Abstract
Chitosan and chitooligosaccharides were extracted from white-leg shrimp shells by chemical treatment. Low molecular weight (13 kDa) and a high degree of deacetylation (54.83%) in chitooligosaccharides led to high water solubility compared to chitosan. Antimicrobial assays indicated that chitosan and chitooligosaccharides exhibited marked inhibitory activity against food-borne pathogenics, spoilage bacterial, and fungal strains tested. However, chitooligosaccharides revealed greater inhibitory effects than chitosan on tested microorganisms. The substitution of flour by chitosan or chitooligosaccharides in bread formulation (1 g/100 g total weight basis) showed antimicrobial effects against Bacillus cereus and Rhizopus sp. growth. Also, the fruity odor in bread containing chitosan or chitooligosaccharides was delayed. Interestingly, the bread containing chitooligosaccharides showed a stronger inhibitory effect against B. cereus and Rhizopus sp. compared to bread containing chitosan and control, where B. cereus and Rhizopus sp. were observed growing on the surface of bread after 4 days of incubation at 30 °C.
Keywords: Chitosan, Chitooligosaccharides, Antimicrobial agents, Pathogenic microorganisms, Bread
Introduction
Nowadays, consumers greater recognize the importance of food safety, especially when concerning contamination by food-borne pathogenic microorganisms. Microbial contamination in food not only results in a reduction of a product’s shelf life and food deterioration, but can also lead to disease and economic loss. Therefore, synthetic additives are used in the food industries and play an important role in maintaining the quality of food as well as inhibiting the growth of various spoilage and pathogenic bacteria. However, the formation of carcinogenic by-products from these substances has raised concerns about the effect of food additives on health. Thus, the study and development of new antimicrobial substances from natural products in order to control microbial safety and extend the shelf life of food products would be advantageous.
Bread products have become more favorable worldwide for consumption. One issue though is the loss of bread quality over time, which is generally associated with microbial spoilage, it becoming stale, and moisture loss [1]. The most common sources of microbial spoilage in bread are mold and bacterial growth, especially the natural mold growth that occurs during storage [1, 2]. Rope spoilage is another common cause of decomposition in bread. Normally, spoilage is caused by contamination by Bacillus species (B. subtilis, B. licheniformis, B. pumilus, and B. cereus) in flour and results in the rope formation of bacteria, which causes a sticky texture and fruity odor in bread [3].
Chitosan is a natural biopolymer composed of a deacetylated form of chitin with d-glucosamine repeating units linked by (1–4) glycosidic bonding, which is a natural resource derived from the exoskeletons of arthropods and the cell walls of fungi [2]. Some reports have pointed out that chitosan is a potentially useful and indirect antimicrobial material for food protection [4, 5]. However, the application of chitosan in food products remains difficult due to its high molecular weight, which results in a poor solubility at neutral pH and a high solution viscosity. Such factors limit its use in food applications [6, 7]. Chitooligosaccharides (COS) are the degradation products from chitosan or chitin, and have recently been produced by several methods, such as enzymatic and acidic hydrolysis. They are short polymers composed of free d-glucosamine [8], which results in water-soluble properties at neutral pH and low viscosity [6]. Some research results indicated that COS has shown markedly antibacterial activity against important food-borne pathogenic bacteria and other microorganisms [9, 10]. According to the various properties of COS, a study of the physicochemical properties and antimicrobial activity of COS would provide useful information for their further application in many fields, especially food and nutrition, to improve the quality of products and to promote health benefits.
Thus, the aim of this study was to extract chitosan and COS from shrimp-shell waste. The obtained COS was characterized and compared with chitosan in terms of physicochemical properties, antimicrobial activity, and potential application in bread products for microbial inhibition.
Materials and methods
Raw materials
White-leg shrimp shells were obtained from Thai Union Frozen Co., Ltd. The shrimp shells were packed in polyethylene bags, placed in ice, and transported to the research laboratory. Upon arrival, the shrimp shells were washed twice with water and then dried in a drying oven at 60 °C for 4 h. After drying, the shells were ground down and then stored at − 20 °C until used.
Chitin, chitosan, and COS preparation
For the demineralization process, 100 g of shrimp shells were placed into 1000 mL HCl (1.27 N) at room temperature under constant stirring for 1 h to remove the calcium carbonate. The decalcified shells were filtered and washed to neutrality with ionized water, and then oven-dried at 80 °C overnight. After that, the dried shells were added into 1000 mL of 3% (w/w) NaOH at 100 °C for 15 min to remove the protein from the shells. At the end of this process, the material was filtrated and rinsed with distilled water until neutralized and dried, as previously described in the demineralization process. This was followed by adding 250 mL of 95% ethanol into the chitin residue to remove some ethanol soluble substances [6].
The conversion of chitin into chitosan involved a deacetylation process, with 1 g of chitin added into 50 mL of 50% (w/w) NaOH, as a deacetylation reagent, at 60 °C for 8 h with constant stirring. After that, the solid was filtered and washed with water and 80% (v/v) alcohol until the filtrate was neutral. Afterward, it was oven-dried at 80 °C overnight. To prepare COS, 1 g of crude chitosan was dissolved in 50 mL HCl (6.25 N) at 56 °C for 3 h. The solid was filtered and oven-dried at 50 °C.
Infrared spectroscopic analyses and degree of deacetylation
The infrared spectra of the commercial chitosan, extracted chitosan, and COS were analyzed with Fourier transform infrared spectroscopy (FTIR). Each sample was thoroughly mixed with KBr. The dried mixture was then pressed to create a homogenous sample/KBr disk. FTIR was performed in the frequency range of 400 to 4000 cm−1 and the results for the extracted chitosan and COS were compared with the commercial standard chitosan.
The degree of deacetylation (DD) was calculated using Baxter’s equation [11]:
where A1655 and A3450 are the absorbance at 1655 and 3450 cm−1, respectively.
Water solubility and weight-average molecular weight
To estimate the solubility, 1 g of extracted chitosan and COS was mixed with 100 mL of distilled water, stirred for 3 h, and then filtrated through 0.45 µm filter paper. The solubility was estimated from the change in paper weight and reported as g/100 mL [12].
The weight-average molecular weight (Mw) was measured by gel permeation chromatography (GPC) with 0.5 M CH3COOH/0.5 M CH3COONa (acetate buffer pH 4) used as the eluent. The flow rate was maintained at 0.6 ml/min. The sample concentration was approximately 2 mg/mL, which was filtrated using a nylon 66 membrane (pore size 0.45 µm) before injection. The standards used to calibrate the column were pullulans (Mw 5900–708,000).
Antimicrobial activity
The bacterial strains used to determine the antibacterial activities were Staphylococcus aureus, Bacillus subtilis TISTR 1248, Enterococcus faecalis TISTR 379, Bacillus cereus, Escherichia coli TISTR 527, Vibrio parahaemolyticus, Vibrio cholerae, Enterobacter aerogenes TISTR 1540, Pseudomonas aeruginosa, and Salmonella typhimurium. The fungal strains used to determine the antifungal activities were Rhizopus oligosporus and Aspergillus niger. The antimicrobial activities of chitosan and COS were assayed using a disk diffusion method adapted from Bauer et al. [13]. The chitosan solutions (1 mg/mL) were dissolved in 0.1% (v/v) acetic acid, while the COS solutions (1 mg/mL) were dissolved in deionized water. Then, 100 µl of bacterial cells (107 CFU/mL) from the bacterial and fungal strains (108 spores/mL) was spread on nutrient agar and potato dextrose agar media, respectively. Subsequently, a sterilized filter disk paper (0.4 cm) was placed onto the inoculated agar and impregnated with 60 µL of the sample. At the end of the incubation time (24 h at 37 °C for bacterial strains or 72 h at 30 °C for fungal strains), the antimicrobial activities were measured by the diameter of the clear zone of growth inhibition compared with a positive (ampicillin and tetracyclin, 10 µg/mL) and negative control (0.1% (v/v) acetic acid and deionized water).
Antimicrobial activity of chitosan and COS bread
The antimicrobial activities of chitosan and COS additions to bread were studied according to Lafarga et al. [14] with a slight modification to detect the inhibition of natural mold growth and intentional rope development on the control, chitosan-, and COS-containing bread. The B. cereus strain was inoculated into 25 mL of nutrient broth and incubated at 37 °C for 24 h. The overnight culture was serially diluted in nutrient broth to give the required number of 106 CFU/mL in the solution used for inoculation of the bread. Then, 1 g/100 g on a total weight basis of chitosan and COS were mixed with the flour and the breads were prepared according to Kerch et al. [15]. Three slices of each bread (10 mm thickness) for the control, chitosan-, and COS-containing bread were inoculated with 1 mL of the diluted B. cereus solution, while another three slices of the control, chitosan-, and COS-containing breads were set up as uninoculated. All the samples were placed in plastic bags and incubated at 30 °C for 4 days.
Statistical analysis
The antimicrobial activities of chitosan and COS were analyzed. All the results were presented as the average of three replications. Data were analyzed using an analysis of variance (ANOVA) and Duncan’s multiple range test with the statistical significance determined at P < 0.05.
Results and discussions
Physicochemical properties of chitosan and COS
The yields of chitin, chitosan, and COS were found to be 20.20, 17.13, and 14.56 g/100 g of weight of shrimp shells respectively. The water solubility, degree of deacetylation, and molecular weight of chitosan and COS are shown in Table 1. COS presented a high water solubility (0.97 g/100 g water). The extracted COS showed a higher solubility than reported in Chung et al. [12], who reported a maximum solubility of water-soluble chitosan produced by the Maillard reaction of 0.60 g/100 g water. However, the extracted chitosan in this study was slightly dissolved in water (0.13 g/100 g water). The application of chitosan is limited because it is normally insoluble in neutral or basic pH conditions, while being soluble in acidic media, such as acetic and formic acid [16]. Thus, the development of COS has been shown to be advantageous for use in many fields, including food, cosmetics, medicine, and agriculture, due to its water solubility [6].
Table 1.
Properties of chitosan and chitooligosaccharides (COS) extracted from white-legs shrimp shell waste
| Properties | Chitosana | COSa |
|---|---|---|
| Water solubility (g/100 mL) | 13.00 ± 0.3 | 97.00 ± 0.2 |
| Molecular weight (kDa) | 650.00 ± 1.2 | 13.00 ± 3.2 |
| Degree of deacetylation (%) | 30.81 ± 4.5 | 54.83 ± 2.3 |
aValues are reported as the mean ± SD of three separate experiments
The weight-average molecular weight (Mw) is one parameter that can be used to characterize chitosan materials because a low Mw is appropriate for antibacterial, antioxidant, and antitumor applications, while a high Mw is responsible for the low solubility of chitosan in water [6]. The Mw of the chitosan obtained in this study was 650 kDa, whereas that of COS was 13 kDa. The degree of deacetylation (DD) vales were calculated according to the formula given in the materials and methods section and were found to be 30.81 and 54.83% for chitosan and COS, respectively. Normally, the DD ranges from 30 to 95% depending on the source and preparation procedures [17]. The properties of chitosan and COS are dependent on the removal of the acetyl group. A high DD shows that the acetyl groups contain the substance in low amounts. Less chitosan acetyl groups means the interaction between the ions and hydrogen bonds of chitosan will be stronger. Therefore, the solubility of substances will be increased due to shorter chains [18].
Structural characterization
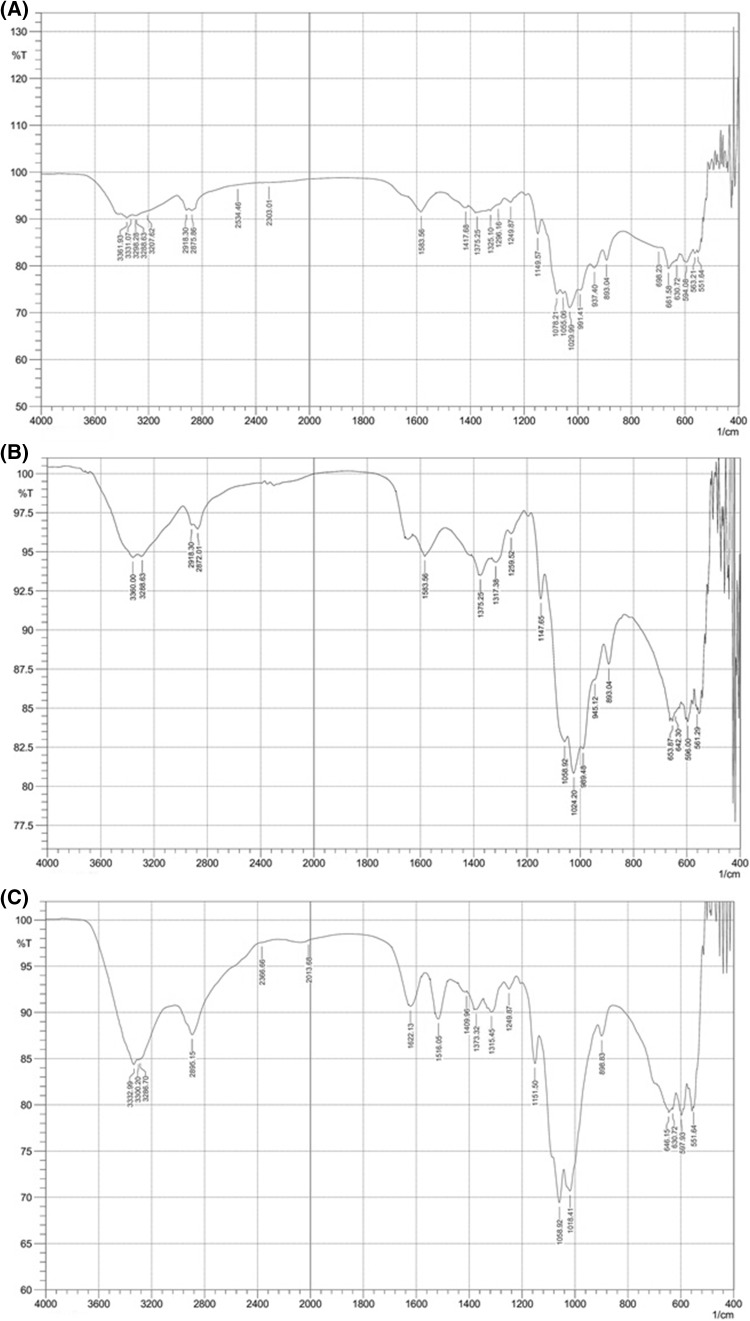
The IR spectra of the commercial chitosan, extracted chitosan, and COS are shown in Fig. 1(A)–(C), respectively. The IR spectrum of the extracted chitosan [Fig. 1(A)] was similar to that of commercial chitosan [Fig. 1(B)], whereas COS exhibited different peaks [Fig. 1(C)]. The absorption band at 1590 cm−1 was assigned to the amide II band (amine ν(NH2) tensions), which is the characteristic peak for chitosan and could be clearly observed in the extracted chitosan. The IR spectra of the chitosan and COS showed peaks around 1000–1200 cm−1, which were attributed to the saccharide structure due to the stretching of C–O–C [19]. An increase in the peak of COS around 1315–1320 cm−1, which referred to the amide III group [19] was compared for the extracted chitosan and the commercial chitosan, which confirmed the increase in the DD due to the removal of the acetyl group and the leaving behind of the amino group. The ammonium NH4+ bands at 1516 cm−1 [20], together with the amide I band around 1600–1650 cm−1 [19], clearly appeared in the IR spectrum of COS, but were not observed in those of the commercial and extracted chitosans. The region between 1420 and 1460 cm−1 is considered conformation sensitive for polysaccharides, which is the orientation of the -CH2OH group and was shifted in COS compared to the commercial and extracted chitosans, indicating a change in the secondary structural environment [21].
Fig. 1.
Infrared spectrum of (A) commercial chitosan, (B) extracted chitosan and (C) chitooligosaccharides
Antimicrobial activities
In this study, the antibacterial and antifungal activities of chitosan and COS from white-leg shrimp-shell waste were investigated against eleven bacteria and two fungi (spoilage and pathogenic microorganisms). Based on the results from the disk diffusion method, as listed in Table 2, chitosan and COS exhibited lower antimicrobial activity compared to all the other tested microbial strains and to ampicillin and tetracycline, which were used as the positive control. However, no inhibition zone appeared on the negative control (water and 0.1% acetic acid).
Table 2.
Antimicrobial activities of chitosan and COS extracted from white-legs shrimp shell waste
| Test microorganisms | Inhibition zone diametera (mm) | |||||
|---|---|---|---|---|---|---|
| Chitosan | COS | Ampicillin | Tetracyclin | H2O | Acetic acid | |
| Bacteria, Gram negative | ||||||
| Escherichia coli TISTR 527 | 7.3 ± 0.4e | 12.5 ± 0.3c | 14.0 ± 0.4cde | 14.5 ± 0.1e | – | – |
| Vibrio parahaemolyticus | 7.5 ± 0.3d | 14.0 ± 0.2a | 16.5 ± 0.5bc | 14.5 ± 0.4e | – | – |
| Vibrio cholerae | 6.2 ± 0.1i | 12.5 ± 0.1c | 19.0 ± 0.4a | 15.3 ± 0.3c | – | – |
| Enterobacter aerogenes TISTR 1540 | 6.8 ± 0.2g | 10.8 ± 0.3e | 13.1 ± 0.1def | 16.0 ± 0.4b | – | – |
| Pseudomonas aeruginosa | 6.5 ± 0.4h | 11.0 ± 0.2d | 11.7 ± 0.4ef | 14.5 ± 0.2e | – | – |
| Salmonella typhimurium | 7.9 ± 0.3c | 13.7 ± 0.3b | 16.5 ± 0.2bc | 16.5 ± 0.2a | – | – |
| Bacteria, Gram positive | ||||||
| Staphylococcus aureus | 7.1 ± 0.2f | 8.5 ± 0.5i | 18.3 ± 0.2ab | 15.0 ± 0.2d | – | – |
| Bacillus subtilis TISTR 1248 | 8.2 ± 0.3b | 9.5 ± 0.1h | 14.7 ± 0.2cd | 10.5 ± 0.4g | – | – |
| Bacillus cereus | 8.0 ± 0.1c | 10.5 ± 0.2f | 13.5 ± 0.2def | 9.5 ± 0.5h | – | – |
| Enterococcus faecalis TISTR 379 | 8.5 ± 0.2a | 9.8 ± 0.1g | 15.1 ± 0.4cd | 14.1 ± 0.2f | – | – |
| Fungi | ||||||
| Rhizopus oligosporus | 6.5 ± 0.1h | 7.5 ± 0.2j | 11.5 ± 0.4f | 10.5 ± 0.2g | – | – |
| Aspergillus niger | 5.3 ± 0.1j | 7.0 ± 0.2k | 11.5 ± 0.3f | 10.5 ± 0.3g | – | – |
aDiameter of inhibition zone including disc diameter. Values are reported as the mean ± SD of three separate experiments. Values with similar superscripts in a column are not significant different (P < 0.05)
According to the results of the antibacterial activity tests, the inhibition zone diameters of chitosan varied between 6.2 and 8.5 mm, whereas COS varied between 8.5 and 14.0 mm. Chitosan and COS were observed to be effective against all the tested bacterial strains. Considering the inhibition zone given in Table 2, the lowest inhibitory effect of chitosan was observed on V. cholerae (6.2 mm) and it showed the highest inhibitory effect against Enterococcus faecalis TISTR 379 (8.5 mm). In addition, chitosan presented higher antibacterial activity against Gram-positive bacteria than Gram-negative bacteria. Similar results were obtained by Jeon et al. [22], who observed that chitosan was more effective against Gram-positive bacteria than Gram-negative bacteria. This is probably due to Gram-positive bacteria lacking an outer membrane, which enables the penetration of chitosan into the nuclei of microorganisms, which then leads to the inhibition of mRNA and protein. However, Kaya et al. [23] found that extracted chitosan from two grasshopper species showed stronger antibacterial effectiveness against Gram-negative bacteria than Gram-positive bacteria. Thus, the antibacterial activity of chitosan depends on the species of the microorganisms and the characteristics of the tested chitosan [24].
As shown in Table 2, COS presented a lower inhibitory effect against S. aureus (8.5 mm) and showed the greatest inhibitory effect against V. parahaemolyticus (14.0 mm). In addition, COS presented higher antibacterial activity against Gram-negative bacteria than Gram-positive bacteria. Likewise, No et al. [9] found that COS was more effective against Gram-negative bacteria than Gram-positive bacteria. This was possibly due to COS possessing a high number of cationic molecules that could interact with negatively charged carbohydrates, lipids, and proteins located on the surface of bacterial cells, which inhibit the growth of the bacterial cell [25].
Moreover, the research results in this study revealed that COS was more effective against the tested bacterial strains than chitosan. Similar to this study, Du et al. [6] found that COS was more effective against E. coli, B. subtilis, and S. aureus than chitosan. It could be suggested that the considerable antibacterial activity of chitosan and COS is due to the high DD value and low Mw. As shown in Table 1, COS possessed a higher DD than chitosan. This might cause an increase in positive charge in COS, thereby leading to a stronger binding to the bacterial cell walls as a result. These results are in agreement with Gerasimenko et al. [26], who found that the death rate of bacterial cells tended to increase with an increase in the DD of chitosan. Moreover, the low molecular weight of COS might enable greater flexibility and allow it to bind with bacteria in more than one cell, resulting in the rapid formation of a bridge between the bacterial cells and polymer chains, resulting in the inactivation of bacterial cells.
However, the exact mechanism of chitosan and its derivatives against microorganisms is not clearly understood. Several mechanisms have been suggested as the main cause of the inhibition of microbial cells by chitosan. Chitosan has the ability to interact with bacterial compounds on the cell surface and can be absorbed on the cell surface [4, 27]. It can then precipitate inside the cell, leading to the formation of layers around the bacterial cells and ultimately causing blocking of the channels. This layer is expected to prevent the essential solutes from entering the cell and thus will destabilize the repair process of bacterial cells. Finally, a severe leakage of cell constituents occurs, thereby causing cell death [28].
The antifungal activities of the extracted chitosan and COS were also investigated (Table 2). Chitosan and COS were active on the two tested fungal strains, with the following order of sensitivity: R. oligosporus > A. niger. Meanwhile, chitosan showed less effect on A. niger. Likewise, Arancibia et al. [29] reported that chitosan solutions could not inhibit A. niger. Similar to the results of the antibacterial testing, COS showed a higher effectiveness against the tested fungi. Both tested samples showed higher antibacterial than antifungal properties. This was consistent with a previous study by Tsai et al. [30], who reported that the antimicrobial activity of chitosan from shrimp (Solenocera melantho) shells was stronger against bacteria than fungi. The difference in components of the bacterial and fungal cell walls might have an effect on the interaction and penetration of the extracted chitosan and COS.
Antimicrobial activity of chitosan and COS bread
The addition of chitosan and COS caused the delay of Rhizopus sp. growth and the development of ropiness as well as a fruity odor (Fig. 2). The difference in growth, under the same conditions, was easily observed in the sample after 24 h of incubation at 30 °C. The addition of chitosan was shown to exhibit high antimicrobial activity against Rhizopus sp. and B. cereus growth compared to the control. The ropiness formation and fruity odor began to develop after 3 days of incubation in chitosan bread, while the control showed a significant notification of ropiness and fruity odor after only 24 h of incubation. Moreover, Rhizopus sp. growth was also inhibited by the addition of chitosan in bread and showed no evidence of Rhizopus sp. growth until 4 days of incubation compared to the control bread, where a higher Rhizopus sp. growth was observed on the bread surface and crust. Interestingly, COS significantly inhibited the ropiness formation and Rhizopus sp. growth in bread when monitored over a 4-day period. It also presented less fruity odor compared to the control and chitosan bread. Thus, this study confirmed the antimicrobial effect of chitosan and COS against the tested microorganisms and the remaining antimicrobial properties after treatment with high temperature during the baking process, resulting in a shelf-life extension of the bread. Moreover, the breads containing chitosan and COS showed less effect on the bread’s physical characteristics compared with the control (data not shown).
Fig. 2.
Inhibitory effects of chitosan and chitooligosaccharides bread against B. cereus, rope formation and Rhizopus sp.
In this research, the authors showed that extracted COS exhibited a low Mw, high DD, high solubility, and high antimicrobial effects against all the tested microorganisms compared to chitosan. Moreover, the results showed the potential use of COS in bread, which presented antimicrobial activities against food-borne pathogens and Rhizopus sp. growth, resulting in a prolonged shelf life of the bread. However, further research into COS activity should be performed to gain an additional understanding of the mechanisms and factors involved in food protection as well as the various applications for food products.
Acknowledgements
The authors wish to thank Dr. Adchara Phadermchoke for kindly proofreading the physicochemical section. Thanks are also due to Thai Union Frozen Co., Ltd (Samutsakhon, Thailand) for supplying the white-legs shrimp-shell waste. This study was supported by Srinakharinwirot University grant (Grant Number: 735/2558). The authors have no conflict of interest to declare.
References
- 1.Leuschner RGK, O’Callaghan MJA, Arendt EK. Moisture distribution and microbial quality of part baked breads as related to storage and rebaking conditions. J. Food Sci. 1999;64:543–546. doi: 10.1111/j.1365-2621.1999.tb15081.x. [DOI] [Google Scholar]
- 2.No HK, Meyers SP, Prinyawiwatkul W, Xu Z. Applications of chitosan for improvement of quality and shelf life of foods: a review. J. Food Sci. 2007;72:87–100. doi: 10.1111/j.1750-3841.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JM, Dodd CER, Waites WM. Spoilage of bread by bacillus. Int. Biodeterior. Biodegradation. 1993;32:55–66. doi: 10.1016/0964-8305(93)90039-5. [DOI] [Google Scholar]
- 4.Helander I, Nurmiaho Lassila EL, Ahvenainen R, Rhoades J, Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001;71:235–244. doi: 10.1016/S0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 5.Roller S, Covill N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999;47:67–77. doi: 10.1016/S0168-1605(99)00006-9. [DOI] [PubMed] [Google Scholar]
- 6.Du Y, Zhao Y, Dai S, Yang B. Preparation of water-soluble chitosan from shrimp shell and its antibacterial activity. Innov. Food Sci. Emerg. Technol. 2009;10:103–107. doi: 10.1016/j.ifset.2008.07.004. [DOI] [Google Scholar]
- 7.Xia W, Liu P, Zhang J, Chen J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011;25:170–179. doi: 10.1016/j.foodhyd.2010.03.003. [DOI] [Google Scholar]
- 8.Jeon YJ, Shahidi F, Kim SK. Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Rev. Int. 2000;16:159–176. doi: 10.1081/FRI-100100286. [DOI] [Google Scholar]
- 9.No HK, Young Park N, Ho Lee S, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002;74:65–72. doi: 10.1016/S0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- 10.Barboza Corona JE, Gutierrez Acosta OB, Imperial Cervantes M, Bideshi DK, de la Fuente Salcido N, Bautista Justo M, Salcedo Hernández R. Generation of antibacterial oligosaccharides derived from chitin using heterologous endochitinase synthesized in Escherichia coli. J. Appl. Microbiol. 2008;105:1511–1520. doi: 10.1111/j.1365-2672.2008.03904.x. [DOI] [PubMed] [Google Scholar]
- 11.Baxter A, Dillon M, Anthony Taylor KD. Roberts GAF. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992;14:166–169. doi: 10.1016/S0141-8130(05)80007-8. [DOI] [PubMed] [Google Scholar]
- 12.Chung YC, Tsai CF, Li CF. Preparation and characterization of water-soluble chitosan produced by Maillard reaction. Fish. Sci. 2006;72:1096–1103. doi: 10.1111/j.1444-2906.2006.01261.x. [DOI] [Google Scholar]
- 13.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 14.Lafarga T, Gallagher E, Walsh D, Valverde J, Hayes M. Chitosan-containing bread made using marine shellfishery byproducts: functional, bioactive, and quality assessment of the end product. J. Agric. Food Chem. 2013;61:8790–8796. doi: 10.1021/jf402248a. [DOI] [PubMed] [Google Scholar]
- 15.Kerch G, Zicans J, Meri RM. The effect of chitosan oligosaccharides on bread staling. J. Cereal Sci. 2010;52:491–495. doi: 10.1016/j.jcs.2010.08.007. [DOI] [Google Scholar]
- 16.Lodhi G, Kim YS, Hwang JW, Kim SK, Jeon YJ, Je JY, Ahn CB, Moon SH, Jeon BT, Park PJ. Chitooligosaccharide and its derivatives: preparation and biological applications. Biomed Res. Int. 10.1155/2014/654913 (2014) [DOI] [PMC free article] [PubMed]
- 17.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Shahidi F, Arachchi JKV, Jeon YJ. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999;10:37–51. doi: 10.1016/S0924-2244(99)00017-5. [DOI] [Google Scholar]
- 19.Mourya VK, Inamdar NN, Choudhari YM. Chitooligosaccharides: synthesis, characterization and applications. Polym. Sci. Ser. A. 2011;53:583–612. doi: 10.1134/S0965545X11070066. [DOI] [Google Scholar]
- 20.Ritthidej GC, Phaechamud T, Koizumi T. Moist heat treatment on physicochemical change of chitosan salt films. Int. J. Pharm. 2002;232:11–22. doi: 10.1016/S0378-5173(01)00894-8. [DOI] [PubMed] [Google Scholar]
- 21.Characterization of solid state structure Focher B, Naggi A, Torri G, Cosani A, Terbojevich M. Chitosans from Euphausia superba. 2. Carbohydr. Polym. 1992;18:43–49. doi: 10.1016/0144-8617(92)90186-T. [DOI] [Google Scholar]
- 22.Jeon YJ, Park PJ, Kim SK. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001;44:71–76. doi: 10.1016/S0144-8617(00)00200-9. [DOI] [Google Scholar]
- 23.Kaya M, Baran T, Asan-Ozusaglam M, Cakmak YS, Tozak KO, Mol A, Mentes A, Sezen G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta) Biotechnol. Bioprocess Eng. 2015;20:168–179. doi: 10.1007/s12257-014-0391-z. [DOI] [Google Scholar]
- 24.Guo Z, Ren J, Dong F, Wang G, Li P. Comparative study of the influence of active groups of chitosan derivatives on antifungal activity. J. Appl. Polym. Sci. 2013;127:2553–2556. doi: 10.1002/app.37747. [DOI] [Google Scholar]
- 25.Jung EJ, Youn DK, Lee SH, No HK, Ha JG, Prinyawiwatkul W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. Technol. 2010;45:676–682. doi: 10.1111/j.1365-2621.2010.02186.x. [DOI] [Google Scholar]
- 26.Gerasimenko DV, Avdienko ID, Bannikova GE, Zueva OI, Varlamov VP. Antibacterial effects of water-soluble low-molecular-weight chitosans on different microorganisms. Prikl. Biokhim. Mikrobiol. 2014;40:301–306. [PubMed] [Google Scholar]
- 27.Xiaoyan L, Yi L, Peng L, Songsheng Q, Ziniu Y. Microcalorimetric investigation on the growth model and the protein yield of Bacillus thuringiensis. J. Biochem. Biophys. Methods. 2004;59:267–274. doi: 10.1016/j.jbbm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Sagoo SK, Board R, Roller S. Chitosan potentiates the antimicrobial action of sodium benzoate on spoilage yeasts. Lett. Appl. Microbiol. 2002;34:168–172. doi: 10.1046/j.1472-765x.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 29.Arancibia MY, López Caballero ME, Gómez Guillén MC, Fernández García M, Fernández Martín F, Montero P. Antimicrobial and rheological properties of chitosan as affected by extracting conditions and humidity exposure. LWT - Food Sci. Technol. 2015;60:802–810. doi: 10.1016/j.lwt.2014.10.019. [DOI] [Google Scholar]
- 30.Tsai GJ, Su WH, Chen HC, Pan CL. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish. Sci. 2002;68:170–177. doi: 10.1046/j.1444-2906.2002.00404.x. [DOI] [Google Scholar]