Abstract
The aim of this study was to investigate the effect of material type (artichoke leave, lemon peel, flaxseed meal), extraction temperature (50, 100, 120, 140, 160, 180, 200 °C) and static extraction time (5, 15, 30, 45 min) on 5-hydroxymethylfurfural (5-HMF) formation during subcritical water extraction. 5-HMF content of artichoke leave and lemon peel extracts increased 7.2 and 26.1 times with the rise of extraction temperature from 160 to 180 °C for 5 min during subcritical water extraction, respectively. Besides, 5-HMF content of artichoke leave, lemon peel and flaxseed meal extracts increased 1.4, 2.0 and 4.5 times as static extraction time increased from 15 to 45 min at 180 °C during subcritical water extraction, respectively. The highest 5-HMF content of artichoke leave and lemon peel extracts were obtained as 58.83 and 231.21 mg/L at 180 °C and 45 min, respectively. However, for flaxseed meal, the highest 5-HMF content (222.94 mg/L) was obtained at 200 °C and 15 min during subcritical water extraction.
Keywords: 5-Hydroxymethylfurfural, Subcritical water extraction, Lemon peel, Flaxseed meal, Artichoke leave
Introduction
Subcritical water extraction is carried out between 100 and 273 °C and also, high pressure is applied to keep water in the liquid state. Dielectric constant of water decrease with increasing extraction temperature during subcritical water extraction process. So, apolar compounds can be extracted with pressurized hot water or subcritical water [1, 2]. In the literature, it was reported that various phenolic compounds and other antioxidant compounds were extracted from plants, foods and food-by products with subcritical water [1, 3–7].
On the other hand, Maillard reaction (non-enzymatic browning reaction) is occurred during subcritical water extraction [8]. Furtherly, 5-hydroxymethyl furfural (HMF) is formed at high temperatures during thermal processing of foods. Carbohydrates such as fructose, glucose, sucrose, inulin, hemicellulose and cellulose and also, other carbonyl groups as polyphenols and ascorbic acid can be used during 5-HMF formation via the Maillard reaction [9, 10]. During subcritical water extraction, decomposition of oligosaccharides and polysaccharides are occurred at high temperatures under high pressure [11, 12] so, food by-products, which have high amount of inulin, hemicellulose, cellulose and lignin, are potential sources for 5-HMF formation during subcritical water extraction. Because of that, material type is an important factor to form corresponding 5-HMF during subcritical water extraction. Besides, extraction temperature is the most important parameter during subcritical water extraction of bioactive compounds and also, static extraction time is an effective parameter to extract bioactive compounds with subcritical water [3, 4, 13].
However, there are no studies about the effect of material type and static extraction time on 5-HMF formation during subcritical water extraction in the literature and also, there are not sufficient studies [14] about the effect of extraction temperature on 5-HMF formation during subcritical water extraction. The aim of this study was to investigate potential formation of 5-HMF during subcritical water extraction process. For this aim, the effect of material type (different food by-products), extraction temperature (50, 100, 120, 140, 160, 180, 200 °C) and static extraction time (5, 15, 30, 45 min) were investigated. As food by-products, artichoke (Cynara scolymus L.) leave, lemon (C. limon L.) peel and flaxseed (Linum usitatissimum L.) meal were used in this study.
Materials and methods
Material
Artichokes (C. scolymus L.), Sakız variety, were obtained from a natural farm in Aydın and dark green coloured artichoke leaves were used as material. Interdonato Lemons (C. limon L.) were obtained from a natural farm in Muğla and yellow coloured lemon peels were used as material. Lemons were peeled with a stainless steel knife. Artichokes leaves and lemon peels were dried in the vacuum air oven at 50 °C until constant weight. Besides, flaxseed (L. usitatissimum L.) meal sticks, obtained from Bükaş in İzmir, were used as material.
The materials were vacuum packed and stored at – 20 °C. Just before extraction process, the materials were ground with a coffee grinder (Bosch, KM 13) and the particle size range between 600 and 1500 μm was used during subcritical water extraction process.
5-Hydroxymethyl furfural and methanol (Sigma), used for the chromatographic determination of 5-HMF, was of HPLC grade.
Method
Subcritical water extraction process
Subcritical water extraction process was carried out by a Dionex accelerated solvent extractor (ASE) Model 350 equipped with a Solvent Controller (Dionex Corp., Sunnyvale, CA, USA). All extractions were performed in 34 mL extraction cells, containing 1 g of sample for 1500 psi, 5% fresh water and one cycle. Subcritical water extraction process by accelerated solvent extractor is as follows: (1) sample is loaded into the cell; (2) the cell is filled with water up to a pressure of 1500 psi; (3) initial heat-up time is applied; (4) a static extraction with all system valves closed is performed; (5) the cell is rinsed (with 60% cell volume using water); (6) water is purged from the cell with N2 gas and (7) depressurization takes place. After each extraction process, a rinse of the complete system was made in order to overcome any extract carry-over.
Extraction temperature is the most important parameter during subcritical water extraction by accelerated solvent extractor. Static extraction time is also an effective parameter during subcritical water extraction but, it is not just as effective as temperature. Particle size is also an important parameter because, small particle size increases surface area and so, extraction efficiency increases. However, particle size, which was smaller than 65 μm, cause to shut down accelerated solvent extractor because of clogging problem [13]. The particle size range between 600 and 1500 μm was used in this study.
Extraction temperature and static extraction time were independent factors during subcritical water extraction (Table 1). The effect of independent factors on system response was determined by changing the level of one factor and keeping the other factors constant and, it was also reported by Çam and Hışıl [13] and Özkaynak Kanmaz and Ova [3]. The aim of this study was to investigate the effect of material type (different food by-products), extraction temperature (50, 100, 120, 140, 160, 180, 200 °C) and static extraction time (5, 15, 30, 45 min) on 5-HMF formation during subcritical water extraction. As food by-products, artichoke (C. scolymus L.) leave, lemon (C. limon L.) peel and flaxseed (L. usitatissimum L.) meal were used (Table 1).
Table 1.
The research design and subcritical water extraction parametersa for accelerated solvent extractor, ASE 350
| Materials | Extraction temperature (°C) | Static extraction time (min) | Pressure (psi) | Fresh water (%) | Sample amount (g) | |
|---|---|---|---|---|---|---|
| The first part of the study | Artichoke leave | 50 | 5 | 1500 | 5 | 1 |
| 15 | ||||||
| 100 | 5 | 1500 | 5 | 1 | ||
| 15 | ||||||
| 120 | 5 | 1500 | 5 | 1 | ||
| 15 | ||||||
| Lemon peel | 140 | 5 | 1500 | 5 | 1 | |
| 15 | ||||||
| 160 | 5 | 1500 | 5 | 1 | ||
| 15 | ||||||
| 180 | 5 | 1500 | 5 | 1 | ||
| 15 | ||||||
| The second part of the study | Artichoke leave | 180 | 15 | 1500 | 5 | 1 |
| 180 | 30 | 1500 | 5 | 1 | ||
| Lemon peel | 180 | 45 | 1500 | 5 | 1 | |
| Flaxseed meal | 200 | 15 | 1500 | 5 | 1 |
aAll runs were carried out at one cycle
After each ASE run, the extracts were adjusted to 25 mL volume with distilled water and stored at – 40 °C until analyzed. All subcritical water extractions were performed in duplicate.
Determination of 5-hydroxymethyl furfural (HMF) by ultra performance liquid chromatography
5-Hydroxymethyl furfural (HMF) content of subcritical water extracts were analysed by the method of Rada-Mendoza et al. [15] with some modifications. Ultra performance liquid chromatography (UPLC) (Thermo Scientific Accela), which equipped with Photodiode Array (PDA) detector (Thermo Scientific Accela) at 283 nm, was used. The UPLC column was ODS (Inertsil ODS-4, GL Sciences; 250 mm * 4.6 mm * 5 μm) and column temperature was 30 °C.
Before HPLC analysis, all subcritical water extracts were diluted 5 times and filtered through 0.45 μm PVDF syringe filter (Millex). Injection volume was 20 μL. Flow rate of mobile phase was 0.8 mL/min. Mobile phase had a linear gradient from methanol:water (5:95) to methanol:water (80:20) in 6 min and this isocratic elution was applied for 9 min. Then, initial condition (methanol/water: 5/95) was reestablished in 10 min and this isocratic elution was applied for 13 min.
5-HMF (Sigma) was used as an external standard. 5-HMF content of subcritical water extracts were calculated against the calibration standard curve, which has concentrations of 0, 1, 2, 5 and 10 ppm (R2: 0.9995). Besides, LOD (limit of detection) and LOQ (limit of quantification) were determined as 0.15 and 0.45 ppm, respectively. 5-HMF content of all subcritical water extracts were analysed in triplicate.
Statistical analysis
The study results were interpreted by analysis of variance (ANOVA) with post hoc test, least significant difference (LSD) test and t test using SPSS software package. The statistical significance was evaluated at p < 0.05 level.
Results and discussion
The study results suggested that material type, extraction temperature and static extraction time had statistically significant (p < 0.05) effect on 5-HMF formation during subcritical water extraction (Tables 2, 3). 5-HMF content of artichoke leave and lemon peel extracts were determined as 1.28 and 0.64 mg/L at 140 °C during subcritical water extraction, respectively. However, 5-HMF content of artichoke leave and lemon peel extracts increased 5.4 and 20.8 times with the rise of extraction temperature from 140 to 160 °C for 15 min during subcritical water extraction, respectively (Table 2).
Table 2.
5-HMF content of subcritical water extracts at different extraction temperatures for artichoke leave and lemon peel
| Extraction temperature (°C) | Static extraction time (min) | 5-HMF content of artichoke leave (mg/L) | 5-HMF content of lemon peel (mg/L) |
|---|---|---|---|
| 50 | ND | ND | |
| 100 | ND | ND | |
| 120 | ND | ND | |
| 140 | 5 | ND | ND |
| 160 | 1.43 ± 0.01b | 0.97 ± 0.01b | |
| 180 | 10.32 ± 0.06a | 25.27 ± 0.19a | |
| 50 | ND | ND | |
| 100 | ND | ND | |
| 120 | ND | ND | |
| 140 | 15 | 1.28 ± 0.01c | 0.64 ± 0.01c |
| 160 | 6.87 ± 0.06b | 13.33 ± 0.03b | |
| 180 | 42.71 ± 1.17a | 115.13 ± 0.93a |
Values are means ± standard deviations of three (n = 3) measurements
ND not determined
Values with different superscript letters within a column are significantly different at p < 0.05
Table 3.
5-HMF content of subcritical water extracts obtained from food-by products at 180 and 200 °C
| Extraction temperature (°C) | Static extraction time (min) | 5-HMF content of artichoke leave (mg/L) | 5-HMF content of lemon peel (mg/L) | 5-HMF content of flaxseed meal (mg/L) |
|---|---|---|---|---|
| 180 | 15 | 42.71 ± 1.17c | 115.13 ± 0.93d | 47.49 ± 0.11c |
| 180 | 30 | 48.90 ± 0.18b | 203.39 ± 1.32b | 122.73 ± 4.23b |
| 180 | 45 | 58.83 ± 0.18a | 231.21 ± 3.14a | 215.02 ± 13.20a |
| 200 | 15 | 44.49 ± 0.10c | 181.45 ± 2.76c | 222.94 ± 13.67a |
Values are means ± standard deviations of three (n = 3) measurements
Values with different superscript letters within a column are significantly different at p < 0.05
180 °C was obtained as a critical extraction temperature for 5-HMF formation during subcritical water extraction of lemon peel (Table 2). 5-HMF content of lemon peel extracts increased 26.1 and 8.6 times as extraction temperature increased from 160 to 180 °C for 5 and 15 min during subcritical water extraction, respectively. The noticeably rise of 5-HMF formation at 180 °C could be explained with decomposition of hemicellulose, which was found in lemon peel as 8.09% in dry weight [16]. Because, hemicellulose is formed by different sugar units such as glucose, galactose, mannose, xylose and arabinose so, hemicellulose is hydrolysed faster than cellulose [9]. Also, Xiao et al. [17] reported that hemicellulose components such as glucose, galactose, xylose and arabinose reached to the highest level at 160 °C and began to decrease at 180 °C during hot compressed water pretreatment of woody biomass. As static extraction time increased from 15 to 30 min at 180 °C, 5-HMF content of lemon peel extract increased 1.8 times and also, 5-HMF content of lemon peel extracts increased 1.6 times with the rise of extraction temperature from 180 to 200 °C for 15 min during subcritical water extraction (Table 3). The rise of 5-HMF formation at high extraction temperatures could be also explained with high flavonoid content (12.54%) of lemon peel [18]. Also, Pourali et al. [11] reported that eleven phenolic compounds were identified after rice bran thermally decomposed during subcritical water extraction. Also, rise of 5-HMF formation at high extraction temperatures could be explained with decomposition of cell wall at high temperatures under high pressure during subcritical water extraction and so, high lignin content (7.56%) of lemon peel [16]. Wahyudiono et al. [12] also reported that lignin decomposed into phenolic compounds during subcritical and supercritical water extraction because, secondary metabolites such as flavonoids (luteolin and apigenin) and phenolic acids (chlorogenic acid and dicaffeoylquinic acids) had precursor role during biosynthesis of lignin in plants.
For artichoke leave, 180 °C was also obtained as a critical extraction temperature for 5-HMF formation during subcritical water extraction (Table 2). 5-HMF content of artichoke leave extracts increased 7.2 and 4.8 times as extraction temperature increased from 160 to 180 °C for 5 and 15 min during subcritical water extraction, respectively (Table 2). The noticeably rise of 5-HMF formation at 180 °C could be explained with decomposition of inulin, which was found in artichoke leave as 11.86% in dry weight [19]. Besides, the brown coloured bubbles first occurred at 180 °C for 5 and 15 min during subcritical water extraction of artichoke leave (Fig. 1). The highest 5-HMF content of artichoke leave and lemon peel extracts were obtained as 58.83 and 231.21 mg/L at 180 °C and 45 min, respectively However, for flaxseed meal, the highest 5-HMF content (222.94 mg/L) was obtained at 200 °C and 15 min during subcritical water extraction (Table 3). The noticeably low 5-HMF content of artichoke leave could be explained with high cellulose content of artichoke leave (53.78%) as compared with lemon peel (23.06%) and flaxseed meal (21.89%) [16, 19, 20]. Because, decomposition of cellulose occurred at higher extraction temperatures above 200 °C during subcritical water extraction. Also, Sasaki et al. [21] reported that cellulose was hydrolyzed to sugars and 5-HMF at high extraction temperatures around critical point (273 °C) during subcritical water extraction and supercritical water extraction. Also, Bahari [22] reported that cellulose hydrolysis was fast during alkali hydrolysis whereas, acidic and hydrothermal conditions had slow reaction rate.
Fig. 1.
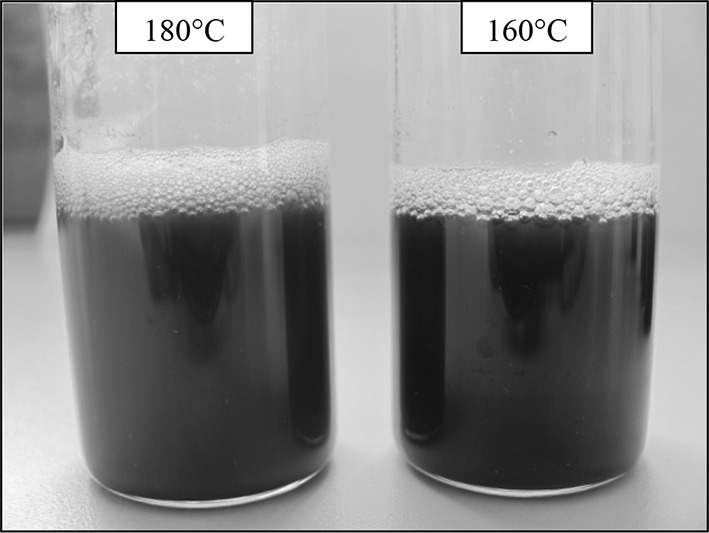
Subcritical water extracts from artichoke leave at 160 and 180 °C for 15 min
During subcritical water extraction of flaxseed meal, 5-HMF content of water extract increased 2.6 times with the rise of static extraction time from 15 to 30 min at 180 °C. Also, 5-HMF content of flaxseed meal extract increased 1.8 times with the rise of static extraction time from 30 to 45 min at 180 °C and reached to 222.94 mg/L at 200 °C, 15 min during subcritical water extraction (Table 3). The rise of 5-HMF formation at 180 °C could be explained with high secoisolariciresinol diglucoside (SDG) lignan content of flaxseed meal. Özkaynak Kanmaz [4] also reported that SDG lignan content of flaxseed meal extract decreased 1.2 times with the rise of static extraction time from 15 to 30 min at 180 °C whereas, total flavonoid content increased 1.2 times during subcritical water extraction. Also, the rise of 5-HMF formation could be explained with phenolic compounds, which were reported as potential sources for 5-HMF formation by Manzocco et al. [10].
This study results also suggested that there was not a relationship between 5-HMF content and the colour of subcritical water extracts. In the literature, Ramírez-Jiménez et al. [23] also reported that a linear correlation was not found between 5-HMF content and 100-L* (r2 = 0.004) value of the selected bakery products. During subcritical water extraction of flaxseed meal, that dark coloured extracts occurred with the rise of extraction temperature and it was also reported by Özkaynak Kanmaz and Ova [3]. However, subcritical water extracts from lemon peel had the same colour such as dark brown-black at 160 and 180 °C for 15 min and also at 180 °C, 5 min (Fig. 2). On the other hand, the colour of artichoke leave extracts was dark green-black at each extraction temperature (from 50 to 200 °C) for 5 and 15 min during subcritical water extraction. It could be explained with high polyphenoloxidase enzyme activity and so, enzymatic browning reaction. In the literature, it was reported that polyphenoloxidase catalyzed the hydroxylation of monophenols to o-diphenols and o-quinones and so, peroxidase occurred by oxidation of o-diphenols [24] and then quinones polymerized to dark pigments [25]. Besides, Tuncay and Yagar [25] reported that thermal stability of polyphenoloxidase enzyme in artichoke head was better than artichoke leaves.
Fig. 2.

Subcritical water extracts from lemon peel at 160 and 180 °C for 5 and 15 min, respectively
In the literature, it was reported that instant coffee had 5-HMF content between 91.3 and 3060 mg/kg by Husøy et al. [26]. Czerwonka et al. [27] also reported that instant coffee had 5-HMF content between 854.5 and 6099.3 mg/kg. However, 5-HMF content of subcritical water extracts obtained from artichoke leave, flaxseed meal and lemon peel were noticeably lower than instant coffee. Besides, Ramírez-Jiménez et al. [23] reported that 5-HMF content of bakery products varied between 4.1 to 151.2 mg/kg. On the other hand, Rosatella et al. [9] and Abraham et al. [28] reported that 5-HMF did not have toxic effect on human health. However, 5-HMF was metabolized to more toxic molecule such as 5-sulfooxymethylfurfural (5-SMF) [28].
Acknowledgements
The present study was financially supported as two project (Project Nos. 2014.M80.02.03, 2014.M80.02.04) by Artvin Çoruh University Scientific Research Project Unit. The author also thank to “Food Safety and Agricultural Research Center” in Akdeniz University for technical help using Accelerated Solvent Extractor, ASE 350 and also UPLC.
References
- 1.Ko MJ, Cheigh CI, Chung MS. Relationship analysis between flavonoids structure and subcritical water extraction (SWE) Food Chem. 2014;143:147–155. doi: 10.1016/j.foodchem.2013.07.104. [DOI] [PubMed] [Google Scholar]
- 2.Bubalo MC, Vidović S, Redovniković IR, Jokić S. Green solvents for green technologies. J Chem. Technol. Biotechnol. 2015;90:1631–1639. doi: 10.1002/jctb.4668. [DOI] [Google Scholar]
- 3.Özkaynak Kanmaz E, Ova G. The effective parameters for subcritical water extraction of SDG lignan from flaxseed (Linum usitatissimum L.) using accelerated solvent extractor. Eur. Food Res. Technol. 2013;237(2):159–166. doi: 10.1007/s00217-013-1974-1. [DOI] [Google Scholar]
- 4.Özkaynak Kanmaz E. Subcritical water extraction of phenolic compounds from flaxseed meal sticks using accelerated solvent extractor (ASE) Eur. Food Res. Technol. 2014;238:85–91. doi: 10.1007/s00217-013-2088-5. [DOI] [Google Scholar]
- 5.Duba KS, Casazza AA, Mohamed HB, Perego P, Fiori L. Extraction of polyphenols from grape skins anddefatted grape seeds using subcritical water: Experiments and modeling. Food Bioproducts Process. 2015;94:29–38. doi: 10.1016/j.fbp.2015.01.001. [DOI] [Google Scholar]
- 6.Vergara-Salinas JR, Vergara M, Altamirano C, Gonzalez A, Correa JR. Characterization of pressurizedhot water extracts of grape pomace: chemical andbiological antioxidant activity. Food Chem. 2015;171:62–69. doi: 10.1016/j.foodchem.2014.08.094. [DOI] [PubMed] [Google Scholar]
- 7.Yu XM, Zhu P, Zhong QP, Li MY, Ma HR. Subcritical water extraction of antioxidant phenolic compounds from XiLan olive fruit dreg. J Food Sci Technol. 2015;52(8):5012–5020. doi: 10.1007/s13197-014-1551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kodama S, Shoda T, Machmudah S, Wahyudiono KH, Goto M. Extraction of β-glucan by hydrothermal liquidization of barley grain in a semi-batch reactor. Separation Sci. Technol. 2016;51(2):278–289. doi: 10.1080/01496395.2015.1086377. [DOI] [Google Scholar]
- 9.Rosatella AA, Simeonov SP, Frade RFM, Afonso CAM. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011;13:754–793. doi: 10.1039/c0gc00401d. [DOI] [Google Scholar]
- 10.Manzocco L, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2001;11:340–346. doi: 10.1016/S0924-2244(01)00014-0. [DOI] [Google Scholar]
- 11.Pourali O, Asghari FS, Yoshida H. Production of phenolic compounds from rice bran biomass under subcritical water conditions. Chem. Eng. J. 2010;160(1):259–266. doi: 10.1016/j.cej.2010.02.057. [DOI] [Google Scholar]
- 12.Sasaki M, Goto M. Recovery of phenolic compounds through the decomposition of lignin in near and supercritical water. Chem. Eng. Process. 2008;47(9–10):1609–1619. [Google Scholar]
- 13.Çam M, Hışıl Y. Pressurized water extraction of polyphenols from pomegranate peels. Food Chem. 2010;123:878–885. doi: 10.1016/j.foodchem.2010.05.011. [DOI] [Google Scholar]
- 14.Herrero M, Castro-Puyana M, Rocamora L, Ferragut JA, Cifuentes A, Ibáñez E. Formation and relevance of 5-hydroxymethylfurfural in bioactive subcritical water extracts from olive leaves. Food Res. Int. 2012;47(1):31–37. doi: 10.1016/j.foodres.2012.01.008. [DOI] [Google Scholar]
- 15.Rada-Mendoza M, Olano A, Villamiel M. Determination of hydroxymethylfurfural in commercial jams and in fruit-based infant foods. Food Chem. 2002;79:513–516. doi: 10.1016/S0308-8146(02)00217-0. [DOI] [PubMed] [Google Scholar]
- 16.Marín FR, Soler-Rivas C, Benavente-García O, Castillo J, Pérez-Alvarez JA. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007;100:736–741. doi: 10.1016/j.foodchem.2005.04.040. [DOI] [Google Scholar]
- 17.Xiao LP, Sun ZJ, Shi ZJ, Xu F, Sun RC. Impact of hot compressed water pretreatment on the structural changes of woody biomass for bioethanol production. Bioresources. 2011;6(2):1576–1598. [Google Scholar]
- 18.Wang YC, Chuang YC, Hsu HW. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008;106:277–284. doi: 10.1016/j.foodchem.2007.05.086. [DOI] [Google Scholar]
- 19.Mayor L, Calvo A, Moreira R, Fito P, Garcia-Castello E. Water sorption isotherms of globe artichoke leaves. Agric. Sci. 2013;4:63–69. [Google Scholar]
- 20.Filipović J, Košutić M, Filipović V, Razmovski R. Chemical composition of fatty acids in spelt and flaxseed pasta. J. Process Energy Agric. 2016;20(3):140–142. [Google Scholar]
- 21.Sasaki M, Kabyemela B, Malalulan R, Hirose S, Takeda N, Adschiri T, Arai K. Celluluse hydrolysis in subcritical and supercritical water. J. Supercritical Fluids. 1998;13:261–268. doi: 10.1016/S0896-8446(98)00060-6. [DOI] [Google Scholar]
- 22.Bahari A. Subcritical Water Mediated Hydrolysis of Cider Lees as a Route for Recovery of High Value Compounds. Doctor of philosophy thesis. School of Chemical Engineering College of Engineering and Physical Sciences University of Birmingham. 235 p. (2010)
- 23.Ramírez-Jiménez A, García-Villanova B, Guerra-Hernández E. Hydroxymethylfurfural and methylfurfural content of selected bakery products. Food Res. Int. 2000;33(10):833–838. doi: 10.1016/S0963-9969(00)00102-2. [DOI] [Google Scholar]
- 24.Jiang Y, Duan X, Joyce D, Zhang Z, Li J. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004;88:443–446. doi: 10.1016/j.foodchem.2004.02.004. [DOI] [Google Scholar]
- 25.Tuncay D, Yagar H. Comparison of polyphenol oxidases prepared from different parts of artichoke (Cynara scolymus L.) Int. J. Food Properties. 2011;14(4):809–821. doi: 10.1080/10942910903453363. [DOI] [Google Scholar]
- 26.Husøy T, Haugen M, Murkovic M, Jobstl D, Stolen LH, Bjellaas T, Ronningborg C, Glatt H, Alexander J. Dietary exposure to 5-hydroxymethylfurfural from Norwegian food and correlations with urine metabolites of short-term exposure. Food Chem. Toxicol. 2008;46:3697–3702. doi: 10.1016/j.fct.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 27.Czerwonka M, Opiłka J, Tokarz A. Evaluation of 5-hydroxymethylfurfural content in non-alcoholic drinks. Eur. Food Res. Technol. 2018;244:11–18. doi: 10.1007/s00217-017-2933-z. [DOI] [Google Scholar]
- 28.Abraham K, Gürtler R, Berg K, Heinemeyer G, Lampen A, Appel KE. Toxicology and risk assessment of 5-hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011;55:667–678. doi: 10.1002/mnfr.201000564. [DOI] [PubMed] [Google Scholar]


