Abstract
The effect of different extraction methods i.e. extraction with alkali (AEDF), enzyme (EEDF) and enzyme plus shear emulsifying hydrolysis (SEDF) on structure, physiochemical as well as the functional characteristics of dietary fiber (DF) from defatted walnut flour were studied. AEDF process showed significantly higher (P < 0.05) amount of water retention capacity (WRC; 5.39 g/g), water swelling capacity (WSC; 3.16 g/mL), and particle size; while, shown lower value of oil adsorption capacity (OAC; 29 g/g) amongst all. Compared to AEDF, no major differences were observed in network except the matrix in EEDF and SEDF was more porous and honey comb like. DF extracted through AEDF, EEDF and SEDF showed good viscosity and emulsifying activity however, less stability indices. The results from this study suggest that AEDF and EEDF and SEDF had specific effects on the structure-functional properties of DF from defatted walnut flour, which has great potential in food applications.
Keywords: Defatted walnut flour, Dietary fiber, Structural, Physicochemical and functional properties, Extraction methods, Proximate composition
Introduction
Dietary fiber (DF) is one of the most important edible parts of plants that remain undigested in human small intestine followed by complete fermentation in the large intestine, where it continues to impart its functionality [1]. The complete digestion of DF promotes healthy benefits including weight control, colon cancer prevention, cholesterol and glycemic index reductions [22]. Various vegetables, fruits and seeds have been used for extraction of DF e.g. deoiled cumin, coconut kernel, barley grains, soya meal and rice bran [2, 20, 26, 33].
Recently, chemical, enzymatic and chemical-enzymatic processing showed safe processing for extraction of DF [20]. However, each method has advantageous and adverse effects on DF properties [22].The effects of different extraction conditions on physicochemical and functional properties of DF have recently been reported for cumin seeds, rice bran and soy pods [7, 20, 24]. Many different methodologies have been adopted for extraction of DF, either directly or from by-product [6].
Walnut flour consists of high protein content, fiber, copper, zinc, iron and vitamin E [17].Walnut flour has been used in desserts, cakes and confectionary, as well as in many savory dishes such as soups, sauces, traditional bakery, ice cream, chocolate baked products such as walnut brownies and many more. The increasing market need of walnut lipids because of its beneficial health applications results in the large amount of the by-product: defatted walnut flour, which contains nutritional fiber and protein. It is essential to improve the economic value of the defatted walnut flour, which is incorporated in food products development or used as feeds for animals [16]. The remaining residue after extraction of oil from walnut can be used to isolate DF.
According to literature no or little information have been reported for extraction and utilizations of DF from defatted walnut flour. Therefore, this study is aimed to extract DF from defatted walnut flour with recently reported technologies e.g. AEDF, EEDF, and SEDF. The results of this study will provide potential application of DF from byproduct in walnut oil industries.
Methods and materials
Defatted walnut flour preparation
Defatted walnut flour was prepared according to [29]. Simply, walnuts were ground to paste, followed by mixing with n-hexane with ratio of 1:10 (w/v). The slurry was magnetically stirred for 3 h at ambient temperature followed by vacuum filtration. The filtrates were again dissolved in methanol with same ratio for complete removal of oil and were vacuum filtered. The resultant flour was dried in fume hood for 24 h at 45 °C and then was ground into fine powder.
DF extraction
Three different methods were used for the extraction of DFs from defatted walnut flour: AEDF, EEDF, and SEDF.
AEDF 100 g of defatted walnut flour was mixed in 1.5 L of 50 mM NaOH and was stirred at 100 rpm at 45 °C. The slurry pH was adjusted to 7 with 50 mM HCl and was centrifuged with speed of 10,000 g for 30 min [13].
EHDF 100 g of defatted walnut flour was mixed with deionized water (3.5 L), followed by hydrolyzation with alcalase (Sigma-Aldrich) 4.5% (w/w, pH 7.7) for 3 h. The mixture was heated for 15 min to terminate hydrolysis and was centrifuged at 10,000 g for 30 min [16].
SEDF 100 g of defatted walnut flour was mixed with 3.5 L of deionized water at 25 °C, shear emulsified at 22,000 rpm for 15 min, and hydrolyzed like EHDF.
The three extracts were washed and dried in fume hood at 50 °C for 24 h. All of the extracts were smash into fine powder for further analysis.
Proximate composition
Recommended methods [4] were used for determination of moisture, protein, fat, starch, ash, total DF, insoluble DF and soluble DF.
Scanning electron microscopy (SEM)
The measurements of surface and microstructure of DFs were carried out using SEM, Model VEGA TESCON with variable pressure. Gold sputtering of samples was carried out and images were taken at different magnifications.
Fourier-transformed infrared spectroscopy (FTIR)
DFs were thoroughly mixed with KBr (1:250 w/w) and pelletized. IR spectra of the DFs of three extracts were taken on a Thermo-Nicolet 6700 FTIR Spectrometer at the frequency range of 4000–650 cm−1 on ATR mode with 256 scans at 8 cm−1 resolution.
Particle size (D3,2 (µm))
Particle Size Analyzer (Model; BT-9300, Dandong Baite Instrument Co., Ltd., Dandong, Liaoning, China) was used in the measurements of particle size D3,2 (µm) of the selected samples (AEDF, EEDF and SEDF) according to the standard method [20].
Physicochemical properties
The hydration properties
The hydration properties are generally fiber specific and can explain various biological effects such as induction of colonic fermentation and increase in stool weight. These could also alter the texture and aspect of food products. The hydration properties include parameters such as water retention capacity (WRC) and water swelling capacity (WSC). Water retention capacity is the measurements of the amount of water held after application of an external force such as centrifugation. On the other hand, “Water swelling capacity is used to quantify the volume occupied by hydrated fibers or the volume occupied by known weight of fiber under the conditions used; measured as settled bed volume” [27]. An increased WRC and WSC vales in DFs result in slowing down the gastric emptying, increase in stomach distension thus causing satiation [30]. DF with enhanced WRC and SWC reduce the intestinal absorption of carbohydrates, and re-absorption of bile acids thus preventing the risk of cardiovascular diseases as well as diabetes [11].
Water retention capacity (WRC)
The WRC parameter was measured according to the modified method (18). One gram of DFs was dissolved in 30 mL distilled water at ambient temperature for 24 h followed by centrifugation at 3000 g for 20 min. The weight of the follow-on filtrate was noted before and after drying at 100 °C. Following Eq. (1) was used for the calculation of WRC
| 1 |
mf = weight of the fresh filtrate (g) and md = weight of the dry filtrate (g).
Water swelling capacity (WSC)
Standard method was used to determine the WSC was [28]. According to this method, 0.2 g DFs was hydrolyzed in distilled water (10 mL) for 18 h. The settled volume in graduated cylinder was recorded and following Eq. (2) was used for the calculation of WSC
| 2 |
υ1 = volume of the hydrated DF, υ0 = volume of DF earlier to hydration, and W0 = weight of DF earlier to hydration.
Oil adsorption capacity (OAC)
The OAC parameter was measured according to the method [2], with minor amendment. DFs (0.2 g) were hydrated with 30 mL sunflower oil for 18 h followed by centrifugation at 2000 g for 20 min. The amount of oil hold by DF was calculated as OAC using Eq. (3).
| 3 |
mr = residue weight, which contained the oil (g) and md = original weight of DF (g).
Functional properties
Emulsifying properties
Emulsifying properties of the samples were determined using emulsifying activity index (EAI) and stability index (ESI) according to the method [34]. In EAI measurements, DFs (7 g) was soaked in slurry of water and sun flower oil (100 mL each), followed by emulsification in homogenizer (D-160 DLAB, China) for 1 min at 10,000 rpm. The resulted emulsion was separately taken in 50 mL centrifuge tubes and re-centrifuged for 5 min at 2000 rpm. Emulsifying activity was obtained from the following Eq. (4).
| 4 |
For the determination of ESI, same protocol was adopted as previously in EAI with addition to heat the emulsion at 70 °C for 40 min followed by cooling with water and centrifuged afterwards for 5 min at 2000 rpm. ESI measurements were carried out using Eq. (5).
| 5 |
Viscosity
Viscosities of the DFs were determined using the method [10]. Simply 1, 3, 5 and 7% (w/v) concentration of DFs were mixed with water at high speed in a blender for 60 s. The mixture was allowed to settle down at 25 °C for 24 h before viscosity measurements. The viscosity was determined using a NDJ-8S Digital rotary viscometer at 60 rpm at room temperature.
Statistical analysis
All the experiments were performed in triplicate. The means were compared by the Duncan multiple-range test at P < 0.05 using SAS 8.1 software.
Results and discussion
Proximate composition
The proximate composition of defatted walnut flour and DFs extracted by AEDF, EEDF and SEDF technologies are shown in Table 1. The data in the table were expressed as g/100 g dry basis except for moisture (count in %) and minerals (count in ppm). Major composition of defatted walnut flour included: TDF (49.31 g/100 g), fat (0.24 g/100 g), protein (32.61 g/100 g) and ash (1.39/100 g). By comparing the composition of defatted walnut flour and AEDF, EEDF and SEDF extracted DFs shown significantly higher content (P < 0.05) of TDF (58.11 g/100 g, 61.73 g/100 g and 69.59 g/100 g); while significant decrease was observed for fat (0.21 g/100 g, 0.22 g/100 g and 0.19 g/100 g) and protein contents (8.67 g/100 g, 23.56 g/100 g and 14.81 g/100 g, P < 0.05). Among extraction methods, the lower protein content was reported for AEDF, probably due to the denaturation of protein in long extraction method of DFs with NaOH. Furthermore, TDF reported for SEDF remained lower as compared to mango, lemon and cumin seeds DFs respectively (74 g/100 g, 70.76 g/100 g and 84.14 g/100 g) [12, 15, 20].
Table 1.
Proximate composition of defatted walnut flour and dietary fiber (DF) obtained by three different methods
| AEDF | EEDF | SEDF | Defatted walnut flour | |
|---|---|---|---|---|
| Moisture | 2.41 ± 0.13C | 2.46 ± 0.05C | 3.55 ± 0.07B | 5.11 ± 0.44A |
| Protein | 8.67 ± 0.12D | 23.56 ± 0.28B | 14.81 ± 0.04C | 32.61 ± 0.08A |
| Fat | 0.21 ± 0.01BC | 0.22 ± 0.02B | 0.19 ± 0.02C | 0.24 ± 0.83A |
| Ash | 1.61 ± 0.05C | 2.5 ± 0.14A | 1.85 ± 0.03B | 1.39 ± 0.07D |
| TDF | 58.11 ± 0.53C | 61.73 ± 0.22B | 69.59 ± 0.93A | 49.31 ± 0.61D |
| SDF | 0.88 ± 0.07D | 3.85 ± 0.26C | 6.53 ± 0.91B | 9.36 ± 0.22A |
| IDF | 72.61 ± 0.33C | 75.59 ± 0.29B | 78.39 ± 0.41A | 29.77 ± 0.51D |
| Cu | 19.00 ± 1.41BC | 21.00 ± 0.70B | 21.00 ± 0.16B | 24.00 ± 0.39A |
| Fe | 22.00 ± 0.34D | 24.00 ± 0.53C | 25.00 ± 0.09B | 29.00 ± 0.87A |
| Zn | 28.00 ± 0.87D | 32.00 ± 0.88C | 35.00 ± 0.10B | 39.00 ± 0.17A |
| Ni | 00.20 ± 0.11BC | 00.18 ± 0.17C | 00.21 ± 0.51B | 00.27 ± 0.37A |
| Pb | 00.20 ± 0.71D | 00.22 ± 0.19C | 0.24 ± 0.88B | 00.29 ± 0.71A |
| D3,2 µm | 86.03 ± 1.22A | 70.27 ± 1.79B | 70.83 ± 1.42B |
Values were mean ± SD of three determinations. Data were expressed as g/100 g dry basis except for moisture content count in (%) and minerals count in (parts per million, ppm). Values of A–D indicate significant difference among each row (P < 0.05). AEDF, dietary fiber obtained by alkali extraction; EHDF, dietary fiber obtained by enzymatic hydrolysis; SEDF, dietary fiber obtained by shear emulsifying assisted enzymatic hydrolysis; TDF, total dietary fiber; SDF, soluble dietary fiber; IDF, insoluble dietary fiber; Particle size distribution D3,2 µm
Higher content of SDF was observed for SEDF (6.53 g/100 g) with ratio of 12.00 which was significantly lower (P < 0.05) as compared to AEDF and EEDF (82.52 and 19.64), maybe due to the breakage of glycosidic bonds in DF which results in loss of SDF, and thus affects the ratio between IDF and SDF [18]. Ma and Mu [20] reported similar results for SEDF in cumin seeds. Additionally, the SDF content of SEDF was observed as higher than that of coconut, rice and oat DFs respectively [9, 25]. Consumption of SDF results in reduction of blood cholesterol as well as helps in maintaining proper insulin level [11]. Increase in SDF can reduce oil absorption, thus imparts beneficial properties to the fibers incorporated traditional food products [32]. Consequently, all the extracts could be used as valuable additives in various food products.
Scanning electron microscopy (SEM)
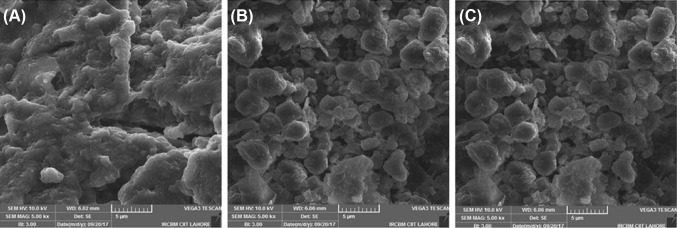
The structural characterization of AEDF, EEDF and SEDF by SEM are shown in Fig. 1. The structures reveal no major differences in network except the matrix in EEDF and SEDF is more porous (holes and crakes) and honey comb like while in AEDF, the matrix is not such intact (Fig. 1(A)). This may be attributed to the fact that alkali removes less protein than enzymatic hydrolysis. Under the same alkaline conditions, the micro structures were not much destroyed as predicted by Ma and Mu [20] however, the matrix vanished. These results reveal that the network in DF of defatted walnut flour is not much affected by alkali extraction method due to the possible resistance. Moreover, the SEDF has not affected the matrix structure, since the honey comb structure in both EEDF and SEDF look alike (Fig. 1(B )and (C)).
Fig. 1.
Scanning electron microscopy (SEM) of dietary fiber obtained from defatted walnut flour by three different methods. (A) AEDF, dietary fiber obtained by alkali extraction; (B) EHDF, dietary fiber obtained by enzymatic hydrolysis; (C) SEDF, dietary fiber obtained by shear emulsifying assisted enzymatic hydrolysis
Ftir
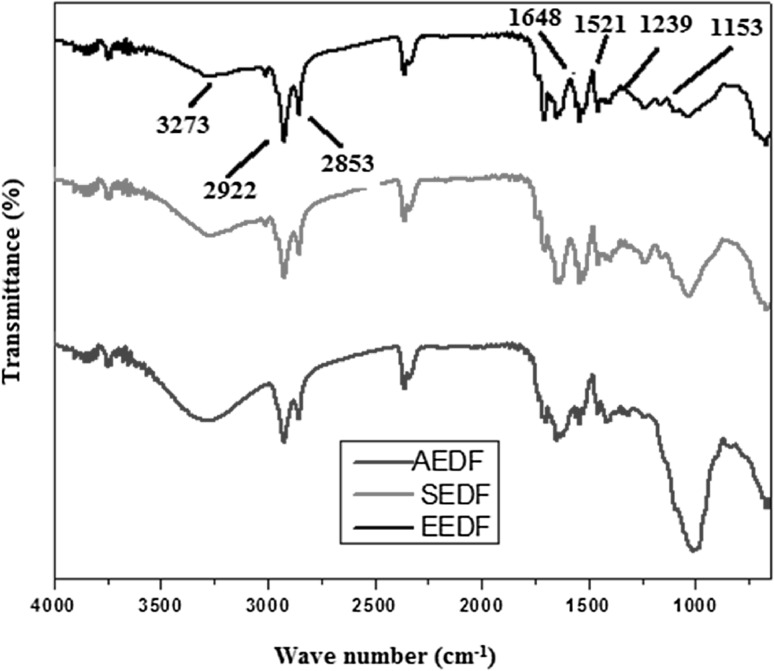
The FTIR spectrum (600–4000 cm−1) of each DF shows various functionalities in AEDF, SEDF and EEDF (Fig. 2). Characteristic bands for hydroxyl stretching vibrations (OH in polysaccharides) were observed at 3273 cm−1. The C-H stretching absorption originating from methylenic group (in polysaccharide) was observed at 2922 cm−1 [31] while bands for aromatic C–H stretching appeared at 2853 cm−1 along with overtones (1800–2000 cm−1), however, the characteristic absorption at 1648 cm−1 (aromatic lignin) was not observed in AEDF showing reduction in aromatic components. Moreover, the band at 3273 and 1040 cm−1(OH stretching) were broaden in AEDF, while C–O bending absorptions at 1,248,898 and 811 cm−1(β-glycosidic linkages in polysaccharides) were not observed thus reveal a reduction in intermolecular hydrogen bonding in the cellulose and hemicellulose components [31], possibly due to the alkali solution effect. The results of IR spectral data confirm that over all structural matrixes in AEDF is disturbed as compared to the EEDF and SEDF, observed in SEM results earlier.
Fig. 2.
The FTIR spectra of the dietary fiber obtained from defatted walnut flour by three different method
Particle size (D3,2 (µm))
Particle sizes of AEDF, EEDF and SEDF have been shown in Table 1. Particle size is one of the major factors which effect various physiochemical and functional properties of DFs [32]. The smallest particle size was observed for EEDF (70.27 µm) followed by SEDF (70.83 µm) and AEDF (88.58 µm) respectively. The results reveal that by comparing EEDF and shear emulsifying treatment before enzymatic hydrolysis in SDEF has no effect on particle size. However, particle size of AEDF was significantly higher (P < 0.05) compared to EEDF and SEDF. The smaller particle size distribution may be attributed to the high-speed emulsifying pretreatment or probably the proteins in defatted walnut flour changed into smaller monomers in the presence of protease [8].
Physicochemical properties
Wrc
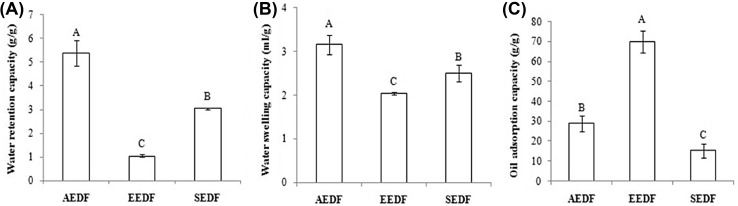
Figure 3(A) shows WRC of AEDF, EEDF and SEDF. WRC of AEDF was significantly higher (P < 0.05) (5.39 g/g) than that of EEDF (1.05 g/g) and SEDF (3.04 g/g). The lower level of WRC in EEDF and SEDF may be due to hard procedure of grinding and denaturation of polysaccharide linkage, which leads to the reduction of WRC [20]. Furthermore, WRC for AEDF was observed higher compared to grapefruits (2.09 g/g), citrus fruits (1.65 g/g), apples (1.87 g/g), and bananas (1.71 g/g), but lower than that of mango peel (11.40 g/g) and coconut kernels (10.71 g/g; [6, 14, 32, 33]. These results reveal that, DFs from defatted walnut flour can bind/entrap more water than DFs of grapefruits, citrus fruits, apples and bananas since DF WRC is dependent on D3,2, extraction methods, and surface morphology [6]. WRC is one of the major key parameter which has been studied in functional food. Most significant changes that happen during baking i.e. gelatinization of starch, denaturation of protein, flavor and color formation are due to water contents [23]. It has been observed that the WRC is directly proportional to the amount of SDF [19]. Since AEDF has higher IDF/SDF ratio (82.52) as compared to EEDF and SEDF, therefore can bind to more water.
Fig. 3.
Effect of extraction methods on (A) water retention capacity, (B) water swelling capacity and (C) oil adsorption capacity of dietary fiber from defatted walnut flour. Values in each figure followed by the different letters are significantly (P < 0.05) different
Wsc
Figure 3(B) shows WSC of AEDF, EEDF and SEDF. WSC is the measure of DF hydration after 18 h of dispersion in water. Figure 3(B) reveals that there is significant difference in WSC among AEDF, EEDF and SEDF (P < 0.05). The highest WSC was observed for AEDF (3.16 mL/g) followed by SEDF (2.5 mL/g) and EEDF (2.04 mL/g). WRC and WSC are directly proportional, i.e. higher the value of WRC, higher will be WSC [33]. AEDF shown significantly (P < 0.05) higher value of WRC and WSC compared to that of EEDF and SEDF (Fig. 3(A) and (B)). Our WSC results of DFs are lower than that of coconut kernel [25, 33] probably due to the methods of preparation and purification of DF. However, our results are similar to that of Ma and Mu [20], related to WSC measurements for de-oiled cumin. The maximum amount of water that a fiber can bind depends on its chemical, physical and structural characteristics [25]. DFs extracted through AEDF, EEDF and SEDF have strong effect on free polar groups which tend to minimize the hydrophilic sites; results in less binding of water. AEDF showed maximum swelling capacity and better chemical, physical and structural characteristics as compared to EEDF and SEDF. WSC results are in contradiction with the structural results in the present study (Figs. 1 and 2). López et al. [15] reported that IDF/SDF ratio is sensitive for physical treatments and may possibly lead to an increase or a decrease in the WSC.
Oac
Figure 3(C) shows OAC of AEDF, EEDF and SEDF. The results indicate that OAC is significantly different among three different extracted DFs (P < 0.05). The highest amount of OAC was observed for EEDF (70 g/g) followed by AEDF (29 g/g) and SEDF (15.3 g/g). OAC is the capability of DF to adsorb fat. During food processing, the reduction of cholesterol level in blood is linked with higher value of OAC [19]. Our results suggest a significantly higher value of OAC than that of deoiled cumin, coconut kernel, lime residue and grapefruits [20, 33]. According to Navarro-González et al. [21], the lower value of OAC are related to absence or limited amount of lignin in DF, therefore; our study reveals that DFs extracted through AEDF, EEDF and SEDF from defatted walnut flour had higher amount of lignin. Additionally, OAC is related to the DF chemical composition, however; it is more of the porosity of the fiber structure rather than the attraction of the fiber molecule to oil [5]. The structure of EEDF is more porous as most of the non-cellular low molecular weight components are removed during enzymatic hydrolysis. Therefore, adsorption of oil is easier in EEDF compared to AEDF and SEDF.
Functional properties
Emulsifying properties
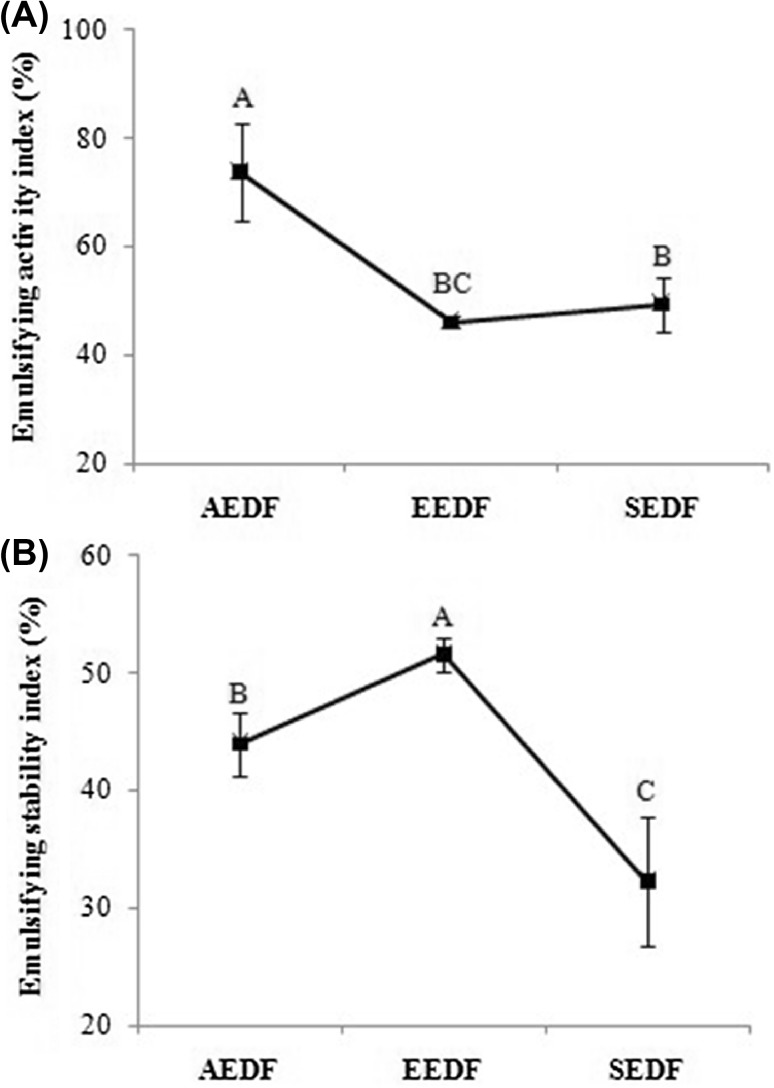
Figure 4(A) and (B) shows emulsifying activity index (EAI) and emulsifying stability index (ESI) of AEDF, EEDF and SEDF. Figure 4(A) and (B) indicates that there is significant (P < 0.05) difference among EAI and ESI values of DFs. The higher value of EAI was observed for AEDF (73.67%) followed by SEDF (49.33%) and EEDF (46%). While higher value of ESI was observed for EEDF (51.56%) followed by AEDF (43.94%) and SEDF (32.22%) respectively. Our results of EAI and ESI were significantly higher than that of rice bran and soybean products [2, 34]. The DF extracted through AEDF, EEDF and SEDF shows good emulsifying activity however, shown less stability indices of less than 50%. The stability index of any emulsion having 94% was considered an excellent value previously, while 50% was categorized as poor stability index [34]. In the present study the lower ESI value obtained probably due to the lower amount of protein in AEDF, EEDF and SEDF possibly because of the alkali and enzymatic hydrolysis of protein. Even though AEDF, EEDF and SEDF exhibited low ESI value, it may still aid as a stabilizer in emulsion based food product, along with high-quality source of DF.
Fig. 4.
Effect of extraction methods on (A) emulsifying activities index and (B) stability index of dietary fiber from defatted walnut flour
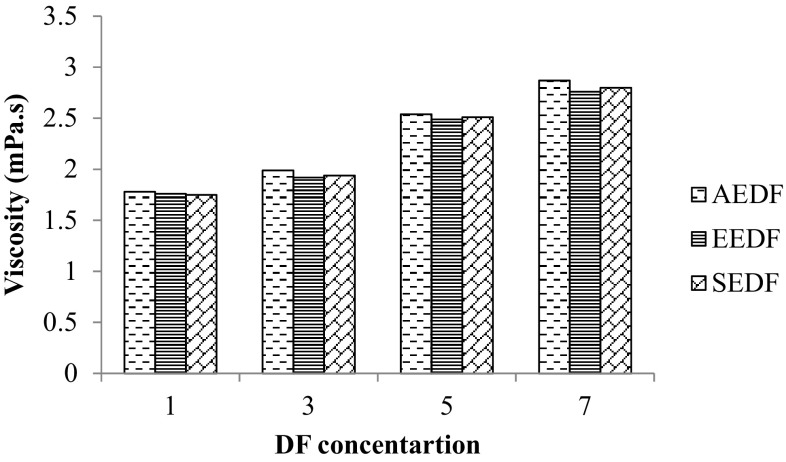
Viscosity
Figure 5 shows viscosity of AEDF, EEDF and SEDF. There was no significant difference (P < 0.05) was observed in viscosities among all of the extracted DFs at different concentration. The concentration of DF is the major component that would increase the viscosity of a solution which lead to the viscous solution, and consequently increase viscosity in the intestine and thus health benefits are related to viscous fibers [3]. The viscosity values of AEDF, EEDF and SEDF was observed higher compared to that of rice bran [2]. However, similar pattern was observed in increasing of viscosity with increase in DF concentration.
Fig. 5.
Effect of extraction methods on viscosities of dietary fiber from defatted walnut flour. The data for each concentration of DF has almost negligible difference, thus error bars were not plotted for the data
The result of this study reveals that the structural, physicochemical and functional properties of DF are dependable on extraction methods from defatted walnut flour. AEDF shows better structural and physiochemical properties, however, no significant difference was observed in emulsifying ability and viscosities of AEDF, EEDF and SEDF. OAC was the main parameter for all of the extracted DFs (70 g/g, 29 g/g and 15.3 g/g), which shown higher values compared to other available DF from different sources. During food processing and reduction of cholesterol level in blood are linked with higher value of OAC. The results from this study recommended that DF from defatted walnut flour have enormous potential in food applications, especially as a food supplement or in development of functional foods. However, further study need to investigate in vivo physiological effects of AEDF, EEDF and SEDF, including its anti-diabetics, anti-cancer effects.
Acknowledgements
The authors are thankful to Nuclear Institute of Food and Agriculture (NIFA) Pesharwar, Pakistan for determination of proximate composition of samples. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Gul Mali Khan, Email: gulmalikhan@gmail.com.
Nasir Mehmood Khan, Email: nasir@sbbu.edu.pk.
Zia Ullah Khan, Email: ziakhujari@yahoo.com.
Farman Ali, Email: alinuml@gmail.com.
Abdul Khaliq Jan, Email: abdukhaliq@gmail.com.
Nawshad Muhammad, Email: nawshadmuhammad@ciitlahore.edu.pk.
Rizwan Elahi, Email: malikrizwan@foxmail.com.
References
- 1.AACC. Report of the dietary fiber definition submitted to the committee board of director of American association of cereal chemists submitted January 10. (2001).
- 2.Abdul-Hamid A, Luan YS. Functional properties of dietary fiber prepared from defatted rice bran. Food Chem. 2000;68(1):15–19. doi: 10.1016/S0308-8146(99)00145-4. [DOI] [Google Scholar]
- 3.Anderson JW, Chen WJL. Cholesterol-lowering properties of oat products. In: Webster FH, editor. Oats chemistry and technology. St. Paul, MN: American Association of Cereal Chemists; 1986. pp. 309–333. [Google Scholar]
- 4.AOAC. Official methods of analysis. Washington, DC: Association of Official Analytical Chemists (2000).
- 5.Biswas AK, Kumar V, Bhosle S, Sahoo J, Chatli MK. Dietary fibre as functional ingredients in meat products and their role in human health. Int J Livest Prod. 2009;2(4):45–54. [Google Scholar]
- 6.Chau CF, Wang YT, Wen YL. Different micronization methods significantly improve the functionality of carrot insoluble fiber. Food Chem. 2007;100(4):1402–1408. doi: 10.1016/j.foodchem.2005.11.034. [DOI] [Google Scholar]
- 7.Daou C, Zhang H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J Food Sci Technol. 2013 doi: 10.1007/s13197-013-0925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong X, Zhao M, Shi J, Yang B, Li J, Luo D, Jiang G, Jiang Y. Effects of combined high-pressure homogenization and enzymatic treatment on extraction yield, hydrolysis and function properties of peanut proteins. Innov Food Sci Emer Technol. 2011;12(4):478–483. doi: 10.1016/j.ifset.2011.07.002. [DOI] [Google Scholar]
- 9.Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H. Dietary fiber and fiber-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011;124(2):411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- 10.Frost J, Hegedus EF, Glicksman M. Objective characterization of hydrocolloid organoleptic properties. Food Technol. 1984;38(1):118–122. [Google Scholar]
- 11.Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nut Biochem. 2008;19(2):71–84. doi: 10.1016/j.jnutbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Gourgue CMP, Champ MMJ, Lozano Y. Dietary fiber from mango byproducts: Characterization and hypoglycemic effects determined by in vitro methods. J Agric Food Chem. 1992;40(10):1864–1868. doi: 10.1021/jf00022a027. [DOI] [Google Scholar]
- 13.Lan GS, Chen HX, Chen SH, Tian JG. Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food ResInter. 2012;49(1):406–410. doi: 10.1016/j.foodres.2012.07.047. [DOI] [Google Scholar]
- 14.Larrauri JA, Ruperez P, Borroto B. Mango peel as a new tropical fiber: Preparation and characterization. Lebensmittel-Wissenschaft und-Technologie. 1996;29(8):729–733. doi: 10.1006/fstl.1996.0113. [DOI] [Google Scholar]
- 15.López G, Ros G, Rincón F. Relationship between physical and hydration properties of soluble and insoluble fiber of artichoke. J Agric Food Chem. 1996;44(9):2773–2778. doi: 10.1021/jf9507699. [DOI] [Google Scholar]
- 16.Ma MM, Mu T, Sun H, Zhang M, Chen J, Yan Z. Optimization of extraction efficiency by shear emulsifying assisted enzymatic hydrolysis and functional properties of dietary fiber from deoiled cumin (Cuminum cyminum L.) Food Chem. 2015;179:270–277. doi: 10.1016/j.foodchem.2015.01.136. [DOI] [PubMed] [Google Scholar]
- 17.Mao X, Hua Y. Composition, structure and functional properties of protein concentrates and isolates produced from Walnut (Juglans regia L.) Int J Mol Sci. 2012;13(2):1561–1581. doi: 10.3390/ijms13021561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margareta E, Nyman GL. Modification of physicochemical properties of dietary fiber of carrots by mono-and divalent cations. Food Chem. 2002;76:273–280. doi: 10.1016/S0308-8146(01)00271-0. [DOI] [Google Scholar]
- 19.Martin-Carron N, Goni I, Larrauri JA, Garcia-Alonson A, Saura-Calixio F. Reduction in serum total and LDL cholesterol concentration by a dietary fiber and polyphenol rich grapes products in hypercholesterolemic rats. Nutr Res. 1999;19:1371–1381. doi: 10.1016/S0271-5317(99)00094-9. [DOI] [Google Scholar]
- 20.Ma M-M, Mu T-H. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016;194:237–246. doi: 10.1016/j.foodchem.2015.07.095. [DOI] [PubMed] [Google Scholar]
- 21.Navarro-González I, García-Valverde V, García-Alonso J, Periago J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res Int. 2011;44(5):1528–1535. doi: 10.1016/j.foodres.2011.04.005. [DOI] [Google Scholar]
- 22.Peerajit P, Chiewchan N, Devahastin S. Effects of pretreatment methods on health-related functional properties of high dietary fiber powder from lime residues. Food Chem. 2012;132(4):1891–1898. doi: 10.1016/j.foodchem.2011.12.022. [DOI] [Google Scholar]
- 23.Pomeranz Y. Functional properties of food components. New York: Academic Press, Inc; 1985. [Google Scholar]
- 24.Qi BK, Jiang LZ. Extract dietary fiber from the soy pods by chemistry enzymatic methods. Procedia Eng. 2011;15:4862–4873. doi: 10.1016/j.proeng.2011.08.907. [DOI] [Google Scholar]
- 25.Raghavarao KSMS, Raghavendra SN, Rastogi NK. Potential of coconut dietary fiber. Indian Coconut J. 2008;6:2–7. [Google Scholar]
- 26.Redgwell R, Trovato V, Merinat S, Curti D, Hediger S, Manez A. Dietary fibre in cocoa shell: characterization of component polysaccharides. Food Chem. 2003;81(1):103–112. doi: 10.1016/S0308-8146(02)00385-0. [DOI] [Google Scholar]
- 27.Robertson JA, de Monredon FD, Dysseler P, Guillon F, Amado R, Thibault JF. Hydration properties of dietary fibre and resistant starch: a European collaborative study. LWT-Food Sci Technol. 2000;33(2):72–79. doi: 10.1006/fstl.1999.0595. [DOI] [Google Scholar]
- 28.Sowbhagya HB, Suma PF, Mahadevamma S, Tharanathan RN. Spent residue from cumin: a potential source of dietary fiber. Food Chem. 2007;104(3):1220–1225. doi: 10.1016/j.foodchem.2007.01.066. [DOI] [Google Scholar]
- 29.Sze-Tao KWC, Sathe SK. Walnuts (Juglans regia L): proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J Sci Food Agric. 2000;80:1393–1401. doi: 10.1002/1097-0010(200007)80:9<1393::AID-JSFA653>3.0.CO;2-F. [DOI] [Google Scholar]
- 30.Tan C, Wei H, Zhao X, Xu C, Peng J. Effects of dietary fibers with high water-binding capacity and swelling capacity on gastrointestinal functions, food intake and body weight in male rats. Food Nutr Res. 2017;61(1):1308118. doi: 10.1080/16546628.2017.1308118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao YJ. Study on the modification and application of wheat bran dietary fiber. Wuxi: Jiangnan University; 2008. [Google Scholar]
- 32.Tosh SM, Yada S. Dietary fibers in pulse seeds and fractions: characterization, functional attributes, and applications. Food Res Int. 2010;43(2):450–460. doi: 10.1016/j.foodres.2009.09.005. [DOI] [Google Scholar]
- 33.Yalegama LLWC, Karunaratne DN, Sivakanesan R, Jayasekara C. Chemical and functional properties of fiber concentrates obtained from byproducts of coconut kernel. Food Chem. 2013;141(1):124–130. doi: 10.1016/j.foodchem.2013.02.118. [DOI] [PubMed] [Google Scholar]
- 34.Yasutmasu K, Sawada K, Moritaka S, Nafisaki M, Toda J, Wada T, Ishi K. Whipping and emulsifying properties of soybean products. Agric Biol Chem. 1972;36:719–737. doi: 10.1080/00021369.1972.10860321. [DOI] [Google Scholar]