Abstract
Novel strategies toward the use of low-cost media to produce food-grade microbial products have been considerably attended in recent years. In this study, date syrup obtained from low-quality date fruits was implemented for biosurfactant production by the probiotic bacterium, Lactobacillus rhamnosus PTCC 1637. The most level of biosurfactant was achieved through fermentation in a bioreactor with a lactose feeding phase, up to 24 h. Critical micelle concentration of the cell-bound biosurfactant was found to be 6.0 mg/ml with a minimum surface tension value of 39.00 mN/m and a maximum emulsifying index of 42%. The spectrum of Fourier transform infrared spectroscopy taken from the cell-bound biosurfactant suggests that it should be a multi-component mixture of protein and polysaccharides associated with phosphate groups. The results indicated the potential for developing strategies toward the low-cost production of food-grade biomaterials by probiotic microorganisms.
Keywords: Biosurfactant, Date syrup, Fed-batch fermentation, Lactic acid bacteria
Introduction
Biosurfactants are amphiphilic compounds with pronounced surface and emulsifying activities generated by microorganisms extracellularly or intracellularly [17, 29]. These surface active molecules because of their low toxicity, biodegradability, and effectiveness at a wide range of pH and temperature values, are regarded as the adequate choice for environmentally friendly technologies [3, 6]. In recent years, biosurfactants have been attended as they are potential candidates for many commercial applications in the petroleum, pharmaceuticals, and food processing industries [7].
Lactic acid bacteria (LAB), which are generally regarded as safe (GRAS, the United States Food and Drug Administration), are recognized for usage in the food industry and medicine. Accordingly, production of biosurfactant from these non-pathogenic microorganisms has received a lot of attention so as not to happen pathogenicity. The strains of Lactobacillus rhamnosus, which are shown to have probiotic values, are increasingly being applied in novel-type foods [28]. A composite culture medium such as MRS, which is prevalently employed for the growth of LAB, due to its high amount of expensive nutrients such as meat extract, peptone and yeast extract, is not economically interesting to utilize in industrial microbiology [4, 13, 16]. An effective production process develops an economic system that makes use of low-cost materials while it provides a high-product yield. Date syrup obtained from low-quality date fruits can be examined as a low-cost alternative medium for growth of LAB.
Dates (Phoenix dactylifera L.) are an important crop in the Middle Eastern and many Persian Gulf countries. Date palm fruits comprise typically carbohydrate (total sugars, 44–88%), fat (0.2–0.5%), protein (2.3–5.6%), dietary fiber (6.4–11.5%), minerals (0.1–916 mg/100 g date), and vitamins such as vitamin C, B1, B2 and A [1, 2]. Whereas date fruit is becoming a valuable commercial crop in the producing countries by taking up modern technological approaches to improve product yield, date processing industries have not developed at the same rate. Furthermore, a large amount of the harvested dates due to persistent rain and stormy conditions, become dusty and are damaged by birds and insects [20]. Because of high concentrations of sugars present in date syrup, it is important to develop new uses of these sugars, especially in fermentation sectors.
In the current study, date syrup obtained from low-quality date fruits was used as a low-cost medium to produce biosurfactant by L. rhamnosus PTCC 1637. Afterward, physical and structural properties of the synthesized biosurfactant were investigated and examined.
Materials and methods
Strain and culture conditions
Lactobacillus rhamnosus was obtained from the Persian Type Culture Collection (PTCC), strain number 1637. The strain was stored at − 80 °C in MRS broth containing 15% (v/v) glycerol solution until it was used. Whenever required, frozen stocks were streaked on MRS agar plates and incubated overnight at 37 °C for further culturing. The agar plates were stored at 4 °C, no longer than 2 weeks. To prepare subcultures, MRS broth was inoculated with a colony from the plate and incubated overnight under the same conditions. It was inferred from the various screening assays that the strain L. rhamnosus PTCC 1637 was graded as the efficient biosurfactant-producing LAB, which has the ability to produce large amounts of cell-bound biosurfactants. Thus, in the current study, the capability of L. rhamnosus PTCC 1637 as the selected strain to utilize date syrup for biosurfactant synthesis was additionally investigated.
Extraction and composition of date syrup
Date syrup was extracted using a modified method described by Moosavi-Nasab et al. [21]. In brief, stone-free and low-quality date fruits were soaked in warm distilled water (50 °C) for 30 min. The soaked dates were then mixed thoroughly, using an electric drill (Ronix, Model 2106C, China) containing a double-edged auger for 2 min at high speed. The homogenized extract was filtered through a double-layer cheesecloth, and the residue was washed with hot water (85 °C). pH and °Brix (total soluble solid) of resultant solution were adjusted to 6.7 and 10, respectively. All media were sterilized by autoclaving at 121 °C for 15 min. In order to remove sludges, the extracted solutions were centrifuged (SORVALL, RC-5; USA) at 5000×g for 15 min in sterile conditions.
The composition of date syrup was determined according to the AOAC methods, pH, °Bx, nitrogen, total sugar, potassium and sodium by flame photometric, magnesium and manganese by atomic absorption [14].
Shake flask experiments
Biosurfactant production was examined using several supplemented date syrup media as described in Table 1. The bacterial strain was cultured in shake flasks containing 100 ml supplemented date syrup medium. Each culture broth was inoculated with 1 ml of an overnight subculture and incubated for 48 h at 37 °C and 120 rpm. After 48 h, cells were harvested by centrifugation (10,000×g, 5 min, 10 °C), washed twice in demineralized water, and resuspended in 25 ml of phosphate-buffered saline (PBS: 10 mM KH2PO4/K2HPO4 and 150 mM NaCl with pH adjusted to 7.0). In order to release cell-bound biosurfactants, the cell-suspension was left at room temperature for 8 h with gentle stirring. Subsequently, the cells were removed by centrifugation (10,000×g, 5 min, 10 °C) and the remained supernatant liquid was assayed for the biosurfactant presence [30].
Table 1.
The compounds used for supplementing of date syrup to produce biosurfactant by strain PTCC 1637
| Medium | Compounds used for supplementing date syrup |
|---|---|
| 1 | Meat extract (2 g/l) |
| 2 | Yeast extract (2 g/l) |
| 3 | Meat extract (1 g/l) + yeast extract (1 g/l) |
| 4 | Lactose (5 g/l) |
| 5 | MgSO4 (0.1 g/l) |
| 6 | FeSO4 (0.1 g/l) |
| 7 | None |
Scale up studies
In order to produce biosurfactant by the use of date syrup medium, two fermentation processes (batch and fed-batch) were implemented. A batch fermentation process was put into practice in a 5 l capacity fermentor (Infors HT, Minifors, Switzerland) containing 2.5 l production medium (date syrup). Also, a fed-batch fermentation process was carried out in two phases: the first phase was growth and biosurfactant production phase, up to 24 h (biosynthesis of biosurfactant was found to be growth-associated), and the second phase was a satiety phase, up to 48 h. In the first phase, 180 ml of 7.5% lactose solution was fed into the date syrup during 24 h of fermentation at speed of 7.5 ml/h. pH was maintained at 6.7 by automatic addition of 2.5 N sodium hydroxide solution, and the agitation speed was set at 120 rpm.
At the end of the fermentation processes, the cells were harvested by centrifuging (10,000×g, 5 min, 10 °C), washed twice in demineralized water, and resuspended in 150 ml of phosphate-buffered saline (pH adjusted to 7.0). The cell-suspension was left at room temperature for 8 h with gentle stirring. Subsequently, the bacterial cells were pelleted using centrifugation, and the supernatant was filtered through a 0.22-mm filter (Millipore). The solution containing the cell-bound biosurfactants was dialyzed against demineralized water at 4 °C in a Cellu-Sep© membrane (molecular weight cut-off 6000–8000 Dalton; Membrane Filtration Products, Inc., USA) for 24 h. Finally, the dialysate was freeze-dried and stored at − 20 °C [30].
Biomass concentration
Bacterial growth was inspected by measuring of the broth optical density at a wavelength of 600 nm. A conventional method of cell dry weight measurement was employed for biomass analysis. A 10-ml sample was poured into pre-weighed tubes and centrifuged at 8000×g for 10 min. Finally, the cell pellet was dried in an oven at 105 °C for 24 h and its dry weight was measured subsequently.
Investigation physical characteristics of biosurfactant
The surface tension of cell-bound biosurfactant solutions was measured with a tensiometer (Nanometric, Contact angle-101, Iran) working on the principles of Wilhelmy plate method [9]. The accuracy of the surface tension readings was verified with pure water (72.80 ± 0.10 mN/m) before each reading. All measurements were repeated three times and their average values were reported. PBS solution was used as the negative control. The surface activity of the produced biosurfactant was also expressed as a percentage of the reduction in surface tension (%STR) which was calculated as follows:
where γm is the surface tension of the control (PBS) and γc is the surface tension of the cell-bound biosurfactant solutions.
The emulsification index (%E24) was determined by adding 2 ml of n-hexadecane, to the same volume of cell-bound biosurfactant solution [in the value of critical micelle concentration (CMC)] mixed with a vortex for 2 min and left to stand for 24 h. The emulsification index was determined as the percentage of the height of the emulsified layer (mm) divided by the total height of the liquid column (mm) and 10 g/l Sodium dodecyl sulfate (SDS) solution was used as the positive control.
The CMC was determined by plotting the surface tension as a function of the logarithm of biosurfactant concentration, and it was found at the point of intersection between the two lines that best fit through the pre- and post-CMC data. CMC is defined as the concentration of an amphiphilic compound in solution at which the formation of micelles is initiated. Concentrations ranging from 0.001 to 100 mg/ml of the biosurfactant obtained from L. rhamnosus PTCC 1637 were prepared in PBS (pH 7.0) and the surface tension of each sample was determined by the plate method at room temperature (20 °C) as described before. All measurements were performed in triplicates.
Fourier transform infrared (FT-IR) spectroscopy
The FT-IR spectroscopy analysis can be used to illustrate the chemical composition of biosurfactants by identifying the types of chemical bonds or the functional groups present in their chemical structures. The FT-IR spectrum of the cell-bound biosurfactant produced by L. rhamnosus PTCC 1637 in the date syrup medium was taken in the wavelength range of 400–4000 cm−1 with a scan speed of 2 mm/s on an FT-IR system (Perkin-Elmer Spectrum RX I, USA).
Statistical analysis
The incomes of the obtained results were analyzed statistically via SPSS software (version 22.0). They were compared using one-way ANOVA and Duncan test to reveal any significant difference among the parameters and the variables. The result was considered significant if p < 0.05.
Results and discussion
Biosurfactant production using date syrup
As described above, the ingredients of the utilized date syrup (°Brix = 10) were assayed, and it was found that the substrate is composed of 8% total sugar, 0.7% protein, 196 mg/l magnesium, 0.136 mg/l manganese, 46 mg/l sodium and 45.5 mg/l potassium. It is expected that date syrup will be the adequate substrate for LAB growth; for the reason that the LAB are exceedingly fastidious microorganisms and require complex growth factors.
Several sets of supplemented date syrup were used to study the biosurfactant production by L. rhamnosus PTCC 1637 (see Table 1) and the outcomes compiled in Table 2. Surface tension values are related to the solutions of cell-bound biosurfactant samples extracted by PBS (pH adjusted to 7.0). A decrease in surface tension of the PBS was observed for all media in the range of 20–30 mN/m. The lowest surface tension value resulted from the cell-bound biosurfactant solution which its cells were produced in the date syrup supplemented by lactose (medium 4). Consequently, the %STR value of biosurfactant produced in medium 4 was maximal, and the statistical analysis demonstrated that the differences between this value and the other ones are significant. There was no statistically significant difference between the %STR value of biosurfactant produced in the date syrup supplemented by nitrogen sources separately (medium 1, 2), whereas simultaneous supplementing of the nitrogen sources (medium 3) showed significant statistical differences with the other ones. Although statistical differences between the %STR value of biosurfactant produced in media supplemented by elements (Fe2+, Mg2+) and the other ones are significant, there was no statistically significant difference between the %STR value of biosurfactant produced in them (p < 0.05).
Table 2.
Surface tension values (mN/m) and %STR resulted from biosurfactant solutions
| Medium | Surface tension (mN/m) | %STR |
|---|---|---|
| 1 | 47.91 ± 0.06 | 34.18c ± 0.09 |
| 2 | 48.12 ± 0.05 | 33.89cd ± 0.08 |
| 3 | 52.78 ± 0.30 | 27.50e ± 0.42 |
| 4 | 42.24 ± 0.20 | 41.97a ± 0.28 |
| 5 | 45.75 ± 0.07 | 37.15b ± 0.09 |
| 6 | 45.48 ± 0.02 | 37.52b ± 0.02 |
| 7 | 48.42 ± 0.52 | 33.47d ± 0.71 |
Values are representatives of mean ± SD (n = 3). Values followed by different superscripts in a column are significantly different (p < 0.05)
The ingredients of date syrup rely on the type of date, but in general fructose and glucose (invert) are the main sugars [22]. LAB ferment sugars via different pathways and are also capable to accumulate various products, e.g. flavors such as diacetyl and acetoin, bacteriocins or biosurfactants. The different carbon sources give altering amounts of by-products [13]. However different utilized substrates influence the quantity of biosurfactant produced, it may result in the synthesis of products with different composition and characteristics [16]. As described before, the addition of lactose into the date syrup fermentative medium as a surplus carbon source, yielded a higher biosurfactant production. Hence, it can be surmised that the utilization of lactose as the carbon source instead of invert sugar induced the cells to employ another metabolic pathway, and accordingly produce different amounts of biosurfactant. Gudiña et al. [12] reported that increase in initial lactose concentration in conventional media yielded higher biomass and biosurfactant produced by different lactobacilli.
It was found that the simultaneous supplementing of the nitrogen sources (yeast extract and meat extract) in date syrup resulted in a lower biosurfactant production when compared to the other media. These effects may have been caused related to the variation of carbon/nitrogen (C/N) ratio and or due to the nature of nitrogen sources. It is obviously seen in the literature that the C/N ratio plays an undeniable role in the production of rhamnolipid [15, 27] and other biosurfactants [10, 11]. In contrast to our outcomes, Gudiña et al. [11] reported that a combination of peptone and meat extract in conventional media resulted in a higher biosurfactant production as compared to the standard medium.
The trace elements play a key role as co-factors in the enzymes that are involved in the biosurfactants synthesis [8]. Onur [23] indicated that the supplementation of low-cost media with elements, Fe2+, Mn2+, and Mg2+, could be beneficial for the production of biosurfactants by B. subtilis PX573.
Biosurfactant production in bioreactor
LAB drastically decrease pH of the fermentative media by producing lactic acid and other metabolites during fermentation processes. Hence, the most important advantage of fermentation in the bioreactor was the lack of formation any sludges in the date syrup medium due to controlling the value of pH.
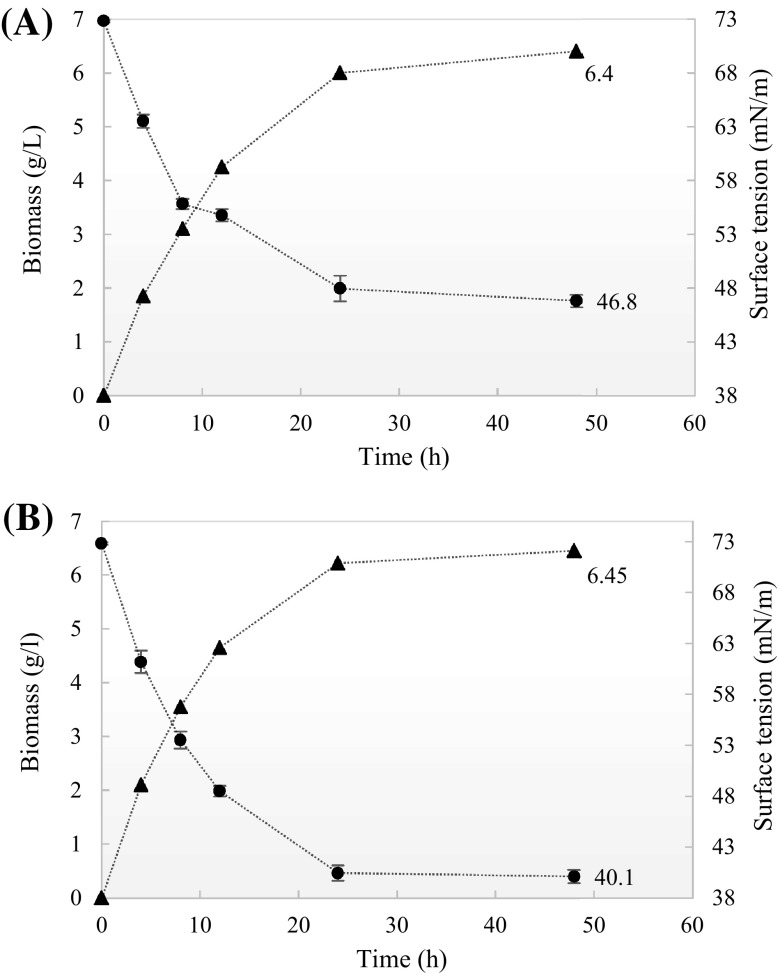
As inferred above, medium 4 (date syrup supplemented by lactose) was selected for the further studies. At the end of both fermentation processes the surface tension values of the cell-bound biosurfactant solutions got reduced to 46.8 and 40.1 mN/m from 72.8 mN/m for batch and fed-batch fermentation processes, respectively, while the biomass concentrations were obtained to reach up to about 6.4 g/l (the measured surface tension values were below of the CMC value) (see Fig. 1). Accordingly, the production yield of biosurfactant has been developed by feeding lactose up to end of the exponential growth phase. Although the variation of the carbon sources has not been a significant influence on the biomass concentration, they have affected the amount of cell-bound biosurfactant produced. In other words, the feeding of lactose as a surplus carbon source into the date syrup medium induced the cells to produce more biosurfactant. Therefore, two culture media containing the same biomass concentrations, necessarily do not have equal cell-bound biosurfactant concentrations.
Fig. 1.
Surface tension variation (mN/m) and biomass concentrations (g/l) obtained from fermentations carried out in the bioreactor with date syrup medium through (A) batch process, (B) two-stepped Fed-batch process. Results represent the average of three independent experiments. Biomass (dotted triangle), Surface Tension (dotted circle)
In this study, a direct relation was observed between biosurfactant production (shown by a decrease in the surface tension) and cell growth during both fermentation processes. Thus, the biosurfactant biosynthesis was found to be growth-associated that is in accordance with the earlier reports by Rodrigues et al. [12, 26].
The synthesis of microbial products from various agro-industrial wastes is an encouraging route to attain low-cost bioprocesses. Supplemented cheese whey and molasses media were successfully utilized as relatively inexpensive media for biosurfactant production by probiotic strains, Lactococcus lactis 53 and Streptococcus thermophilus A [25]. In another study, monomeric hemicellulosic sugars obtained from diluted acid hydrolysis of distilled grape marc were used to efficiently convert to biosurfactants by Lactobacillus pentosus after nutrients supplementation [24]. Other examples, where hemicellulosic sugars obtained from agricultural residues such as trimming vine shoots or detoxified E. globulus wood hydrolyzates were successfully used as carbon sources for the production of biosurfactants using LAB [18].
Very few studies have been contributed toward using of date syrup as a substrate to economically synthesis of microbial products. Succinoglycan biogums produced from low-quality date syrup using Agrobacterium radiobacter were three to five times higher than those from sucrose medium [21]. Also, in other studies, levan exopolysaccharide [19] and xanthan gum [20] were significantly produced in date syrup medium. The current study seems to be an interesting approach for developing new strategies of biosurfactant production by enrichment of unconventional media to implement low-cost and high-yielded processes.
Physical characterizations of biosurfactant
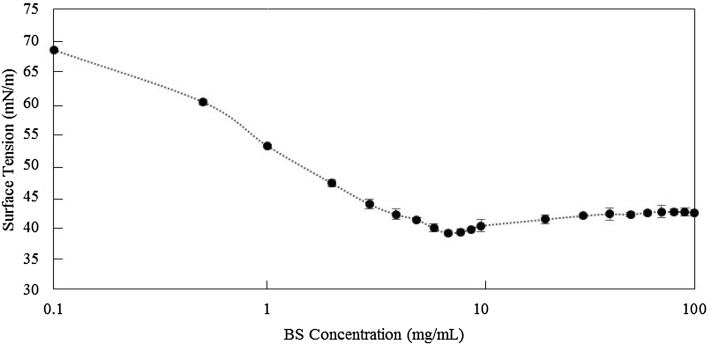
The freeze-dried biosurfactant was dissolved in PBS (pH 7.0) at different concentrations ranging from 0.001 to 100 mg/ml (see Fig. 2). As can be seen a gradual decline in surface tension was observed following by raising the quantity of biosurfactant concentration; for biosurfactant concentrations higher than 6.0 mg/ml, the surface tension turned into a relatively constant state; hence, this value is considered as the CMC for this biosurfactant with a minimum surface tension value of 39.00 mN/m and a maximum emulsifying index of 42%.
Fig. 2.
Progressive decrease in surface tension with increase in concentration of biosurfactant up to 6.0 mg/ml
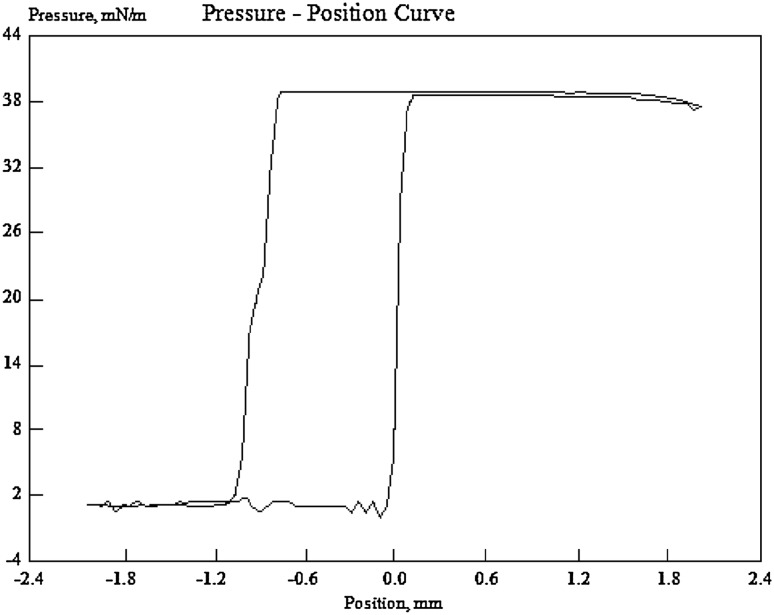
The biosurfactant showed a significant reduction in surface tension of PBS from 72.80 to 39.00 mN/m. As indicated in Fig. 3, as soon as the platinum plate contacted with the surface of the biosurfactant solution (the zero point), the value of surface tension abruptly achieved to 39.00 and then precipitously decreased to zero after exiting the platinum plate from the cell-bound biosurfactant solution.
Fig. 3.
Surface tension value of 39.00 calculated at distance between − 2 and 2 of the biosurfactant solution. The Zero point is just the surface of biosurfactant solution obtained from strain PTCC 1637
FT-IR spectroscopy
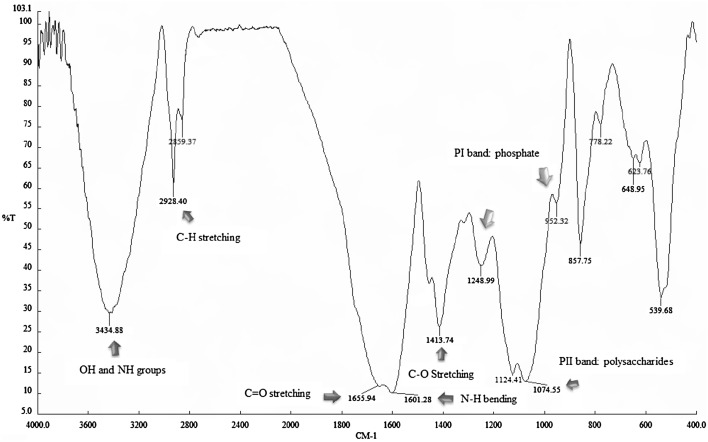
The molecular characterization of the cell-bound biosurfactant produced by L. rhamnosus PTCC 1637 in date syrup medium was carried out using FT-IR spectroscopy. The FT-IR spectrum (see Fig. 4) indicated that the produced biosurfactant is proteinaceous in nature. The absorbance bands at 3434, 1655, and 1601 cm−1 respectively, correspond to OH and NH groups, C=O stretching in proteins (AmI band) and NH bending in proteins (AmII band), that are revealing the presence of proteins in the extracted biosurfactant sample. Furthermore, excitation in the spectrum at 2928 cm−1 corresponding to C–H band (CH2–CH3 stretching) and the small peak at 1454 cm−1 corresponding to CH (scissor), suggest the presence of bonds occurring in aliphatic chains. The peak at 1075 cm−1 corresponds to the PII polysaccharide band that is typical of bond vibrations in the C–O–C group. Excitation at the wavelength of 1413 cm−1 corresponds to C–O stretching in sugars. Moreover, the absorbance bands at 1248 (PI phosphate bond) and 952 cm−1 relate to the stretching bonds formed by phosphorus and oxygen atoms (P–O–C) in aromatic and aliphatic molecules.
Fig. 4.
FT-IR absorption spectrum of the cell-bound biosurfactant produced by strain PTCC 1637
The spectrum obtained from the cell-bound biosurfactant produced by L. rhamnosus PTCC 1637 suggests that it should be a multi-component mixture of protein and polysaccharides associated with phosphate groups.
Several reports have been published about biosurfactant produced by LAB, but inadequate information about their chemical structure is known. LAB-derived biosurfactants were initially characterized as multi-component mixtures consisting of protein fractions, polysaccharides and phosphate groups [5, 30]. Similar to our results, Brzozowski et al. [5] reported that the structure of the biosurfactant made from MRS broth using L. rhamnosus CCM 1825 inoculation is a three-component of protein, polysaccharide and phosphate in different ratio.
Acknowledgements
This work was supported by Shiraz University Grant No. GR-56 (Shiraz, Iran).
References
- 1.Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F. Compositional and sensory characteristics of three native sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005;53:7586–7591. doi: 10.1021/jf050578y. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shahib W, Marshall RJ. The fruit of the date palm: its possible use as the best food for the future? Int. J. Food Sci. Nutr. 2003;54:247–259. doi: 10.1080/09637480120091982. [DOI] [PubMed] [Google Scholar]
- 3.Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010;87:427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- 4.Batish V, Lal R, Chander H. Effect of nutritional factors on the production of antifungal substance by Lactococcus lactis subsp. lactis biovar diacetylactis. Aust. J. Dairy Technol. 1990;45:74–76. [Google Scholar]
- 5.Brzozowski B, Bednarski W, Golek P. The adhesive capability of two Lactobacillus strains and physicochemical properties of their synthesized biosurfactants. Food Technol. Biotechnol. 2011;49:177. [Google Scholar]
- 6.Cameotra SS, Makkar RS, Kaur J, Mehta S. Synthesis of biosurfactants and their advantages to microorganisms and mankind. In: Biosurfactants. Springer, pp 261–280 (2010). [DOI] [PubMed]
- 7.Desai JD, Banat IM. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes ECR. Study of biosurfactant “cocktails” with enhanced properties. Diss. (2013).
- 9.Fernandes PAV, Arruda IRd, Santos AFABd, Araújo AAd, Maior AMS, Ximenes EA. Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Braz. J. Microbiol. 2007;38:704–709. doi: 10.1590/S1517-83822007000400022. [DOI] [Google Scholar]
- 10.Fontes GC, Amaral F, Filomena P, Nele M, Coelho Z, Alice M. Factorial design to optimize biosurfactant production by Yarrowia lipolytica. BioMed. Res. Int. 1–8 (2010). [DOI] [PMC free article] [PubMed]
- 11.Ghribi D, Ellouze-Chaabouni S. Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol. Res. Int. 2011:1–6 (2011). 10.4061/2011/653654. [DOI] [PMC free article] [PubMed]
- 12.Gudiña E, Teixeira J, Rodrigues L. Biosurfactant-producing Lactobacilli: screening, production profiles, and effect of medium composition. Appl. Environ. Soil Sci. 2011;10:1155. [Google Scholar]
- 13.Hofvendahl K, Hahn-Hägerdal B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000;26:87–107. doi: 10.1016/S0141-0229(99)00155-6. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz W. Official methods of analysis of the AOAC International. No. C/630.240 O3/2000 (2000).
- 15.Hospinal M, Martínez D, Valladares K, Gutierrez S, Merino F. Effect of carbon/nitrogen and carbon/phosphorus ratio on the production of rhamnolipid biosurfactant by pseudomonas aeruginosa 6k11 isolated from soil contaminated with oil. p. 1–8 (2015).
- 16.Jensen PR, Hammer K. Minimal requirements for exponential growth of Lactococcus lactis. Appl. Environ. Microbiol. 1993;59:4363–4366. doi: 10.1128/aem.59.12.4363-4366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maneerat S. Production of biosurfactants using substrates from renewable-resources. Songklanakarin J. Sci. Technol. 2005;27:675–683. [Google Scholar]
- 18.Moldes AB, Torrado AM, Barral MT, Domínguez JM. Evaluation of biosurfactant production from various agricultural residues by Lactobacillus pentosus. J. Agric. Food Chem. 2007;55:4481–4486. doi: 10.1021/jf063075g. [DOI] [PubMed] [Google Scholar]
- 19.Moosavi-Nasab M, Layegh B, Aminlari L, Hashemi MB. Microbial production of levan using date syrup and investigation of its properties. World Acad. Sci. Eng. Technol. 2010;44:1248–1254. [Google Scholar]
- 20.Moosavi-Nasab M, Shekaripour F, Alipoor M. Use of date syrup as agricultural waste for xanthan production by Xanthomonas campestris. Iran Agric. Res. 2010;27:89–98. [Google Scholar]
- 21.Moosavi-Nasab M, Taherian AR, Bakhtiyari M, Farahnaky A, Askari H. Structural and rheological properties of succinoglycan biogums made from low-quality date syrup or sucrose using agrobacterium radiobacter inoculation. Food Bioprocess Technol. 2012;5:638–647. doi: 10.1007/s11947-010-0407-4. [DOI] [Google Scholar]
- 22.Mostafazadeh AK, Sarshar M, Javadian S, Zarefard M, Haghighi ZA. Separation of fructose and glucose from date syrup using resin chromatographic method: experimental data and mathematical modeling. Sep. Purif. Technol. 2011;79:72–78. doi: 10.1016/j.seppur.2011.03.014. [DOI] [Google Scholar]
- 23.Onur G. Screening of biosurfactant producing and diesel oil degrading bacteria from petroleum hydrocarbon contaminated surface waters. Dissertation, Middle East Technical University. Diss. (2015).
- 24.Rivera OMP, Moldes AB, Torrado AM, Domínguez JM. Lactic acid and biosurfactants production from hydrolyzed distilled grape marc. Process Biochem. 2007;42:1010–1020. doi: 10.1016/j.procbio.2007.03.011. [DOI] [Google Scholar]
- 25.Rodrigues L, Teixeira J, Oliveira R. Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem. Eng. J. 2006;32:135–142. doi: 10.1016/j.bej.2006.09.012. [DOI] [Google Scholar]
- 26.Rodrigues L, Teixeira J, Oliveira R, Van Der Mei HC. Response surface optimization of the medium components for the production of biosurfactants by probiotic bacteria. Process Biochem. 2006;41:1–10. doi: 10.1016/j.procbio.2005.01.030. [DOI] [Google Scholar]
- 27.Santa Anna L, Sebastian G, Menezes E, Alves T, Santos A, Pereira N, Jr, Freire D. Production of biosurfactants from Pseudomonas aeruginosa PA 1 isolated in oil environments. Braz. J. Chem. Eng. 2002;19:159–166. doi: 10.1590/S0104-66322002000200011. [DOI] [Google Scholar]
- 28.Schillinger U. Isolation and identification of lactobacilli from novel-type probiotic and mild yoghurts and their stability during refrigerated storage. Int. J. Food Microbiol. 1999;47:79–87. doi: 10.1016/S0168-1605(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 29.Van Hamme JD, Singh A, Ward OP. Physiological aspects: part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006;24:604–620. doi: 10.1016/j.biotechadv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Velraeds MM, van der Mei HC, Reid G, Busscher HJ. Physicochemical and biochemical characterization of biosurfactants released by Lactobacillus strains. Colloids Surf., B. 1996;8:51–61. doi: 10.1016/S0927-7765(96)01297-0. [DOI] [Google Scholar]