Abstract
Ultraviolet B (UVB)-induced cyclooxygenase (COX)-2 and matrix metalloproteinase (MMP)-1 are representative markers for skin inflammation and photoaging, respectively. To evaluate compounds that may counteract the effects of UVB-induced skin damage, we developed an immortalized human keratinocyte (HaCaT) cell line with an MMP-1 reporter construct. Among the 30 botanical extracts screened, we selected Ephedra sinica extract (ESE) as a promising candidate and confirmed that ESE significantly suppresses UVB-induced COX-2 and MMP-1 expression in HaCaT cells. Treatment with ESE also potently suppressed UVB-induced ERK1/2 phosphorylation, as well as UVB-induced MEK1/2 and Raf phosphorylation in HaCaT cells. These findings suggest that our MMP-1 reporter system can be used to evaluate compounds with anti-inflammatory and anti-photoaging effects. We also report that ESE has potent suppressive effects against COX-2 and MMP-1 expression, which occurs via downregulation of Raf/MEK1/2/ERK1/2 phosphorylation.
Keywords: Cyclooxygenase-2, Ephedra sinica extract, Matrix metalloproteinase-1, Mitogen-activated protein kinase
Introduction
Excessive ultraviolet (UV) exposure can cause acute skin inflammation and chronic exposure has been linked to skin cancer [1]. While also dependent on genetic factors and lifestyle choices, repeated exposure to UV irradiation generally results in the appearance of irregular brown spots and wrinkle formation, collectively referred to as photoaging [2, 3]. In contrast to some acute skin diseases including sunburn, photoaging is not generally associated with severe pain. However, the deterioration of the skin’s aesthetic qualities due to photoaging can cause considerable mental stress. Therefore, interest in new agents that can counteract the effects of photoaging is growing.
Analysis of UV-irradiated human skin samples has shown that collagen breakdown and reductions in collagen synthesis within the dermis are major causes of UV-induced photoaging [2]. Additionally, acute and chronic UV irradiation of human skin can cause skin inflammation [4]. The transcription factor AP-1 plays a critical role in these processes via regulation of mmp-1 and cox-2 gene expression, respectively [5, 6]. Due to the fact that AP-1 is a central mediator of UV-induced photoaging and inflammation, the development of analytical tools that efficiently screen compounds that can inhibit AP-1 activity represents a potential strategy to develop new anti-photoaging and anti-inflammatory agents.
Ephedra sinica (Ephedraceae) has long been cultivated in eastern China, Mongolia, and Russia [7] and is used in Chinese medicine (Chinese name: Ma Huang) for the treatment of colds, arthralgia, edema, and asthma [8, 9]. Additionally, the roots of ES are known to possess antisudorific properties [10]. A recent study has shown that consumption of a water extract of E. sinica reduces body weight and causes changes to gut microbiota [9]. Although there are some differences in content between the root and stem [10], ephedrine alkaloids are the primary bioactive components of E. sinica that are likely to be useful for the treatment of asthma and colds [11, 12]. Accumulating evidence indicates that ES may be an effective ingredient for nutraceuticals, but to date, there has been no direct evidence that ES has any effect on UV-induced inflammation or the expression of genes relevant to photoaging.
In the present study, we developed a reporter gene assay to evaluate natural extracts that elicit an inhibitory effect on UVB-induced COX-2 and MMP-1 expression. Using the new system, we screened 30 natural extracts to determine which has the strongest inhibitory effect on UVB-induced MMP-1 promoter binding activity.
Materials and methods
Materials
Chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), gentamicin, l-glutamine, penicillin–streptomycin and fetal bovine serum (FBS) were obtained from Thermo Scientific HyClone (Logan, UT, USA). The antibodies against MMP-1 and β-actin were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). The antibodies against COX-2, p44/42 MAP Kinase, SAPK/JNK, p38 MAPK, phospho-p44/42 MAPK (Erk 1/2) (Thr202/Tyr204), phospho-SAPK/JNK (Thr183/Tyr185), MEK1/2, phospho-MEK1/2 (Ser217/221), B-Raf, and phosphor-B-Raf were purchased from Cell Signaling Biotechnology (Beverly, MA, USA). The antibody against phosphorylated p38 MAPK (pT180/pY182) was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Sample preparation and extraction procedure
Thirty samples were obtained from the Korea Plant Extract Bank, Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). Ephedra sinica specimens were purchase from Kyungdong Market (Seoul, Korea). The specimen was deposited in the Plant Resources and Environment Department, Cheju National University, Korea and identified by a botanist, Dr. Ji-Hum Kim (Department of Plant Resources and Environment, Cheju National University). The samples were ground with a blender (Wonder Blender, Osaka Chemical Co., Osaka, Japan) to obtain a fine powder. Powdered materials were stored in plastic bags at room temperature for use in the extraction experiments. One hundred grams of dried powder were extracted with an Ultrasonic Processor VCX 750 (Sonics & Materials, Inc., Newtown, USA) with 1000 mL of 95% (v/v) ethanol and incubated at room temperature for 24 h. After precipitate removal, the extracts were concentrated to 100 mL with an IKA RV 10 Rotary Evaporator (IKA® Works, Guangzhou, China), then freeze-dried.
Cell culture, UVB exposure
Human epidermal keratinocyte HaCaT and 293T cells were maintained in DMEM containing 10% FBS, 100 units/mL of penicillin and 100 mg/mL of streptomycin at 37 °C in a 5% CO2 humidified incubator (Thermo Scientific, Heraeus BB15, MA, USA). UVB irradiation was conducted using a bank of four Westinghouse F520 lamps (National Biological, Twinsburg, OH, USA) at 6 J/s/m light in the UVB range. Approximately 10% of the additional radiation from the F520 lamp is in the UVA spectrum (320 nm). A UVB exposure chamber was fitted with a Kodak Kodacel K6808 filter eliminates all wavelengths below 290 nm. UVB radiation was measured using a UVX radiometer (UVX-31).
MMP-1 promoter assay
To evaluate the ability of various botanical extracts to activate the MMP-1 promoter, we constructed a pGreenFire (pGF1) vector containing the MMP-1 promoter [13]. For stable expression of pGF1 with the MMP-1 promoter, 293T cells were transfected with the pGF1 plasmid using Lipofectamine (ThermoFisher Scientific, MA, USA), following the manufacturer’s instructions. The transfection medium was changed 4 h after transfection, and the cells were then cultured for 36 h. Virus particles were harvested by filtration using a 0.45-μm syringe filter, and then combined with 8 mg/mL of polybrene (Millipore) and infected into HaCaT cells for 24 h. The cell99 culture media was replaced with fresh culture medium and the cells were further cultured for 24 h, prior to selection with puromycin (1 μg/mL) for 36 h. Selected HaCaT cells (8 × 103 cells/well) were seeded into 96-well plates, which were incubated at 37 °C in a 5% CO2 incubator. When the cells reached 80–90% confluence, they were starved by culturing in serum-free DMEM for a further 24 h. Cells were treated with ESE for 1 h prior to UVB (0.04 J/cm2) exposure and then incubated for 5 h. The cells were disrupted with 100 μL of lysis buffer [0.1 M potassium phosphate buffer (pH 7.8), 1% Triton X-100, 1 mM dithiothreitol (DTT), and 2 mM EDTA], after which luciferase activity was measured with a luminometer (SpectraMax L, Molecular Devices, Sunnyvale, CA, USA).
Cell viability assay
To assess cell viability, HaCaT cells were seeded (1 × 103 cells/well) in 96-well plates and incubated at 37 °C in a 5% CO2 incubator. After the cells were treated with E. sinica extract (ESE) for 24 h, 20 μL of MTS reagent (Promega, Madison, WI, USA) was added to each well. After 1 h of incubation, absorbance levels for formazan at 490 and 690 nm were measured using a microplate reader (Bio-Rad Inc., Hercules, CA, USA).
Western blot assay
For Western blot assays, cells (1.5 × 106 cells/mL) were cultured in 10 cm dishes for 24 h, followed by starvation in serum-free DMEM for 24 h. Cells were then treated with ESE for 1 h and irradiated with UVB (0.04 J/cm2). After incubation, the cells were collected and washed twice with cold PBS, before lysis in Cell Lysis Buffer (Cell Signaling Biotechnology, Beverly, MA, USA) and maintained on ice for 30 min. The lysate protein was washed via centrifugation and the concentration was determined using a DC Protein Assay kit (Bio-Rad Laboratories) following manufacturer’s instructions. The lysate was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Immobilon®-P transfer membrane, Bedford, MA, USA). Subsequently, the membranes were incubated in TBST with 5% skim milk for 1 h at room temperature. The membranes were incubated in the specific primary antibody dilution buffer with gentle agitation at 4 °C overnight. After rinsing, the membranes were incubated in HRP-conjugated secondary antibody dilution buffer for 1 h at room temperature. Protein bands were visualized using a chemiluminescence detection kit (ATTO, Tokyo, Japan) after hybridization with a horseradish peroxidase (HRP)-conjugated secondary antibody.
Statistical analysis
As required, data are expressed as mean values ± SD with significant differences determined by one-way ANOVA (Analysis Of Variance). Statistical analysis was performed with GraphPad Prism 5.01 (GraphPad Software, Inc.) for Windows. A probability value of p < 0.05 was used as the criterion for statistical significance.
Results and discussion
Generation of MMP-1 promoter expressing human keratinocyte HaCaT cells
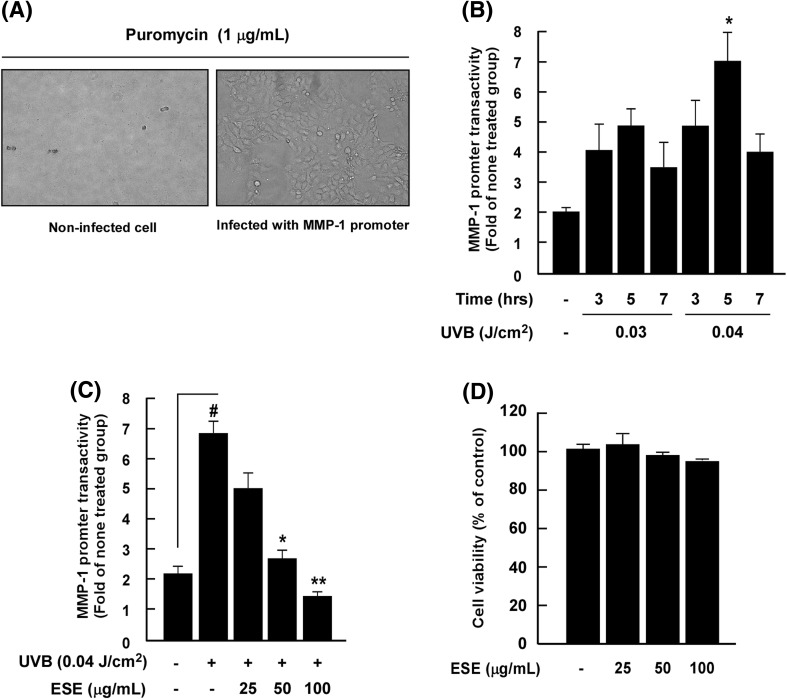
Chronic exposure to UV-irradiation causes collagen damage in human skin, a hallmark of skin aging [3]. Abnormal expression of MMP-1 induced by UV directly degrades collagen in the dermis of skin, and multiple lines of evidence have shown that UV-induced MMP-1 expression is closely linked to photoaging [3, 5, 14]. Moreover, AP-1 play a critical role in photoaging by regulating mmp-1 gene expression [15]. Therefore, targeting the MMP-1 signaling pathway represent a promising strategy for the development of anti-photoaging agents. To screen effective anti-photoaging agents, we generated immortalized human keratinocyte HaCaT cells stably transfected with an MMP-1 promoter [13]. For stable expression of pGF1 with the MMP-1 promoter, transfection media from 293T cells harboring the pGF1 plasmid were used to treat human keratinocytes. After selection with puromycin, we confirmed that HaCaT cells were successfully transfected with the pGF1 vector containing the MMP-1 promoter [Fig. 1(A)].
Fig. 1.
Development of MMP-1 promoter-expressing human keratinocyte HaCaT cells and measurement of ESE on UVB-induced MMP-1 promoter activity. (A) HaCaT cells were transfected with pGF1 vector containing the MMP-1 promoter and selected with puromycin treatment (1 μg/mL). Left figure: non-infected cell with puromycin; right figure: infected with MMP-1 promoter. (B) Optimization of MMP-1 promoter transactivity using varying UV dosages and incubation times. Asterisks (*) indicate a significant difference (p < 0.05) relative to the control group. Data are presented as mean ± SD of three independent experiments. (C) Effect of ESE on UVB-induced MMP-1 promoter activity in HaCaT cells. Promoter activity is presented as the mean ± SD of three independent experiments. Hash symbols (#) indicate a significant difference (p < 0.05) between the control group and the group exposed to UVB alone; asterisks [(*) and (**)] indicate significant differences [(p < 0.05) and (p < 0.01)] between groups irradiated with UVB and ESE and the group exposed to UVB alone. Data are presented as mean ± SD of three independent experiments. (D) ESE exhibits no detectable cell cytotoxicity up to 100 μg/mL in HaCaT cells. Cell viability was measured by MTS assay as described in “Materials and methods”
Optimization of MMP-1 promoter transactivity and screening of inhibitory botanical extracts
To determine the optimal conditions needed to assess MMP-1 promoter activity, we investigated different doses of UV and incubation times. The reporter gene assay revealed that 0.04 J/cm2 and 5 h were the optimal conditions for activating the MMP-1 promoter [Fig. 1(B)]. Using these cells, we screened 30 botanical extracts of interest. ESE was identified as the most potent anti-photoaging material, suppressing UVB-induced MMP-1 promoter binding activity by 79.0% (Table 1). Previous studies have reported that E. sinica elicits multiple biological effects, including the inhibition of pigmentation formation [16], as well as an anti-inflammatory [17], and immune modulatory effects [18]. However, the effect of ESE on UVB-induced skin inflammation and photoaging has not previously been investigated.
Table 1.
Screening of 30 botanical extracts for effects on MMP-1 promoter activity
| Botanical extract | MMP-1 promoter activity (% inhibition) | Botanical extract | MMP-1 promoter activity (% inhibition) |
|---|---|---|---|
| Aconitum carmichaeli | 36.02 ± 3.81 | Fraxinus rhynchophylla | 6.96 ± 14.15 |
| Acronychia pedunculata | 39.85 ± 0.55* | Imperata cylindrica | − 15.69 ± 12.73 |
| Aloe ferox | 38.38 ± 10.25 | Loranthus parasticus | 3.66 ± 7.26 |
| Castanea crenata | − 2.16 ± 4.48 | Pinus densiflora | − 24.09 ± 3.70 |
| Chelidonium majus | − 45.39 ± 1.62 | Playtcodon grandiflorum | − 39.65 ± 9.06 |
| Citrus unshiu | 26.02 ± 12.40 | Polygala tenuifolia | − 6.55 ± 7.96 |
| Codonopsis pilosula | 1.21 ± 20.07 | Quisqualis indica | − 23.34 ± 13.54 |
| Dalbergia odorifera | 39.85 ± 18.06 | Rehmannia glutinosa | 6.64 ± 1.44 |
| Dendrobium nobile | 50.20 ± 3.43* | Rosa laevigata | 44.49 ± 1.09* |
| Desmodium styracifolium | 27.87 ± 11.84 | Sinapis alba | − 40.24 ± 7.33 |
| Dianthus chinensis | − 3.96 ± 7.50 | Sophora japonica | 7.94 ± 3.86 |
| Dimocarpus longan | 12.50 ± 6.77 | Sterculia scaphigera | 17.12 ± 6.92 |
| Dioscorea tokora | 9.40 ± 6.27 | Strychnos ignatii | − 7.39 ± 4.70 |
| Ephedra sinica | 79.00 ± 0.79* | Strychnos nux-vomica | − 15.89 ± 6.97 |
| Eucommia ulmoides | 54.50 ± 4.49* | Trachelospermum asiaticum | 7.11 ± 2.14 |
Data are presented as mean ± SD of three independent experiments
Asterisk symbols (*) indicate a significant difference (p < 0.05) between groups irradiated with UVB and botanical extracts and the group exposed to UVB alone
Ephedra sinica extract (ESE) inhibits UVB-induced MMP-1 promoter activity in HaCaT cells
Evaluation of varying ESE concentrations on UVB-induced MMP-1 promoter binding activity and cell cytotoxicity revealed that ESE inhibits UVB-induced MMP-1 promoter binding activity in a dose-dependent manner [Fig. 1(C)]. The effective concentrations of ESE tested did not affect the viability of immortalized human keratinocyte HaCaT cells [Fig. 1(D)].
ESE inhibits UVB-induced MMP-1 and COX-2 expression in HaCaT cells
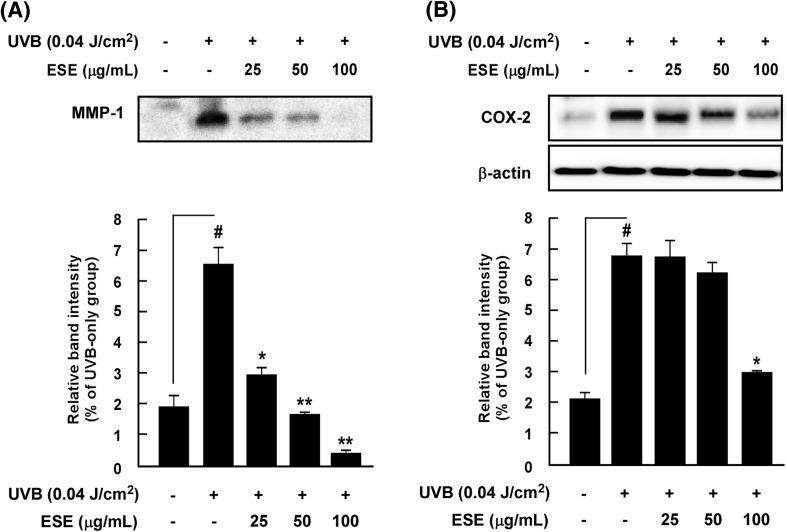
Because MMP-1 transcription is primarily regulated by AP-1 [19] and COX-2, which are two representative enzymes for inflammatory regulation [5], we hypothesized that ESE may influence both MMP-1 and COX-2 expression by mediating AP-1 activity. Western blot results showed that ESE significantly suppresses UVB-induced MMP-1 and COX-2 expression in HaCaT cells (Fig. 2), indicating that the MMP-1 promoter assay was indeed working as intended and suitable for identifying novel anti-photoaging and skin inflammation agents. Our previous study results indicated that the suppression of MMP-1 and COX-2 expression was closely related to UVB-induced skin inflammation [1, 20] and photoaging [21, 22], respectively. In vivo experiment using an SKH-1 hairless mouse model are needed to confirm that ESE measurably attenuates UV-mediated skin inflammation and photoaging.
Fig. 2.
Effect of ESE on UVB-induced MMP-1 and COX-2 expression in HaCaT cells. (A) ESE inhibits UVB-induced MMP-1 expression in HaCaT cells. (B) ESE inhibits UVB-induced COX-2 expression in HaCaT cells. Expression levels of MMP-1, COX-2 and β-actin were determined by Western blot assay. Hash symbols (#) indicate a significant difference (p < 0.05) between the control group and the group exposed to UVB alone; asterisks [(*) and (**)] indicate significant differences [(p < 0.05) and (p < 0.01)] between groups irradiated with UVB and ESE and the group exposed to UVB alone. Data are presented as mean ± SD of three independent experiments
ESE inhibits UVB-induced Raf/MEK/ERK1/2 phosphorylation in HaCaT cells
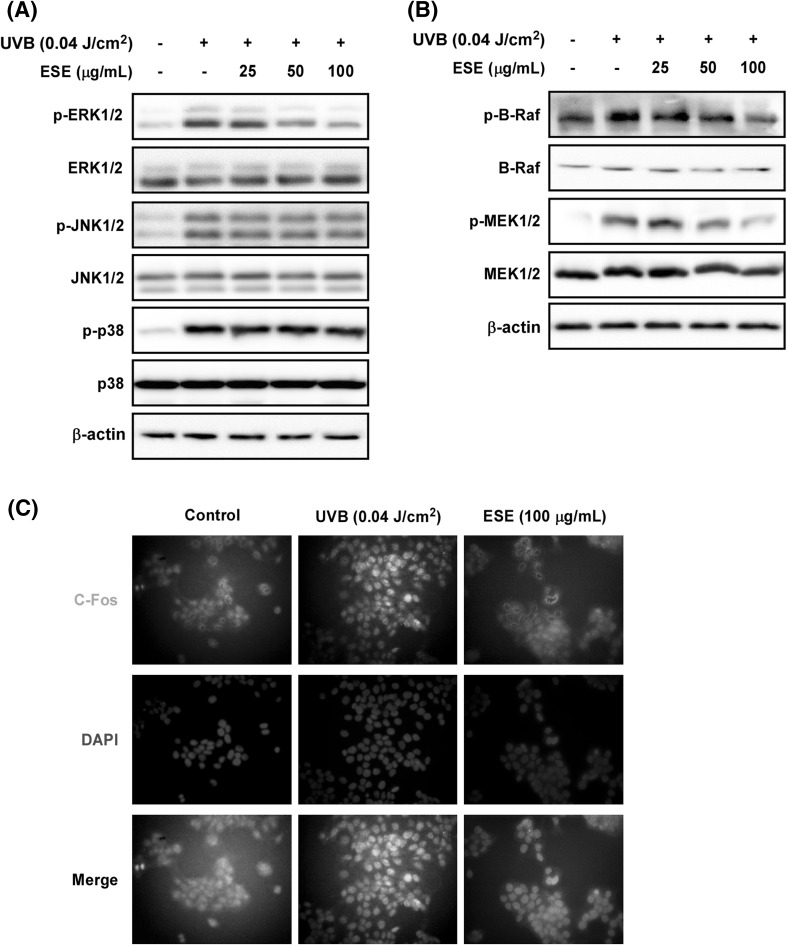
Multiple lines of evidence have shown that MAPKs play a central role in UV-induced MMP-1 and COX-2 expression by mediating AP-1 activity [5, 23]. Our Western blot analysis showed that ESE suppresses UVB-induced ERK1/2 phosphorylation, but did not affect JNK1/2 or p38 [Fig. 3(A)]. A previous study showed that ERK phosphorylation plays a critical role in the regulation of AP-1 activity via its effect on Fra-1, a constituent of the AP-1 complex [24]. Our own study results have also shown that UVB-induced activation of ERK signaling plays an important role in AP-1 activity and MMP-1 expression by mediating Fra1 stability. Based on our previous study and in our current study results, phosphorylation of c-Jun and c-Fos stability by ERK activation plays a major role in AP-1 activity and MMP-1 expression [22]. Because ESE only affects ERK phosphorylation, we hypothesized that ESE may affect Fos-dependent protein stability. The results showed that ESE suppressed UVB-induced C-Fos nuclear translocation in HaCaT cells [Fig. 3(C)]. These findings suggest that suppression of Raf/MEK1/2/ERK1/2 phosphorylation by ESE subsequently inhibits UVB-induced COX-2 and MMP-1 expression via regulation of AP-1 activity.
Fig. 3.
Effect of ESE on UVB-induced Raf/MEK/ERK1/2 phosphorylation and C-Fos nuclear translocation in HaCaT cells. (A) ESE inhibits UVB-induced ERK1/2 phosphorylation; and (B) Raf and MEK phosphorylation in HaCaT cells. Phosphorylation status and total expression were assessed by Western blot assay with specific antibodies. Data are presented as mean ± SD of three independent experiments. (C) ESE suppresses UVB-induced C-Fos nuclear translocation in HaCaT cells
Taken together, our results show that ESE is a potent inhibitor of UVB-induced MMP-1 promoter binding activity and significantly inhibits UVB-induced COX-2 and MMP-1 expression. This inhibition occurs via the suppression of UVB-induced Raf/MEK1/2/ERK1/2 phosphorylation and subsequent suppression of AP-1 activity. This represents the first report describing the anti-inflammatory and -photoaging effect of ESE and sheds light on the mechanisms of action responsible.
Acknowledgements
This research was supported by a grant from the Korea Food Research Institute, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jung SK, Lee KW, Byun S, Kang NJ, Lim SH, Heo YS, Bode AM, Bowden GT, Lee HJ, Dong Z. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008;68:6021–6029. doi: 10.1158/0008-5472.CAN-08-0899. [DOI] [PubMed] [Google Scholar]
- 2.Fisher GJ. The pathophysiology of photoaging of the skin. Cutis 75: 5–8; discussion 8–9 (2005) [PubMed]
- 3.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer M, Werner S. Nrf2—A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015;88:243–252. doi: 10.1016/j.freeradbiomed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 6.Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016;17:868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Zheng S. Whole fossil plants of Ephedra and their implications on the morphology, ecology and evolution of Ephedraceae (Gnetales) Chinese Sci. Bull. 2010;55:1511–1519. doi: 10.1007/s11434-010-3069-8. [DOI] [Google Scholar]
- 8.Wei P, Huo H-l, Ma Q-h, Li H-c, Xing X-f, Tan X-m, Luo J-b. Pharmacokinetic comparisons of five ephedrine alkaloids following oral administration of four different Mahuang–Guizhi herb-pair aqueous extracts ratios in rats. J. Ethnopharmacology 155: 642–648 (2014) [DOI] [PubMed]
- 9.Kim B-S, Song M-y, Kim H. The anti-obesity effect of Ephedra sinica through modulation of gut microbiota in obese Korean women. J. Ethnopharmacology 152: 532–539 (2014) [DOI] [PubMed]
- 10.Lv M, Sun J, Wang M, Huang W, Fan H, Xu F, Zhang Z. GC–MS based metabolomics study of stems and roots of Ephedra sinica. J. Pharmaceut. Biomed. 2015;114:49–52. doi: 10.1016/j.jpba.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Tseng YL, Hsu H-R, Kuo F-H, Shieh M-H, Chang C-F. Ephedrines in over-the-counter cold medicines and urine specimens collected during sport competitions. J. Anal. Toxicol. 2003;27:359–365. doi: 10.1093/jat/27.6.359. [DOI] [PubMed] [Google Scholar]
- 12.Lee M, Cheng B, Che C, Hsieh D. Cytotoxicity assessment of Ma-huang (Ephedra) under different conditions of preparation. Toxicol. Sci. 2000;56:424–430. doi: 10.1093/toxsci/56.2.424. [DOI] [PubMed] [Google Scholar]
- 13.Kim MK, Shin JM, Eun HC, Chung JH. The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PLoS One. 2009;4:e4864. doi: 10.1371/journal.pone.0004864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung SK, Ha SJ, Kim YA, Lee J, Lim TG, Kim YT, Lee NH, Park JS, Yeom MH, Lee HJ. MLK3 is a novel target of dehydroglyasperin D for the reduction in UVB-induced COX-2 expression in vitro and in vivo. J. Cell. Mol. Med. 2015;19:135–142. doi: 10.1111/jcmm.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Ma WY, Hanenberger D, Cleary MP, Bowden GT, Dong Z. Inhibition of ultraviolet B-induced activator protein-1 (AP-1) activity by aspirin in AP-1-luciferase transgenic mice. J. Biol. Chem. 1997;272:26325–26331. doi: 10.1074/jbc.272.42.26325. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Cho YD, Leem KH, Lee DN, Kim EH, Kim MG, Kim DK, Shin TY, Boo Y, Lee JH, Kim HK. Effects of Ephedrae Herba on melanogenesis and gene expression profiles using cDNA microarray in b16 melanocytes. Phytotherapy Res. 2006;20:748–754. doi: 10.1002/ptr.1947. [DOI] [PubMed] [Google Scholar]
- 17.Yeom MJ, Lee HC, Kim GH, Lee HJ, Shim I, Oh SK, Kang SK, Hahm DH. Anti-arthritic effects of Ephedra sinica STAPF herb-acupuncture: inhibition of lipopolysaccharide-induced inflammation and adjuvant-induced polyarthritis. J. Pharmacol. Sci. 2006;100:41–50. doi: 10.1254/jphs.FP0050637. [DOI] [PubMed] [Google Scholar]
- 18.Wang QH, Shu ZP, Xing N, Xu BQ, Wang CF, Sun GB, Sun XB, Kuang HX. A pure polysaccharide from Ephedra sinica treating on arthritis and inhibiting cytokines expression. Int. J. Biol. Macromol. 2016;86:177–188. doi: 10.1016/j.ijbiomac.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 20.Ha SJ, Lee J, Kim H, Song K-M, Lee NH, Kim YE, Lee H, Kim YH, Jung SK. Preventive effect of Rhus javanica extract on UVB-induced skin inflammation and photoaging. J. Funct. Foods. 2016;27:589–599. doi: 10.1016/j.jff.2016.10.011. [DOI] [Google Scholar]
- 21.Jung SK, Lee KW, Kim HY, Oh MH, Byun S, Lim SH, Heo YS, Kang NJ, Bode AM, Dong Z, Lee HJ. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010;79:1455–1461. doi: 10.1016/j.bcp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung SK, Ha SJ, Jung CH, Kim YT, Lee HK, Kim MO, Lee MH, Mottamal M, Bode AM, Lee KW, Dong Z. Naringenin targets ERK2 and suppresses UVB-induced photoaging. J. Cell. Mol. Med. 2016;20:909–919. doi: 10.1111/jcmm.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003: RE2 (2003) [DOI] [PubMed]
- 24.Basbous J, Chalbos D, Hipskind R, Jariel-Encontre I, Piechaczyk M. Ubiquitin-independent proteasomal degradation of Fra-1 is antagonized by Erk1/2 pathway-mediated phosphorylation of a unique C-terminal destabilizer. Mol. Cell. Biol. 2007;27:3936–3950. doi: 10.1128/MCB.01776-06. [DOI] [PMC free article] [PubMed] [Google Scholar]