hosseini et al. [18], respectively. Pseudoplastic flow behavior in high concentrations is explained to arise from disentanglement of the polymer network and the partial chain orientation in the direction of the shear flow with a relative increase in shear rate [22].
Fig. 2.
The apparent viscosity of different concentrations of pectin solutions versus shear rate
FTIR spectroscopy
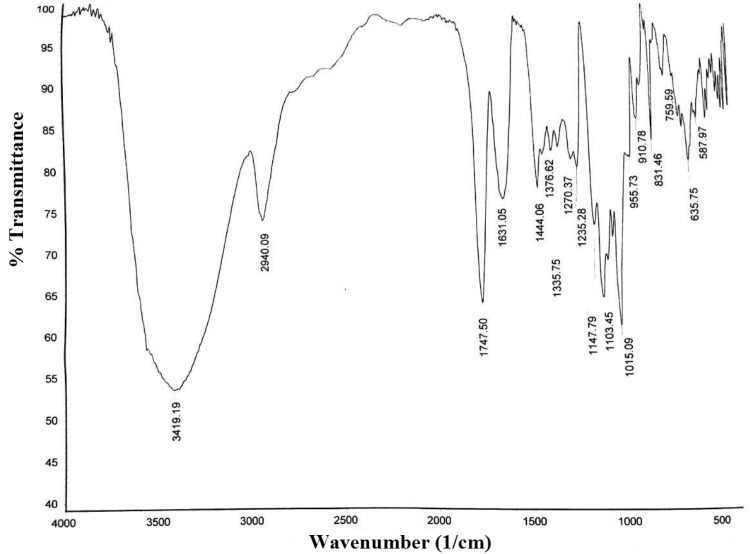
The FTIR spectrum of the pectin obtained under optimum extraction conditions (extraction time of 90 min, temperature of 95 °C and pH of 1.5) is depicted in Fig. 3. The peak between 3200 and 3600 cm−1 is referred to OH groups. The peak at around 2940 cm−1 is related to C–H of CH, CH2 and CH3 groups [7]. Besides, the peak about 1747 cm−1 is attributed to the vibrating of CO group of OCH3. Carboxylate groups have two peaks: one peak is due to asymmetrical vibrating at 1631.05 cm−1, and another peak is referred to weaker symmetric vibrating at 1444.06 cm−1. The two intense absorption at 1015.09 and 1103.45 cm−1 are related to glycosidic linkage between sugar units [14]. Commonly, the total peak area between 800 and 1200 cm−1 is represented “Finger print” region which is exclusive and its interpretation also is difficult [21]. Based on the above statements, it can be express that the achieved precipitate is rich in polygalacturonic acid [38].
Fig. 3.
Fourier transform infrared spectrum of extracted pectin under optimal extraction conditions
Acknowledgements
This study was a part of a Master’s degree research work, and the authors would like to thank all the members of Food Science and Technology Department, University of Tehran.
References
- 1.Balasubramani P, Palaniswamy PT, Visvanathan R, Thirupathi V, Subbarayan A, Prakash Maran J. Microencapsulation of garlic oleoresin using maltodextrin as wall material by spray drying technology. Int. J. Biol. Macromol. 2015;72:210–217. doi: 10.1016/j.ijbiomac.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Bitaraf MS, Khodaiyan F, Mohammadifar MA, Mousavi SM. Application of response surface methodology to improve fermentation time and rheological properties of probiotic yogurt containing Lactobacillus reuteri. Food Bioprocess Technol. 2012;5:1394–1401. doi: 10.1007/s11947-010-0433-2. [DOI] [Google Scholar]
- 4.Canteri-Schemin MH, Fertonani HCR, Waszczynskyj N, Wosiacki G. Extraction of pectin from apple pomace. Brazilian Arch. Biol. Technol. 2005;48:259–266. doi: 10.1590/S1516-89132005000200013. [DOI] [Google Scholar]
- 5.Chaharbaghi E, Khodaiyan F, Hosseini SS. Optimization of pectin extraction from pistachio green hull as a new source. Carbohydr. Polym. 2017;173:107–113. doi: 10.1016/j.carbpol.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Xie MY, Gong XF. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007;81:162–170. doi: 10.1016/j.jfoodeng.2006.10.018. [DOI] [Google Scholar]
- 7.Chen Y, Zhang JG, Sun HJ, Wei ZJ. Pectin from Abelmoschus esculentus: Optimization of extraction and rheological properties. Int. J. Biol. Macromol. 2014;70:498–505. doi: 10.1016/j.ijbiomac.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Cui SW, Chang YH. Emulsifying and structural properties of pectin enzymatically extracted from pumpkin. LWT - Food Sci. Technol. 2014;58:396–403. doi: 10.1016/j.lwt.2014.04.012. [DOI] [Google Scholar]
- 9.Einhorn-Stoll U, Kastner H, Drusch S. Thermally induced degradation of citrus pectins during storage - Alterations in molecular structure, colour and thermal analysis. Food Hydrocoll. 2014;35:565–575. doi: 10.1016/j.foodhyd.2013.07.020. [DOI] [Google Scholar]
- 10.El-Nawawi SA, Shehata FR. Effect of the extraction temperature on the quality characteristics of pectin extracted from Egyptian Orange Peel. Biol. Wastes. 1988;24:307–311. doi: 10.1016/0269-7483(88)90116-4. [DOI] [Google Scholar]
- 11.Essien EP, Essien JP, Ita BN, Ebong GA. Physicochemical properties and fungitoxicity of the essential oil of Citrus medica L. against groundnut storage fungi. Turk. J. Botany. 2008;32:161–164. [Google Scholar]
- 12.Faravash RS, Ashtiani FZ. The effect of pH, ethanol volume and acid washing time on the yield of pectin extraction from peach pomace. Int. J. Food Sci. Technol. 2007;42:1177–1187. doi: 10.1111/j.1365-2621.2006.01324.x. [DOI] [Google Scholar]
- 13.de Oliveira Freitas. C, Giordani D, Lutckemier R, Gurak PD, Cladera-Olivera F, Ferreira Marczak LD. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT - Food Sci. Technol. 2016;71:110–115. doi: 10.1016/j.lwt.2016.03.027. [DOI] [Google Scholar]
- 14.Gnanasambandam R, Proctor A. Determination of pectin degree of esterication by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000;68:327–332. doi: 10.1016/S0308-8146(99)00191-0. [DOI] [PubMed] [Google Scholar]
- 15.Happi Emaga T, Garna H, Paquot M, Deleu M. Purification of pectin from apple pomace juice by using sodium caseinate and characterisation of their binding by isothermal titration calorimetry. Food Hydrocoll. 2012;29:211–218. doi: 10.1016/j.foodhyd.2012.02.019. [DOI] [Google Scholar]
- 16.Homayoonfal M, Khodaiyan F, Mousavi M. Modelling and optimising of physicochemical features of walnut-oil beverage emulsions by implementation of response surface methodology: Effect of preparation conditions on emulsion stability. Food Chem. 2015;174:649–659. doi: 10.1016/j.foodchem.2014.10.117. [DOI] [PubMed] [Google Scholar]
- 17.Hosseini SS, Khodaiyan F, Yarmand MS. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016;140:59–65. doi: 10.1016/j.carbpol.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Hosseini SS, Khodaiyan F, Yarmand MS. Aqueous extraction of pectin from sour orange peel and its preliminary physicochemical properties. Int. J. Biol. Macromol. 2016;82:920–926. doi: 10.1016/j.ijbiomac.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Jafari F, Khodaiyan F, Kiani H, Hosseini SS. Pectin from carrot pomace: Optimization of extraction and physicochemical properties. Carbohydr. Polym. 2017;157:1315–1322. doi: 10.1016/j.carbpol.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Jamsazzadeh Kermani Z, Shpigelman A, Kyomugasho C, Van Buggenhout S, Ramezani M, Van Loey AM, Hendrickx ME. The impact of extraction with a chelating agent under acidic conditions on the cell wall polymers of mango peel. Food Chem. 2014;161:199–207. doi: 10.1016/j.foodchem.2014.03.131. [DOI] [PubMed] [Google Scholar]
- 21.Kozarski M, Klaus A, Niksic M, Jakovljevic D, Helsper JPFG, Van Griensven LJLD. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011;129:1667–1675. doi: 10.1016/j.foodchem.2011.06.029. [DOI] [Google Scholar]
- 22.Kumbar V, Nedomova S, Pytel R, Kilian L, Buchar J. Study of rheology and friction factor of natural food hydrocolloid gels. Potravin. Slovak J. Food Sci. 2017;11:203–209. [Google Scholar]
- 23.Lota ML, de Rocca Serra D, Tomi F, Bessiere JM, Casanova J. Chemical composition of peel and leaf essential oils of Citrus medica L. and C. limonimedica Lush. Flavour. Fragr. J. 1999;14:161–166. doi: 10.1002/(SICI)1099-1026(199905/06)14:3<161::AID-FFJ801>3.0.CO;2-8. [DOI] [Google Scholar]
- 24.Ma S, Yu SJ, Zheng XL, Wang XX, Bao QD, Guo XM. Extraction, characterization and spontaneous emulsifying properties of pectin from sugar beet pulp. Carbohydr. Polym. 2013;98:750–753. doi: 10.1016/j.carbpol.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 25.Maran JP, Swathi K, Jeevitha P, Jayalakshmi J, Ashvini G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015;123:67–71. doi: 10.1016/j.carbpol.2014.11.072. [DOI] [PubMed] [Google Scholar]
- 26.Menichini F, Tundis R, Bonesi M, de Cindio B, Loizzo MR, Conforti F, Statti GA, Menabeni R, Bettini R, Menichini F. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation, cold-pressing and supercritical carbon dioxide extraction. Nat. Prod. Res. 2011;25:789–799. doi: 10.1080/14786410902900085. [DOI] [PubMed] [Google Scholar]
- 27.Moorthy IG, Maran JP, Surya SM, Naganyashree S, Shivamathi CS. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int. J. Biol. Macromol. 2015;72:1323–1328. doi: 10.1016/j.ijbiomac.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Mort AJ, Qiu F, Maness NO. Determination of the pattern of methyl esterification in pectin. Distribution of contiguous nonesterified residues. Carbohydr. Res. 1993;247:21–35. doi: 10.1016/0008-6215(93)84238-2. [DOI] [PubMed] [Google Scholar]
- 29.Nicolosi E, La Malfa S, El-Otmani M, Negbi M, Goldschmidt EE. The search for the authentic citron (Citrus medica L.): Historic and genetic analysis. HortScience. 2005;40:1963–1968. [Google Scholar]
- 30.Pagan J, Ibarz A, Llorca M, Pagan A, Barbosa-Canovas GV. Extraction and characterization of pectin from stored peach pomace. Food Res. Int. 2001;34:605–612. doi: 10.1016/S0963-9969(01)00078-3. [DOI] [Google Scholar]
- 31.Pasandide B, Khodaiyan F, Mousavi ZE, Hosseini SS. Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydr. Polym. 2017;178:27–33. doi: 10.1016/j.carbpol.2017.08.098. [DOI] [PubMed] [Google Scholar]
- 32.Pereira PHF, Oliveira TIS, Rosa MF, Cavalcante FL, Moates GK, Wellner N, Waldron KW, Azeredo HMC. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016;88:373–379. doi: 10.1016/j.ijbiomac.2016.03.074. [DOI] [PubMed] [Google Scholar]
- 33.Prakash Maran J, Manikandan S, Thirugnanasambandham K, Vigna Nivetha C, Dinesh R. Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013;92:604–611. doi: 10.1016/j.carbpol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Prakash Maran J, Mekala V, Manikandan S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohydr. Polym. 2013;92:2018–2026. doi: 10.1016/j.carbpol.2012.11.086. [DOI] [PubMed] [Google Scholar]
- 35.Prakash Maran J, Sivakumar V, Thirugnanasambandham K, Sridhar R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013;97:703–709. doi: 10.1016/j.carbpol.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 36.Raji Z, Khodaiyan F, Rezaei K, Kiani H, Hosseini SS. Extraction optimization and physicochemical properties of pectin from melon peel. Int. J. Biol. Macromol. 2017;98:709–716. doi: 10.1016/j.ijbiomac.2017.01.146. [DOI] [PubMed] [Google Scholar]
- 37.Samavati V, Manoochehrizade A. Polysaccharide extraction from Malva sylvestris and its anti-oxidant activity. Int. J. Biol. Macromol. 2013;60:427–436. doi: 10.1016/j.ijbiomac.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 38.Santos JDG, Espeleta AF, Branco A, De Assis SA. Aqueous extraction of pectin from sisal waste. Carbohydr. Polym. 2013;92:1997–2001. doi: 10.1016/j.carbpol.2012.11.089. [DOI] [PubMed] [Google Scholar]
- 39.Tang PY, Wong CJ, Woo KK. Optimization of pectin extraction from peel of dragon fruit (Hylocereus polyrhizus) Asian J. Biol. Sci. 2011;4:189–195. doi: 10.3923/ajbs.2011.189.195. [DOI] [Google Scholar]
- 40.Verzera A, Trozzi A, Zappala M, Condurso C, Cotroneo A. Essential oil composition of Citrus meyerii Y. Tan. and Citrus medica L. cv. Diamante and Their Lemon Hybrids. J. Agric. Food Chem. 2005;53:4890–4894. doi: 10.1021/jf047879c. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Zhai W. Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC-MS. Innov. Food Sci. Emerg. Technol. 2010;11:470–476. doi: 10.1016/j.ifset.2010.03.003. [DOI] [Google Scholar]
- 42.Yapo BM. Lemon juice improves the extractability and quality characteristics of pectin from yellow passion fruit by-product as compared with commercial citric acid extractant. Bioresour. Technol. 2009;100:3147–3151. doi: 10.1016/j.biortech.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 43.Yapo BM, Robert C, Etienne I, Wathelet B, Paquot M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007;100:1356–1364. doi: 10.1016/j.foodchem.2005.12.012. [DOI] [Google Scholar]
- 44.Zheng X, Yin F, Liu C, Xu X. Effect of Process Parameters of Microwave Assisted Extraction (MAE) on Polysaccharides Yield from Pumpkin. J. Northeast Agric. Univ. (English Ed) 2011;18:79–86. doi: 10.1016/S1006-8104(12)60014-2. [DOI] [Google Scholar]