Abstract
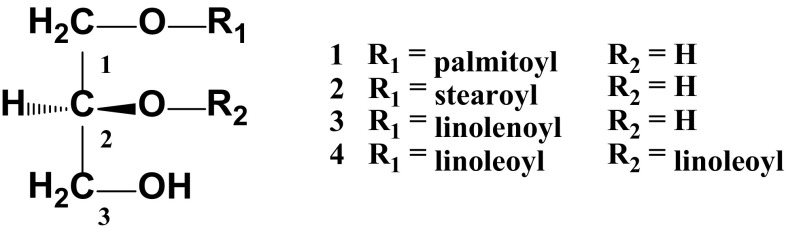
A preliminary study revealed that a 10 µg/mL n-BuOH fraction of Malva verticillata aerial parts significantly enhanced splenocyte proliferation and induced significant enhancement of natural-killer (NK) cell activity against tumor cells (YAC-1). This study was initiated to identify the principal components that exhibited these activities, and four glycerides were isolated through repeated SiO2 and ODS column chromatography. Structures of compounds 1–4 were determined to be (2S)-1-O-palmitoyl glyceride, (2S)-1-O-stearoyl glyceride, (2S)-1-O-linolenoyl glyceride, and (2S)-1,2-di-O-linoleoyl glyceride, respectively. Compounds 1–3 showed potential immune-enhancing activity in murine splenocyte and natural-killer (NK) cells at 10 µM. In contrast, compound 4 showed weak activity, indicating the monoacyl glycerides (1–3) are more effective than diacyl glyceride (4). Also, the longer the carbon number of the fatty acid in monoacyl glyceride, the better the activity, and the monoacyl glyceride including an unsaturated fatty acid (3) is more effective than the glycerides including the saturated fatty acids (1–2).
Keywords: Malva verticillata, Glyceride, Splenocyte, Natural killer cells, Immunotherapy
Introduction
Known as herbal therapy or phytomedicine, the therapeutic use of plants, plant parts, or plant-derived substances is generally considered a form of complementary medicine [1, 2]. Although the side effect profile of current therapeutic approaches necessitates the development of new treatments [3], the low toxicity of herbs and a long history of empirical support for the use of these herbs as immunostimulants may have therapeutic applications in integrative medicine. There is some evidence that natural immune mechanisms can be modulated to impede the development and progression of certain infectious and neoplastic diseases [4, 5]. Chemoprevention can slow, block, or reverse the disease process through the use of natural materials including foods and a variety of foods have been indicated to be potential therapeutics in past research [6]. Recently, the safety of Malva verticillata was verified by the Korea Ministry of Food and Drug Safety (KMFDS) and the Codex Alimentarius Commission (CAC).
Malva verticillata (Chinese mallow), is a popular leafy vegetable in East Asia that has been used as an herbal tea and as a medicine [7]. In the past few decades, the use of M. verticillata as a food product has spread from East Asia and consumers can easily find it in markets globally. Malva verticillata seeds have also been used in traditional Chinese medicinal formulae as a diuretic, laxative, and galactopoietic material [8]. Despite its medicinal uses, the chemical constituents and the biological activity of the aerial parts of the M. verticillata plant are not well understood. Raw vegetable oils are known to contain partial acylglycerides, such as diacylglycerols or monoacylglycerols, in high quantity [9]. Lipid droplets (LDs), such as acylglycerides, are dynamic organelles that govern the storage and turnover of lipids [10] and they play important roles in membrane and lipid trafficking, protein storage, protein degradation, and replication of hepatitis C and dengue viruses [11–14]. Furthermore, acylglycerides were reported to have anti-cancer [15], anti-neuroinflammatory [16], and anti-tumor [17] activities.
In a preliminary study, an n-BuOH fraction (Fr) of M. verticillata aerial parts showed significant natural killer (NK) cell cytotoxicity against tumor cells (YAC-1), and TLC analysis of the n-BuOH Fr indicated the presence of many glycerides. NK cells play an important role in the first response against viruses and tumors and the fact that NK cells function in innate immunity is crucial to their ability to combat viral infection and destroy cancer [18, 19]. Recently, some studies have suggested that NK cells have characteristics of both the innate and adaptive immune systems. NK cells exert direct cytotoxic activity against tumor targets and can regulate the adaptive immune response by cytokine production [20–22]. Therefore, this study was focused on isolate glycerides from M. verticillata aerial parts to investigate their potential as immunomodulation treatments. Four glycerides were isolated from the n-BuOH Fr of M. verticillata aerial parts and identified on the basis of spectroscopic analysis. Solvent Frs and the isolated glycerides were evaluated for their immunomodulation effects.
Materials and methods
General experimental procedures
Previously, most of experimental procedures for isolation and identification were reported [23].
GC–MS spectra were recorded on a Shimadzu GC–MS-QP2010 Plus spectrometer (Kyoto, Japan).
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent was purchased from Dutchefa Biochemie B.V. (Haarlem, The Netherlands). Dimethyl sulfoxide (DMSO) was purchased Daejung Chemicals (Gyeonggi-do, Korea). Dulbecco’s Modified Eagle’s Medium (DMEM), RPMI 1640 medium, fetal bovine serum (FBS), phosphate-buffered saline (PBS), l-glutamine, and penicillin–streptomycin were obtained from Invitrogen Life Technologies Inc. (Carlsbad, CA, USA). Unless identified otherwise, all reagents were used of analytical grade.
Plant materials
The M. verticillata aerial parts were obtained at a commercial farm, Namyangju City, Korea, in April 2016, and the voucher specimen (KHU20160419) is deposited at Kyung Hee University (Laboratory of Natural Products Chemistry), Yongin, Korea.
Isolation of glycerides
The dried aerial parts of M. verticillata (3.1 kg) were extracted using 80% MeOH (54.0 L × 5) at room temperature (Temp) for 24 h. The extracts (Exts) were filtered through filter paper (6 μm, 70 mm) and evaporated under reduced pressure at 43 °C to yield 794 g of Ext. The obtained MeOH Exts were suspended in H2O (2 L) and then successively extracted with ethyl acetate (EtOAc, 2 L × 4) and n-butanol (n-BuOH, 2 L × 4). Each layer was concentrated to obtain EtOAc (MVE, 80 g), n-BuOH (MVB, 75 g), and H2O (MVW, 637 g) Frs. The n-BuOH Fr (MVB, 77 g) was applied to a SiO2 column chromatography (SCC, φ 10 × 15 cm) and eluted with CHCl3–MeOH–H2O (25:3:1 → 22:3:1 → 20:3:1 → 18:3:1 → 15:3:1 → 12:3:1, 18.8 L of each) to yield 14 Frs (MVB-1 to MVB-14). Fr MVB-9 (4.0 g, Ve/Vt 0.384–0.557) was applied to OCC (φ 4 × 6 cm) and eluted with MeOH–H2O (3:2 → 2:1 → 2.5:1 → 3:1 → 5:1, 3.6 L of each) to yield 16 Frs (MVB-9-1 to MVB-9-16). Fr MVB-9-5 (383.9 mg, Ve/Vt 0.116–0.263) was applied to OCC (φ 2.5 × 6 cm) and eluted with acetone-H2O (2:3, 8.0 L) to yield 14 Frs (MVB-9-5-1 to MVB-9-5-14) along with purified compound (Com) 1 [MVB-9-5-4, 22.8 mg, Ve/Vt 0.038–0.053, Rf 0.20, TLC (Kie 60 F254), CHCl3–MeOH–H2O (8:3:1), Rf 0.83, TLC (RP-18 F254S), acetone-H2O (2:1)]. Fr MVB-9-2 (115.8 mg, Ve/Vt 0.004–0.009) was applied to SCC (φ 2.5 × 15 cm) and eluted with CHCl3–MeOH–H2O (20:3:1 → 18:3:1 → 15:3:1 → 12:3:1, 1.7 L of each) to yield 17 Frs (MVB-9-2-1 to MVB-9-2-17) along with purified Com 2 [MVB-9-2-11, 16.1 mg, Ve/Vt 0.745–0.794, Rf 0.65, TLC (Kie 60 F254), CHCl3–MeOH–H2O (65:35:10), Rf 0.71, TLC (RP-18 F254S), MeOH–H2O (3:1)]. Fr MVB-8 (3.5 g, Ve/Vt 0.128–0.383) was applied to ODS column chromatography (OCC, φ 4.5 × 8 cm) and eluted with MeOH–H2O (3:1 → 4:1 → 5:1 → 6:1, 1.0 L of each) to yield 17 Frs (MVB-8-1 to MVB-8-17) along with purified Com 3 [MVB-8-5, 71.5 mg, Ve/Vt 0.055–0.647, Rf 0.81, TLC (Kie 60 F254), CHCl3–MeOH–H2O (8:3:1), Rf 0.52, TLC (RP-18 F254S), MeOH–H2O (5:1)]. Fr MVB-5 [2.4 g, elution volume/total volume (Ve/Vt) 0.036–0.051] was applied to SCC (φ 3.5 × 15 cm) and eluted with EtOAc-n-BuOH-H2O (30:3:1, 2.4 L) to yield 13 Frs (MVB-5-1 to MVB-5-13) along with purified Com 4 [MVB-5-12, 76.4 mg, Ve/Vt 0.766–0.874, Rf 0.19, TLC (Kieselgel (Kie) 60 F254), EtOAc-n-BuOH–H2O (8:3:1), Rf 0.65, TLC (RP-18 F254S), acetone-H2O (1:4)].
(2S)-1-O-palmitoyl glyceride (MVB-9-5-4, 1), pale yellow wax; [α]D − 1.2° (c 0.10, CHCl3); positive FAB-MS m/z 331 [M + H]+; IR (KBr, ν) 3383, 1723 cm−1; 1H-NMR (CD3OD, 400 MHz, δH) 0.88 (3H, t, J = 7.2, H-16′), 1.27–1.34 (24H, overlapped, H-4′-15′), 1.59 (2H, m, H-3′), 2.33 (2H, t, J = 8.0, H-2′), 3.87 (2H, m, H-3), 3.96 (1H, m, H-2), 4.10 (1H, dd, J = 11.4, 6.0, H-1b), 4.17 (1H, dd, J = 11.4, 4.4, H-1a); 13C-NMR (100 MHz, CD3OD, δC) 14.4 (C-16′), 23.6 (C-15′), 25.9 (C-3′), 29.9–30.1 (C-4′-13′), 33.0 (C-14′), 34.9 (C-2′), 66.1 (C-1), 67.4 (C-3), 69.8 (C-2), 175.1 (C-1′); GC–MS tR = 10.27 min (palimic acid methyl ester).
(2S)-1-O-stearoyl glyceride (MVB-9-2-11, 2), pale yellow wax; [α]D +1.5° (c 0.10, CHCl3); positive FAB-MS m/z 359 [M + H]+; IR (KBr, ν) 3386, 1724 cm−1; 1H-NMR (CD3OD, 400 MHz, δH) 0.88 (3H, t, J = 7.2, H-18′), 1.27–1.34 (28H, overlapped, H-4′-17′), 1.59 (2H, m, H-3′), 2.34 (2H, t, J = 8.0, H-2′), δ: 3.90 (2H, m, H-3), 3.96 (1H, m, H-2), 4.10 (1H, dd, J = 11.4, 6.0, H-1b), 4.17 (1H, dd, J = 11.4, 4.4, H-1a); 13C-NMR (100 MHz, CD3OD, δC) 14.2 (C-18′), 22.7 (C-17′), 25.1 (C-3′), 29.4–30.0 (C-4′-15′), 31.9 (C-16′), 34.3 (C-2′), 66.1 (C-1), 67.4 (C-3), 69.6 (C-2), 173.6 (C-1′); GC–MS tR = 14.37 min (stearic acid methyl ester).
(2S)-3-O-linolenoyl glyceride (MVB-8-5, 3), dark green wax; [α]D +3.5° (c 0.10, CHCl3); positive FAB-MS m/z 353 [M + H]+; IR (KBr, ν) 3386, 1722, 1612 cm−1; 1H-NMR (CD3OD, 400 MHz, δH) 0.96 (3H, t, J = 7.2, H-18′), 1.30–1.35 (8H, overlapped, H-4′-7′), 1.60 (2H, m, H-3′), 2.07 (4H, m, H-8′,17′), 2.34 (2H, t, J = 8.0, H-2′), 2.80 (4H, overlapped, H-11′,14′), 3.90 (2H, m, H-3), 3.96 (1H, m, H-2), 4.10 (1H, dd, J = 11.4, 6.0, H-1b), 4.17 (1H, dd, J = 11.4, 4.4, H-1a), 5.27–5.40 (6H, overlapped, H-9′,10′,12′,13′,15′,16′); 13C-NMR (100 MHz, CD3OD, δC) 14.6 (C-18′), 21.5 (C-17′), 26.0 (C-3′), 26.4 (C-11′), 26.5 (C-14′), 28.1 (C-8′), 30.2–30.7 (C-4′-7′), 34.9 (C-2′), 66.2 (C-1), 67.4 (C-3), 69.9 (C-2), 128.2 (C-15′), 128.8 (C-10′), 129.2 (× 2, C-12′,13′), 131.0 (C-9′), 132.7 (C-16′), 175.3 (C-1′); GC–MS tR = 13.76 min (linolenic acid methyl ester).
(2S)-1,2-di-O-linoleoyl glyceride (MVB-5-12, 4), pale yellow wax; [α]D − 2.0° (c 1.00, CHCl3); negative FAB-MS m/z 615 [M–H]−; IR (KBr, ν) 3389, 1725, 1610 cm−1; 1H-NMR (CD3OD, 400 MHz, δH) 0.89 (6H, t, J = 6.8, H-18′, 18′′), 1.28–1.32 (28H, overlapped, H-4′-7′,4′′-7′′,15′-17′,15′′-17′′), 1.60 (4H, m, H-3′,3′′), 2.06 (8H, overlapped, H-8′, 8′′, 14′, 14′′), 2.30 (2H, t, J = 7.6, H-2′), 2.33 (2H, t, J = 7.6, H-2′′), 2.80 (4H, m, H-11′,11′′), 3.96 (2H, m, H-3), 4.17 (1H, dd, J = 12.0, 6.4, H-1b), 4.43 (1H, dd, J = 12.0, 2.8, H-1a), 5.20 (1H, m, H-2), 5.32–5.36 (8H, overlapped, H-9′,9′′,10′,10′′,12′,12′′,13′,13′′); 13C-NMR (100 MHz, CD3OD, δC) 14.5 (× 2, C-18′,18′′), 21.8 (× 2, C-17′,17′′), 23.8 (× 2, C-17′,17′′), 26.0 (× 2, C-3′,3′′), 26.5 (× 2, C-11′,11′′), 28.2 (× 4, C-8′,8′′,14′,14′′), 30.2–30.8 (C-4′-7′,4′′-7′′,15′-16′,15′′-16′′), 34.9 (C-2′), 35.1 (C-2′′), 63.9 (C-3), 64.7 (C-1), 72.0 (C-2), 129.2 (× 4, C-10′,10′′,12′,12′′), 131.1 (× 4, C-9′,9′′,13′,13′′), 174.6 (C-1′′), 174.9 (C-1′); GC–MS tR = 13.99 min (linoleic acid methyl ester).
GC–MS analysis of fatty acids in glycerides
Each glyceride (1.0 mg) was dissolved in 2 mL 20% KOH/MeOH and heated in an 80 °C water bath for 60 min. After cooling to room Temp, the reaction mixture was tested for disappearance of the starting material on TLC plates (n-hexane: EtOAc = 1:1), and it was neutralized by adding the acidic cation-exchange resin (Dowex 50 W, H+ form) and filtered. The filtrate was evaporated in vacuo and partitioned into EtOAc and H2O Frs. Each EtOAc Fr was then evaporated in vacuo, dissolved in 200 μL EtOAc, and filtered, respectively, through a syringe filter (0.2 μm, 13 mm). The filtrate was stored at − 4 °C until GC–MS analysis.
A DB-5 column (0.25-μm film thickness × 0.25-mm diameter × 30-m length) was used for GC–MS experiments. Helium was used as the Mobile phase at a flow rate of 24.2 mL/min. The injector and detector Temps were both set at 280 °C. The oven Temp was programmed as follows: from 160 to 320 °C (rate of 4 °C/min) and held for 15 min. Sample solution (1 μL) was injected into the GC column with a 10:1 split ratio. Detection was performed by electron ionization (70 eV) and quadrupole mass spectrometry. Fatty acids were identified by comparing the retention time with those of authentic fatty acids and their mass spectra with those of a library (Wiley Library, version 2008; John Wiley & Sons Inc., Hoboken, NJ, USA).
Splenocyte isolation
All of the experimental procedures were performed in accordance with the Principles of Laboratory Animal Care (NIH publication, #80-23, revised 1996) and the Animal Care and Use Guidelines of Kyung Hee University, Korea. Adult male ICR mice (7–10 weeks old) were obtained from Young Bio Lab Co. (Osan, Korea). The mice were maintained in a standard laboratory animal facility under 12-h light/dark cycles with food and water ad libitum.
For splenocyte isolation, each spleen was removed aseptically from ICR mice and kept in cooled HBSS. A single cell suspension was prepared by gently homogenizing the spleens with the distal end of a syringe in a cell strainer and washed with HBSS. The cell suspension was centrifuged at 400×g and further incubated with red blood cell lysis buffer for 15 min. The cells were then centrifuged at 400×g, washed, and cultured in RPMI 1640 medium (supplemented with 10% FBS). Primary mouse splenocytes were cultured in RPMI 1640 media with 10% FBS at 37 °C in 5% CO2 atmosphere incubator.
Cell culture
YAC-I (KCLB no. 40160) cell lines were purchased from the Korean Cell Line Bank (Seoul, Korea). The cell lines were grown in RPMI 1640 medium or DMEM with 10% FBS and 1% penicillin–streptomycin, and incubated at 37 °C in 5% CO2. Cell cultures with less than 5 passages were used in all experiments.
In vitro splenocyte proliferation assay
The MTT assay was used to measure splenocyte proliferation. In summary, 50 µg of 4 × 106 cells/well were seeded in 96-well culture plates. After 2 h, cells were treated with 10 μg/mL of each Fr and 10 µM of each Com, respectively, for 48 h. MTT solution was added and the cells were incubated for another 4 h. After removing the media, DMSO was added to each well to dissolve MTT-formazan product. The resulting absorbance was measured using a microtiter plate reader (Synergy HT, Multi-mode microplate reader, BioTek Instruments, Inc., Winooski, VT, USA) at 570 and 630 nm. Cell proliferation is expressed as the percentage of viable treated sample cells to control cells. All tests were performed in quadruplicate.
Cytotoxicity NK cell assay
To evaluate NK cell activity, the non-adherent splenocytes were used as the effector cells and YAC-1 as the target cells [50 µL effector (E) at 4 × 106 cell/well was added to 100 µL of 2 × 105 target (T) cells/well to give an E:T ratio of 10:1] were cultured in 96-well plates in the presence or absence of the Frs (10 µg/mL) or the Coms (10 µM/mL) and incubated at 37 °C in a 5% CO2 incubator for 24 h. Afterwards, tumor killing activity of NK cell was evaluated by the MTT assay. NK cell activity is shown as cell viability compared to control according to the following equation: cell viability = (ODsample − ODeffector control)/ODtarget control × 100.
Statistical analysis
Data were analyzed using the Prism 5 Statistical Software package (GraphPad, San Diego, CA, USA). All data are expressed as the mean ± standard error mean (SEM). Statistical comparisons between the groups were performed using one-way repeated measures ANOVA with Tukey’s post hoc test. Values of p < 0.05, 0.01, and 0.001 were considered statistically significant.
Results and discussion
Repeated column chromatography for the n-BuOH Fr of M. verticillata aerial parts led to the isolation of four glycerides (1–4), which were identified as (2S)-1-O-palmitoyl glyceride [24], (2S)-1-O-stearoyl glyceride [25], (2S)-1-O-linolenoyl glyceride [26], and (2S)-1,2-di-O-linoleoyl glyceride [27], respectively, based on NMR, IR, FAB-MS, GC–MS, and FAB-MS analyses in addition to comparison of the data with those in literatures (Table 1).
Table 1.
GC–MS analysis for fatty acids in glycerides 1–4
| Fatty acids | Retention time (min) | Molecular weight | Molecular formula | MS product ions (m/z) | Glyceridesa |
|---|---|---|---|---|---|
| Palmitic acid methyl ester | 10.27 | 270 | C17H34O2 | 270 [M]+, 227 [M-(CH2)2CH3]+, 143 [M-(CH2)8CH3]+, 87 [M-(CH2)12CH3]+, 74 [M-(CH2)13CH3 + H]+ | 1 |
| Linolenic acid methyl ester | 13.76 | 292 | C19H32O2 | 236 [M–H(CH)2CH2CH3]+, 108 [M-(CH)(CH2)8COOCH3]+, 95 [C7H11]+, 79 [C6H7]+, 67 [C5H7]+ | 3 |
| Linoleic acid methyl ester | 13.99 | 294 | C19H34O2 | 294 [M]+, 263 [M-OCH3]+, 109 [M–H(CH)(CH2)8COOCH3]+, 95 [C7H11]+, 81 [C6H9]+, 67 [C5H7]+ | 4 |
| Stearic acid methyl ester | 14.37 | 298 | C19H38O2 | 298 [M]+, 255 [M-(CH2)2CH3]+, 199 [M-(CH2)6CH3]+, 143 [M-(CH2)10CH3]+, 87 [M-(CH2)14CH3]+, 74 [M-(CH2)15CH3 + H]+ | 2 |
aGlycerides include the corresponding fatty acids. Each glyceride (1.0 mg) was dissolved in 2 mL of 20% KOH/MeOH and heated 80 °C in water bath for 60 min. After cooling at room temperature, the reaction mixture was neutralized by adding the acidic cation-exchange resin (Dowex 50 W, H+ form) and filtered. The filtrate was evaporated in vacuo and partitioned into EtOAc and H2O fractions. Each EtOAc fraction was then evaporated in vacuo, dissolved in EtOAc 200 μL and filtered through a syringe filter (0.2 μm, 13 mm). The filtrate was used to GC–MS analysis
Com 1 was detected as a yellow color on TLC plates by spraying with 10% sulfuric acid (SA) and heating. The molecular weight (MW) and the molecular formula (MF) were determined to be 330 and C19H38O4, respectively, from the molecular ion peak (MIP) m/z 331 [M + H]+ in the positive FAB-MS. In the IR spectrum, Com 1 showed the hydroxyl (3383 cm−1) and ester (1723 cm−1) absorbance bands. The 1H-NMR spectrum showed one terminal methyl (δH 0.88, 3H, t, J = 7.2), one allyl methylene (δH 2.33, 2H, t, J = 8.0), and thirteen methylene (δH 1.27–1.34, 24H) proton signals due to a hexadecanoic acid. The oxygenated methine (δH 3.96, 1H, H-2), and two oxygenated methylene (δH 4.17, 1H, H-1a; δH 4.10, 1H, H-1b; δH 3.87, 2H, H-3) proton signals due to a glycerol moiety were also observed. Observation of the oxygenated methylene (δH 4.17, 1H, H-1a; δH 4.10, 1H, H-1b) proton signals in the lower magnetic field compared with that of the glycerol confirmed Com 1 to have an ester bond at C-1. Based on these results, Com 1 was assumed to be a monoglyceride with a saturated fatty acid. The 13C-NMR spectrum showed an oxygenated methylene (δC 66.1, C-1), an oxygenated methine (δC 69.8, C-2), and an oxygenated methylene (δC 67.4, C-3) carbon signals were confirmed as the signals of a glycerol moiety. An ester (δC 175.1), one terminal methyl (δC 14.4), an allyl methylene (δC 34.9) and thirteen methylene (δC 25.9–30.1) carbon signals were observed as signals of a hexadecanoic acid moiety. The fatty acid methyl ester obtained by alkaline hydrolysis and solvent fractionation appeared as a clear peak at 10.27 min on the GC–MS spectrum, which was identified as a palmitic acid methyl ester by comparing the mass spectrum of the peak with the reference value (Wiley 9 Library). In the gradient heteronuclear multiple-bond connectivity (gHMBC) spectrum, 3J correlations were observed between the oxygenated methylene proton signal of the glycerol H-1a, 1b (δH 4.17, 4.10) and the ester carbonyl carbon signal C-1′ (δC 175.1) confirming that palmitic acid was linked to the C-1 of the glycerol. On the basis of the positive optical rotation value of Com 1 ([α]D − 1.2°, c 0.10, CHCl3), which was similar to that of (2S)-1-O-palmitoyl glyceride ([α]D − 1.2°, c 0.10, CHCl3) [28], and a biogenetic perspective of the plant glyceride, the absolute configuration of C-2 in Com 1 was identified to be S. Therefore, the chemical structure (CS) of Com 1 was identified to be (2S)-1-O-palmitoyl glyceride (Fig. 1).
Fig. 1.
Strutures of compounds 1–4 isolated from the aerial parts of M. verticillata. 1: (2S)-1-O-palmitoyl glyceride, 2: (2S)-1-O-stearoyl glyceride, 3: (2S)-1-O-linolenoyl glyceride, 4: (2S)-1,2-di-O-linoleoyl glyceride
Com 2 was appeared yellow in color on TLC plates after spraying the plate with 10% SA and heating. The MW and the MF were determined to be 358 and C21H42O4, respectively, from the MIP m/z 359 [M + H]+ in the positive FAB-MS. In the IR spectrum, Com 2 showed the hydroxyl (3386 cm−1) and ester (1724 cm−1) absorbance bands. The 1H-NMR spectrum showed one terminal methyl (δH 0.88, 3H, t, J = 7.2), one allyl methylene (δH 2.34, 2H, t, J = 8.0), and fifteen methylene (δH 1.27–1.34, 28H) proton signals due to an octadecanoic acid. The oxygenated methine (δH 3.96, 1H, H-2) and two oxygenated methylene (δH 4.17, 1H, H-1a; δH 4.10, 1H, H-1b; δH 3.90, 2H, H-3) proton signals due to a glycerol moiety were also observed. Observation of the oxygenated methylene (δH 4.17, 1H, H-1a; δH 4.10, 1H, H-1b) proton signals in the lower magnetic field compared with that of glycerol confirmed Com 2 to have an ester bond at C-1. Based on these results, Com 2 was assumed to be a monoglyceride with a saturated acid. The 13C-NMR spectrum showed an oxygenated methylene (δC 66.1, C-1), an oxygenated methine (δC 69.6, C-2), and an oxygenated methylene (δC 67.4, C-3) carbon signals were confirmed as the signals of a glycerol moiety. An ester (δC 173.6), one terminal methyl (δC 14.2), an allyl methylene (δC 34.3) and fifteen methylene (δC 25.1-30.0) carbon signals indicated the presence of an octadecanoic fatty acid moiety. The fatty acid methyl ester obtained by alkaline hydrolysis and solvent fractionation appeared as a clear peak at 14.37 min on the GC–MS spectrum, which was identified as a stearic acid methyl ester by comparing the mass spectrum of the peak with that in the library (Wiley 9 Library). In the gHMBC spectrum, 3J correlations were observed between the oxygenated methylene proton signal of the glycerol H-1a, 1b (δH 4.17, 4.10) and the ester carbonyl carbon signal C-1′ (δC 173.6) confirming that stearic acid was linked to the C-1 of the glycerol. On the basis of the positive optical rotation value of Com 2 ([α]D + 1.5°, c 0.10, CHCl3), which was similar to that of (2S)-1-O-stearoyl glyceride ([α]D +1.3°, c 0.10, CHCl3) [27], and a biogenetic perspective of the plant glyceride, the absolute configuration of C-2 in Com 2 was identified to be S. Therefore, the CS of Com 2 was identified to be (2S)-1-O-stearoyl glyceride.
Com 3 was dark yellow in color on TLC plates after spraying the plate with 10% SA and heating. The MW and the MF were determined to be 352 and C21H36O4, respectively, from the MIP m/z 353 [M + H]+ in the positive FAB-MS. In the IR spectrum, Com 3 showed the hydroxyl (3386 cm−1), ester (1722 cm−1), and double bond (1612 cm−1) absorbance bands. The 1H-NMR spectrum showed one terminal methyl (δH 0.96, 3H, t, J = 7.2), five allyl methylene (δH 2.07–2.80, 10H), five methylene (δH 1.30–1.60, 10H), six olefin methine (δH 5.27–5.40, 6H) proton signals due to an octadecatrienoic acid. The oxygenated methine (δH 3.96, 1H, H-2) and two oxygenated methylene (δH 4.17, 1H, H-1a; δH 4.10, 1H, H-1b; δH 3.90, 2H, H-3) proton signals due to a glycerol moiety were also observed. Observation of the oxygenated methylene (δH 4.17, 1H, H-1a; δH 4.10, 1H, H-1b) proton signals in the lower magnetic field compared with that of glycerol confirmed Com 3 to have an ester bond at C-1. Based on these results, Com 3 was assumed to be a monoglyceride with an unsaturated fatty acid. The 13C-NMR spectrum showed an oxygenated methylene (δC 66.2, C-1), an oxygenated methine (δC 69.9, C-2), and an oxygenated methylene (δC 67.4, C-3) carbon signals as the signals of a glycerol moiety. An ester (δC 175.3), one terminal methyl (δC 14.6), six olefin methine (δC 128.2–132.7), five allyl methylene (δC 21.5–34.9), and five methylene (δC 26.0–30.0) carbon signals indicated the presence of an octadecatrienoic acid moiety. The fatty acid methyl ester obtained by alkaline hydrolysis and solvent fractionation appeared as a clear peak at 13.76 min on the GC–MS spectrum, which was identified as a linolenic acid methyl ester by comparing the mass spectrum of the peak with that in the library (Wiley 9 Library). In the gHMBC spectrum, 3J correlations were observed between the oxygenated methylene proton signal of the glycerol H-1a, 1b (δH 4.17, 4.10) and the ester carbonyl carbon signal C-1′ (δC 175.3) indicating that the linolenic acid was linked to the C-1 of the glycerol. On the basis of the positive optical rotation value of Com 3 ([α]D +3.5°, c 0.10, CHCl3), which was similar to that of (2S)-1-O-linolenoyl glyceride ([α]D +3.8°, c 0.42, CHCl3) [25], and a biogenetic perspective of the plant glyceride, the absolute configuration of C-2 in Com 3 was identified to be S. Therefore, the CS of Com 3 was identified to be (2S)-1-O-linolenoyl glyceride.
Com 4 was detected as dark yellow in color on TLC plates by spraying the plate with 10% SA and heating. The MW and the MF were determined to be 616 and C39H68O5, respectively, from the MIP m/z 615 [M–H]− in the negative FAB-MS. In the IR spectrum, Com 4 showed the hydroxyl (3389 cm−1), ester (1725 cm−1), and double bond (1610 cm−1) absorbance bands. The 1H-NMR spectrum showed two terminal methyl (0.89, 6H, t, J = 6.8), eight allyl methylene (δH 2.06–2.80, 16H), eleven methylene (δH 1.28–1.60, 22H), eight olefin methine (δH 5.32–5.36, 8H) proton signals due to two octadecadienoic acids. The oxygenated methine (δH 5.20, 1H, H-2), and two oxygenated methylene (4.43, 1H, H-1a; δH 4.17, 1H, H-1b; δH 3.96, 2H, H-3) proton signals due to a glycerol moiety were also observed. Observation of the oxygenated methylene (δH 4.17, 1H, H-1a; δH 4.10, 1H, H-1b) and oxygenated methine (δH 5.20, 1H, H-2) proton signals in the lower magnetic field compared with those of the glycerol confirmed Com 4 to have ester bonds at C-1 and C-2. Based on these results, Com 4 was assumed to be a diacylglyceride with two unsaturated fatty acids. The 13C-NMR spectrum showed two oxygenated methylene (δC 64.7, C-1; δC 63.9, C-3), an oxygenated methine (δC 72.0, C-2) carbon signals were confirmed to be the signals of a glycerol moiety. Two ester (δC 174.9, 174.6), two terminal methyl (δC 14.5, × 2), eight olefin methine (δC 129.2–131.1), eight allyl methylene (δC 21.8–35.1), and eleven methylene (δC 21.8–30.8) carbon signals were observed indicating the presence of two octadecadienoic acids moieties. The fatty acid methyl ester obtained by alkaline hydrolysis and solvent fractionation appeared as a clear peak at 13.99 min on the GC–MS spectrum, which was identified as linoleic acid methyl ester by comparing the mass spectrum of the peak with that in the library (Wiley 9 Library). In the gHMBC spectrum, 3J correlations were observed between the oxygenated methylene proton signal of the glycerol H-1a, 1b (δH 4.43, 4.17) and the ester carbonyl carbon signal C-1′ (δC 174.6), as well between the oxygenated methine proton signal of glycerol H-2 (δH 5.20) and the ester carbonyl carbon signal C-1″ (δC 174.9) indicating that two linoleic acids were linked to the C-1 and C-2 of the glycerol, respectively. On the basis of the positive optical rotation value of Com 4 ([α]D − 2.0°, c 1.00, CHCl3), which was similar to that of (2S)-1,2-di-O-linoleoyl glyceride ([α]D − 2.0°, c 1.10, CHCl3) [29], and a biogenetic perspective of the plant glyceride, the absolute configuration of C-2 in Com 4 was identified to be S. Therefore, the CS of Com 4 was identified to be (2S)-1,2-di-O-linoleoyl glyceride.
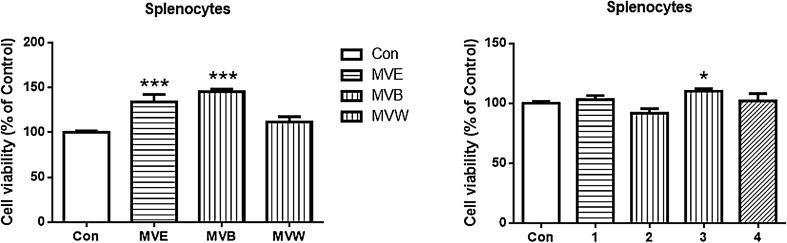
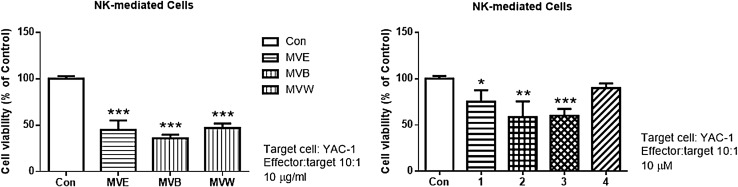
All Frs and Coms 1–4 obtained from M. verticillata aerial parts showed no significant toxicity on splenocytes at 10 µg/mL or 10 µM/mL, respectively, therefore, these concentrations were used for all assays. Treatment with some Frs and a glyceride showed a significant effect on splenocyte proliferation. As shown in Fig. 2, the EtOAc Fr, the n-BuOH Fr, and Com 3 induced splenocyte proliferation approximately 1.5- and 1.2-fold versus the negative (only cells) control. Splenocyte cytotoxicity was also examined against NK-sensitive tumor cells (YAC-1). NK cell cytotoxicity was significantly increased after exposure to all Frs (10 µg/mL) and Coms 1–3 (10 µM/mL), at approximately 65 and 50% versus the control, which indicated that the all Frs and Coms 1–3 can modulate the innate immune response (Fig. 3).
Fig. 2.
The effect of M. verticillata fractions and compounds 1–4 on splenocyte proliferation. *p < 0.05 and ***p < 0.001 indicate significant differences from normal group using one-way ANOVA). The results shown are representative of four independent experiments. MVE: the EtOAc fraction of M. verticillata aerial parts, MVB: the n-BuOH fraction of M. verticillata aerial parts, MVW: the H2O fraction of M. verticillata aerial parts. 1: (2S)-1-O-palmitoyl glyceride, 2: (2S)-1-O-stearoyl glyceride, 3: (2S)-1-O-linolenoyl glyceride, 4: (2S)-1,2-di-O-linoleoyl glyceride
Fig. 3.
The effect of M. verticillata fractions and compounds 1–4 on the NK cell activity (*p < 0.05, **p < 0.01, and ***p < 0.001). The results shown are representative of four independent experiments. MVE: the EtOAc fraction of M. verticillata aerial parts, MVB: the n-BuOH fraction of M. verticillata aerial parts, MVW: the H2O fraction of M. verticillata aerial parts
In this study, the immune modulation activity of M. verticillata Frs and Coms 1–4 were investigated. Several studies have investigated and indicated the immunomodulatory and antitumor activity of some medicinal plants [30, 31]. The immunomodulatory effect of M. verticillata Frs and Coms 1–4 were evaluated for their effects on splenocyte proliferation. The results indicated that the Frs (10 µg/mL) and Coms 1–4 (10 µM/mL) did not show any significant toxicity in the splenocytes for 48 h. In addition, NK cell cytotoxic activity was evaluated to determine the immune modulation effect of the Frs and Coms 1–4 on innate immunity. The results indicated that all tested Frs and Coms 1–3 significantly increased NK cell cytotoxic activity. The NK cell cytotoxicity against NK-sensitive tumor cells (YAC-1) significantly increased after exposure to all Frs and Coms 1–3, indicating increased the innate immunity response against tumor cells in the presence of these Coms.
In conclusion, we isolated four glycerides from the n-BuOH Fr of M. verticillata aerial parts, and it was found that monoacyl glycerides significantly enhanced splenocyte proliferation and induced significant enhancement of NK cell activity against tumor cells (YAC-1). In particular, the n-BuOH Fr is the most effective in immune modulation, and Coms 1–3, which were isolated from the n-BuOH Fr, also showed immune modulation effects. In contrast, Com 4 showed very weak activity, indicating the monoacyl glycerides are more effective than diacyl glycerides. Also, the longer the carbon number of the fatty acid in monoacyl glyceride, the better the activity, and the monoacyl glyceride including an unsaturated fatty acid (3) is more effective than the glycerides including the saturated fatty acids (1–2). When monoacylglycerides and diacylglyceride were structurally compared with each other, it was confirmed that the activity varies greatly depending on the presence or absence of fatty acid bond in 2-OH of glycerol moiety. The fact that natural killer cells were very stable to monoglycerides induced death was reported [32], the role of NK cell activator against tumor cells (YAC-1) was first introduced in this study. These findings suggest that M. verticillata could inhibit cancer cell growth as well as generate an effective cell-mediated immune response through the activation of immune cell function.
Acknowledgements
This work was supported by the Korea Evaluation Institute of Industrial Technology (Development of novel materials based on dual functions for improvement of atopic dermatitis and photoaging by regulating NFAT Grant number: 10076337), Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jonas WB. Alternative medicine. J. Fam. Pract. 1997;45:34–37. [PubMed] [Google Scholar]
- 2.Israelsen LD. Phytomedicines: the greening of modern medicine. J. Altern. Comp. Med. 1995;1:245–248. doi: 10.1089/acm.1995.1.245. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu DT, Lepe-Zuniga J, Wong WL, LaPushin R, Mavligit GM. Fractionated extract of Astragalus membranaceus, a Chinese medicinal herb, potentiates LAK cell cytotoxicity generated by a low dose of recombinant interleukin-2. J Clin. Lab. Immunol. 1988;26:183–187. [PubMed] [Google Scholar]
- 5.Chu DT, Lin JR, Wong W. The in vitro potentiation of LAK cell cytotoxicity in cancer and AIDS patients induced by F3-a fractionated extract of Astragalus membranaceus. Chung Hua Chung Liu Tsa Chih. 1994;16:167–171. [PubMed] [Google Scholar]
- 6.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer letters. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Odontuya G, Enkhmaa G, Batbayar N, Naran R, Inngjerdingen KT, Michaelsen TE, Paulsen BS. Pharmacological activities of a mongolian medicinal plant, Malva mohileviensis Down. Eur. J. Med. Plants. 2012;2:230–241. doi: 10.9734/EJMP/2012/1280. [DOI] [Google Scholar]
- 8.Gonda R, Tomoda M, Shimizu N, Kanari M. Characterization of an acidic polysaccharide from the seeds of Malva verticillata stimulating the phagocytic activity of cells of the RES1. Planta Medica. 1990;56:73–76. doi: 10.1055/s-2006-960888. [DOI] [PubMed] [Google Scholar]
- 9.Franke K, Strijowski U, Fleck G, Pudel F. Influence of chemical refining process and oil type on bound 3-chloro-1,2-propanediol contents in palm oil and rapeseed oil. LWT Food Sci. Technol. 2009;42:1751–1754. doi: 10.1016/j.lwt.2009.05.021. [DOI] [Google Scholar]
- 10.Farese RV, Walther TC. Lipid droplets finally get a little RESPECT. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nature Rev. Mol. Cell Biol. 2006;7:373. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 12.Welte MA. Proteins under new management: lipid droplets deliver. Trends Cell Biol. 2007;17:363–369. doi: 10.1016/j.tcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nature Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 14.Samsa MM, Mondotte JA, Iglesias NG, Assunção-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathogens. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Hamid NM, Fawzy MA, El-Moselhy MA. Evaluation of hepatoprotective and anticancer properties of aqueous olive leaf extract in chemically induced hepatocellular carcinoma in rats. Am. J. Med. Med. Sci. 2011;1:15–22. [Google Scholar]
- 16.Wu XY, Xiong J, Liu XH, Hu JF. Chemical constituents of the rare cliff plant Oresitrophe rupifraga and their antineuroinflammatory activity. Chem. Biodivers. 2016;13:1030–1037. doi: 10.1002/cbdv.201500357. [DOI] [PubMed] [Google Scholar]
- 17.Ramos-Bueno RP, González-Fernández MJ, Guil-Guerrero JL. Various acylglycerols from common oils exert different antitumor activities on colorectal cancer cells. Nutri. Cancer. 2016;68:518–529. doi: 10.1080/01635581.2016.1152382. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Zhang HG. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer. 1999;80:880–888. doi: 10.1002/(SICI)1097-0215(19990315)80:6<880::AID-IJC14>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Hicks AM, Riedlinger G, Willingham MC, Alexander-Miller MA, Von Kap-Herr C, Pettenati MJ, Sanders AM, Weir MH, Du W, Kim J, Simpson AJG, Old LJ, Cui Z, Simpson AJ. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. Proc. Natl. Acad. Sci. 2006;103:7753–7758. doi: 10.1073/pnas.0602382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 23.Thi NN, Song HS, Oh EJ, Lee YG, Ko JH, Kwon JE, Kang SC, Baek NI. Phenylpropanoids from Lilium Asiatic hybrid flowers and their anti-inflammatory activities. Appl. Biol. Chem. 2017;60:527–533. doi: 10.1007/s13765-017-0307-7. [DOI] [Google Scholar]
- 24.Minn CV, Kiem PV, Huong LM, Kim YH. Cytotoxic constituents of Diadema setosum. Arch. Pharm. Res. 2004;27:734–737. doi: 10.1007/BF02980141. [DOI] [PubMed] [Google Scholar]
- 25.Han L, Wang T. Preparation of glycerol monostearate from glycerol carbonate and stearic acid. RSC Advances. 2016;6:34137–34145. doi: 10.1039/C6RA02912D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogihara T, Amano N, Mitsui Y, Fujino K, Ohta H, Takahashi K, Matsuura H. Determination of the absolute configuration of a monoglyceride antibolting compound and isolation of related compounds from Radish leaves (Raphanus sativus) J. Nat. Prod. 2017;80:872–878. doi: 10.1021/acs.jnatprod.6b00746. [DOI] [PubMed] [Google Scholar]
- 27.Gaffney PR, Reese CB. Preparation of 2-O-arachidonoyl-1-O-stearoyl-sn-glycerol and other di-O-acyl glycerol derivatives. Tetrahedron letters. 1997;38:2539–2542. doi: 10.1016/S0040-4039(97)00395-X. [DOI] [Google Scholar]
- 28.Kodali DR. Improved method for the synthesis of 1-or 3-acyl-sn-glycerols. J. Lipid Res. 1987;28:464–469. [PubMed] [Google Scholar]
- 29.Duralski AA, Spooner PJ, Watts A. Synthesis of optically active polyunsaturated diacylglycerols. Tetrahedron letters. 1989;30:3585–3588. doi: 10.1016/S0040-4039(00)99448-6. [DOI] [Google Scholar]
- 30.Katayama S, Nishio T, Kishimura H, Saeki H. Immunomodulatory properties of highlyviscous polysaccharide extract from the Gagome alga (Kjellmaniella crassifolia) Plant Foods Hum. Nutr. 2012;67:76–81. doi: 10.1007/s11130-011-0271-z. [DOI] [PubMed] [Google Scholar]
- 31.Zhao M, Wang Q, Ouyang Z, Han B, Wang W, Wei Y, Wu Y, Yang B. Selective fraction of Atractylodes lancea (Thunb.) DC. and its growth inhibitory effect on human gastric cancer cells. Cytotechnol. 2014;66:201–208. doi: 10.1007/s10616-013-9559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philippoussis F, Arguin C, Fortin M, Steff AM, Hugo P. Cellular specificity related to monoglyceride-induced cell death. Immunol. Lett. 2002;83:221–230. doi: 10.1016/S0165-2478(02)00117-7. [DOI] [PubMed] [Google Scholar]