Abstract
A Box–Behnken design (Extraction-time, pulse-cycle, sonication-amplitude) was employed to extract phenolic compounds from Justicia spicigera leaves by ultrasonic-assisted extraction. The muicle leaves extracts were analyzed measuring total phenolic compounds and antioxidant capacity. According to response surface methodology the optimal conditions of ultrasonic-assisted extraction to obtain the highest soluble phenolic content were 2 min (extraction time) for 0.7 s (pulse cycle) at 55% of sonication amplitude. Under these optimal conditions, the total phenolic content was higher when was used ultrasonic-assisted extraction (54.02 mg/g) than stirring (46.46 mg/g) and thermal decoction (47.76 mg/g); however, the antioxidant capacity from J. spicigera extracts did not increase by ultrasonic-assisted extraction. The extracts or aqueous infusions from J. spicigera leaves are used for therapeutic proposes, therefore the ultrasonic-assisted extraction is a useful technology to improve the extraction of phytochemicals from J. spicigera leaves.
Keywords: Justicia spicigera, Polyphenols, Ultrasonic-assisted extraction, Response surface methodology, Optimization
Introduction
The Justicia spicigera plant belong to the Acanthaceae family, native to Mexico and South America, and mostly grow in wild conditions [1]. Fresh J. spicigera (J. spicigera) leaves have a bright green color, but aqueous extracts from J. spicigera leaves produce a deep purple-reddish hue due to the release of colored compounds [2]. Infusions from J. spicigera leaves had been used in Mexico for people suffering from dengue considering [3]. Justicia spicigera leaves are considered to be useful in treatment of diabetes, as antihypertensive as antipyretics, and antitumoral [4–6]. These effects might be related to the high content of polyphenols and antioxidant capacity of J. spicigera leaf extracts [7, 8]. Therefore, this plant could serve for possible industrial and pharmaceutical applications as a good source for extracting natural antioxidant compounds [9].
Traditionally, the extraction of antioxidant compounds has been achieved by thermal processes (heating, boiling and reflux), maceration or combination of these procedures; using water or solvents as ethanol, methanol, hexane or chloroform [6, 8]. However, these processes may affect the stability and yield of polyphenols and the antioxidant capacity, mainly due to the effect of temperature and long extractions times [10]. Alternative technologies, such as ultrasound, have been applied for polyphenols extraction in starfruit (Averroha carambola L.) leaves [11] and Ocimum tenuiflorum leaves [12] with positive effects during ultrasonic-assisted extraction.
Ultrasound is a special type of sound wave that causes physical and chemical phenomena; the enhancement of extraction obtained by the use of ultrasound is mainly attributed to the effect of acoustic cavitation (mechanical and chemical effect) produced in the solvent by the passage of ultrasound waves. Ultrasonic-assisted extraction is reported to offer an inexpensive, environmentally friendly, less time consuming and efficient alternative to conventional extraction technique as mentioned by Baqueiro-Peña and Guerrero-Beltrán [13] who applied ultrasound at 42 kHz for 30 min at 20 °C for the extraction of phenolic compounds from J. spicigera leaves, and samples were extracted using distilled water and water–ethanol at different concentrations. Polyphenols content and antioxidant capacity of J. spicigera leaf extracts were affected by the extraction conditions, and therefore, it is important to investigate the optimum operating conditions when ultrasound (amplitude, pulse cycle and extraction time) is applied in order to minimize the losses of the extracted compounds. Box–Behnken design, one of response surface methodology tool, has been widely used in pharmaceuticals, processing, food engineering, agrochemicals and other industries, to extract biological active compounds intended for human use [14]. The main advantages of this design is that it provides a large amount of information, and is an economical approach due to a small number of experiments are performed for monitoring the interaction of the independent variables on the response [15].
The aim of this study was to employ response surface methodology to optimize the extraction parameters of polyphenols from J. spicigera leaves. Optimization of extraction time, pulse cycle and sonication amplitude for extraction of soluble polyphenols from J. spicigera leaves were carried out using ultrasonic-assisted extraction through a Box–Behnken design. Additionally, we compared the changes in phenolic compounds and antioxidant capacity from J. spicigera leaves using the extraction optimal conditions of ultrasound-assisted extraction and two conventional methods (stirring and thermal decoction).
Materials and methods
This work was carried out in two stages. The initial stage was conducted to obtain the combination of experimental conditions for the optimization of ultrasonic-assisted extraction of soluble phenolic compounds from J. spicigera leaves using response surface methodology. The final stage consisted in a comparison of different extraction methods (ultrasonic-assisted extraction, stirring and thermal extraction), measuring soluble, hydrolysable and total phenolic compounds; as well as antioxidant capacity from J. spicigera leaf extracts.
Plant material
Justicia spicigera leaves (4–7 cm of long and 2.5–3.0 cm of wide) of light green color were collected from wild trees in Tepic, Nayarit, México in June 2016. Leaves were washed with distilled water and dried in a conventional oven (Memmert GmbH, Schwabach, Germany) at 60 °C for 48 h. They were grounded in a food processor (NB-101B, Los Angeles, California, USA) and sieved with a 500 µm mesh.
Extraction procedures
Ultrasonic-assisted extraction was adapted according to the methodology proposed by Baqueiro-Peña and Guerrero-Beltrán [13] with some modifications. For the experimental setup, a 400 Watts and 24 kHz frequency ultrasonic processor (Hielscher UP400S, Teltow, Germany) with sonication amplitude scale of 20–100% and pulse cycle scale of 0–1, where set value equals the acoustic power time in seconds, the difference to 1 s as pause time (e.g. 0.6 equals to power discharge 0.6 s, pause 0.4 s) was used. The probe (H7 Tip 7, Hielscher, Teltow, Germany) with maximum amplitude of 175 µm and acoustic power density of 300 W/cm2 was immersed at 2 cm in the water solution and the sonication was started immediately. The J. spicigera leaves dried powder (0.5 g) was sonicated with distilled water (50 mL) and kept in ice bath (Firstek Scientific B401L, New Taipei City, Taiwan). The initial temperature of extract was 20 °C and the extraction average temperature was controlled in a range of 20–24 ± 3 °C with a cool water bath.
Stirring extraction
Powder (0.5 g) was mixed with 50 mL of distilled water into amber glass bottles, covered with aluminum foil and stirred at room temperature (25 ± 2 °C) at a moderate speed for 2 h using a magnetic stirrer [16].
Thermal decoction
Powder (0.5 g) was mixed with 50 mL of distilled water into amber glass bottles, and the solution was slowly boiled at 60 °C for 5 min as recommended by García-Márquez et al. [16]. The extracts obtained were filtered and the supernatants were collected and stored at 4 °C in amber glass bottles wrapped with aluminum foil until analysis. The experiments were performed three times in triplicate.
Experimental design
The optimization of extraction parameters of soluble phenolic compounds from J. spicigera leaves was carried out by response surface methodology. A Box–Behnken design was employed to determine the optimal ultrasonic-assisted extraction conditions from J. spicigera leaves with three levels for each factor. The study was designed to evaluate the individual and interactive effects of pulse cycle (X1, s), sonication amplitude (X2, %) and extraction time (X3, min) on soluble phenolic compounds (SP; mg gallic acid equivalent per gram). The factors and their levels were X1 (0.4, 0.7 and 1 s), X2 (40, 70 and 100%) and X3 (2, 7 and 12 min). The design consisted of fifteen different combinations, including 3 replicates at the central point. A second-order polynomial Eq. (1), which includes all terms, was used to calculate the predicted response:
| 1 |
where Y is the predicted response (SP), Xi are the uncoded or coded values for the factors (X1, X2 and X3), β0 is a constant, βi is the main effect coefficients for each variable, and βij are the interaction effect coefficients. Model adequacy was evaluated using F ratio. Lack of fit test was used to determine significant interactions in the model and coefficient of determination (R-square and R-Adjust) represented at 1% level of significance [17]. Results of the experimental design were fitted with a second order polynomial equation by a multiple regression technique. Statistical analyses were performed using Statistic software (v. 10 Statsoft®, Tulsa, Oklahoma, USA).
Soluble, hydrolysable and total phenolic compounds
For the aqueous-organic extraction, the methodology proposed by Pérez-Jiménez et al. [18] was used. The soluble phenolic content was determined with the Folin–Ciocalteu’s reagent using Montreau [19] procedure with slight modifications. Briefly, 250 µL of extract were mixed with 1000 µL of sodium carbonate solution (75 g/L) and 1250 µL of Folin–Ciocalteu reagent. Absorbance was measured at 750 nm in a microplate reader (Biotek Synergy HT, Winooski, Vermont, USA). The results were expressed in mg of gallic acid equivalents per gram of J. spicigera leaves (mg GAE/g dry basis, db). Hydrolysable polyphenols were determined by hydrolysis with methanol/H2SO4, 90:10 (v/v) at 85 °C for 20 h, on the residues obtained in SP [20]. Samples were centrifuged (15 min, 25 °C, 3000 g) and hydrolysable polyphenols were determined in the supernatants by the Folin–Ciocalteu’s reagent as was described above. Data were reported in mg GAE/g db. Total phenolic compounds were determined with the sum of soluble and hydrolysable phenols.
Antioxidant capacity
The antioxidant capacity analysis was measured as described below from the aqueous-organic extracts.
2,2´-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS·+) radical cation scavenging activity, were analyzed using a modified methodology reported by Re et al. [21]. ABTS (7 mM) was dissolved in 2.45 mM potassium persulfate, stored in the dark at room temperature overnight (12–16 h). solution was first diluted with phosphate buffer to an absorbance of 0.7 (± 0.02) at 734 nm. The sample or standard (30 µL) of Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) was mixed with 255 µL of (30 °C, 7 min). The decrease in absorbance was measured at 734 nm.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was done according to Prior et al. [22] method with some modifications. The sample or Trolox standard (30 µL) were reacted with 200 µL of DPPH solution (190 µM) and the absorbance was measured at 517 nm after 10 min.
Ferric-ion reducing antioxidant power (FRAP) assay was performed according to the methods of Benzie and Strain [23] with some modifications. FRAP solution 10:1:1 (v/v/v) of sodium acetate buffer (0.3 M, pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) and 20 mM ferric chloride hexahydrated was warmed to 37 °C before mixing with the samples. For the reaction, 24 µL sample extract or Trolox standard were added to 96-well microplate and each was mixed with 180 µL of FRAP solution using multi-channel dispenser. Absorbance was measured at 595 nm after 30 min.
A microplate reader (Bio-Tek Synergy HT, Winooski, Vermont, USA) was used for all antioxidant capacity methods and results were expressed as mmol Trolox equivalent (mmol TE/g db).
Yield
Extraction yield was defined as the percentage of the extracted soluble polyphenols from the total weight of the sample (g). The extraction yield was calculated using the Eq. (2) as suggested by Aydar et al. [17].
| 2 |
Statistical analysis
Data were expressed as mean ± SD (n = 9). One-way ANOVA and Tukey test were used to examine the difference between samples (p < 0.01). All statistical analyses were done with Statistic software (v. 10 Statsoft®. Tulsa, USA).
Results and discussions
Ultrasonic-assisted extraction of soluble polyphenols from J. spicigera leaves
The experimental treatments and the observed and predicted data for soluble phenolic compounds from J. spicigera leaf extracts are shown in Table 1. Statistical differences (p < 0.01) were observed between treatments. Results showed that the yield ranged from 4.65 to 7.42% and the maximum extraction of soluble phenolic content (36.85 mg GAE/g) from ultrasonic-assisted extraction was obtained at lower extraction time and sonication amplitude. These values were relatively lower than the values reported by Baqueiro-Peña and Guerrero-Beltrán [13], who reported a total phenolic content of 52.6 mg GAE/g from J. spicigera leaf extracts after applying ultrasound-assisted extraction (42 kHz, for 30 min at 20 °C) using water as a solvent. However, the extraction time in the treatment of 2 min at 20 °C was reduced in 15 times compared to those of Baqueiro-Peña and Guerrero-Beltrán [13]. The same authors reported that a combined solution of water–ethanol at different concentrations (70:30, 50:50, 30:70) decreased the extraction of polyphenols (47.6, 36.8 and 47.7 mg GAE/g, respectively). On the other hand, the soluble phenolic content by ultrasonic-assisted extraction was higher than the obtained using a traditional extraction method after 12 h (maceration and solvents at 60 °C) from J. spicigera leaves [16]. These comparisons show the importance of the selection and management of the different variables in the ultrasonic-assisted extraction optimization. Some studies have demonstrated that ultrasonic-assisted extraction could be used as a useful method for the extraction of bioactive compounds [24]. Additionally, several authors have discussed regarding the disruptive effect (mechanical effect) of ultrasound on cell walls, increasing the content of several bioactive compounds [12]. The increase of phenolic content is attributable to the cavitation phenomena increasing the contact surface area between solid and liquid phases to improve extraction performance [17].
Table 1.
Experimental matrix used for response surface methodology with experimental and predicted values for the independent variables, error rate, yield and final temperature after ultrasonic-assisted extraction of J. spicigera leaf extracts
| Run | Predictors1 | Response variable | Error rate (%) | Yield (%) | Final temperature (°C) | |||
|---|---|---|---|---|---|---|---|---|
| XPC (s) | XSA (%) | XET (min) | Experimental SP2 | Predicted SP3 | ||||
| 1 | 0.4 | 40 | 7 | 23.89 ± 2.16d | 23.91 | − 0.08 | 4.65 | 20 ± 0.5 |
| 2 | 1 | 40 | 7 | 25.43 ± 0.42cd | 25.37 | 0.23 | 5.08 | 24 ± 0.5 |
| 3 | 0.4 | 100 | 7 | 24.47 ± 0.79d | 23.85 | 2.53 | 4.78 | 24 ± 0.5 |
| 4 | 1 | 100 | 7 | 25.87 ± 0.20d | 23.20 | 10.32 | 4.74 | 26 ± 0.5 |
| 5 | 0.4 | 70 | 2 | 24.55 ± 1.05cd | 24.27 | − 0.08 | 4.91 | 19 ± 0.5 |
| 6 | 1 | 70 | 2 | 32.60 ± 0.90b | 32.88 | − 0.08 | 6.52 | 20 ± 0.5 |
| 7 | 0.4 | 70 | 12 | 25.21 ± 0.96cd | 24.93 | 1.11 | 5.04 | 23 ± 0.5 |
| 8 | 1 | 70 | 12 | 23.98 ± 0.21d | 24.26 | − 1.16 | 4.79 | 27 ± 0.5 |
| 9 | 0.7 | 40 | 2 | 37.12 ± 0.70a | 36.85 | 0.72 | 7.42 | 20 ± 0.5 |
| 10 | 0.7 | 100 | 2 | 27.19 ± 0.56c | 27.47 | − 1.02 | 5.43 | 24 ± 0.5 |
| 11 | 0.7 | 40 | 12 | 24.51 ± 1.48cd | 24.23 | 1.14 | 4.90 | 21 ± 0.5 |
| 12 | 0.7 | 100 | 12 | 24.42 ± 0.12cd | 24.70 | − 1.14 | 4.88 | 26 ± 0.5 |
| 13 | 0.7 | 70 | 7 | 33.44 ± 0.74b | 33.85 | − 1.12 | 6.68 | 20 ± 0.5 |
| 14 | 0.7 | 70 | 7 | 33.87 ± 0.76b | 33.85 | 0.05 | 6.77 | 20 ± 0.5 |
| 15 | 0.7 | 70 | 7 | 34.24 ± 0.18b | 33.85 | 1.13 | 6.85 | 20 ± 0.5 |
All values are mean ± SD of three determinations by triplicate (n = 9). Different letters in each file indicate significant statistical differences between treatments (α = 0.01)
1Pulses cycle (XPC); Sonication Amplitude (XSA) and Extraction Time (XET)
2Gallic acid equivalents (mg GAE/g dry basis)
3The values were predicted using a secondary polynomial equation, R2 = 0.97. Gallic acid equivalents (mg GAE/g dry basis)
To show the significant (p < 0.01) interaction effects of ultrasonic extraction parameters on the soluble polyphenol content [Fig. 1(A-C)] 3D response surface plots were created. Additionally, the Pareto chart [Fig. 1(D)] shows the effect of independent variables on extraction of the soluble phenolic compounds at a confidence level of 99%; where all linear and quadratic parameters had a statistically significant effect (p < 0.01) on the extraction of soluble phenolic compounds. Khan et al. [25] reported that the ultrasound power is the most important factor involved in the polyphenolic extraction by ultrasonic-assisted extraction, followed by the temperature. They also reported an interaction between the ultrasound power and temperature. In this study, the extraction of soluble phenolic compounds from J. spicigera leaves was observed in the whole experimental domain, independent of the ultrasound-assisted extraction conditions. However, the lower soluble phenolic content was obtained when the highest processing times of > 7 min, sonication amplitude of 100% and pulse cycles of 1 s were applied; similar trends were reported previously during the extraction of soluble phenolic compounds by ultrasonic-assisted extraction [13]. According to Guitescu et al. [26] when ultrasonic-assisted extraction is applied with longer processing times (> 30 min) polyphenol degradation can also occur. Nonetheless, a decrease in the polyphenol (~ 10%) content from J. spicigera leaf extracts with respect to extraction temperature (25–60 °C) was observed by García-Márquez et al. [16]. For this reason, the extracting conditions, ultrasound power and extraction time are important factors to be considered to obtain the maximum yield of polyphenols during the extraction process when ultrasound-assisted extraction is applied [17, 27].
Fig. 1.
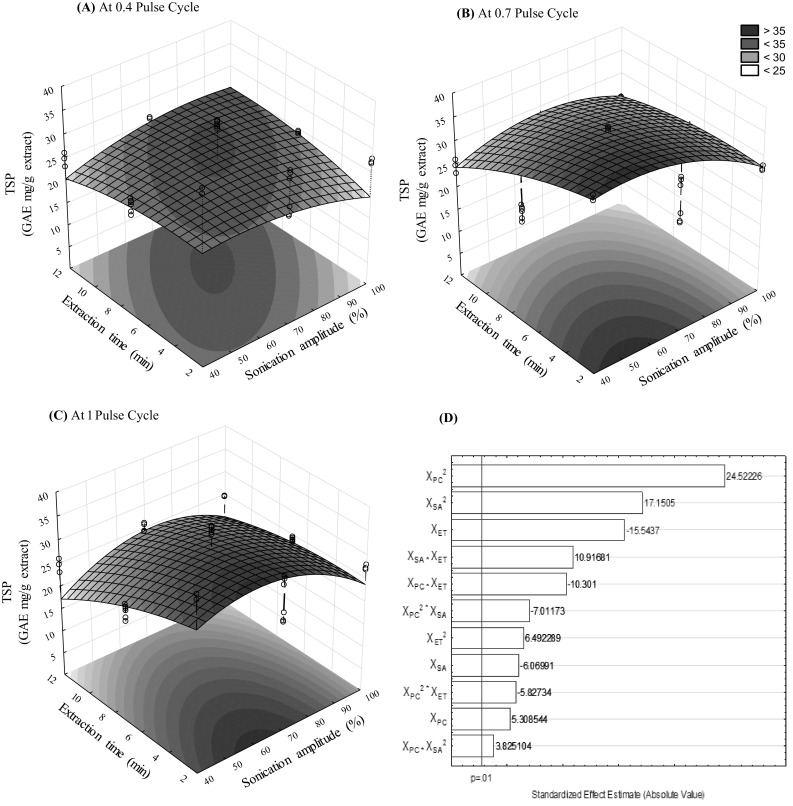
Response surface plots indicating the effect of ultrasonic-assisted extraction on the soluble polyphenols (SP) content using at 0.4 (A), 0.7 (B) and 1.0 (C) of pulse cycle (PC) and Pareto chart (D). ET extraction time, SA sonication amplitude, V lineal and Q quadratic interaction
The analyses of variance of soluble phenolic compounds showed that the experimental data have a correlation coefficient (R2) of 0.978, and adequate adjustment of the experimental data to the models was observed (lack of fit, p > 0.01). The lack of fit showed the fitness of the model. All parameters of the model were significant (p < 0.01) as shown in Table 2. The estimated effects of each variable as well as their interactions on the extracted soluble phenols are listed in Table 2. Also, according to the regression model, the soluble phenolic content from J. spicigera leaf extracts using ultrasonic-assisted extraction can be predicted with the following polynomial equation [3] (R2 = 0.97, R2 Adj. = 0.96 with 99% confidence level):
| 3 |
where X1 = Pulse cycle (s); X2 = Sonication amplitude; X3 = Extraction time.
Table 2.
Analysis of variance and regression coefficients of predicted quadratic polynomial models with the ultrasonic-assisted extraction conditions on the soluble phenolic content from J. spicigera leaf extracts
| Source1 | Analysis of variance | Regression coefficients | |||
|---|---|---|---|---|---|
| SS2 | DF3 | MS4 | F Value | Soluble phenolic content β–coefficient | |
| Mean/intercept | – | – | – | – | − 31.489* |
| X1 | 17.15 | 1 | 17.15 | 38.18* | 179.609* |
| 365.93 | 1 | 365.93 | 601.34* | − 136.002* | |
| X2 | 22.42 | 1 | 22.42 | 36.84* | 0.134** |
| 178.99 | 1 | 178.99 | 294.12* | 0.001** | |
| X3 | 147.02 | 1 | 147.02 | 241.60* | 2.039* |
| 25.64 | 1 | 25.64 | 42.14* | − 0.061* | |
| X1 * | 8.90 | 1 | 8.90 | 14.63* | − 0.007* |
| * X2 | 29.918 | 1 | 29.91 | 49.16* | 0.618* |
| X1 * X3 | 64.57 | 1 | 64.57 | 106.11* | − 7.320* |
| * X3 | 20.66 | 1 | 20.66 | 33.95* | 4.124* |
| X2 * X3 | 72.52 | 1 | 72.52 | 119.17* | 0.016* |
| Lack of Fit | 4.15 | 1 | 4.15 | 6.83** | |
| Pure Error | 19.47 | 32 | 0.608 | ||
| R-square | 0.978 | ||||
| R-Adjust | 0.969 | ||||
| Total SS | 1015.92 | ||||
1Pulses cycle (XPC); Sonication Amplitude (XSA) and Extraction Time (XET)
2SS, sum of square
3DF, degree of freedom
4MS, means square
*Significant (p < 0.01); **nonsignificant (p > 0.01)
The coefficients X2 and were not significant to the model (Table 2). Similar values of soluble phenolic content were obtained between experimental and predicted data when ultrasonic-assisted extraction was used (R2 = 0.97). Comparable results were reported by Bashi et al. [27] during optimization of ultrasonic-assisted extraction (extraction temperature, pH, liquid/solid ratio and extraction time) of phenolic compounds (8.06–11.79 mg GAE/g db) from Achillea beibrestinii using RSM (R2 = 0.85). Pan et al. [28] obtained a second order model (R2 = 0.99) when applied ultrasonic-assisted extraction for the polyphenol extraction from pomegranate peel and reported that the intensity level (59.2 W/cm2) and pulse duration (5 s) had prominent effect on the polyphenolic yield; while a long extraction time (> 10 min) had a negative effect. The same authors reduced the extraction time by 87% compared to thermal extraction. Additionally, an increase in the temperature up to 7 °C (Table 1) in some ultrasonic-assisted extraction-runs was observed (2, 3, 4, 7, 8, 10 and 12). This temperature increase was attributable to the ultrasonic intensity and the processing time used during the treatment. The heat transference from cavitation bubbles may cause a gradual temperature increases in the medium and the cavitation effect on extraction by this increase can be minimized [29]. Also, the temperature may cause the degradation of heat-sensitive compounds such as polyphenols as it was suggested by Guo et al. [30].
Optimization of the extraction process and the validation model
In order to optimize the ultrasonic-assisted extraction variables which resulted in the most desirable response numerical optimizations were applied as recommended by Aydar et al. [17]. The optimal ultrasonic-assisted extraction conditions for the extraction of soluble phenolic compounds from J. spicigera leaves are shown in Table 3. The extraction time of 2 min, pulse cycle of 0.7 s and sonication amplitude of 55% produced the predicted optimal response of 37.51 mg GAE/g, with a significant reduction (p < 0.01) of the extraction time compared with the conditions (42 kHz, for 30 min at 20 °C) reported by Baqueiro-Peña and Guerrero-Beltrán [13].
Table 3.
Optimal conditions of ultrasonic-assisted extraction on the soluble phenolic content from J. spicigera leaf extracts obtained by the predicted model
| Parameter | Soluble phenolic content (mg GAE/g) |
|---|---|
| Extraction time (min) | 2 |
| Pulse cycle (s) | 0.7 |
| Sonication amplitude (%) | 55 |
| Optimal response | 37.51 |
| − 95% confidence limit | 36.58 |
| + 95% confidence limit | 38.45 |
To verify the reliability of the models under the optimal conditions (Table 3), experiments were performed. The experimental value of the soluble phenolic content was 37.50 mg GAE/g (Table 4). This result is in agreement with the predicted value (37.51 mg GAE/g) under the optimal conditions. Table 1 shows the error rates between the experimental and predicted values which in all cases are smaller than 3% (except for run 4, 10%). Similar trends on the error rates were reported previously using response surface methodology as tool to optimize an extraction process [17]. Therefore, the predicted conditions can be used in further analysis to obtain a high extraction of polyphenols from J. spicigera leaves using ultrasonic-assisted extraction. Response surface methodology has been used to optimize the extraction of polyphenols by ultrasonic-assisted extraction from starfruit leaves [11] and orange peel [25].
Table 4.
Soluble polyphenols, hydrolysable polyphenols, total polyphenols and antioxidant capacity from Muicle leaves extracts using the optimal condition of ultrasound-assisted extraction (UAE), stirring and thermal decoction
| Parameter | UAE3 | Stirring4 | Thermal decoction5 |
|---|---|---|---|
| Total soluble polyphenols1 | 37.50 ± 0.39a | 32.25 ± 0.28c | 34.41 ± 0.53b |
| Hydrolysable polyphenols1 | 16.51 ± 0.35a | 14.21 ± 0.18b | 13.35 ± 0.45c |
| Total polyphenols1 | 54.01 ± 0.17a | 46.46 ± 0.46c | 47.76 ± 0.45b |
| ABTS2 | 294.69 ± 1.01b | 292.13 ± 1.43b | 495.16 ± 0.90a |
| DPPH2 | 43.00 ± 0.96a | 43.86 ± 0.37a | 132.32 ± 0.58b |
| FRAP2 | 120.35 ± 1.45c | 135.86 ± 2.60b | 239.05 ± 2.73a |
All values are mean ± SD of three determinations. Different letters in each file indicate significant statistical differences between treatments (α = 0.01)
1Gallic acid equivalent, GAE mg/g dry basis
2Trolox equivalent, Trolox mmoL/g extract dry basis
3UAE: 0.7 s for XPC, 55% for XSA and 2 min for XET
4Stirring: 2 h using a magnetic stirrer at room temperature
5Thermal decoction: 60 °C for 5 min
Effect of ultrasound-assisted extraction and conventional extraction methods on phenolic compounds and AOX
The soluble, hydrolysable and total phenolic compounds and antioxidant activity contents by ultrasonic-assisted extraction (0.7 s for XPC, 55% for XSA and 2 min for XET) and conventional extraction methods (stirring and thermal decoction) are shown in Table 4. Significant differences (p < 0.01) were observed between the three extraction methods; these findings are in agreement with those obtained by Baqueiro-Peña and Guerrero-Beltrán [13]. In general, more compounds were detected in fresh J. spicigera extracts obtained with the ultrasound method compared with stirring extraction [13]. Furthermore, similar trends were reported by Michelon et al. [31] when different methods for extraction (ultrasound, chemical and enzymatic) of carotenoids from Phaffia rhodozyma were compared. They reported an increase in polyphenolic content near of 13% when samples were treated by ultrasonic-assisted extraction (40 kHz, 10 min) compared to the traditional treatments. In the J. spicigera leaf extracts a higher concentration of soluble, hydrolysable and total phenolic compounds was observed when ultrasonic-assisted extraction was used (37.50, 16.51 and 54.01 mg GAE/g, respectively) compared to stirring (32.25, 14.21 and 46.46 mg GAE/g, respectively) and thermal decoction (34.41, 13.35 and 47.76 mg GAE/g, respectively). The polyphenolic content from J. spicigera leaf extracts using the stirring extraction and thermal decoction was similar to data reported [8, 16]. Also, as it was mentioned above Baqueiro-Peña and Guerrero-Beltrán [13] reported a total polyphenolic content of 52.6 mg GAE/g from J. spicigera leaf extracts when they used ultrasonic-assisted extraction at 40 kHz for 30 min at 20 °C, coinciding with the total phenolic content (54.01 mg GAE/g) quantified in this experiment but with less extraction time (2 min). The long extraction times might cause an oxidation of polyphenols as was demonstrated by Nafar et al. [32], who found that the importance of independent variables on the effect on bioactive compounds could be ranked in the following order: ultrasonic frequency > temperature > exposure time. On the other hand, our results are in agreement with Upadhyay et al. [12], who compared ultrasonic-assisted (30 kHz, 40 °C, 15 min) extraction and conventional solvent extraction for extraction of phenolic compounds from Ocimum tenuiflorum leaves. They reported that the polyphenolic yield obtained by ultrasonic-assisted extraction was higher (6.83 mg GAE/g) than by conventional (3.84 mg GAE/g) solvent extraction, and indicated that ultrasonic-assisted extraction is preferred on the basis of high extraction yields, short extraction time, and less energy consumption.
Hydrolysable phenolic compounds are considered as non-extractable polyphenols [17] and our results are in agreement with the other reports. Yang et al. [33] reported an increase in naphthoquinone pigments from Purple gromwell when applying ultrasonic-assisted extraction (20 kHz, pulse cycle 0.6 s, 20 min) compared with other extraction methods (Sohxlet extraction for 6 h and supercritical CO2 extraction at 42 °C and 23 MPa for 2 h). Kim et al. [24] reported an increase of quercetin from Houttuynia cordata Thunb after ultrasonic-assisted extraction. Ultrasound is well known for extracting some components caused by its disrupting effect on cell walls [29]. In the present study, ultrasound-assisted extraction treatment showed higher hydrolysable phenolic content compared to the heat treatment. Sousa et al. [34] observed that the polyphenols profile in extracts of Phyllanthus amarus treated with ultrasonic-assisted (19 kHz, 5 min) extraction presented five times more gallic acid (monomers) compared to a conventional treatment (water extraction at 85 °C) that presented mainly ellagitannins (a class of hydrolysable polyphenols). The authors found that the ultrasonic-assisted extraction was not efficient for the extraction of hydrolysable polyphenols. This suggests that the heat treatment could further hydrolyze the high molecular weight polyphenols producing smaller fractions. The difference in polyphenols profiles could be responsible for the greater antioxidant capacity found for the heat treatment [34]. Significant differences (p > 0.01) were not found in antioxidant capacity values using ABTS and DPPH assays, when the extracts were obtained by ultrasonic-assisted extraction (294 and 43 mmol TE/g db, respectively) and stirring (292 and 43.86 mmol TE/g db, respectively). However, the thermal decoction presented the greatest antioxidant capacity (495 and 132 mmol TE/g db, for ABTS and DPPH, respectively). Baqueiro-Peña and Guerrero-Beltrán [13] reported statistical differences in antioxidant capacity between treatments when compared stirring method (2 h) and ultrasonic-assisted extraction (42 kHz for 30 min at 20 °C). They reported that a higher antioxidant capacity in the J. spicigera leaf extracts was found by the ABTS method, when used stirring extraction (14.3 mg TE/g) compared to those obtained by ultrasonic-assisted extraction (7.6 mg TE/g). On the other hand, the same authors reported an increase of AOX from J. spicigera leaf extracts by DPPH in ultrasonic-assisted extraction (0.78 mg TE/g) compared to stirring method (0.66 mg TE/g). The antioxidant capacity in this experiment was higher than those reported in the literature for J. spicigera leaf extracts [8, 13, 16].
With respect to the antioxidant capacity by FRAP assay, significant differences (p < 0.01) were observed between treatments and similar trends have been reported by other authors applying ultrasonic-assisted extraction for the extraction of bioactive compounds from different plant materials [11]. Differences between treatments may be attributable to the quantification of the antioxidant capacity exerted by compounds derived from oxidation reactions during thermal treatment [13] or by the presence of diverse phenolic compounds. The extracted compounds are directly related to the compatibility of the compounds with the solvent system or by the extraction conditions [4, 16]. Recently, it was found that ultrasound induced significant degradation of cyanidin-3-glucosylrutinoside causing a change of its UV–Vis spectra, visual color and antioxidant capacity evaluated by FRAP and DPPH. These changes were correlated with the ·OH generation [35]. Thus, phenolic acids present in a food matrix can interact among them and these interactions can affect the antioxidant capacity by synergistic, neutral, or antagonistic mechanisms [8]. Studies on identification, isolation and evaluation of compounds and their interactions in model systems could be carried out to check their effects on the antioxidant activity using ultrasonic-assisted extraction.
Response surface methodology was successfully employed to optimize the ultrasonic-assisted extraction conditions. Pulse cycle exerted a maximum influence in the extraction process followed by the extraction time and sonication amplitude. Ultrasonic-assisted extraction reduced the extraction time and increased the total polyphenolic content from J. spicigera leaves; however, the antioxidant activity not increased. In our study, there was not significant differences between the experimental values and predicted values confirmed the model effectiveness. These results indicate that J. spicigera leaves can be considered a recommended source of polyphenols with a potential application in the pharmaceutical and food industries.
Acknowledgements
This research was financially supported by Tecnólogico Nacional de Mexico (Grant No. 5611.15-P).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Sengupta SD, Paul R. Exploration of knowledge on new ethno-botanical value of Justicia spicigera Schltdl. Global J Res. Med. Plants Indigen. Med. 5: 261–266 (2016).
- 2.Pavón-García LMA, Pérez-Alonso C, Orozco-Villafuerte J, Pimentel-González DJ, Rodríguez-Huezo ME, Vernon-Carter EJ. Storage stability of the natural colourant from Justicia spicigera microencapsulated in protective colloids blends by spray-drying. Int. J. Food Sci. Technol. 2011;46:1428–1437. doi: 10.1111/j.1365-2621.2011.02634.x. [DOI] [Google Scholar]
- 3.Zapata-Morales JR, Alonso-Castro AJ, Domínguez F, Carranza-Álvarez C, Castellanos LMO, Martínez-Medina RM, Pérez-Urizar J. Antinociceptive Activity of an Ethanol Extract of Justicia spicigera. Drug Dev. Res. 2016;77:180–186. doi: 10.1002/ddr.21307. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz-Andrade R, Cabañas-Wuan A, Arana-Argáez VE, Alonso-Castro AJ, Zapata-Bustos R, Salazar-Olivo LA, Domínguez F, Chávez M, Carranza-Álvarez C, García-Carrancá A. Antidiabetic effects of Justicia spicigera Schltdl (Acanthaceae) J. Ethnopharma. 2012;143:455–462. doi: 10.1016/j.jep.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Esquivel-Gutiérrez ER, Noriega-Cisneros R, Arellano-Plaza M, Ibarra-Barajas M, Salgado-Garciglia R, Saavedra-Molina A. Antihypertensive effect of Justicia spicigera in L-NAME-induced hypertensive rats. Pharmacology. 2013;2:120–127. [Google Scholar]
- 6.Alonso-Castro AJ, Ortiz-Sánchez E, Domínguez F, Arana-Argáez V, Juárez-Vázquez MC, Chávez M, Carranza-Álvarez C, Gaspar-Ramírez O, Espinoza-Reyes G, López-Toledo G, Ortíz-Andrade R, García-Carrancá A. Antitumor and immunomodulatory effects of Justicia spicigera Schltdl (Acanthaceae) J. Ethnopharmacol. 2012;141:888–894. doi: 10.1016/j.jep.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Cassani J, Dorantes-Barrón AM, Novales LM, Real GA, Estrada-Reyes R. Anti-depressant-like effect of Kaempferitrin isolated from Justicia spicigera Schltdl (Acanthaceae) in two behavior models in mice: Evidence for the involvement of the serotonergic system. Molecules. 2014;19:21442–21461. doi: 10.3390/molecules191221442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sepúlveda-Jiménez G, Reyna-Aquino C, Chaires-Martínez L, Bermúdez-Torres K, Rodríguez-Monroy M. Antioxidant activity and content of phenolic compounds and flavonoids from Justicia spicigera. J. Biol. Sci. 2009;9:629–632. doi: 10.3923/jbs.2009.629.632. [DOI] [Google Scholar]
- 9.Baqueiro-Peña I, Guerrero-Beltrán JA. Uses of Justicia spicigera in medicine and as a source of pigments. Funct. Foods Heal. Dis. 2014;4:401–414. [Google Scholar]
- 10.Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 11.Zamora-Gasga VM, Serafín-García MS, Sánchez-Burgos JA, Estrada RMV, Sáyago-Ayerdi SG. Optimization of Ultrasonic-Assisted Extraction of antioxidant compounds from Starfruit (Averroha carambola L) leaves. J. Food Process. Preserv. 2016 [Google Scholar]
- 12.Upadhyay R, Nachiappan G, Mishra HN. Ultrasound-assisted extraction of flavonoids and phenolic compounds from Ocimum tenuiflorum leaves. Food Sci. Biotechnol. 2015;24:1951–1958. doi: 10.1007/s10068-015-0257-y. [DOI] [Google Scholar]
- 13.Baqueiro-Peña I, Guerrero-Beltrán JA. Physicochemical and antioxidant characterization of Justicia spicigera. Food Chem. 2017;218:305–312. doi: 10.1016/j.foodchem.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 14.Zou TB, Xia EQ, He TP, Huang MY, Jia Q, Li HW. Ultrasound-Assisted Extraction of Mangiferin from Mango (Mangifera indica L.) Leaves Using Response Surface Methodology. Molecules. 2014;19:1411–1421. doi: 10.3390/molecules19021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Z, Guan Q, Guo Y, He J, Liu G, Li S, Jaffrin MY. Green ultrasound-assisted extraction of anthocyanin and phenolic compounds from purple sweet potato using response surface methodology. Int. Agrophys. 2016;30(1):113–122. doi: 10.1515/intag-2015-0066. [DOI] [Google Scholar]
- 16.García-Márquez E, Román-Guerrero A, Pérez-Alonso C, Cruz-Sosa F, Jiménez-Alvarado R, Vernon-Carter EJ. Effect of solvent-temperature extraction conditions on the initial antioxidant activity and total phenolic content of muitle extracts and their decay upon storage at different pH. Rev. Mex. Ing. Quim. 2012;11:1–10. [Google Scholar]
- 17.Pérez-Jiménez J, Arranz S, Tabernero M, Díaz-Rubio ME, Serrano J, Goñi I, Saura-Calixto F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008;41:274–285. doi: 10.1016/j.foodres.2007.12.004. [DOI] [Google Scholar]
- 18.Montreau F. Sur le dosage des composés phénoliques totaux dans les vins par la methode Folin-Ciocalteau. Connaiss Vigne Vin. 1972;24:397–404. [Google Scholar]
- 19.Hartzfeld PW, Forkner R, Hunter MD, Hagerman AE. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 2002;50:1785–1790. doi: 10.1021/jf0111155. [DOI] [PubMed] [Google Scholar]
- 20.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 21.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 22.Benzie IF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 23.Aydar AY, Bagdathiglu N, Koseoglu O. Effect on olive oil extraction and optimization of ultrasound-assisted extraction of extra virgin olive oil by response surface methodology (RSM) Grasas y Aceites. 2017;68(2):el89. doi: 10.3989/gya.1057162. [DOI] [Google Scholar]
- 24.Kim HS, Lee AY, Jo JE, Moon BC, Chun JM, Choi G, Kim HK. Optimization of ultrasound-assisted extraction of quercitrin from Houttuynia cordata Thunb. using response surface methodology and UPLC analysis. Food Sci. Biotechnol. 2014;23:1–7. doi: 10.1007/s10068-014-0001-z. [DOI] [Google Scholar]
- 25.Khan MK, Abert-Vian M, Fabiano-Tixier AS, Dangles O, Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119:851–858. doi: 10.1016/j.foodchem.2009.08.046. [DOI] [Google Scholar]
- 26.Ghitescu RE, Volf I, Carausu C, Buhlmann AM, Gilca IA, Popa VI. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015;22(535–541):30. doi: 10.1016/j.ultsonch.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Bashi DS, Mortazavi SA, Rezaei K, Rajaei A, Karimkhani MM. Optimization of ultrasound-assisted extraction of phenolic compounds from yarrow (Achillea beibrestinii) by response surface methodology. Food Sci. Biotechnol. 2012;21:1005–1011. doi: 10.1007/s10068-012-0131-0. [DOI] [Google Scholar]
- 28.Pan Z, Qu W, Ma H, Atungulu GG, McHugh TH. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011;18:1249–1257. doi: 10.1016/j.ultsonch.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Guerrero S, López-Malo A, Alzamora SM. Effect of ultrasound on the survival of Saccharomyces cerevisiae: Influence of temperature, pH and amplitude. Innov. Food Sci. Emerg. Technol. 2001;2:31–39. doi: 10.1016/S1466-8564(01)00020-0. [DOI] [Google Scholar]
- 30.Guo L, Zhu WC, Liu YT, Wu JY, Zheng AQ, Liu YL. Response surface optimized extraction of flavonoids from mimenghua and its antioxidant activities in vitro. Food Sci. Biotechnol. 2013;22:1–8. doi: 10.1007/s10068-013-0214-6. [DOI] [Google Scholar]
- 31.Michelon M, de Matos de Borba T, da Silva Rafael R, Burkert CAV, de Medeiros Burkert JF. Extraction of carotenoids from Phaffia rhodozyma: A comparison between different techniques of cell disruption. Food Sci. Biotechnol. 2012;21:1–8. doi: 10.1007/s10068-012-0001-9. [DOI] [Google Scholar]
- 32.Nafar M, Emam-Djomeh Z, Yousefi S, Hashemi M. An optimization study on the ultrasonic treatments for Saccharomyces cerevisiae inactivation in red grape juice with maintaining critical quality attributes. J. Food Qual. 2013;36:269–281. doi: 10.1111/jfq.12032. [DOI] [Google Scholar]
- 33.Yang RF, Huang PP, Qiu TQ. Ultrasound-enhanced subcritical water extraction of naphthoquinone pigments from purple gromwell (Lithospermum erythrorhizon) to higher yield and bioactivity. Food Sci. Biotechnol. 2013;22:671–676. doi: 10.1007/s10068-013-0130-9. [DOI] [Google Scholar]
- 34.Sousa AD, Maia AIV, Rodrigues THS, Canuto KM, Ribeiro PRV, Pereira RDC, de Brito ES. Ultrasound-assisted and pressurized liquid extraction of phenolic compounds from Phyllanthus amarus and its composition evaluation by UPLC-QTOF. Ind. Crops Prod. 2016;79(91–103):39. [Google Scholar]
- 35.Sun J, Li X, Lin X, Mei Z, Li Y, Ding L, Bai W. Sonodegradation of cyanidin-3-glucosylrutinoside: Degradation kinetic analysis and its impact on antioxidant capacity in vitro. J. Sci. Food Agric. 2017;97:1475–1481. doi: 10.1002/jsfa.7887. [DOI] [PubMed] [Google Scholar]


