Abstract
Baizhi (Angelica dahurica) has been widely used as a traditional Chinese herbal medicine, functional food and cosmetic product ingredient, mostly because of the high furanocoumarin compounds in roots. Because the fresh root is perishable, drying techniques are needed to maintain a higher-quality product. Freeze-drying is the best method but energy-consuming and costly. The aim of this study was to analyze the quality (antioxidant and furanocoumarin content) of Baizhi roots after freeze-drying (the control) and in-the-shade, 40 and 70 °C drying. Antioxidant activity was revealed by 2,2-diphenyl-1-picrylhydrazyl and Fe2+ chelating assay, and the content of six furanocoumarin compounds, including xanthotoxin, bergapten, oxypeucedanin, imperatorin, phellopterin and isoimperatorin, was analyzed by liquid chromatography. Antioxidant activity was greater in roots with in-the-shade, 40 and 70 °C drying than freeze-drying. The furanocoumarin content pattern was similar with 70 °C drying and freeze-drying. A. dahurica roots dried at 70 °C may be an alternative method for maintaining high quality.
Keywords: Angelica dahurica, Drying method, Furanocoumarin, Antioxidant
Introduction
Baizhi (Angelica dahurica BENTH. et HOOK.) is a perennial Apiaceae plant that originated in Taiwan [1] and is abundant in Korea, China, Japan and Russia [2, 3]. The root of Baizhi is a well-known traditional Chinese medicine that has been used for its antipyretic and analgesic properties for thousands of years in Asia. Today, researchers are analyzing the multiple biological functions of Baizhi and investigating its chemical constituents.
The multiple pharmacological effects of Baizhi root extracts include antioxidation [4], anti-inflammatory [5], antiproliferative [6], skin-whitening [7], anti-tumor [8], antimicrobial [9] and anti-Alzheimer disease effects [10]. These effects are believed to be attributed to the plant’s rich furanocoumarin compounds such as imperatorin and isoimperatorin. Imperatorin has anti-inflammatory [11], anticonvulsant [12], hepatoprotective [13], myorelaxant [14], vasodilator [15] and anti-cancer effects [16]. Isoimperatorin has anti-inflammatory [17], antiallergic [18] and antimicrobial effects [19]. Besides the pharmacological effects of Baizhi furanocoumarins, root extracts contain a variety of phenolic compounds that are connected to its strong antioxidant activity [20]. Recently, foods with antioxidant effects have become valuable for their ability to remove reactive oxygen species, which oxidize lipids, DNA, membranes and proteins and are involved in atherosclerosis, cancers and other diseases [21].
Fresh harvested Baizhi root can be easily infected by Macrophomina phaseolina (Tassi) Goid, a kind of fungal disease, causing root rot [22]. Moreover, with the harvesting of Baizhi concentrated in July and August [23], an efficient storage method is required to prevent root rot and maintain an annual supply. Drying is an efficient and easy method to store food, but it may cause the root to deteriorate. For example, Chinese farmers dry Baizhi with vulcanization fumigation to prevent pests and diseases while also decolorizing the root product, but one study showed that Baizhi loses about 58–67% coumarin after vulcanization fumigation [24]. Therefore, a good drying method should dry the product efficiently but also maintain its pharmacological effects.
The drying temperature may be the most influential factor of product quality. The stability of coumarin in Baizhi root extract increases then decreases with drying at increasingly high temperature [25]. Vacuum freeze-drying is the best method of water removal of food, with final products of the highest quality as compared with other drying methods [26, 27]. During the freeze-drying process, the water in the fresh sample is frozen and then sublimated. The low temperature and lack of water prevent sample deterioration and microbiological contamination. However, the cost for freeze-drying is 4–8 times higher than with hot-air drying, so it is little used in the food industry [27].
Generally, contents of imperatorin and isoimperatorin are used to assess the quality of Baizhi root [22, 28]. However, the multiple constituents in herbal drugs may work synergistically and cannot be easily separated into active parts. As well, the chemical constituents in herbs may vary by the drying process used [29].
In this study, to find an alternative drying process for Baizhi root, we used freeze-drying as the control and compared in-the-shade, 40 and 70 °C drying of root extracts. We also tested the content of six furanocoumarins—xanthotoxin, bergapten, oxypeucedanin, imperatorin, phellopterin and isoimperatorin—in roots with the four drying methods, as well as antioxidant activity by measuring the content of phenolic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity and Fe2+ chelating capacity. Drying Baizhi root with 70 °C drying is better than in-the-shade and 40 °C drying.
Materials and methods
Reagents and chemicals
All solvents were purchased from Merck (Darmstadt, Germany) and were of analytical grade or high-performance liquid chromatography (HPLC) grade. Folin–Ciocalteu, Gallic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 3-(2-pyridyl)-5,6, diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt hydrate were from Merck, and Fe2+ chloride tetrahydrate ferrozine, and furanocoumarin compound standards, including xanthotoxin, bergapten, oxypeucedanin, imperatorin, phellopterin and isoimperatorin were from Merck. The standards for HPLC with diode-array detection (HPLC–DAD) were dissolved in methanol to a concentration of 100 μg/mL and were stored at − 20 °C.
Plant materials
Baizhi was cultivated at the Hualien District Agricultural Research and Extension Station (121°33E, 23°58N), Council of Agriculture, Executive Yuan in November, 2014, and harvested in following May. Plantlets were harvested and the roots were then sliced and mixed. Dried roots were crushed to a powder by using a high-speed disintegrator (Solar Energy Co, Taiwan) and stored at − 20 °C.
Drying method
Four drying methods were applied in this study: freeze-drying and in-the-shade, 40 and 70 °C drying. A freeze-drying system (LYPH. LOCK 18, Labconco, USA) was used to dry root slices. For in-the-shade drying, the flattened root slices were placed under a shadowed and ventilated location at 30 °C for 4 h. Also, root slices were dried with a drying oven (Deng Yng, Taiwan) at 40 or 70 °C. All samples were treated by constant weighting till no weight change. For water-content corrections, a small portion of all samples were further treated by 70 °C drying to completely dry.
Root extracts and sample processing
Extraction for HPLC–DAD was performed as described [28] with some modifications. Methanol (20 mL) was added to 0.2 g ground powder, vortexed and let stand for 30 min. The powder with methanol was ultrasonic-shocked (DC600H, Delta, Taiwan) for 30 min, then centrifuged at 3320 rcf for 3 min. The supernatant was collected and the residue was re-extracted under the same conditions for complete extraction. Finally, the supernatant was mixed and filtered through a 0.2-µm Acrodisc Syringe Filter (Pall, USA) before being collected in the Automatic Sampler-approved certified vials (Agilent, USA). Antioxidant assay extraction was performed as described [30] with some modification. Methanol (20 mL) was added to 1.0 g ground powder, vortexed and shaken overnight (50 rpm), then centrifuged at 3320 rcf for 3 min. The supernatant was collected as the sample.
HPLC–DAD analysis
To identify the furanocoumarin compounds in root extracts, 25 µL samples were separated by using an analytical column (Syncrois C18, 250 × 4.6 mm, 5 µm particle; ThermoFisher, USA) with flow rate 1.0 mL/min at 30 °C and absorbance 310 nm monitored by the ThermoFisher LC system (ThermoFisher, USA) equipped with a ThermoFinnigan UV detector and ThermoFisher Automatic Sampler. The column was protected by a C18 Guard column (HI-5C18-10C5, Hichrom, UK). A binary gradient was used for H2O (Solvent A) and methanol (Solvent B). Optimized pigment separation was achieved with a linear gradient of 40–20% Solvent A for 0–5 min, 20–10% Solvent A for 5–7 min, and 10–0% Solvent A for 7–9 min. After linear gradient was settled, the injections of the first sample were repeated until the displayed detector signal was stable, following samples were then injected.
Total polyphenol content
The total polyphenol content in Baizhi root was determined by using the Folin–Ciocalteu colorimetric method [31]. A mixture of 50 µL of aqueous extract (50 mg/mL) and 50 µL of 1 N Folin–Ciocalteu reagent was incubated for 3 min; 1.0 mL of 2% aqueous sodium carbonate was then added. The reaction mixture was incubated for 30 min in the dark and light absorbance was measured at 750 nm against distilled water as blank. Gallic acid was used as the standard to calculate the total polyphenol content of samples.
DPPH assay
DPPH radical scavenging activity was analyzed as described [30] with some modifications. The DPPH solution in methanol (0.4 mM) was prepared daily, and each antioxidant samples were diluted or concentrated to 83.3, 50, 35.7, 25, 17.86, 14.7, 10, 8.33, 6.25, 4.69, 3.13 mg/mL. An amount of 50 µL sample solutions was loaded on a 96-well plate. Each concentration was loaded in six wells. Three were mixed thoroughly with 150 µL fresh DPPH solution (A1) and the others were mixed thoroughly with 150 µL methanol (A2). Pure methanol was used as the control (C1, C2). The loaded 96-well plates were incubated for 30 min in the dark, then the decrease in light absorbance at 517 nm was measured. The DPPH radical scavenging effects were calculated as .
Fe2+ chelating assay
Fe2+ chelating activity was measured as described [32] with some modification. Antioxidant samples were diluted or concentrated to at least seven concentrations. The Fe2+ chloride tetrahydrate solution in methanol (12 mM) was prepared, and 10.5 µL of this solution was mixed thoroughly with 1400 µL sample solution. Ferrozine solution in methanol (5 mM) was prepared. An amount of 200 µL sample solution was loaded on 96-well plates, with each concentration loaded in six wells. Three wells were thoroughly mixed with 20 µL ferrozin solution (A1) and the others were thoroughly mixed with 150 µLmethanol (A2). Pure methanol was used as the control (C1, C2). The loaded 96-well plates were left to stand for 5 min, then the decrease in light absorbance at 562 nm was measured. The Fe2+ chelating effects were calculated as .
Statistical analysis
Data were analysed by using ANOVA. The source of variation was harvest stage. Significant differences by harvest stage were calculated by the Fisher least significant difference (LSD) test (P < 0.05). Data are expressed as mean ± SD. The IC50 value of DPPH radical scavenging activity and Fe2+ chelating activity was predicted by Loess regression. All analyses involved use of R 3.2.2. P < 0.05 was considered statistically significant.
Results and discussion
Characterization of furanocoumarin compounds in Baizhi root
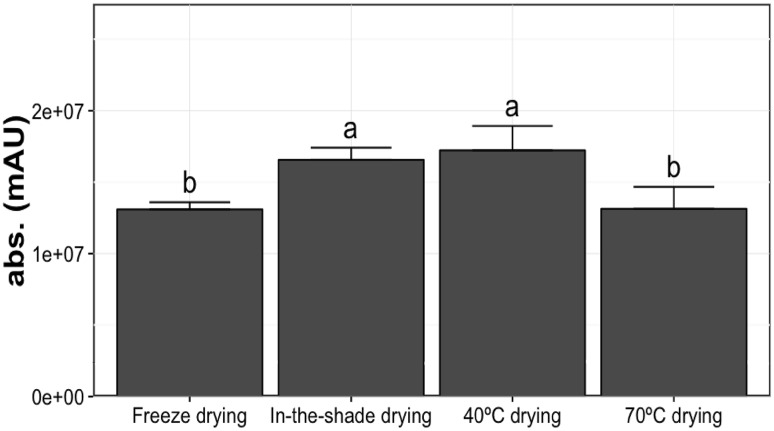
In traditional Chinese medicine, crude Baizhi roots are used as medicine or as functional food for their antipyretic and analgesic activities because of abundant furanocoumarin compounds [3]. Here, we first determined the amount of furanocoumarin compounds in roots by the total coumarin content, then independent active compounds were determined to assess the medical functions [28, 33, 34]. For example, isoimperatorin, imperatorin and oxypeucedanin may be useful for treating Alzheimer disease [10]. Isoimperatorin, imperatorin and phellopterin have potential anti-inflammatory effects [35], whereas xanthotoxin, isoimperatorin, imperatorin and oxypeucedanin have anti-cancer effects [8]. In addition, the synergistic effect of the active compounds may affect the final quality of the dried root. Accordingly, we analyzed the absorbance at 310 nm for root extracts to represent the total coumarin content [36]. Absorbance was slightly greater for roots dried in-the-shade- and at 40 °C than freeze- and 70 °C drying (Fig. 1). Because the freeze-dried roots was used as the control, this result implied that additional coumarin compounds may result from in-the-shade and 40 °C drying.
Fig. 1.
Effect of drying methods on absorbance (310 nm) of dried root extracts, indicating total coumarin content. Different letters indicate difference (P < 0.05) from freeze-drying. Means followed by the same letter(s) are not significantly different at 5% level by Fisher’s least significant difference (LSD) test
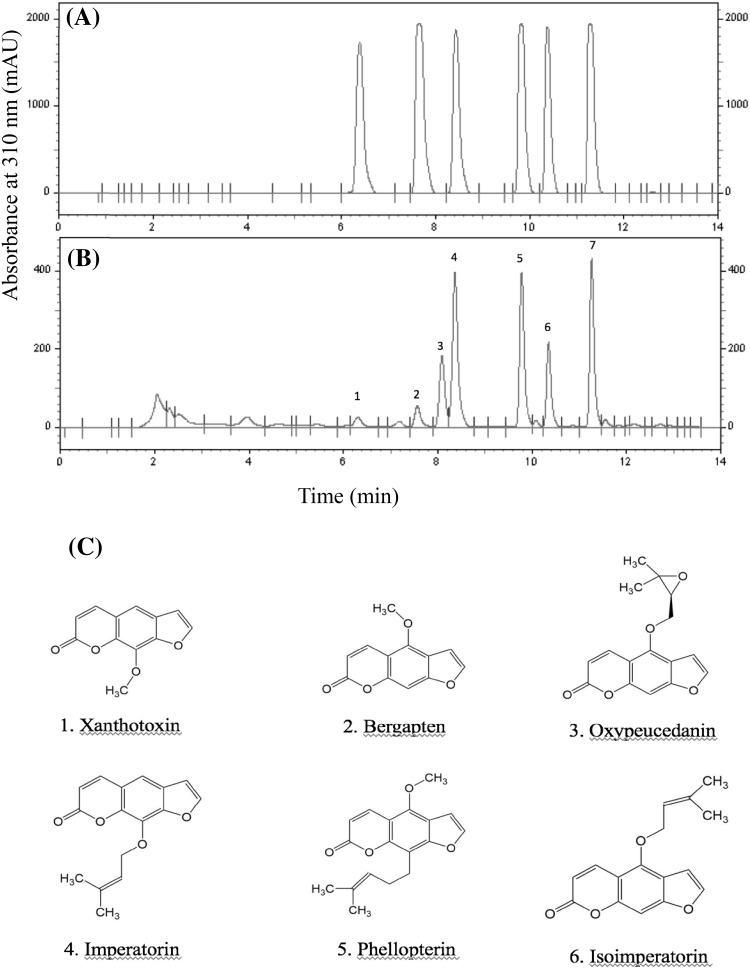
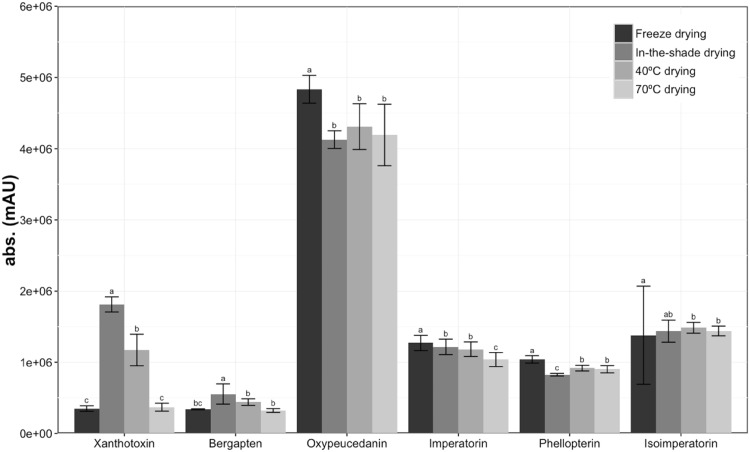
Coumarin in Baizhi is mainly composed of furanocoumarin compounds [3]. We extracted Baizhi root samples for HPLC–DAD chromatography and obtained seven clear peaks. By using reference compounds, we identified peaks 1, 2, 4, 5, 6 and 7 as xanthotoxin, bergapten, oxypeucedanin, imperatorin, phellopterin and isoimperatorin, respectively (Fig. 2). We then analyzed the peak area of six the furancoumarins by using HPLC–DAD at 310 nm to indicate the content of the compounds. We found different patterns of furancoumarin compounds from each dried root. The peak area of xanthotoxin in roots was about 3 and 5 times less with freeze-drying than 40 °C and in-the-shade drying, respectively, and the peak area of bergapten was about 1.3 and 1.6 times less (Fig. 3). The peak areas of the other furanocoumarins, including oxypeucedanin, imperatorin, phellopterin and isoimperatorin, were greater with freeze-drying than the other methods, but the variations were less than 30%.
Fig. 2.
HPLC–DAD chromatogram of standard (A) compared with Baizhi root tissue (B). Peaks 1, 2, 4, 5, 6 and 7 were identified as xanthotoxin, bergapten, oxypeucedanin, imperatorin, phellopterin and isoimperatorin, of which structural formula were shown respectively (C)
Fig. 3.
Xanthotoxin, bergapten, oxypeucedanin, imperatorin, phelllopterin and isoimperatorin content in Baizhi roots dried by four methods. Roots were extracted with methanol and underwent HPLC–DAD analysis (n = 5). Data are mean ± SD absorbance (310 nm). Means within the same treatment followed by the same letter(s) are not significantly different at 5% level by Fisher’s least significant difference (LSD) test
The findings could be explained by the compound stability and the metabolisation of furanocoumarin. In terms of compound stability, xanthotoxin, bergapten, imperatorin and isoimperatorin are relatively stable [37]. Previously, drying under 40 °C did not affect the concentration of imperatorin and isoimperatorin [28]. In addition, the stability was poorer for phellopterin than imperatorin [38], with oxypeucedanin the most unstable compound among the six furanocoumarins. The epoxide ring of oxypeucedanin is easy to hydrolyze [39].
Xanthotoxin and bergapten result from complex furanocoumarin degradation during metabolisation, and imperatorin is demethylated to xanthotoxin [40]. As a result, xanthotoxin and bergapten content was increased with in-the-shade and 40 °C drying. The pattern of furanocoumarin contents changed with different drying processes, which will affect the pharmacological effect of dried root product. Therefore, we analyzed the proportion of peak area for each furanocoumarin compound. The primary difference in peak area proportions was observed for xanthotoxin with freeze-drying, in-the-shade-, 40 and 70 °C-dried root: 2.66 ± 0.21, 10.95 ± 0.21, 6.77 ± 0.78 and 2.82 ± 0.33, respectively (Table 1). The proportions for oxypeucedanin were 36.95 ± 1.45, 24.96 ± 0.75, 24.22 ± 1.75 and 32.13 ± 1.42, respectively. Thus, although the furanocoumarin compounds were degraded in 70 °C-dried root (Fig. 3), the pattern of peak area proportions was more similar with freeze-drying than other drying methods.
Table 1.
Peak area proportion of furanocoumarins compounds in Baizhi roots with four drying methods
| Drying methods | Xanthotoxin | Bergapten | Oxypeucedanin | Imperatorin | Phellopterin | Isoimperatorin |
|---|---|---|---|---|---|---|
| Freeze-dried | 2.66 ± 0.211c | 2.59 ± 0.132b | 36.95 ± 1.45a | 9.72 ± 0.53a | 7.94 ± 0.31a | 12.55 ± 0.64a |
| In-the-shade | 10.95 ± 0.21a | 3.31 ± 0.66a | 24.96 ± 0.75c | 7.33 ± 0.30c | 4.98 ± 0.18c | 8.67 ± 0.52bc |
| 40 °C | 6.77 ± 0.78b | 2.55 ± 0.07b | 24.22 ± 1.75c | 6.99 ± 0.40c | 5.34 ± 0.29c | 8.32 ± 0.86c |
| 70 °C | 2.82 ± 0.33c | 2.46 ± 0.14b | 32.13 ± 1.42b | 7.94 ± 0.21b | 6.91 ± 0.52b | 10.29 ± 2.31b |
1Data are mean ± SD of 5 repeats
2Means within a column followed by the same letter(s) are not significantly different at 5% level by Fisher’s least significant difference (LSD) test
Total phenolic acid
Extracts of Baizhi root have anti-oxidant properties, mostly with high phenolic acid content [20]. We analyzed the total phenolic acid contents of roots by the four drying types. The contents were 1.24 ± 0.26, 2.04 ± 0.20, 2.68 ± 0.20 and 2.02 ± 0.26 mg/g for freeze-, in-the shade, 40 and 70 °C drying, respectively (Table 2). The total phenolic acid content was about 1.6–2.2 times lower with freeze-drying than with other drying methods. Therefore, during in-the-shade, 40 and 70 °C drying, phenolic acid content increased. This increased content may be due to some phenolic acids being produced or released during drying because the tissue structure of the sample was damaged and degrading enzymes, such as for oxidation, hydrolysis or glycolysis, were activated. In addition, damaged tissue structure and enzyme reaction occurs less with freeze-dried samples [41, 42].
Table 2.
Antioxidant activity of Baizhi roots by four drying methods
| Drying methods | Total phenolic acid content (mg/g) | DPPH radical scavenging capacity IC50 value (mg/mL) |
Fe2+ chelating capacity IC50 value (mg/mL) |
|---|---|---|---|
| Freeze-dried | 1.24 ± 0.261c | 23.93 ± 9.392b | 3.12 ± 1.05a |
| In-the-shade | 2.04 ± 0.20b | 3.94 ± 17.15a | 3.85 ± 0.58a |
| 40 °C | 2.68 ± 0.20a | 5.16 ± 14.69a | 3.68 ± 0.69a |
| 70 °C | 2.02 ± 0.26b | 8.35 ± 15.93a | 3.97 ± 0.40a |
Roots were extracted with methanol and underwent Folin–Ciocalteu colorimetric, DPPH and Fe2+ chelating assay
1Data are mean ± SD IC50 (mg/mL) predicted by Loess regression
2Means within a column followed by the same letter(s) are not significantly different at 5% level by Fisher’s least significant difference (LSD) test
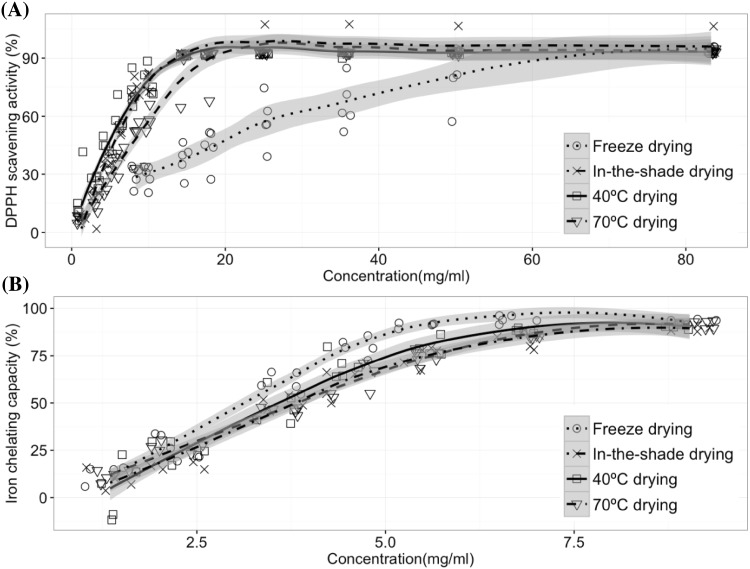
DPPH radical scavenging activity
The variation in total phenolic acid content for roots by drying method should lead to different antioxidant effects. We determined the antioxidant activity of the four drying types by DPPH assay and observed a dose-dependent relation in DPPH scavenging activity of Baizhi root extracts [Fig. 4(A)]. The IC50 value for the extracts was 23.93 ± 9.39, 3.94 ± 17.15, 5.16 ± 14.69 and 8.35 ± 15.93 mg/mL for freeze-, in-the shade, 40 and 70 °C drying, respectively (Table 2). The activity was about 3–6 times less in freeze-drying than with other drying methods, which reflects the variation in phenolic acid content.
Fig. 4.
Antioxidant activities of Baizhi root extracts from roots dried by four methods. Baizhi roots were extracted with methanol, and extracts were diluted or concentrated to at least seven concentrations, and underwent DPPH assay (A) and Fe2+ chelating assay (B)
Fe2+ chelating activity
We also determined antioxidant activity and found a dose-dependent relation on Fe2+ chelating assay of Baizhi root extracts [Fig. 4(B)]. The activity in dried roots did not differ among the drying methods. The IC50 value for the extracts was 3.12 ± 1.05, 3.85 ± 0.58, 3.68 ± 0.69 and 3.97 ± 0.40 mg/mL for freeze-, in-the shade, 40 and 70 °C drying, respectively (Table 2). Therefore, the active compounds for DPPH and Fe2+ chelating mechanisms differ, and the drying method mainly affected a DPPH radical scavenging mechanism instead of Fe2+ chelating.
Quality control for traditional herbs has always been a challenge because multiple factors such as harvest timing, storage condition and drying method affect the pattern of its active compound. Currently, the quality of Baizhi is evaluated by the content of imperatorin, isoimperatorin or total coumarin, which may be inaccurate according to our results. For example, Baizhi is a well-known natural-medicine cosmetic herb with skin-whitening activity with the active compounds imperatorin and isoimperatorin [7], whereas xanthotoxin has a melanogenesis-stimulation effect that may lead to skin pigmentation [43]. We found xanthotoxin content higher in Baizhi roots with 40 °C- and in-the-shade drying than with the other two drying methods. A similar result (high xanthotoxin) was observed by analyzing commercial Baizhi products (data not shown). Therefore, an inappropriate drying method may lead to higher xanthotoxin content and reduce the skin-whitening activity of Baizhi. Thus, in-the-shade and 40 °C drying are not good processes for drying Baizhi root. Also, the phenolic acid content and DPPH scavenging activity was increased in 70 °C-dried root. Hence, 70 °C drying is an appropriate method to reduce Baizhi drying costs.
Acknowledgements
We thank Professor Li-yu D Liu and Chen-An Tsai for description of statistic results. This project was supported by the Council of Agriculture and National Science Council, Executive Yuan, Taiwan, Republic of China.
Abbreviations
- HPLC-DAD
High-performance liquid chromatography with diode-array detection
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
References
- 1.Wang NH, Huang LQ, Yang B, Kimie B, Masahiko T, Yuan CQ, Qin HZ, Shu P. Studies on original plant of traditional chinese drug“Bai Zhi” (Radix Angelicae Dahuricae) and its closely related wild plants IV. discussion on original plant and cultivation history of traditional chinese drug“Bai Zhi”and evolution of its closely related wild plants. China J. Chin. Mater. Med. 26: 733–736 (2001) [PubMed]
- 2.Sarker SD, Nahar L. Natural medicine: The genus Angelica. Curr. Med. Chem. 2004;11:1479–1500. doi: 10.2174/0929867043365189. [DOI] [PubMed] [Google Scholar]
- 3.Li B, Zhang X, Wang J, Zhang L, Gao B, Shi S, Wang X, Li J, Tu P. Simultaneous characterisation of fifty coumarins from the roots of Angelica dahurica by off-line two-dimensional high-performance liquid chromatography coupled with electrospray ionisation tandem mass spectrometry. Phytochem. Analysis. 2014;25:229–240. doi: 10.1002/pca.2496. [DOI] [PubMed] [Google Scholar]
- 4.Piao XL, Park IH, Baek SH, Kim HY, Park MK, Park JH. Antioxidative activity of furanocoumarins isolated from Angelicae dahuricae. J. Ethnopharmacol. 2004;93:243–246. doi: 10.1016/j.jep.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Pervin M, Hasnat MD, Debnath T, Park SR, Kim DH, Lim BO. Antioxidant, anti-Inflammatory and antiproliferative activity of Angelica dahurica root extracts. J. Food Biochem. 2014;38:281–292. doi: 10.1111/jfbc.12046. [DOI] [Google Scholar]
- 6.Yousif AN, Scaman CH, Durance TD, Girard B. Flavor volatiles and physical properties of vacuum-microwave-and air-dried sweet basil (Ocimum basilicum L.) J. Agr. Food Chem. 1999;47:4777–4781. doi: 10.1021/jf990484m. [DOI] [PubMed] [Google Scholar]
- 7.Cho YH, Kim JH, Park SM, Lee BC, Pyo HB, Park HD. New cosmetic agents for skin whitening from Angelica dahurica. J. Cosmet. SCI. 2006;57:11–22. [PubMed] [Google Scholar]
- 8.Kim YK, Kim YS, Ryu SY. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother. Res. 2007;21:288–290. doi: 10.1002/ptr.2043. [DOI] [PubMed] [Google Scholar]
- 9.Kwon YS, Kobayashi A, Kajiyama SI, Kawazu K, Kanzaki H, Kim CM. Antimicrobial constituents of Angelica dahurica roots. Phytochemistry. 1997;44:887–889. doi: 10.1016/S0031-9422(96)00634-6. [DOI] [PubMed] [Google Scholar]
- 10.Marumoto S, Miyazawa M. β-secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phytother. Res. 2010;24:510–513. doi: 10.1002/ptr.2967. [DOI] [PubMed] [Google Scholar]
- 11.García-Argáez AN, Apan TOR, Delgado HP, Velázquez G, Martínez-Vázquez M. Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model. Planta Med. 2000;66:279–281. doi: 10.1055/s-2000-14894. [DOI] [PubMed] [Google Scholar]
- 12.Luszczki JJ, Glowniak K, Czuczwar SJ. Imperatorin enhances the protective activity of conventional antiepileptic drugs against maximal electroshock-induced seizures in mice. Eur. J. Pharmacol. 2007;574:133–139. doi: 10.1016/j.ejphar.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Oh H, Lee HS, Kim T, Chai KY, Chung HT, Kwon TO. Yun YG. Furocoumarins from Angelica dahurica with hepatoprotective activity on tacrine-induced cytotoxicity in Hep G2 cells. Planta Med. 2002;68:463–464. doi: 10.1055/s-2002-32075. [DOI] [PubMed] [Google Scholar]
- 14.Chiou WF, Huang YL, Chen CF, Chen CC. Vasorelaxing effect of coumarins from Cnidium monnieri on rabbit corpus cavernosum. Planta Med. 2001;67:282–284. doi: 10.1055/s-2001-12013. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Wang T, Huang L, Lu W, Zhang J, He H. Synthesis and fluorescent study of 5-phenyl furocoumarin derivatives as vasodilatory agents. Bioorg. Med. Chem. Lett. 2016;26:640–644. doi: 10.1016/j.bmcl.2015.11.056. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Zeng X, Sun J, Li H, Wu P, Fung KP, Liu F. Imperatorin induces Mcl-1 degradation to cooperatively trigger Bax translocation and Bak activation to suppress drug-resistant human hepatoma. Cancer Lett. 2014;348:146–155. doi: 10.1016/j.canlet.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Abad MJ, Heras BDL, Silvan AM, Pascual R, Bermejo P, Rodriguez B, Villar AM. Effects of furocoumarins from Cachrys trifida on some macrophage functions. J. Pharm. Pharmacol. 2001;53:1163–1168. doi: 10.1211/0022357011776432. [DOI] [PubMed] [Google Scholar]
- 18.Ryu SY, Kou NY, Choi HS, Ryu H, Kim TS, Kim KM. Cnidicin, a coumarin, from the root of Angelica koreana, inhibits the degranulation of mast cell and the NO generation in RAW 264.7 cells. Planta Med. 2001;67:172–174. doi: 10.1055/s-2001-11509. [DOI] [PubMed] [Google Scholar]
- 19.Suleimenov EM. Components of Peusedanum morisonii and their antimicrobial and cytotoxic activity. Chem. Nat. Compd. 2009;45:710. doi: 10.1007/s10600-009-9423-x. [DOI] [Google Scholar]
- 20.Wang GH, Chen CY, Tsai TH, Chen CK, Cheng CY, Huang YH, Hsieh MC, Chung YC. Evaluation of tyrosinase inhibitory and antioxidant activities of Angelica dahurica root extracts for four different probiotic bacteria fermentations. J. Biosci. Bioeng. 2017;123:679–684. doi: 10.1016/j.jbiosc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free. Radical. Bio. Med. 2009;47:1673–1706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XU. bai zuo gen fu bing de fa sheng tiao jian yu fang zhi cuo shi. Special Economic Animal and Plant. 2010;13:47. [Google Scholar]
- 23.Chen XF, Lu J, Ding D. bo zhong qi dui bai zuo zao qi chou tai ying xiang de yan jiu. China J. Chin. Mater. Med. 1999;24:211. [Google Scholar]
- 24.Li HY, Dai YJ, Xie CK. zhong yao bai zuo liu xun qian hou xiang dou su cheng fen han liang bi jiao. China J. Chin. Mater. Med. 1991;16:27–28. [Google Scholar]
- 25.He SS, Wu WJ, Tan R. Study on the stability of Angelica dahurica (Fisch ex.Hoffm.) extract. J. North Sichuan Med. Coll. 2013;28:6–9. [Google Scholar]
- 26.Litvin S, Mannheim CH, Miltz J. Dehydration of carrots by a combination of freeze drying, microwave heating and air or vacuum drying. J. Food Eng. 1998;36:103–111. doi: 10.1016/S0260-8774(98)00054-5. [DOI] [Google Scholar]
- 27.Ratti C. Hot air and freeze-drying of high-value foods: a review. J. Food Eng. 2001;49:311–319. doi: 10.1016/S0260-8774(00)00228-4. [DOI] [Google Scholar]
- 28.Shiau YJ, Jeng TL, Kao JL, Lai JS, Huang CH. Extraction of imperatorin from root slices and explants of Angelica dahurica. Journal of Taiwan Agricultural Research. 2011;60:101–114. [Google Scholar]
- 29.Liang YZ, Xie P, Chan K. Quality control of herbal medicines. J. Chromatogr. B. 2004;812:53–70. doi: 10.1016/S1570-0232(04)00676-2. [DOI] [PubMed] [Google Scholar]
- 30.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agr. Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- 31.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 32.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 33.Jia L, Sun HC, Yang TX, Bai BZB, Li CD. Dynamics and correlation analysis of physiology and medicinal components of Angelica dahurica. J. Jilin Agric. Univ. 2008;30:122–127. [Google Scholar]
- 34.Zhai JY, Wu W, Liao K, Zhang X, Hou K. Effects of soil factors on yield and quality of Angelica dahurica var. formosana. Chin. Tradit. Herb. Drugs. 2010;41:984–988. [Google Scholar]
- 35.Deng GG, Wei W, Yang XW, Zhang YB, Xu W, Gong NB, Lü Y, Wang FF. New coumarins from the roots of Angelica dahurica var. formosana cv. Chuanbaizhi and their inhibition on NO production in LPS-activated RAW264.7 cells. Fitoterapia. 2015;101:194–200. doi: 10.1016/j.fitote.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Frérot E, Decorzant E. Quantification of total furocoumarins in citrus oils by HPLC coupled with UV, fluorescence, and mass detection. J. Agr. Food. Chem. 2004;52:6879–6886. doi: 10.1021/jf040164p. [DOI] [PubMed] [Google Scholar]
- 37.Chang Y, Zhang QH, Li J, Zhang L, Guo X, He J, Zhang P, Ma L, Deng Y, Zhang B, Gao X. Simultaneous determination of scopoletin, psoralen, bergapten, xanthotoxin, columbianetin acetate, imperatorin, osthole and isoimperatorin in rat plasma by LC–MS/MS for pharmacokinetic studies following oral administration of Radix Angelicae Pubescentis extract. J. Pharmaceut. Biomed. 2013;77:71–75. doi: 10.1016/j.jpba.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Jiang M, Lu X, Qin F, Song Y, Wen J, Li F. Simultaneous determination of pimpinellin, isopimpinellin and phellopterin in rat plasma by a validated UPLC–MS/MS and its application to a pharmacokinetic study after administration of Toddalia asiatica extract. J. Chromatogr. B. 2012;891–892:102–108. doi: 10.1016/j.jchromb.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Feger W, Brandauer H, Gabris P, Ziegler H. Nonvolatiles of commercial lime and grapefruit oils separated by high-speed countercurrent chromatography. J. Agr. Food Chem. 2006;54:2242–2252. doi: 10.1021/jf052267t. [DOI] [PubMed] [Google Scholar]
- 40.Zhao G, Peng C, Du W, Wang S. Simultaneous determination of imperatorin and its metabolites in vitro and in vivo by a GC-MS method: application to a bioavailability and protein binding ability study in rat plasma. Biomed. Chromatogr. 2014;28:947–956. doi: 10.1002/bmc.3100. [DOI] [PubMed] [Google Scholar]
- 41.Díaz-Maroto M, Pérez-Coello M, Cabezudo M. Effect of different drying methods on the volatile components of parsley (Petroselinum crispum L.) Eur. Food Res. Technol. 2002;215:227–230. doi: 10.1007/s00217-002-0529-7. [DOI] [Google Scholar]
- 42.Que F, Mao L, Fang X, Wu T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Tech. 2008;43:1195–1201. doi: 10.1111/j.1365-2621.2007.01590.x. [DOI] [Google Scholar]
- 43.Matsuda H, Hirata N, Kawaguchi Y, Yamazaki M, Naruto S, Shibano M, Taniguchi M, Baba K, Kubo M. Melanogenesis stimulation in murine B16 melanoma cells by umberiferae plant extracts and their coumarin constituents. Biol. Pharm. Bull. 2005;28:1229–1233. doi: 10.1248/bpb.28.1229. [DOI] [PubMed] [Google Scholar]