Abstract
Many edible plant extracts exhibit biological activities. For example, the ethanol extract of Pueraria montana var. lobata (P. montana) inhibits acetylcholinesterase (AChE), and red ginseng is well known for promoting health. In this study the authors investigated the synergistic effect of P. montana and red ginseng extracts on AChE activity in vitro and in mouse brain tissues and trimethyltin (TMT)-induced cognitive impairment in a mouse model of TMT-induced neurodegeneration. A diet containing a mixture of P. montana and red ginseng extracts reversed learning and memory impairments in Y-maze and passive avoidance behavioral tests. In addition, the mixture inhibited AChE activity and lipid peroxidation synergistically.
Keywords: Alzheimer’s disease, Learning, Memory, Pueraria montana var. lobata, Red ginseng
Introduction
Alzheimer’s disease (AD) is one of the most common forms of dementia in elderly people and is characterized by a progressive dysfunction of learning and memory [14]. The pathogenesis of AD has been linked to a lack of acetylcholine (ACh) [28]. More specifically, it has been commonly observed that AD patients present a decrease in ACh levels in the basal forebrain [4]. ACh plays a critical role in cognitive processes of memory and learning. In central cholinergic systems, ACh is synthesized from acetyl-CoA and choline by choline acetyltransferase (ChAT) [23]. ChAT and acetylcholinesterase (AChE) are respectively responsible for the synthesis and hydrolysis of ACh in cholinergic neurons. One of the most consistent neurotransmitter alterations found in AD is the loss of cholinergic functions via ChAT and/or AChE [26]. Moreover, it is known that such abnormal cholinergic functions are related to the severity of AD [24, 34].
Red ginseng, the root of Panax ginseng C.A. Meyer, has been used as a traditional herbal medicine in Eastern Asian cultures for thousands of years. Ginseng has pharmacological effects on not only the central nervous system (CNS) but also the endocrine, cardiovascular, and immune systems. Multiple lines of evidence have demonstrated the benefits of bioactive compounds from three major ginseng species: Panax ginseng (commonly called as Korean ginseng), Panax japonicus (Japanese ginseng), and Panax quinquefolius (American ginseng). Panax ginseng is treated with steaming and drying, which make the roots turn red. The processed product is called Korean red ginseng (KRG). KRG, the most commonly used and researched ginseng species, was studied on the CNS [20], Alzheimer’s disease, cerebral blood flow, inhibition of superoxide production [11], and learning and memory [16, 25]. The principle active ingredients found in most ginseng species include ginsenosides, peptides, polysaccharides, fatty acids, and polyacetylenic alcohols [1]. Ginseng elicits both inhibitory and stimulatory effects on the CNS, thereby modulating neurotransmission. The ginsenosides, Rb1, Rg1, and Rg3, are thought to play a major role in these effects [3, 29]. Specifically, several animal studies have shown that Rg1, Rb1, Rg3, and Rd can reverse scopolamine-induced memory deficits loss [2, 33]. Rb1 increases the uptake of choline in central cholinergic nerve endings and facilitates the release of ACh from hippocampal slices [2]. Furthermore, Rg1 and Rb1 can prevent scopolamine-induced amnesia by increasing cholinergic activity [31].
Pueraria montana var. lobata (PM; also known as Pueraria thunbergiana, or kudzu in Korea) is a trailing vine native to Korea, Japan, and China that belongs to the Fabaceae family. Its roots have been used for centuries in oriental herbal medicines as spasmolytic and antipyretic agents for the treatment of influenza, acute dysentery, diarrhea, colds, and cerebrovascular and cardiovascular diseases [5, 35]. PM contains a number of bioactive compounds, including daidzein (4′,7-dihydroxy isoflavone) [6]. The ethyl acetate fraction of PM roots protects rat pheochromocytoma cells (PC12) against amyloid β (Aβ)-induced toxicity [7]. In a previous study, we demonstrated that daidzein from PM improves drug-induced amnesia via ChAT activation in MC-IXC cells [12]. Therefore, in the current study, the synergistic effect of Korean red ginseng (KRG) and PM on AChE inhibition and subsequent restoration of ACh levels in synaptic clefts in trimethyltin (TMT)-injected mice was investigated. TMT decreases in ACh release in brain and increases AChE activity in rat hippocampus [32]. Many researchers have reported that injection of TMT in rodents causes neuropathological consequences and marked behavioral change [19].
Materials and methods
Materials
Acetylthiocholine iodide, 5,5-o-dithiobis-(2-nitrobenzoic acid) (DTNB), 9-amino-1,2,3,4-tetrahydroacridine (tacrine), dimethyl sulfoxide (DMSO), and TMT chloride were purchased from Sigma (St. Louis, MO, USA). All other chemicals used were of analytical grade.
Sample preparation
KRG was purchased from Korea Ginseng Corporation (Daejeon, Republic of Korea). The dried roots of PM (1.0 kg) were purchased at the Kyung-dong herbal market in Seoul, Republic of Korea. The dried roots were ground into a fine powder and then extracted with 10 volumes of 80% ethanol.
Cell culture
The PC12 cell line was purchased from ATCC (CRL Number: 1721) and cultured as previously detailed [18].
Measurement of AChE activity
AChE activity was measured by Ellman’s modified method [10, 17]. Briefly, PC12 cells were homogenized with Tris–HCl buffer (20 mM Tris–HCl, pH 7.5 containing 150 mM NaCl, 10 mM MgCl, and 0.5% Triton X-100), and then the samples were centrifuged at 10,000×g for 15 min. The supernatant was used as an enzyme source, and ACh iodide was used as the reaction substrate. DTNB was selected to quantify the thiocholine produced from the hydrolysis of AChE. A 10-μL aliquot of each sample was mixed with 10 μL of enzyme solution and subsequently added to 70 μL of reaction mixture (50 mM sodium phosphate buffer, pH 8.0, containing 1 mM DTNB and 0.5 mM ACh iodide). This mix was then incubated at 37 °C for 15 min. The final enzyme reactions were monitored at a wavelength of 405 nm using a 96-well microplate reader (Bio-Rad, Hercules, CA, USA).
Animals
ICR mice (male, 5 weeks old) were purchased from Samtako Bio Korea (Osan, Republic of Korea). Animals were housed in an animal room that was maintained with a 12 h day–night cycle, 55% humidity, and 20–23 °C. Prior to the intervention, the mice were fed a commercial diet (Purina Korea, Seoul, Republic of Korea) for 1 week. The KRG and PM extracts were then mixed into the commercial diet in a 40:60 ratio. The final concentrations of the KRG and PM extracts were 800 and 1200 mg/kg body weight per day (0.50 and 0.75%, respectively). Animals were fed ad libitum for 4 weeks and then injected with TMT dissolved in a sodium chloride solution (subcutaneous injection; 2.5 mg/kg body weight; injection volume: 100 μL). Control mice were injected with sodium chloride solution. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Korea University (KUIACUC-2017-142).
Y-maze test
A Y-maze test was carried out 48 h after the TMT administration to assess immediate working memory performance, as described previously [18]. Each session lasted 8 min and the percentage alternation was calculated as the ratio of actual alternation, multiplied by 100.
Passive avoidance test
A passive avoidance test was carried out 48 h after the TMT treatment to assess the passive avoidance response, as described previously [18]. Two compartments (illuminated/dark) are connected to each other to allow the animals to move freely from one side to the other. During the acquisition trial, mice were individually placed in the illuminated compartment and then an electronic shock was given as soon as a mouse entered the dark compartment. After 24 h, mice were again placed in the illuminated compartment. The time required to enter the dark compartment was recorded. The maximum limit for test session was 300 s.
Quantification of AChE content in mouse brain tissue
The brain tissues were homogenized with homogenization buffer in order to measure AChE content, as described above [8].
Quantification of ACh content in mouse brain tissue
Brain ACh content was measured using Hestrin’s method, as described previously [13]. The method is based on the reaction of ACh and hydroxylamine. The brain tissues were homogenized in cold phosphate-buffered solution (PBS). The homogenates were directly centrifuged at 33,600×g for 10 s (twice at a 30 s interval). Subsequently, 1 mL of homogenate was mixed with 2 mL of alkaline hydroxylamine reagent. After more than 1 min, the pH was adjusted to 1.2 ± 0.2 with 1 mL of hydrochloride solution and 1 mL of iron solution. The density of the purple–brown color was determined at 540 nm.
Determination of lipid peroxidation in mouse brain tissue
The malondialdehyde (MDA) level was measured for lipid peroxidation products using a previously described method [8]. The brain tissues were homogenized in cold PBS and then centrifuged at 33,600×g for 10 s. The supernatants were carried for measurement of the MDA level and protein content in the brain. In brief, the homogenate was mixed with phosphoric acid (1% [v/v]), and then added to TBA solution (0.67% [w/v]). After mixing, the samples were heated at 95 °C in a water bath during 45 min and then they were left to cool and n-butanol was added. The absorbance was read at 532 nm.
Determination of catalase activity in mouse brain tissue
Catalase activity was assayed using a previously described method [8]. Briefly, phosphate buffer (50 mM; pH 7.0) 0.65 and a 50 mL sample were added into a quartz cuvette. The reaction was started by mixing 300 mL of H2O2 (30 mM). The decomposition capability of H2O2 was monitored at 240 nm at 25 °C. Catalase activity was expressed as nM H2O2 consumed/mg of tissue protein.
Statistical analysis
Results were expressed as the mean ± standard deviation (SD). Statistical analyses were performed using Duncan’s multiple range tests. All statistical analyses were performed using SAS software (Cary, NC, USA).
Results and discussion
Inhibitory activity of KRG and PM extracts on AChE
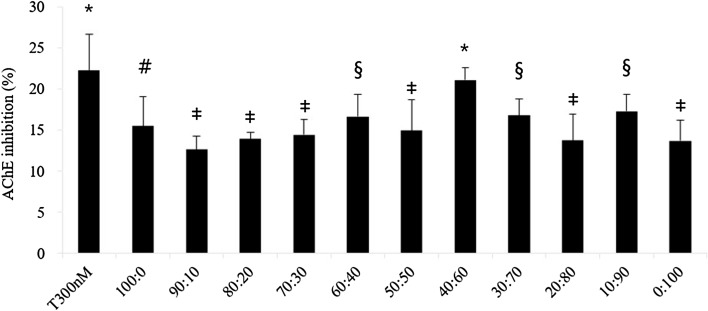
To confirm the synergistic effect of KRG and PM extracts, the AChE inhibitory effect was tested by Ellman’s method. KRG (15 ± 3.6%) and PM (14 ± 3.1%) inhibited AChE activity (Fig. 1). The mixture was tested at various ratios, with 40:60 showing the highest effect (21 ± 1.3%). This effect was similar to that of 300 nM tacrine. This study demonstrated that KRG and PM extracts inhibited AChE in vitro, and that a mixture of KRG and PM showed a synergistic effect. KRG and PM extracts, with their bioactive compounds, are a potent source of AChE inhibitors, which might be beneficial for the cholinergic basal forebrain system.
Fig. 1.
Acetylcholinesterase (AChE) inhibition by Korean red ginseng (KRG) and Pueraria montana var. lobata (PM) extracts. The sample groups were treated with various mixtures of KRG and PM extracts (100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, and 0:100 [w/w]). The concentration of all samples was 1 mg/mL, and the concentration of tacrine was 300 nM. Each value represents the mean ± SD (n = 4), p < 0.05. Different superscript symbols (*, #, ‡, and §) represent statistical differences between groups
Effects of KRG and PM extracts on in vivo behavior test
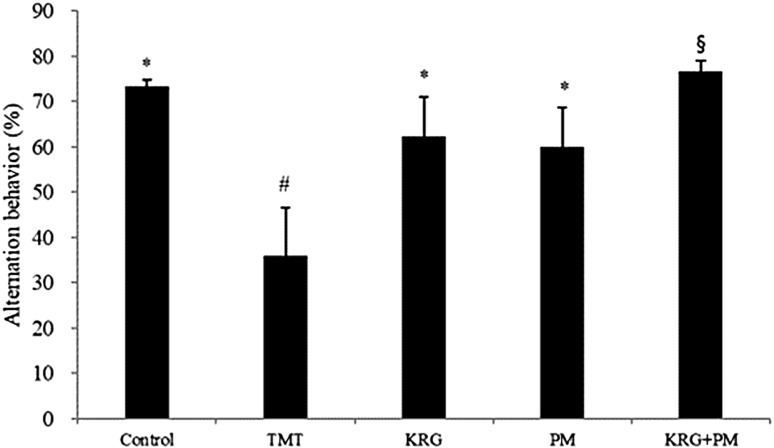
To determine the anti-amnesic effects of KRG and PM extracts in vivo, a Y-maze spontaneous alternation test was performed with TMT-treated mice (Fig. 2). When compared with the control group, the TMT group exhibited significantly lower (about 38%) spontaneous alternation behavior. However, this TMT-induced decrease was significantly improved by pretreatment with KRG and PM extracts, as compared with the control group. No significant differences in the total number of arm entries were observed among the groups. This indicates that KRG and PM extracts had beneficial effects in TMT-treated mice without affecting motor function (data not shown).
Fig. 2.
The effect of Korean red ginseng (KRG) and Pueraria montana var. lobata (PM) extracts on trimethyltin (TMT)-induced memory impairment in mice as measured by the Y-maze test. Spontaneous alternation behavior was measured over 8 min. The control group was injected with a sodium chloride solution. The TMT group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with KRG extract (800 mg/kg of body weight per day), PM extract (800 mg/kg of body weight per day), or KRG + PM extract (800 mg/kg of body weight per day). Each value represents the mean ± SD (n = 8), p < 0.05. Different superscript symbols (*, #, and §) represent statistical differences between groups
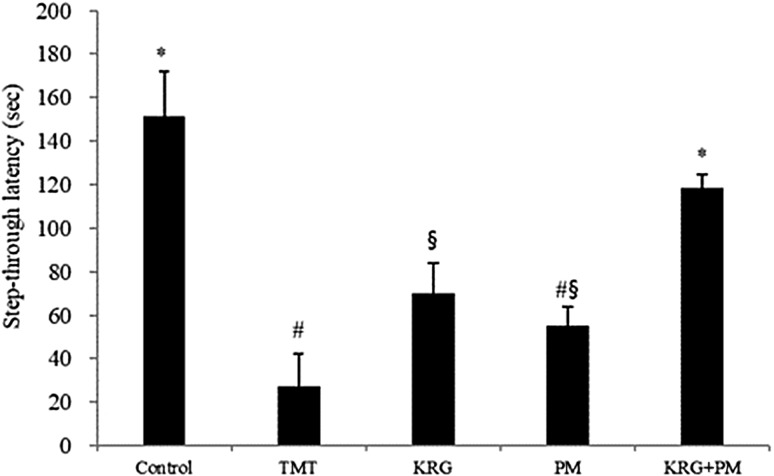
Learning and memory impairment was assessed using a passive avoidance test (Fig. 3). The step-through latency of TMT-treated mice was shorter than that of the control group. However, mice treated with a mixture of KRG and PM extracts reversed TMT-induced impairment further. After performing the behavioral tests, the serum of mice was collected to evaluate the acute toxicity of KRG and PM extracts using a serum transaminase test kit. Serum aminotransferases were not significantly different among the experimental groups, indicating that KRG and PM extracts did not induce liver toxicity (data not shown).
Fig. 3.
The effect of KRG and PM extracts on TMT-induced memory impairment in mice as assessed by the passive avoidance test. Step-through latency was measured over 5 min. The control group was injected with a sodium chloride solution. The TMT group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with KRG extract (800 mg/kg of body weight per day), PM extract (800 mg/kg of body weight per day), or KRG + PM extract (800 mg/kg of body weight per day). Each value represents the mean ± SD (n = 8), p < 0.05. Different superscript symbols (*, #, and §) represent statistical differences between groups
AChE activity and ACh content in mouse brain tissue
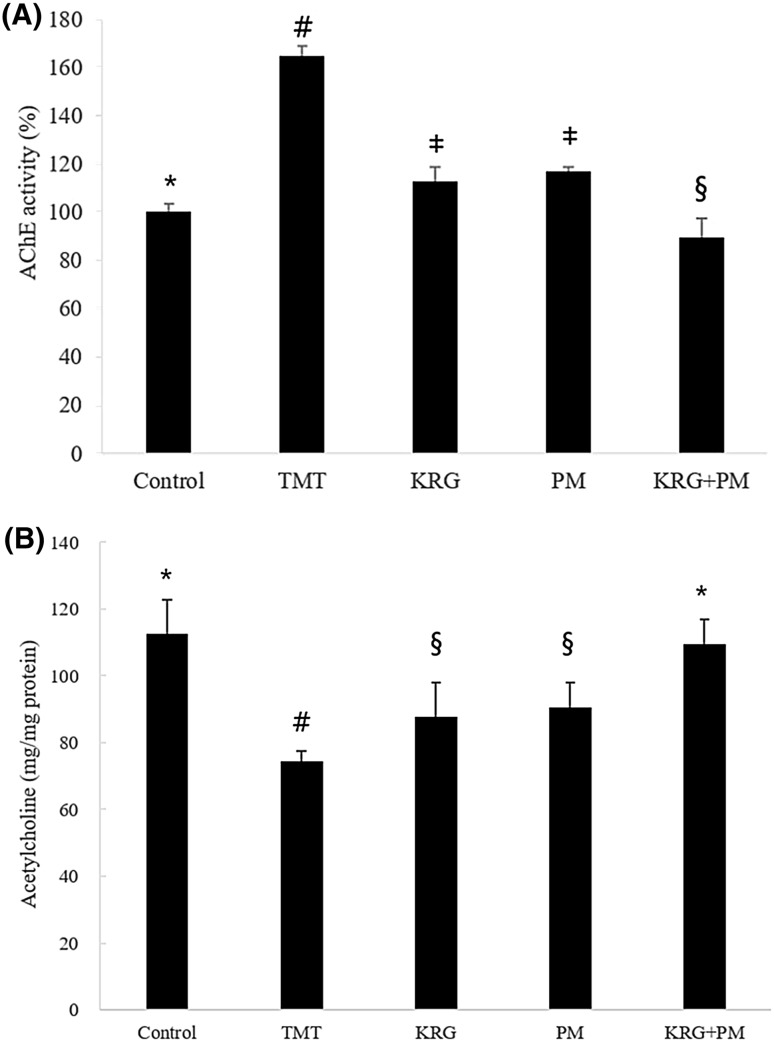
TMT induces neuronal degeneration in the hippocampus, and results in behavioral alterations, including cognitive impairment. TMT has been widely used as a neurotoxin based on its in vivo behavioral changes and biochemical effects in the brain [9]. In agreement with many other previous studies, our study confirmed that TMT induced learning and memory impairment in mice. Our findings showed that a diet containing KRG and PM extracts protected TMT-treated mice against learning and memory impairment. AChE activity was significantly increased in the TMT group (about 60%) [Fig. 4(A)]. However, AChE activity was reduced to the level of the control groups when the mice were fed a diet containing KRG and PM extracts. The ACh content in TMT-treated mouse brain tissue was determined by a spectrophotometric analysis. The ACh level was lower in TMT-treated mice compared with the control group [Fig. 4(B)]. In contrast, administration of KRG and PM extracts reversed the decrease in ACh content compared to mice treated with TMT only. One of the mechanisms of TMT-induced neurodegeneration is an increase in AChE activity. These changes were correlated with degeneration of cholinergic axons and changes in ACh levels and their receptors. TMT toxicity causes a decrease of ACh release from cerebral tissue [22]. KRG and PM extracts protected TMT-treated mice against TMT-induced learning and memory impairment (Figs. 2, 3). Therefore, it is possible that KRG and PM extracts improve learning and memory in TMT-treated mice by AChE inhibition, thereby restoring these functions via enhancement of ACh levels.
Fig. 4.
Effect of KRG and PM extracts on AChE activity and ACh contents in the mouse brain. The control group was injected with a sodium chloride solution. The TMT group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with KRG extract (800 mg/kg of body weight per day), PM extract (800 mg/kg of body weight per day), or KRG + PM extract (800 mg/kg of body weight per day). Each value represents the mean ± SD (n = 8), p < 0.05. Different superscript symbols (*, #, ‡, and §) represent statistical differences between groups
MDA levels and catalase activity in mouse brain tissue
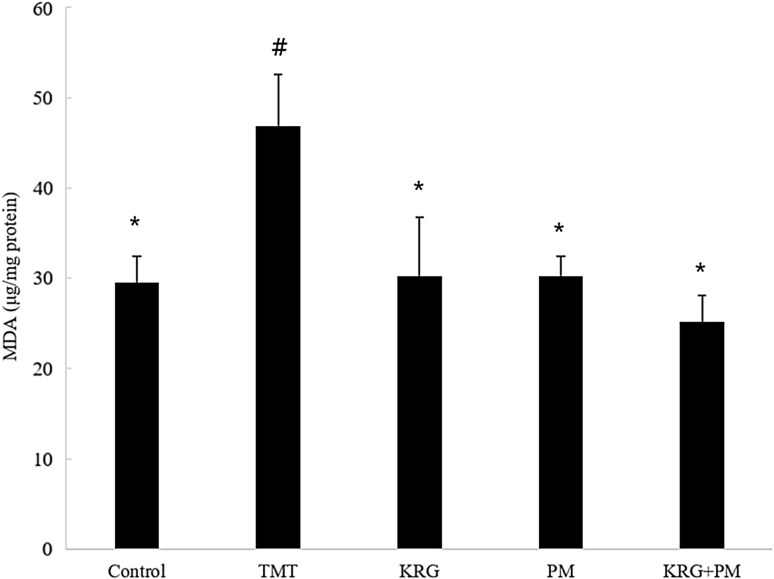
As mentioned above, TMT-induced neurodegeneration causes an increase in AChE activity. Another possible mechanism is TMT-induced production of reactive oxygen species. A previous study reported that TMT promotes the formation of a major product of lipid peroxidation, 4-hydroxynonenal [27, 30]. The MDA levels increased significantly (17.29 mg/mg protein) in the TMT-treated group in comparison with the control group. The increase in MDA levels demonstrated an elevation of lipid peroxidation in the brain of TMT-treated mice. Diets containing KRG and PM extracts reversed the effects of TMT-induced lipid peroxidation (Fig. 5). It is possible that these ACh and oxidative stresses are mechanisms via which learning and memory ability can be improved in mice.
Fig. 5.
Effect of KRG and PM extracts on lipid peroxidation in the mouse brain. The control group was injected with a sodium chloride solution. The TMT group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with KRG extract (800 mg/kg of body weight per day), PM extract (800 mg/kg of body weight per day), or KRG + PM extract (800 mg/kg of body weight per day). Each value represents the mean ± SD (n = 8), p < 0.05. Different superscript symbols (*, #, and §) represent statistical differences between groups
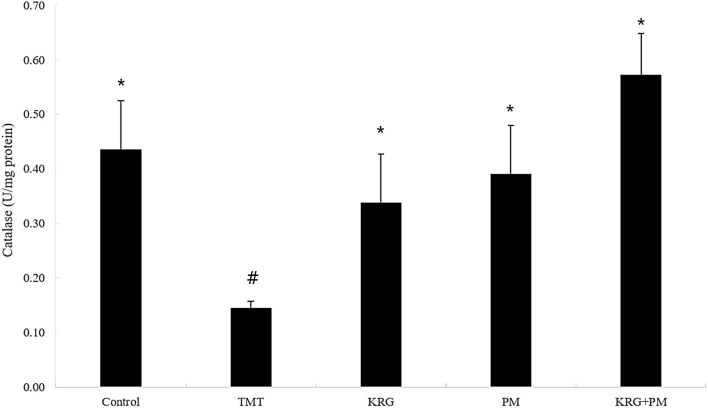
To determine TMT-induced damage to the mouse brain, the activity of catalase was measured. Catalase activity was significantly decreased in TMT-treated mice (0.3 U/mg protein) in comparison with the control group (Fig. 6). In contrast, no significant decrease in catalase activity was observed in the mice fed with KRG and PM extracts. Moreover, the KRG and PM mixture exhibited a synergistic effect. This study showed the potential role of free radical toxicity and cholinergic neuronal damage in a mouse model of learning and memory impairment. Pretreatment with KRG and PM extracts significantly reduced catalases and AChE activities and reversed the elevated MDA in vivo.
Fig. 6.
Effect of KRG and PM extracts on catalase activity in the mouse brain. The control group was injected with a sodium chloride solution. The TMT group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with KRG extract (800 mg/kg of body weight per day), PM extract (800 mg/kg of body weight per day), or KRG + PM extract (800 mg/kg of body weight per day). Each value represents the mean ± SD (n = 8), p < 0.05. Different superscript symbols (*, #, and §) represent statistical differences between groups
With the long successful use of herbal drug mixtures in traditional medicine, it has become necessary to elucidate the rationale for their pharmacological and therapeutic superiority compared to single constituents. In this study, the main active ginsenoside compounds of the KRG extract were identified by HPLC. The extract contained various compounds. By comparison with known standards, ginsenoside Rg1 (1.14 ± 0.03 mg/g), ginsenoside Rb1 (4.88 ± 0.10 mg/g), and ginsenoside Rg3s (2.12 ± 0.04 mg/g) were identified as the main ginsenosides (data not shown). These ginsenosides may contribute to the effects of KRG against learning and memory impairment. Ginsenosides Rg1 and Rb1 ameliorate cognition deficiency in mice with dementia [31]. In addition, Rg1 and Rb1 have been found to enhance cholinergic function by increasing the density of central M-cholinergic receptors and ACh levels in the central nervous system, which might be the result of ginsenoside-induced enhancement of ChAT activity and inhibition of AChE activity [36].
The main active isoflavone compounds of the PM extract by HPLC were also identified. The extract contained various compounds. Puerarin (76.82 ± 2.29 mg/g), daidzin (10.32 ± 0.12 mg/g), genistin (0.85 ± 0.02 mg/g), daidzein (1.67 ± 0.04 mg/g), and genistein (0.14 ± 0.01 mg/g) were identified as the main isoflavones (data not shown). These isoflavones may contribute to the protective effects of PM against learning and memory impairment. Puerarin has been reported to possess anti-inflammatory, antioxidant, anti-inflammatory, anti-diabetic, and anti-apoptotic properties, which are useful in the treatment of various diseases [21]. Recently, puerarin was found to improve learning and memory performance in animal and humans models. Moreover, puerarin supplement significantly inhibits AChE activity and lowers MDA level, while increasing ChAT and superoxide dismutase (SOD) activities [21].
In the cholinergic system, ginsenosides of KRG and puerarin of PM increase the ACh level in the CNS, enhance ChAT activity, and inhibit AChE activity. In addition, both constituents increase SOD activity [21, 36]. Ginsenosides can also facilitate the release of ACh and reuptake [2]. Moreover, they increase the density of central M-cholinergic receptors, whereas puerarin enhances cholinergic activity via the nicotinic receptor [15]. Similar to the above-mentioned mechanisms of ginsenoside and puerarin in the cholinergic system, KRG and PM might synergistically increase ACh release and reuptake, and PM might enhance the cholinergic activity. Therefore, further research is needed to elucidate whether isolated single compounds affect the memory-ameliorating synergy effect on TMT-induced cognitive impairment in vivo. Moreover, detailed studies are required to determine the possible mechanisms of the synergistic effect on the cholinergic system.
In conclusion, these results demonstrate that multiple administrations of KRG and PM extracts inhibit neuronal cell death caused by TMT-induced damage and improve TMT-induced learning and memory impairment.
Acknowledgements
This work was supported by a Korea University Grant.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 2.Benishin CG, Lee R, Wang LC, Liu HJ. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology. 1991;42:223–229. doi: 10.1159/000138801. [DOI] [PubMed] [Google Scholar]
- 3.Benishin CG. Actions of ginsenoside Rb1 on choline uptake in central cholinergic nerve endings. Neurochem Int. 1992;21:1–5. doi: 10.1016/0197-0186(92)90061-U. [DOI] [PubMed] [Google Scholar]
- 4.Boissiere F, Faucheux B, Agid Y, Hirsch EC. Choline acetyltransferase mRNA expression in the striatal neurons of patients with Alzheimer’s disease. Neurosci. Lett. 1997;225:169–172. doi: 10.1016/S0304-3940(97)00210-3. [DOI] [PubMed] [Google Scholar]
- 5.Cai RL, Li M, Xie SH, Song Y, Zou ZM, Zhu CY, Qi Y. Antihypertensive effect of total flavone extracts from Puerariae Radix. J. Ethnopharmacol. 2011;133:177–183. doi: 10.1016/j.jep.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Cao X, Tian Y, Zhang T, Li X, Ito Y. Separation and purification of isoflavones from Pueraria lobata by high-speed counter-current chromatography. J. Chromatogr. 1999;855:709–713. doi: 10.1016/S0021-9673(99)00715-3. [DOI] [PubMed] [Google Scholar]
- 7.Choi YH, Hong SS, Shin YS, Hwang BY, Park SY, Lee D. Phenolic compounds from Pueraria lobata protect PC12 cells against Abeta-induced toxicity. Arch. Pharm. Res. 2010;33:1651–1654. doi: 10.1007/s12272-010-1014-7. [DOI] [PubMed] [Google Scholar]
- 8.Choi SJ, Kim MJ, Heo HJ, Kim JK, Jun WJ, Kim HK, Kim EK, Kim MO, Cho HY, Hwang HJ, Kim YJ, Shin DH. Ameliorative effect of 1,2-benzenedicarboxylic acid dinonyl ester against amyloid β peptide-induced neurotoxicity. Amyloid. 2009;16:15–24. doi: 10.1080/13506120802676997. [DOI] [PubMed] [Google Scholar]
- 9.Choi SJ, Oh SS, Kim CR, Kwon YK, Suh SH, Kim JK, Park GG, Son SY, Shin DH. Perilla frutescens extract ameliorates acetylcholinesterase and trimethyltin chloride-induced neurotoxicity. J. Med. Food. 2016;19:281–289. doi: 10.1089/jmf.2015.3540. [DOI] [PubMed] [Google Scholar]
- 10.Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 11.Heo JH, Lee ST, Chu K, Oh M, Park HJ, Shim JY, Kim M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer’s disease. Eur. J. Neurol. 2008;15:865–868. doi: 10.1111/j.1468-1331.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 12.Heo HJ, Suh YM, Kim MJ, Choi SJ, Mun NS, Kim HK, Kim EK, Kim CJ, Cho HY, Kim YJ, Shin DH. Daidzein activates choline acetyltransferase from MC-IXC cells and improves drug-induced amnesia. Biosci. Biotechnol. Biochem. 2006;70:107–111. doi: 10.1271/bbb.70.107. [DOI] [PubMed] [Google Scholar]
- 13.Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J. Biol. Chem. 1949;180:249–261. [PubMed] [Google Scholar]
- 14.Hou Y, Yu YB, Liu G, Luo Y. A natural squamosamide derivative FLZ reduces amyloid-beta production by increasing non-amyloidogenic AbetaPP processing. J. Alzheimers Dis. 2009;18:153–165. doi: 10.3233/JAD-2009-1133. [DOI] [PubMed] [Google Scholar]
- 15.Ikeya Y, Takeda S, Tunakawa M, Karakida H, Toda K, Yamaguchi T, Aburada M. Cognitive improving and cerebral protective effects of acylated oligosaccharides in Polygala tenuifolia. Biol. Pharm. Bull. 2004;27:1081–1085. doi: 10.1248/bpb.27.1081. [DOI] [PubMed] [Google Scholar]
- 16.Jin SH, Park JK, Nam KY, Park SN, Jung NP. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J. Ethnopharmacol. 1999;66:123–129. doi: 10.1016/S0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- 17.Kim JK, Choi SJ, Bae H, Kim CR, Cho HY, Kim YJ, Lim ST, Kim CJ, Kim HK, Sabrina P, Shin DH. Effects of methoxsalen from Poncirus trifoliata on acetylcholinesterase and trimethyltin-induced learning and memory impairment. Biosci. Biotechnol. Biochem. 2011;75:1984–1989. doi: 10.1271/bbb.110386. [DOI] [PubMed] [Google Scholar]
- 18.Kim CR, Choi SJ, Kwon YK, Kim JK, Kim YJ, Park GG, Shin DH. Cinnamomum loureirii extract inhibits acetylcholinesterase activity and ameliorates trimethyltin-induced cognitive dysfunction in mice. Biol. Pharm. Bull. 2016;39:1130–1136. doi: 10.1248/bpb.b16-00045. [DOI] [PubMed] [Google Scholar]
- 19.Kim MJ, Choi SJ, Lim ST, Kim HK, Heo HJ, Kim EK, Jun WJ, Cho HY, Kim YJ, Shin DH. Ferulic acid supplementation prevents trimethyltin-induced cognitive deficits in mice. Biosci. Biotechnol. Biochem. 2007;71:1063–1068. doi: 10.1271/bbb.60564. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Kim P, Shin CY. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013;37:8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Mo Y, Gong J, Li Z, Peng H, Chen J, Wang Q, Ke Z, Xie J. Puerarin ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Metab. Brain Dis. 2016;31:417–423. doi: 10.1007/s11011-015-9779-5. [DOI] [PubMed] [Google Scholar]
- 22.Loullis CC, Dean RL, Lippa AS, Clody DE, Coupet J. Hippocampal muscarinic receptor loss following trimethyl tin administration. Pharmacol. Biochem. Behav. 1985;22:147–151. doi: 10.1016/0091-3057(85)90498-8. [DOI] [PubMed] [Google Scholar]
- 23.Ohno K, Tsujino A, Brengman JM, Happer CM, Bajzer Z, Udd B, Beyring R, Robb S, Kirkham FJ, Engel AG. Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc. Natl. Acad. Sci. USA. 2001;98:2017–2022. doi: 10.1073/pnas.98.4.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol. Appl. Neurobiol. 1978;4:273–277. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 25.Petkov VD, Mosharrof AH. Effects of standarized ginseng extract on learning, memory and physical capabilities. Am. J. Chin. Med. 1987;15:19–29. doi: 10.1142/S0192415X87000047. [DOI] [PubMed] [Google Scholar]
- 26.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 27.Shuto M, Higuchi K, Sugiyama C, Yoneyama M, Kuramoto N, Nagashima R, Kawada K, Ogita K. Endogenous and exogenous glucocorticoids prevent trimethyltin from causing neuronal degeneration of the mouse brain in vivo: involvement of oxidative stress pathways. J Pharmacol Sci. 2009;110:424–436. doi: 10.1254/jphs.09107FP. [DOI] [PubMed] [Google Scholar]
- 28.Terry AV, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 29.Tsang D, Yeung HW, Tso WW, Peck H. Ginseng saponins: influence on neurotransmitter uptake in rat brain synaptosomes. Planta Med. 1985;51:221–224. doi: 10.1055/s-2007-969463. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Cai J, Zhang J, Wang C, Yu A, Chen Y, Zuo Z. Acute trimethyltin exposure induces oxidative stress response and neuronal apoptosis in Sebastiscus marmoratus. Aquat. Toxicol. 2008;90:58–64. doi: 10.1016/j.aquatox.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Sun LH, Jia W, Liu XM, Dang HX, Mai WL, Wang N, Steinmetz A, Wang YQ, Xu CJ. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother. Res. 2010;24:1748–1754. doi: 10.1002/ptr.3130. [DOI] [PubMed] [Google Scholar]
- 32.Woodruff ML, Baisden RH. Exposure to trimethyltin significantly enhances acetylcholinesterase staining in the rat dentate gyrus. Neurotoxicol. Teratol. 1990;12:33–39. doi: 10.1016/0892-0362(90)90110-X. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi Y, Haruta K, Kobayashi H. Effects of ginsenosides on impaired performance induced in the rat by scopolamine in a radial-arm maze. Psychoneuroendocrinology. 1995;20:645–653. doi: 10.1016/0306-4530(95)00008-C. [DOI] [PubMed] [Google Scholar]
- 34.Younkin SG, Goodridge B, Katz J, Lockett G, Nafziger D, Usiak MF. Molecular forms of acetylcholinesterases in Alzheimer’s disease. Fed. Proc. 1986;45:2982–2988. [PubMed] [Google Scholar]
- 35.Zhang Z, Lam TN, Zuo Z. Radix Puerariae: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2013;53:787–811. doi: 10.1002/jcph.96. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JT, Liu Y, Qu ZhW, Zhang XL, Xiao HL. Influence of ginsenoside Rb1 and Rg1 on some central neurotransmitter receptors and protein biosynthesis in mouse brain. Acta Pharm. Sin. 1988;23:12–16. [PubMed] [Google Scholar]