Abstract
Sulforaphane is a significant chemopreventive compound which is the predominant glucosinolate in broccoli sprouts. However, the existence of the epithiospecifier protein could direct the hydrolysis of glucosinolates toward sulforaphane nitrile formation instead of sulforaphane. Therefore, the study aimed on improving the yielding of sulforaphane in broccoli sprouts with a new method of the united hydrolysis of cruciferous sprouts. According to the results, the addition of radish, rocket and rape sprouts to broccoli sprouts could promote the hydrolysis of the glucoraphanin to anticancer effective sulforaphane to 2.03, 2.32 and 1.95-fold, respectively, compared to single broccoli sprouts. Meanwhile, the formation of non-bioactive sulforaphane nitrile in these three groups decreased greatly. However, the addition of mustard sprouts had no positive effect. These observations could make a contribution to the potential chemoprotective effects of broccoli sprouts.
Electronic supplementary material
The online version of this article (10.1007/s10068-018-0347-8) contains supplementary material, which is available to authorized users.
Keywords: Broccoli sprouts, Sulforaphane, Glucoraphanin, Addition, Hydrolysis
Introduction
Epidemiological studies have showed that cruciferous vegetables intake was associated with lowering the risk of developing cancer [1]. And several studies revealed that these preventive effects appeared to be related to the unique contents of amounts of glucosinolates and myrosinase [2–4]. When plant tissue is mechanically ground or chopped, glucosinolates and myrosinase are brought into contact, and the β-thioglucoside bond of glucosinolates is hydrolyzed by myrosinase to produce glucose, sulfate and an unstable aglucon intermediate. Then the aglucon intermediate breaks down to form isothiocyanates, thiocyanates, nitriles, epithionitriles and oxazolidine-thiones [5].
Glucoraphanin (4-methylsulfinylbutenyl glucosinolate), the predominant glucosinolate in broccoli, yields sulforaphane (4-methylsulfinylbutyl isothiocyanate) and sulforaphane nitrile (5-methylsulfinylpentane nitrile) as main hydrolysis products [6]. Sulforaphane was strongly associated with anticancer effects and it was initially identified as the inducer of phase II enzymes. Studies revealed that sulforaphane has direct proliferation effect on cancer cells [7–9]. Besides, sulforaphane also shows a number of biological activities such as antihypertensive [10], cardioprotective [11] and supplementary treatment in type 2 diabetes [12]. Nitrile, on the other hand, has no significant cancer prevention activity [13]. However, sulforaphane yield from glucoraphanin is low, and the nitrile production is superior to sulforaphane when broccoli is crushed. Importantly, the existence of the epithiospecifier protein (ESP) could affect the ratio of sulforaphane to nitrile by directing the degradation of the aglucon intermediate to nitrile formation, at the expense of sulforaphane [14]. Therefore, it is necessary to find methods to preferentially direct the hydrolysis of glucoraphanin toward sulforaphane formation.
Wang et al. [15] found that when broccoli was treated with brief heating, it was effective to provide less nitrile and more sulforaphane. Since ESP was more heat sensitive than myrosinase, thus making ESP inactivate firstly. However, myrosinase activity could be partly or totally denatured thus significantly decreasing sulforaphane formation during high temperature heating process. Others reported the addition of exogenous source of myrosinase such as broccoli root, mustard seeds to the processed broccoli could increase the formation of sulforaphane [16].
Cruciferous sprouts gained people’s attention due to their higher abundance in bioactive compounds and perceived health benefits. In the present study, the addition of other appropriate cruciferous sprouts including radish, rocket, rape and mustard to broccoli were investigated, with the aim of improving sulforaphane formation, thus providing an optimum yield of health-promoting compounds for human consumption.
Materials and methods
Plant materials and chemicals
Broccoli seeds (Brassica oleracea L.), radish seeds (Raphanus sativus L. Mantanghong), rocket seeds (Eruca sativa Mill.), rape seeds (Brassica campestris L. Shanghaiqing) and mustard seeds (Brassica juncea L.) were kindly provided by Vegetables and Flowers Institute, China Academy of Agriculture Science. Acetonitrile and trifluoroacetic acid (TFA) were of HPLC grade and purchased from Fisher Scientific Co., LTD (Tustin, CA). Ultra-pure water was obtained by Q Millipore System (Millipore, USA). Sodium hypochlorite solution, dichloromethane were analytical grade and purchased from Beijing Chemical Works (Beijing, China), applied without further purification.
Seeds germination and sprouts cultivation
One hundred grams of broccoli (radish, rocket, rape or mustard) seeds were cleaned with deionized water and subsequently they were immersed in a 0.7% sodium hypochlorite solution for 30 min. Then those seeds were drained and washed with deionized water until the solution reached a neutral pH. Afterwards, they were soaked in 500 ml deionized water overnight. The imbibed seeds germinated on four layers of moist sterile gauzes in culture trays which were placed in 25 °C incubators, watered every 8 h to maintain a constant water content. During first 2 days, all seeds germinated in darkness conditions, after that they were cultivated under photoperiod conditions (16 h light and 8 h darkness). Sprouts were harvested daily during germination for up to 7 days. One half of the harvested sprouts were stored at − 20 °C refrigerator and the others were freeze-dried with lyophilizer.
Extraction of glucosinolates
The extraction of glucosinolates in each cruciferous sprout including broccoli, radish, rocket, rape and mustard sprouts were conducted according to previously published method with minor modifications [17]. Firstly, 10 mg of each freeze-dried sprouts were ground into powder with an analytical grinder and then 3 ml boiling water was added. The mixture was heated in a water bath set at 100 °C for 15 min before being centrifuged at 12,000 rpm for 5 min. Subsequently, the supernatant was decanted and the residue was extracted twice with 3 ml boiling water. Finally, the combined supernatants were concentrated to 3 ml using rotary evaporator and filtered with 0.22 μm nylon membrane.
Glucosinolates analysis by HPLC/Q-TOF/MS
The content of glucosinolate in each cruciferous sprouts extraction filtrate was analyzed by an Agilent 1290 HPLC (Agilent Technologies, Germany), coupled to an Agilent 6540 quadrupole-time of flight mass spectrometer (Agilent Technologies, Germany). The separation was carried out on a reversed phase C18 column (2.1 × 150 mm, 3.5 μm; Agilent Technologies, Germany) set at a flow rate of 0.3 ml/min, eluting with a gradient elution of acetonitrile (mobile phase A) and 0.02% (v/v) TFA aqueous solution (mobile phase B) as follows: linear gradient from 1% A to 15% A for 10 min, 100% A kept for 2 min, then 1% A kept for 8 min. The injection volume was 10 μl and the detection wavelength was set at 235 nm. MS spectrometry data were acquired in the negative ionization mode for glucosinolates, and the separate glucosinolates were identified from the extracted samples according to their [M–H]− fragmentations.
Extraction of formed sulforaphane
Two grams of broccoli sprouts combined with 2 g of radish (rocket, rape or mustard) sprouts were juiced together with 20 ml deionized water for 3 min, using a juice centrifuge (Joyoung model JYL-C18D, China). Two grams of single broccoli sprouts acted as control. The obtained slurry was allowed to hydrolyze at room temperature for 1 h to facilitate product formation. Then the slurry was centrifuged at 12,000 rpm for 5 min and supernatant was extracted twice with 20 ml dichloromethane. Then the combined dichloromethane fraction was dried using rotary evaporator and the residue was dissolved in 20 ml water. Finally, the sample was filtered with 0.22 μm nylon membrane.
Sulforaphane formation analysis by HPLC/Q-TOF/MS
The sulforaphane formation in each combined cruciferous sprouts extraction filtrate was analyzed on an Agilent 1290 HPLC (Agilent Technologies, Germany), coupled to an Agilent 6540 quadrupole-time of flight mass spectrometer (Agilent Technologies, Germany). The separation was carried out on a reversed phase C18 column (2.1 × 150 mm, 3.5 μm; Agilent Technologies, Germany) set at a flow rate of 0.3 ml/min, eluting with a gradient elution of acetonitrile (mobile phase A) and 0.02% (v/v) TFA aqueous solution (mobile phase B) as follows: linear gradient from 20% A to 80% A for 10 min, 100% A kept for 2 min, then 20% A kept for 8 min. The injection volume was 10 μL and the detection wavelength was set at 254 nm [18]. MS spectrometry data were acquired in the positive ionization mode for sulforaphane, and the sulforaphane was identified from the extracted samples according to its [M + H]− fragmentations.
Sulforaphane nitrile formation analysis by GC–MS
The extraction of sample was the same as that of sulforaphane analysis. Then the combined dichloromethane fraction was concentrated to 20 ml under vacuum. After that the dichloromethane concentrate was filtered with 0.22 μm nylon membrane and sulforaphane nitrile in it was analyzed on an Agilent 7890B GC system (Agilent Technologies, Germany), equipped with an Agilent 5977A mass spectrometer (Agilent Technologies, Germany). The separation was carried out on an Agilent HP-5 ms (5% phenyl methyl siloxane) column (30 m × 0.25 mm, 0.1 μm film thickness; Agilent Technologies, Germany). The temperature of injector and detector were set at 210 and 280 °C, respectively. Column oven temperature began at 40 °C for 2 min, then gradient (10 °C/min) increased to and kept at 260 °C for 10 min. Helium was used as carrier gas and the flow rate was 25 psi. Ion source temperature was 230 °C, and electron energy was 70 eV [19].
Statistical analysis
All analytical experiments were carried out in three replicates and the results were presented as a mean ± standard deviation (SD). One way analysis of variance (ANOVA) was employed to identify significant differences (p < 0.05) between data sets using software Origin 8.5.
Results and discussions
Glucosinolate analysis in five cruciferous sprouts
According to HPLC/Q-TOF/MS, there was one predominate glucosinolate whose content was much higher than the others in each cruciferous sprouts (Table 1). In broccoli sprouts (Fig. A1), glucoraphanin, the parent compound of sulforaphane, was the most abundant glucosinolate. The predominant glucosinolate in radish sprouts (Fig. A2) was glucoraphenin. In addition, it was found that glucoerucin content was much higher than other glucosinolates in rocket sprouts (Fig. A3), and sinigrin was the predominant glucosinolate in rape (Fig. A4) and mustard sprouts. From the above results, it was noticed that there was no glucoraphanin to be found in the samples of radish, rocket, rape and mustard sprouts, which could be due to there was no or just little of glucoraphanin in these sprouts [20]. Hence, the yielded sulforaphane after treating with addition of other cruciferous was provided by glucoraphanin in broccoli.
Table 1.
Analysis of the predominant glucosinolate in five cruciferous sprouts
| Cruciferous sprouts | The predominant glucosinolate | |
|---|---|---|
| Name | Peak area | |
| Broccoli | Glucoraphanin | 7,448,534 |
| Radish | Glucoraphenin | 9,173,659 |
| Rocket | Glucoerucin | 783,673 |
| Rape | Sinigrin | 312,597 |
| Mustard | Sinigrin | 596,372 |
Effects of addition of other cruciferous sprouts to broccoli sprouts on sulforaphane formation
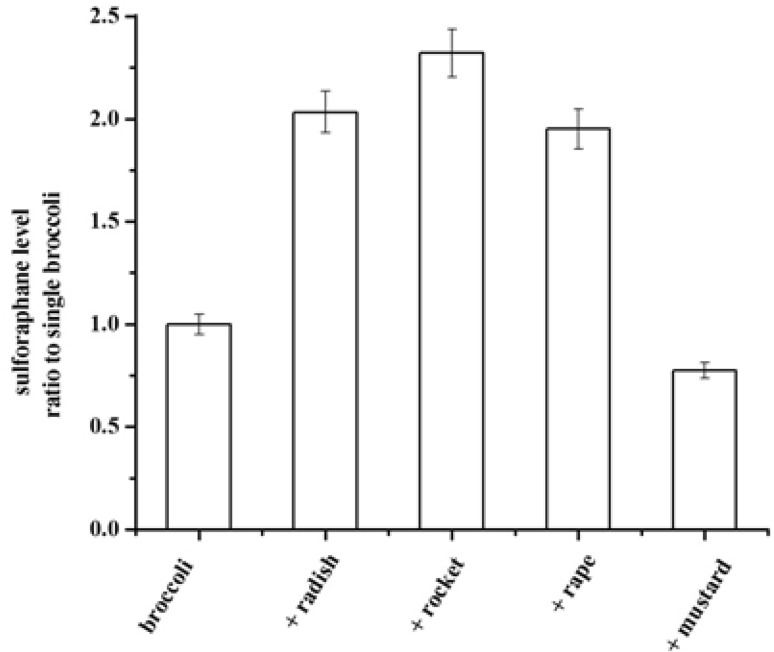
The HPLC/Q-TOF/MS spectrum of the hydrolyzate of single broccoli sprouts was showed in Fig. A5. It was found that the peak with a retention time of 4.88 min was sulforaphane. The same method was used to analysis the sulforaphane content with other cruciferous sprouts additions. Figure 1 showed that the addition of exogenous cruciferous sprouts to broccoli sprouts impacted the sulforaphane formation. The single broccoli acted as control. The addition of radish sprouts significantly increased sulforaphane formation to 2.03-fold compared with single broccoli sprouts (P < 0.05). When adding rocket sprouts to broccoli sprouts, sulforaphane had the highest content level and it significantly increased to 2.32-fold (P < 0.05). Moreover, addition of rape sprouts to broccoli sprouts improved sulforaphane content to 1.95-fold (P < 0.05). While a lower content of sulforaphane for the addition of mustard sprouts was observed, decreasing by 22.5% compared with control. However, it was reported that addition of powdered mustard seeds to thermal processed broccoli could increase sulforaphane formation by more than 5-fold [21]. The decrease of sulforaphane content was caused by the inactivation of myrosinase during the heating process so that it could not catalyze glucoraphanin to generate sulforaphane, whereas the addition of mustard seeds in such condition provided exogenous myrosinase.
Fig. 1.
Effects of the addition of radish, rocket, rape, mustard sprouts to broccoli sprouts on the sulforaphane formation. The experiment was carried out in triplicate. The data were expressed as means ± standard deviations (SD)
The results suggested that the addition of radish, rocket and rape sprouts to broccoli sprouts could make around one-fold increase in sulforaphane formation compared to single broccoli sprouts, while mustard sprouts addition could not improve sulforaphane level. Therefore, consuming broccoli sprouts together with some of radish, rocket and rape sprouts could make broccoli sprouts provide more health-promoting compounds for us.
Effects of addition of other cruciferous sprouts to broccoli sprouts on sulforaphane nitrile formation
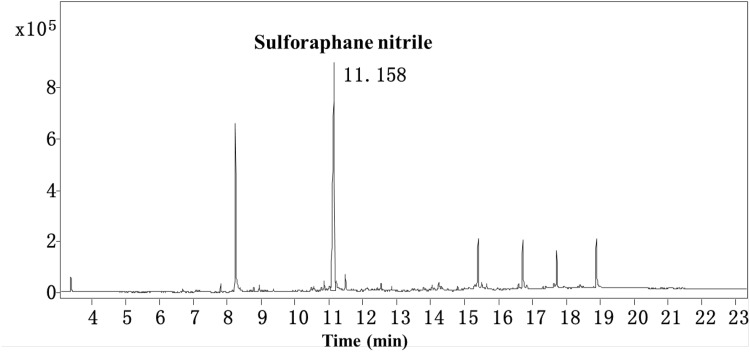
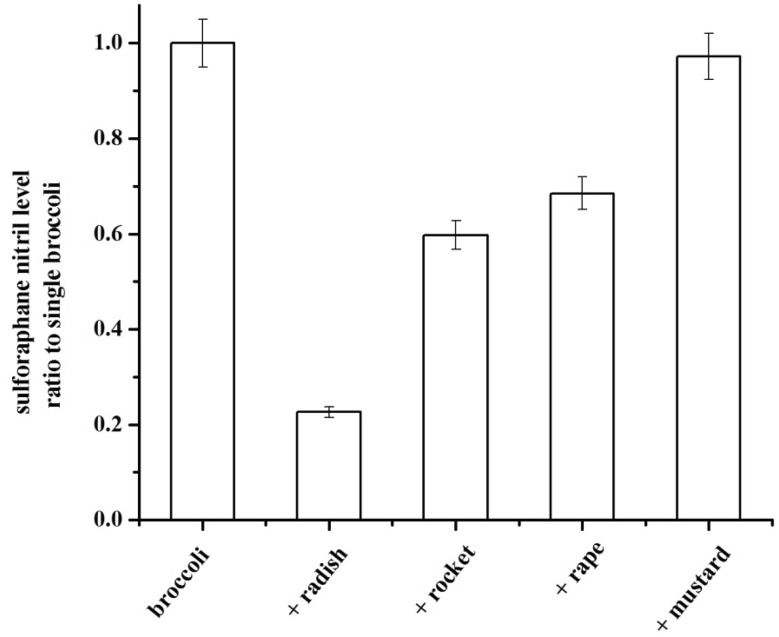
It was clear that exogenous source of myrosinase including broccoli root, radish root and mustard seed could hydrolyze glucoraphanin to sulforaphane, but the formation of sulforaphane nitrile hadn’t been studied yet. In order to explore the origin of the increased sulforaphane after hydrolyzing with other cruciferous sprouts, the sulforaphane nitrile content was examined with GC–MS. The GC spectrum of the hydrolyzate of broccoli sprouts was showed in Fig. 2. The compound, appearing at 11.158 min, was investigated to be sulforaphane nitrile according to its MS analysis (Fig. 3). Then, the sulforaphane nitrile content in other cruciferous sprouts additions were carried out with the same method. As shown in Fig. 4, the addition of radish, rocket and rape sprouts significantly (P < 0.05) decreased sulforaphane nitrile formation by 77.4, 60.3 and 51.5% respectively, compared with single broccoli sprouts. It was might due to the lack of ESP and thus did not generate nitriles. Besides, excess myrosinase would favor sulforaphane formation over nitrile even when a little ESP was present. And there was no significant (P > 0.05) effect on sulforaphane nitrile when the addition of mustard sprouts was conducted. These results were in agreement with the changes of sulforaphane level.
Fig. 2.
The GC spectrum of the hydrolyzate of single broccoli sprouts
Fig. 3.
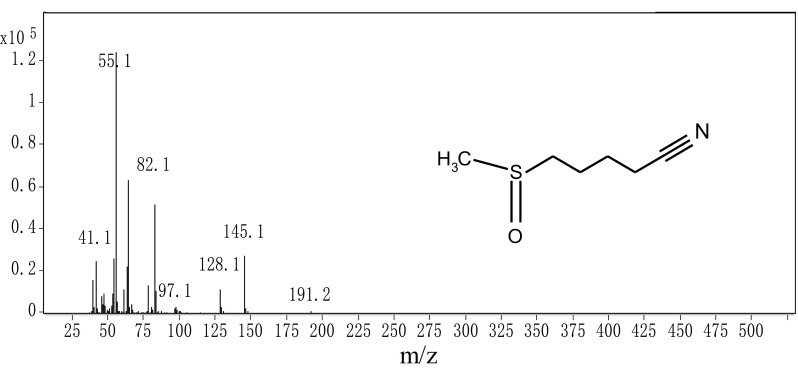
The MS spectrum of the compound whose peak time is 11.158 min
Fig. 4.
Effects of the addition of radish, rocket, rape, mustard sprouts to broccoli sprouts on the sulforaphane nitrile formation. The experiment was carried out in triplicate. The data were expressed as means ± standard deviations (SD)
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the fund of the Beijing Laboratory for Food Quality and Safety (Beijing Technology and Business University), the Beijing Natural Science Foundation (2162030), the Beijing Natural Science Foundation-Beijing Municipal Education Commission Joint Funding project (KZ201710020014), the National Natural Science Foundation of China (21606014), the Double First-rate Program (ylkxj03) and the 111 Project (B13005).
Abbreviations
- ESP
Epithiospecifier protein
- TFA
Trifluoroacetic acid
- HPLC/Q-TOF/MS
High performance liquid chromatography/quadrupole-time of flight mass spectrometer
- GC
Gas chromatography
Contributor Information
Hao Liang, Email: lianghao@mail.buct.edu.cn.
Fuping Zheng, Email: zhengfp@btbu.edu.cn.
References
- 1.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast Cancer Risk in Premenopausal Women Is Inversely Associated with Consumption of Broccoli, a Source of Isothiocyanates, but Is Not Modified by GST Genotype. Nutrition. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 2.Lippmann D, Lehmann C, Florian S, Barknowitz G, Haack M, Mewis I, Wiesner M, Schreiner M, Glatt H, Brigelius-Flohé R, Kipp AP. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food and Function. 2014;5:1073–1081. doi: 10.1039/C3FO60676G. [DOI] [PubMed] [Google Scholar]
- 3.Wang N, Shen L, Qiu S, Wang X, Wang K, Hao J, Xu M. Analysis of the isothiocyanates present in three Chinese Brassica vegetable seeds and their potential anticancer bioactivities. European Food Research and Technology. 2010;231:951–958. doi: 10.1007/s00217-010-1348-x. [DOI] [Google Scholar]
- 4.Hanschen FS, Schreiner M. Isothiocyanates, Nitriles, and Epithionitriles from Glucosinolates Are Affected by Genotype and Developmental Stage in Brassica oleracea Varieties. Front Plant Sci. 2017;8:1095. doi: 10.3389/fpls.2017.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaughn SF, Berhow MA. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Industrial Crops and Products. 2005;21:193–202. doi: 10.1016/j.indcrop.2004.03.004. [DOI] [Google Scholar]
- 6.Radošević K, Srček VG, Bubalo MC, Brnčić SR, Takács K, Redovniković IR. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. Journal of Food Composition and Analysis. 2017;61:59–66. doi: 10.1016/j.jfca.2017.02.001. [DOI] [Google Scholar]
- 7.Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacologica Sinica. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 8.Liang H, Lai B, Yuan Q. Sulforaphane induces cell-cycle arrest and apoptosis in cultured human lung adenocarcinoma LTEP-A2 cells and retards growth of LTEP-A2 Xenografts in Vivo. Journal of Natural Products. 2008;71:1911–1914. doi: 10.1021/np800233q. [DOI] [PubMed] [Google Scholar]
- 9.Li R, Song D, Vriesekoop F, Cheng L, Yuan Q, Liang H. Glucoraphenin, sulforaphene, and antiproliferative capacity of radish sprouts in germinating and thermal processes. European Food Research and Technology. 2017;243:547–554. doi: 10.1007/s00217-016-2764-3. [DOI] [Google Scholar]
- 10.Hoffmann and Bremer, S. Modulation of drug metabolizing enzymes by dietary doses of sulforaphane; role in its anti-hypertensive and anti-oxidant effect in spontaneously hypertensive rats. Journal of Clinical Toxicology. 6: (2016)
- 11.Angeloni C, Leoncini E, Malaguti M, Angelini S, Hrelia P, Hrelia S. Modulation of Phase II Enzymes by Sulforaphane: Implications for Its Cardioprotective Potential. Journal of Agricultural and Food Chemistry. 2009;57:5615–5622. doi: 10.1021/jf900549c. [DOI] [PubMed] [Google Scholar]
- 12.Bahadoran Z, Mirmiran P, Azizi F. Potential Efficacy of Broccoli Sprouts as a Unique Supplement for Management of Type 2 Diabetes and Its Complications. Journal of Medicinal Food. 2013;16:375–382. doi: 10.1089/jmf.2012.2559. [DOI] [PubMed] [Google Scholar]
- 13.Matusheski NV, Jeffery EH. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. Journal of Agricultural & Food Chemistry. 2001;49:5743–5749. doi: 10.1021/jf010809a. [DOI] [PubMed] [Google Scholar]
- 14.Matusheski NV, Swarup R, Juvik JA, Mithen R, Bennett M, Jeffery EH. Epithiospecifier Protein from Broccoli (Brassica oleracea L. ssp. italica) Inhibits Formation of the Anticancer Agent Sulforaphane. Journal of Agriculturaland. Food Chemistry. 2006;54:2069–2076. doi: 10.1021/jf0525277. [DOI] [PubMed] [Google Scholar]
- 15.Wang GC, Farnham M, Jeffery EH. Impact of Thermal Processing on Sulforaphane Yield from Broccoli (Brassica oleracea L. ssp. italica) Journal of Agricultural and Food Chemistry. 2012;60:6743–6748. doi: 10.1021/jf2050284. [DOI] [PubMed] [Google Scholar]
- 16.Dosz EB, Jeffery EH. Modifying the Processing and Handling of Frozen Broccoli for Increased Sulforaphane Formation. Journal of Food Science. 2013;58:1459–1463. doi: 10.1111/1750-3841.12221. [DOI] [PubMed] [Google Scholar]
- 17.Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacological Research. 2011;64:456–463. doi: 10.1016/j.phrs.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song D, Liang H, Kuang P, Tang P, Hu G, Yuan Q. Instability and Structural Change of 4-Methylsulfinyl-3-butenyl Isothiocyanate in the Hydrolytic Process. Journal of Agricultural and Food Chemistry. 2013;61:5097–5102. doi: 10.1021/jf400355d. [DOI] [PubMed] [Google Scholar]
- 19.Guo L, Yang R, Wang Z, Guo Q, Gu Z. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. Journal of Functional Foods. 2014;5:70–77. doi: 10.1016/j.jff.2014.04.015. [DOI] [Google Scholar]
- 20.Judson HG, Barrell GK. The incidence of goitre in newborn lambs from ewes fed fodder radish, rape or Italian ryegrass with or without iodine supplementation. Proceedings of the New Zealand Society of Animal Production. 2011;71:66–70. [Google Scholar]
- 21.Ghawi SK, Methven L, Niranjan K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba) Food Chemistry. 2013;138:1734–1741. doi: 10.1016/j.foodchem.2012.10.119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.