Abstract
The current study investigates the phytochemical and pharmaceutical activities of Sargassium vulgare (SVE) collected from the Suez Canal. The prescreening using cytotoxicity was tested against hepatocellular carcinoma cell lines. Furthermore the SVE inhibit cell growth effectively with IC50 = 20.8 µg/ml. The pharmacological studies revealed high antioxidant capacity at all examined concentrations. On the meantime, anticancer assay carried out using tyrosine kinase (PTK) and sphingosine kinase 1 inhibitor screening assays revealed inhibition with 75.73 and 80.01%; respectively. Furthermore, the anti-inflammatory profiling revealed that the activities against COX1, COX2, IL6 and TNF were 77.39, 88.35, 75.38 and 71.24%; respectively. Additionally, the anti-Alzheimer results showed high activity at 1 mg with 76.33%. Finally the antiviral activities using reverse transcriptase inhibition assay give 92.24%. Consequently, it can be easily conclude that the SVE collected from the Suez Canal are excellent source of natural products for nutritional and pharmaceutical applications.
Keywords: Seaweeds, Suez Canal, Cytotoxic, Anti-oxidant, Anti-Alzheimer, Anti-cancer, Anti-inflammatory, Anti-viral
Introduction
The marine environments are rich in pharmaceutically potent chemicals basically related to polypshenols and sulfated polysaccharides. Recently, significant increase in the number of marine natural products studies have been carried out to extract, isolate and identify new marine and marine-derived natural products [1–4]. Seaweeds are rich sources of protein, iodine, vitamins, and minerals so; their metabolites have shown promising activities as anti-cancer. Edible seaweed like Palmaria palmata is shown to be effective antioxidant, capable of inhibiting cancer cell proliferation. They also contain high amounts of polyphenols such as catechin, epicatechin, epigallocatechin gallate, and gallic acid. Moreover, low-molecular weight fucoidan isolated from Ascophyllum nodosum shows an anti-proliferative effect on both normal and malignant cells, including fibroblasts, sigmoid colon adenocarcinoma cells, and smooth muscle cells [5, 6].
Sargassium species which are one of the most distributed tropical and sub-tropical brown seaweed have great potential to be used in nutraceutical industrial and pharmaceutical areas as they are considered a very rich source of nutritious and bioactive compounds such as vitamins (A, Bl, B2, B3, B12, C, D, E), carotenoids, dietary fibers, proteins, minerals, polyunsaturated fatty acids and amino acids [5, 6]. It has been reported that many pharmacologically biologically active compounds such as sterols, flavonoids, sargaquinoic acids, polyphenols, terpenoids, protein, pheophytine, sulfated polysaccharides, were also extracted, isolated and characterized from different Sargassum species [7, 8].These compounds showed varieties of biological activities such as analgesic, anti-inflammatory, antioxidant, neuroprotective, anti-microbial, anti-tumor, fibrinolytic, immune-modulatory, anti-coagulant, hepatorotective, anti-viral activity, induction of larval settlement of hydrozoan and inhibition of acetylcholine-esterase, and cell toxicity.
The main objective of the present study is to isolate, extract and explore the phytochemical, therapeutic potential, and health benefits of different compounds isolated from Sargassum vulgare as antioxidant, anti-inflammatory, anticancer, antiviral and anti-Alzheimer.
Materials and methods
Area of study
The Suez Canal is an artificial sea-level waterway in Egypt which connects the Mediterranean Sea with the Gulf of Suez, a northern branch of the Red Sea. It expands from the Port Said which located northern terminus to Port Tewfik at the city of Suez which located at the southern terminus. Its length is 193.30 km (120.11 mi), including its northern and southern access channels [Fig. 1(A)].
Fig. 1.
The Sargassium vulgare and the location site, (A) The Suez Canal area of Investigation, (B) Brown algae in the genus Sargassium vulgare
Sampling, identification and prescreening bioassays of the seaweeds
A Sargassum vulgare sample was collected from Suez Canal during spring, 2014 [Fig. 2(B)]. The sample was transferred directly to the laboratory in sterile polyethylene bags under reduced temperature (zero °C).The identification has been done by NIOF team from Hydrobiology lab at the Marine Environment Division.
Fig. 2.
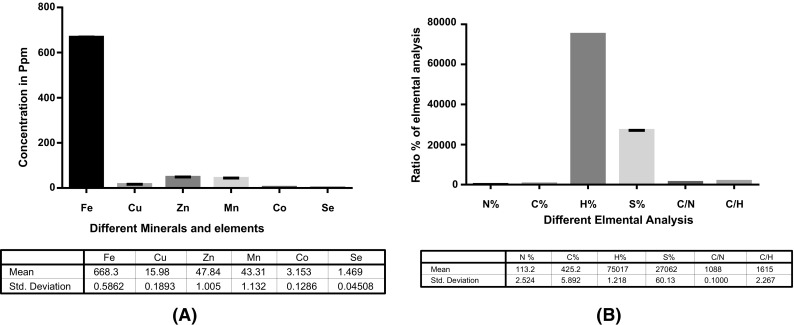
Mineral, elements and elemental analysis of Sargassum vulgare extract, (A) The mineral and elements results, (B) The elemental analysis result of Sargassium, Data analysis using one way ANOVA where p < 0.0001
Chemicals and solvents
Potassium ferricyanide, ferric chloride, sodium hydroxide, chloroform, glacial acetic acid, ferric chloride solution, sulphuric acid, folin–Ciocalteau, vanillin, methanol, hydrochloric acid, n-hexane, hydrogen peroxide, nitric acid, iron, zinc, cobalt, manganese, selenium. etc., β-carotene, catechin, (+)-quercetin, sodium nitrite, aluminum chloride and gallic acid were purchased from Sigma Aldrich.
Instruments
Atomic absorption (AAS and GFASHIMADZU), GC–MS (Thermo, USA).
Preliminary phytochemical screening of Sargassium vulgare marine extract
The ethyl acetate extracts of the Sargassium vulgare samples were subjected to different chemical tests for the detection of different tannins, phlobatannins, saponins, alkaloids, flavonoids, quinines, coumarin, terpenoids and cardiac glycosides phytoconstituents.
Quantitative chemotaxonomy profiling
Determination of total phenolic contents
Total phenolic compounds in the Sargassium vulgare extract were determined [9] as mg gallic acid equivalent in 1 ml of the extract using the standard curve of the gallic acid.
Determination of total flavonoid contents
Total flavonoid contents were determined by a colorimetric method [10] and the results were expressed as mean ml of (+)-Quercetin equivalents.
Determination of total tannins
Tannins (proanthocyanidins) were determined [11] as mg (+)-catechin/g. All samples were analyzed in triplicate using a standard curve.
Determination of total carotenoid
Total carotenoid contents were measured [12]. The results were reported as percentage of total β-carotene equivalents per 100 mg extract using the standard curve.
Preparation and extraction for mineral and metal assessment (Fe, Zn, Co, Mn, Cu and Se)
Wet digestion
A 0.5 gm of dried sample of the marine extract was digested using 5 ml concentrated HNO3, the mixture was heating using hot plate for 1 h and getting semi dried 5 ml of concentrated HNO3 and 2 ml of H2O2 was added and kept on hot plate for 1 h. The semi dried cooled residue, filtered by using Whatman filter paper and the volume was made up to 25 ml with 2 N HNO3.
Determination of minerals and heavy metals using atomic absorption
The determination of minerals and heavy metals concentrations of the powdered sample was determined spectrometry by using AAS (GFASHIMADZU atomic absorption spectrophotometer AA–6800) [13].
Elemental analysis
The total carbon and hydrogen contents of Sargassium vulgare marine extract were determined using CHNO Elemental Analyzer.
Identification and phytochemical screening using gas chromatography-mass spectrometry (GC–MS)
The Sargassium vulgare marine extract was analyzed by GC-ITQ-MS using a Thermo Trace GC Ultra TM gas chromatograph system (Thermo Scientific, USA), equipped with a 30 m × 0.25 mm i.d., 0.25 µm film thickness, non-polar TR-5MS fused silica capillary column, connected to an ion trap quadrupole (ITQ) mass selective detector (unit mass resolution). Split was 1:50, with helium as carrier gas at a flow rate of 1 ml/min, while the damping gas flow was 0.3 ml/min. The initial oven temperature was set to 40 °C for 1 min. The GC oven temperature program was as follows: 40 °C to 220 °C, by ramping at 3 °C, and held at 220 °C for 20 min. The injector temperature was 220 °C and the transfer line was held at 220 °C. The screening of bioactive compounds were complete using Thermo ITQ 900TM mass spectrometer with the EI mode (ionization energy of 70 eV, ion source temperature of 180 °C, emission current of 220 µA). The acquisition was made in full scanning mode (mass range 50–900 m/z; 3 scans/s). Maximum ionization time was 25 ms. A solvent delay time of 5 min (set off) was used to avoid overloading the mass spectrometer with hexane. Data collection, analysis and integration were performed using the software XCaliburTM (version 2.0.7). Areas were recorded for all detectable peaks, and percent composition was calculated by taking area of peak divided by total chromatogram area × 100.
Identification of phytocompounds
The identification and interpretation of the marine extract results of mass-spectrum GC–MS was conducted using the database of the National Institute Standards and Technology (NIST), which having more than 62,000 patterns. The spectrums of the unknown bioactive compounds were identified and compared in the NIST library with the spectrum of known components stored. The name, molecular weight and structure of the components of the test materials were investigated [14].
Prescreening bioassays using in vitro cytotoxicity using cell lines
Different concentrations of different marine extracts μg/ml from all samples were tested for each cell line. Samples were dissolved in DMSO and further diluted with cell culture medium. The final DMSO concentration used was 1% of total volume of the medium in all treatments, including the control group. Cells with no treatment were examined as negative and positive controls, respectively [15].
Primary screening assay
2,2′-Diphenyle–α-picrylhydrazyl (DPPH) radical scavenging assay
DPPH radical scavenging assay of the total marine extract was performed [16]. Assays were performed in flat bottom polystyrene 96 well microtiter plates. To 100 µL of each sample (1–6 mg/ml) in EtOH 25 µL DPPH (1 mM) in ethanol was added. The resultant mixture was briefly shaken and maintained in the dark at room temperature, for 30 min. At the end of this period the absorbance (A) of the mixture was measured at 490 nm, using ELISSA. Scavenging ratio of DPPH assay calculated as follows:
2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) ABTS radical scavenging assay
The ABTS+ free radical decolorization assay [17]. The inhibition percentage of free radicals were measured at absorbance of 734 nm and calculated for each point and the antioxidant capacity of the test samples was expressed as percent inhibition (%). The percentage scavenging of ABTS•+ was calculated by the following formula:
Ax and Ao were the absorbance at 734 nm of samples with and without extract, respectively.
Specialized screening assays
Acetylcholine esterase inhibition (AChEI) assay
Inhibition of AChE by Sargassum vulgare extract was evaluated [18]. The increase in absorbance value due to the spontaneous hydrolysis of the substrate was corrected by subtracting the ratio of the reaction before adding the enzyme from the rate after the enzyme addition.
Percentage inhibition by extracts were calculated using the equation below
Determination of tyrosine kinase inhibitory activity (PTK)
Sample preparation
The dimethylsulfoxide (DMSO) sample solution of the appropriate extract was diluted with H2O (1:1 v/v) to yield corresponding sample solutions (1 mg/mL). Tyrosine kinase (TK) inhibitory activity was determined using a commercial test kit (Tyrosine kinase Assay Kit, non-radioactively, Takara Cat.# MK410). PTK activity of sample was calculated on the basis of the prepared standard curve. The color intensity is stable for 1 h after addition of stop solution at room temperature in a light room.
Determination of sphingosine kinase 1 inhibitor screening assay (SHK1)
Sphingosine kinase inhibitory activity of the crude extract was determined by using the colorimetric sphingosine kinase 1 inhibitor screening assay kit from Cayamen. The plate was covered and the fluorescence was measured using an excitation wavelength between 530 and 540 nm and an emission wavelength between 580 and 590 nm.
Determination of cyclooxygenase 1 (COX1) and cyclooxygenase 2 (COX 2) inhibitor screening assays
Cyclooxygenase inhibitory activity of the crude extract was determined by using the colorimetric COX (Ovine) inhibitor screening assay kit from Cayamen. The absorbance was measured at 590 nm using a plate reader.
Determination of tumor necrosis factor alpha (TNF-α) assay
Tumor necrosis factor alpha (TNF-α) inhibitory activity of the crude extract, was determined by using the KOMA BIOTECH INC colorimetric kit. The absorbance was measured at 450 nm.
Determination of interleukin 6 (IL-6) assays
An Interleukin 6 (IL-6) inhibitory activity of the crude extract was determined by using the KOMA BIOTECH INC colorimetric kit. The absorbance was measured at 450 nm.
Determination of reverse transcriptase enzyme inhibitor screening assay
Reverse transcriptase (RT) inhibitory activity of the crude extract against a purified recombinant HIV1-RT, was determined by using Roche colorimetric kit [19]. HIV-1 protease enzyme and the substrate which is a synthetic peptide that contains a cleavage site Tyr-Pro for HIV protease as well as two covalently modified amino acids for the detection of cleavage. The blank treatment consists of an assay buffer with only the substrate, untreated control of enzyme and substrate was also included where the positive control for HIV inhibition was Acetyl pepstatin (AP). The absorbance was measured at 450 nm.
Statistical analysis
All statistical analyses were performed using the statistical software prism version 6.
Results and discussion
Chemical profiling
Elemental analysis
The results of elemental analysis in 50 mg of marine extract including H%, N%, C%, S%, C/H and C/N [Fig. 2(B)], showed highest ratios of H%, N%, C%, S%, C/H% and C/N%. On the other hand, Fe, Mn, Co, Se, Cu, and Zn which are known to be very important for various metabolic processes in the human body as they are known to be closely linked to growth and health of human being [4]. The results of elemental analysis cleared out high contents of sulfur and selenium, i.e. 1.469 ppm and 27,062%; respectively which are well known to be antioxidant agents that ameliorate oxidative damage. They also have very effective role in activation and regeneration of antioxidants enzymes [4]. The results of mineral and metals analysis the marine extract [Fig. 2(A)] high concentrations of Fe, Cu and Co. Additionally high sulfur and nitrogen contents were also measured.
Phytochemical screening assays
Qualitative and Quantitative phytochemical screening assays including different tests for phlobatannins, saponins, flavonoids, alkaloids, quinones, coumarin, terpenoids and cardiac glycosides are shown in Table 1. The results of Sargassium vulgare marine extract give + vulgare marine extract for all tests. The results of quantitive total β-carotene and total flavonoids content, total tannins content and total phenolic in 100 mg [Fig. 3(A)] revealed the presence of high β-carotene and flavonoids contents in the Sargassium vulgare marine extract.
Table 1.
The qualitative analysis of Sargassium
| Test | Aqueous Extract |
|---|---|
| Test for tannins | ++ |
| Test for phlobatannins | ++ |
| Test for saponins | + |
| Test for flavonoids | +++ |
| Test for alkaloids | +++ |
| Test for quinines | ++ |
| Test for coumarin | ++ |
| Test for terpenoids (Salkowski test) | ++ |
| Test for cardiac glycosides (Keller–Kiliani test) | + |
Fig. 3.
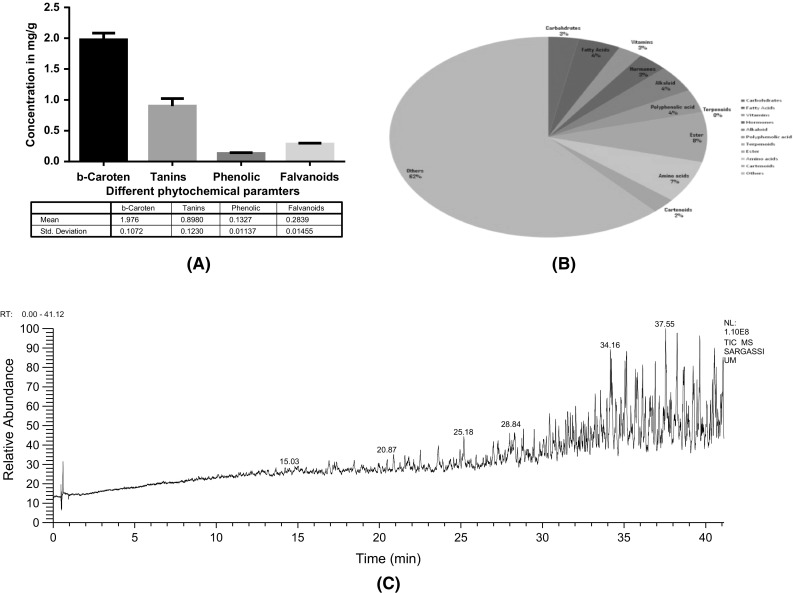
The phytochemical analysis of Sargassium using qualitative and quantitative assay. (A) The Quantitive result of phytochemicals compounds, Data analysis using one way ANOVA where p < 0.0001 (B) The GC–MS profiling percentage distribution of bioactive compounds, (C) The GC–MS chromatogram of Sargassium vulgare extract
Qualitative GC–MS screening
A number of marine natural products have been characterized by using modern chromatography techniques, namely; GC–MS, LC–MS, and IC based on mass–charge ratio (m/z), and retention time of ion spectra of GC–MS. They were further accurately matched with standard data of library NIST [4, 7, 8, 20]. In addition, HPLC–MS was also used to identify the biodegradation products from metabolites. Modern GC–MS, LC–MS-MS and other quantitative assays were applied in the present study for identification of bioactive compounds such as alkaloids, flavonoids, phenols, glycosides, and saponins. The GC–MS profiling of Sargassium vulgare marine extract are shown in Table 2, and are illustrated in Fig. 3(C). The results revealed the presence of different bioactive compounds, such as fatty acids, alkaloid terpenoids, vitamins, hormones, polyphenolic acids, amino acids,…etc. The results revealed also the presence of a chemical diversity and bioactivity [Fig. 3(B), 4(B), Table 2] which are in good agreement with the results of many other researchers [21]. In the present study, a number of bioactive compounds such as tannins, saponins, coumarin, and flavonoids were identified. There are many medicinal applications for these compounds. For example, tannin-containing drugs are used in medicine as astringent. They are also used as healing agents in inflammation, leucorrhoea, gonorrhoea, burns, piles and as antidote. Furthermore, tannins have been found to have antiviral, antibacterial, antiparasitic effects. In addition to their anti-inflammatory, antiulcer and antioxidant properties for possible therapeutic applications. This has been confirmed in the present study (Figs. 4, 5). Saponins and coumarin compounds have, on the other hand, been observed in Sargassium vulgare marine extract. Saponins are known to produce antimicrobial, anti-inflammatory, anti-feeding, and hemolytic effects. Coumarin, on the other hand, has been used as anti-coagulant drugs and to treat lymphedema [22]. On the meantime, flavonoids which are the major group of phenolic compounds were reported for their antimicrobial, antiviral and anticancer activity [4]. Flavonoids ability of scavenging hydroxyl radicals, superoxide anion radicals and lipid proxy radicals highlights many of the flavonoids health-promoting functions in organisms. These abilities are important for prevention of diseases associated with oxidative damage of membrane. Proteins and DNA are also identified in the present study (Figs. 4, 5).
Table 2.
The bioactive and GC–MS profiling of Sargassium vulgare
| Compound name | RT | Prob. | Area | Area % | Compound name | RT | Prob. | Area | Area % |
|---|---|---|---|---|---|---|---|---|---|
| (E)-13-Docosenoic acid | 0.07 | 7.20 | 358,084.25 | 0.10 | |||||
| 9,10-Secocholesta-5,7,10(19)-triene-1,3-diol, 25-[(trimethylsilyl)oxy]-, (3á,5Z,7E)- | 0.17 | 6.64 | 320,935.48 | 0.09 | Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) | 1.36 | 6.16 | 137,212.27 | 0.04 |
| Octadecanoic acid, 4-hydroxy-, methyl ester | 0.17 | 6.64 | 320,935.48 | 0.09 | Ethyl iso-allocholate | 1.52 | 5.48 | 116,195.34 | 0.03 |
| Spiculesporic acid | 0.33 | 7.58 | 29,624.78 | 0.01 | 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl)methyl ester, trans- | 1.89 | 18.65 | 160,341.24 | 0.04 |
| Ursodeoxycholic acid | 1.78 | 18.54 | 129,937.60 | 0.03 | Phorbol 12,13-dihexanoate | 2.05 | 9.08 | 224,162.33 | 0.06 |
| Chenodiol | 1.78 | 12.72 | 129,937.60 | 0.03 | Heptanoic acid, docosyl ester | 2.28 | 9.35 | 136,712.49 | 0.04 |
| Glucobrassicin | 0.75 | 7.06 | 440,260.95 | 0.12 | 2-(2-Azepan-1-yl-2-oxoethyl)-1-hydroxy-1-phenyl-octahydro-pyrido[1,2-a]azepin-4-one | 4.72 | 15.28 | 356,081.28 | 0.10 |
| Retinoic acid, methyl ester | 0.82 | 5.39 | 1,621,521.96 | 0.44 | EPPS | 4.72 | 11.09 | 356,081.28 | 0.10 |
| Morphinan-4,5-epoxy-3,6-di-ol, 6-[7-nitrobenzofurazan-4-yl]amino- | 2.23 | 10.08 | 44,151.57 | 0.01 | 2-(Cyanomethylene)-3,5-dihydroxy-4-methoxycyclohexyl)-á-glucoside | 4.77 | 12.38 | 175,012.00 | 0.05 |
| 11-Heneicosanone | 1.21 | 11.85 | 210,142.95 | 0.06 | Benzaldehyde, 4-methyl-, oxime | 4.84 | 20.85 | 67,640.79 | 0.02 |
| 5,8,11,14-Eicosatetraynoic acid | 3.98 | 5.82 | 83,212.10 | 0.02 | Heptadecane, 9-hexyl- | 6.76 | 27.94 | 840,216.97 | 0.23 |
| Pregn-4-ene-3,20-dione, 17,21-dihydroxy-, bis(O-methyloxime) | 4.08 | 30.27 | 81,015.18 | 0.02 | Octadecane, 3-ethyl-5-(2-ethylbutyl)- | 6.76 | 18.04 | 840,216.97 | 0.23 |
| 1-Oxaspiro[4.4]non-8-ene-4,7-dione, 9-hydroxy-6-(3-methyl-2-butenyl)-2-(1-methylethyl)-8-(3-methyl-1-oxobutyl)- | 4.08 | 6.10 | 81,015.18 | 0.02 | 2,5-Furandione, dihydro-3-octadecyl- | 6.90 | 7.86 | 36,299.75 | 0.01 |
| 2-Chloro-1,3-bis(4-methylpiperazin-1-yl)-4-nitro-benzene | 4.19 | 14.99 | 379,086.59 | 0.10 | 9,19-Cyclolanostan-3-ol, 24,24-epoxymethano-, acetate | 6.90 | 4.29 | 36,299.75 | 0.01 |
| Dodecane, 5,8-diethyl- | 4.34 | 13.93 | 337,434.19 | 0.09 | à-N-Normethadol | 6.93 | 13.07 | 218,379.53 | 0.06 |
| 2,5-Furandione, dihydro-3-isooctadecyl- | 4.65 | 7.13 | 295,458.80 | 0.08 | 9,10-Secocholesta-5,7,10(19)-triene-1,3-diol, 25-[(trimethylsilyl)oxy]-, (3á,5Z,7E)- | 7.06 | 7.14 | 98,889.56 | 0.03 |
| 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol, (3á,5Z,7E)- | 3.11 | 21.68 | 50,947.59 | 0.01 | Propionitrile, 3-[1-[4-[1-(2-cyanoethoxy)cyclohexyl]buta-1,3-diynyl]cyclohexyloxy]- | 7.14 | 6.74 | 249,560.47 | 0.07 |
| 4-Heptadecyne, 1-chloro- | 3.11 | 11.18 | 50,947.59 | 0.01 | l-Lysine, N6-acetyl-N2-[N-[N-[N-(N2-acetyl-N,N,N2-trimethyl-L-asparaginyl)-N-methyl-L-phenylalanyl]-N-methyl-L-phenylalanyl]-N,1-dimethyl-L-tryptophyl]-N2,N6-dimethyl-, methyl ester | 7.22 | 4.81 | 149,066.26 | 0.04 |
| Morphinan-4,5-epoxy-3,6-di-ol, 6-[7-nitrobenzofurazan-4-yl]amino- | 3.19 | 6.63 | 1,059,592.03 | 0.28 | Prost-13-en-1-oic acid, 9-(methoxyimino)-11,15-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester, (8.xi.,12.xi.)- | 7.58 | 13.82 | 5,675,895.92 | 1.52 |
| à-N-Normethadol | 3.26 | 25.19 | 344,333.02 | 0.09 | Octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl- | 9.31 | 15.52 | 6,731,734.92 | 1.81 |
| 2-Methyl-thiobenzamide | 4.84 | 6.13 | 67,640.79 | 0.02 | Remoxipride | 9.98 | 6.59 | 582,702.43 | 0.16 |
| à-N-Normethadol | 4.93 | 19.97 | 362,160.62 | 0.10 | Cyclohexane, 1,1′-dodecylidenebis[4-methyl- | 11.10 | 4.13 | 1,235,479.08 | 0.33 |
| L-Serinamide, 1-methyl-5-oxo-L-prolyl-N,1-dimethyl-L-histidyl-N,1-L-tryptophyl-N,N,N2,O-tetramethyl- | 4.93 | 5.23 | 362,160.62 | 0.10 | [5,9-Dimethyl-1-(3-phenyl-oxiran-2-yl)-deca-4,8-dienylidene]-(2-phenyl-aziridin-1-yl)-amine | 11.16 | 10.90 | 354,576.81 | 0.10 |
| 1-Oxaspiro[4.4]non-8-ene-4,7-dione, 9-hydroxy-6-(3-methyl-2-butenyl)-2-(1-methylethyl)-8-(3-methyl-1-oxobutyl)- | 5.15 | 6.31 | 248,651.71 | 0.07 | Benzene, [3-(2-cyclohexylethyl)-6-cyclopentylhexyl]- | 11.16 | 5.62 | 354,576.81 | 0.10 |
| Heptadecane, 9-hexyl- | 5.27 | 6.58 | 1,297,029.32 | 0.35 | Cholestane-3,7,12,25-tetrol, tetraacetate, (3à,5á,7à,12à)- | 13.12 | 6.40 | 1,192,314.28 | 0.32 |
| Hexadecane, 1,1-bis(dodecyloxy)- | 5.27 | 4.91 | 1,297,029.32 | 0.35 | Ursodeoxycholic acid | 13.39 | 3.71 | 838,378.12 | 0.23 |
| 3-Pentanone, 1,5-diphenyl- | 5.35 | 10.71 | 351,525.71 | 0.09 | Glucobrassicin | 13.77 | 7.55 | 84,398.25 | 0.02 |
| [5,9-Dimethyl-1-(3-phenyl-oxiran-2-yl)-deca-4,8-dienylidene]-(2-phenyl-aziridin-1-yl)-amine | 5.35 | 5.52 | 351,525.71 | 0.09 | 5,8,11,14-Eicosatetraynoic acid | 11.96 | 17.64 | 554,245.64 | 0.15 |
| 4-Hexyl-1-(7-methoxycarbonylheptyl)bicyclo[4.4.0]deca-2,5,7-triene | 5.47 | 8.19 | 66,532.26 | 0.02 | Gibberellic acid | 11.96 | 10.69 | 554,245.64 | 0.15 |
| 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol, (3á,5Z,7E)- | 5.76 | 11.58 | 601,347.56 | 0.16 | Trilostane | 12.19 | 11.74 | 1,276,758.21 | 0.34 |
| 7-Chloro-4-[1-methyl-3-[N-methyl-N1-bromoacetylhydrazinyl]propyl]aminoquinoline | 5.88 | 11.14 | 350,029.26 | 0.09 | l-Galactopyranose, 6-deoxy-1,2-bis-O-(trimethylsilyl)-, cyclic methylboronate | 14.02 | 5.08 | 64,690.61 | 0.02 |
| .psi.,.psi.-Carotene, 1,1′,2,2′-tetrahydro-1,1′-dimethoxy- | 6.36 | 10.52 | 45,278.33 | 0.01 | 1,3-Dioxocane, 2-pentadecyl- | 15.79 | 4.64 | 870,991.45 | 0.23 |
| Photodieldrin | 7.84 | 27.29 | 172,556.63 | 0.05 | .psi.,.psi.-Carotene, 1,1′,2,2′-tetrahydro-1,1′-dimethoxy- | 15.16 | 9.29 | 585,030.76 | 0.16 |
| Ethynodiol Diacetate | 8.17 | 5.63 | 128,311.73 | 0.03 | 8,11,14-Eicosatrienoic acid, methyl ester, (Z,Z,Z)- | 16.56 | 5.77 | 189,229.87 | 0.05 |
| Docosanoic acid, 1,2,3-propanetriyl ester | 8.17 | 4.54 | 128,311.73 | 0.03 | 5,8,11,14-Eicosatetraenoic acid, methyl ester, (all-Z)- | 16.56 | 5.55 | 189,229.87 | 0.05 |
| Ursodeoxycholic acid | 9.57 | 7.16 | 263,257.19 | 0.07 | Curan-17-oic acid, 2,16-didehydro-20-hydroxy-19-oxo-, methyl ester | 16.67 | 4.94 | 537,003.63 | 0.14 |
| Gibberellic acid | 9.66 | 7.18 | 529,204.53 | 0.14 | à-N-Normethadol | 16.74 | 13.79 | 437,226.78 | 0.12 |
| Phorbol | 8.44 | 17.21 | 338,593.24 | 0.09 | Phorbol 12,13-dihexanoate | 18.39 | 12.16 | 600,446.20 | 0.16 |
| Dodecane, 5,8-diethyl- | 8.55 | 4.64 | 297,081.43 | 0.08 | Norvenlafaxine | 17.18 | 5.36 | 388,506.99 | 0.10 |
| Monorden | 8.85 | 35.41 | 173,918.06 | 0.05 | Heptadecane, 9-hexyl- | 17.18 | 4.32 | 388,506.99 | 0.10 |
| Androstan-17-one, 3-ethyl-3-hydroxy-, (5à)- | 10.53 | 20.25 | 111,628.74 | 0.03 | Cyclopropanedodecanoic acid, 2-octyl-, methyl ester | 17.28 | 5.93 | 275,978.22 | 0.07 |
| Benzene, 1,1′-[3-(3-cyclopentylpropyl)-1,5-pentanediyl]bis- | 10.53 | 10.44 | 111,628.74 | 0.03 | Ethyl iso-allocholate | 17.28 | 4.78 | 275,978.22 | 0.07 |
| Methoxyphenamine | 10.65 | 29.36 | 8,624,803.65 | 2.32 | Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) | 17.80 | 27.42 | 214,480.23 | 0.06 |
| Scytalone | 10.65 | 21.90 | 8,624,803.65 | 2.32 | Retinoic acid, methyl ester | 19.22 | 11.99 | 496,739.98 | 0.13 |
| Octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl- | 10.85 | 12.98 | 4,105,007.91 | 1.10 | L-Serinamide, 1-methyl-5-oxo-L-prolyl-N,1-dimethyl-L-histidyl-N,1-L-tryptophyl-N,N,N2,O-tetramethyl- | 19.32 | 22.09 | 450,905.21 | 0.12 |
| Propanoic acid, 2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl)- | 10.85 | 8.64 | 4,105,007.91 | 1.10 | Digoxigenin | 19.32 | 6.61 | 450,905.21 | 0.12 |
| Hexadecanoic acid, ethyl ester | 10.95 | 7.08 | 2,279,096.83 | 0.61 | .psi.,.psi.-Carotene, 1,1′,2,2′-tetrahydro-1,1′-dimethoxy- | 19.38 | 3.40 | 124,040.26 | 0.03 |
| Oxiraneoctanoic acid, 3-octyl-, cis- | 10.95 | 6.53 | 2,279,096.83 | 0.61 | 3-Oxatricyclo[20.8.0.0(7,16)]triaconta-1(22),7(16),9,13,23,29-hexaene | 19.51 | 6.03 | 134,068.87 | 0.04 |
| Oleic Acid | 11.10 | 5.25 | 1,235,479.08 | 0.33 | l-Lysine, N6-acetyl-N2-[N-[N-[N-(N2-acetyl-N,N,N2-trimethyl-L-asparaginyl)-N-methyl-L-phenylalanyl]-N-methyl-L-phenylalanyl]-N,1-dimethyl-L-tryptophyl]-N2,N6-dimethyl-, methyl ester | 19.56 | 7.24 | 430,323.73 | 0.12 |
| 2-Naphthalenamine, 1,2,3,4-tetrahydro-N-(1-methylethyl)-N-(phenylmethyl)- | 18.31 | 5.90 | 324,536.57 | 0.09 | Pregn-4-ene-3,20-dione, 17,21-dihydroxy-, bis(O-methyloxime) | 23.70 | 14.02 | 282,327.29 | 0.08 |
| 17a-Methyl-3á-methoxy-17a-aza-D-homoandrost-5-ene-17-one | 18.39 | 22.29 | 600,446.20 | 0.16 | 2,7-Diphenyl-1,6-dioxopyridazino[4,5:2′,3′]pyrrolo[4′,5′-d]pyridazine | 23.75 | 8.87 | 202,300.27 | 0.05 |
| 5-Aminovaleramide, N-methyl-N-[4-(1-pyrrolidinyl)-2-butynyl]-N’-boc- | 18.76 | 11.02 | 491,379.41 | 0.13 | Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) | 23.75 | 4.57 | 202,300.27 | 0.05 |
| Retinoic acid, methyl ester | 19.22 | 11.99 | 496,739.98 | 0.13 | Gona-1,3,5(10)-trien-17-one, 3-methoxy-, (13à)- | 23.81 | 6.74 | 195,523.47 | 0.05 |
| Pentacosane, 13-phenyl- | 20.85 | 7.40 | 166,888.41 | 0.04 | Gona-1,3,5(10)-trien-17-one, 3-methoxy-, (13à)- | 23.81 | 6.74 | 195,523.47 | 0.05 |
| Cyclopropaneoctanoic acid, 2-octyl-, methyl ester, cis- | 20.90 | 6.65 | 35,392.22 | 0.01 | Tricyclo[4.2.1.1(2,5)]decan-9-one, 10-cyano-10-trimethylsilyloxy- | 23.91 | 4.12 | 250,250.95 | 0.07 |
| Eicosanebioic acid, dimethyl ester | 20.90 | 6.39 | 35,392.22 | 0.01 | 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl)methyl ester, cis- | 23.91 | 4.12 | 250,250.95 | 0.07 |
| Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) | 19.71 | 15.57 | 186,504.69 | 0.05 | N,N’-Pentamethylenebis[s-3-aminopropyl thiosulfuric acid] | 24.12 | 28.24 | 77,633.59 | 0.02 |
| Heptadecane, 9-hexyl- | 19.71 | 8.50 | 186,504.69 | 0.05 | Benzene, (3-octylundecyl)- | 24.12 | 11.16 | 77,633.59 | 0.02 |
| Heptanoic acid, docosyl ester | 19.77 | 20.15 | 401,368.59 | 0.11 | Triazolam | 24.18 | 17.00 | 130,833.04 | 0.04 |
| Cholestane-3,7,12,25-tetrol, tetraacetate, (3à,5á,7à,12à)- | 20.08 | 5.51 | 91,682.18 | 0.02 | Cyclohexane, 1,1′-dodecylidenebis[4-methyl- | 23.00 | 3.23 | 441,978.85 | 0.12 |
| Oleic Acid | 20.45 | 3.72 | 371,362.44 | 0.10 | 1-Naphthalenepropanol, à-ethyldecahydro-4-hydroxy-à,2,5,5,8a-pentamethyl-, [1S-[1à(R*),2à,4à,4aá,8aà]]- | 23.07 | 7.41 | 721,998.42 | 0.19 |
| Folic Acid | 20.76 | 12.01 | 560,147.31 | 0.15 | Chromane-3-carbonitrile, 2,3-dehydro-5,6,7,8-tetrahydro-2-amino-5-oxo-4-(3-pyridyl)- | 23.07 | 5.97 | 721,998.42 | 0.19 |
| Pregna-6,16-diene-11,20-diol, 3,9-epoxy-18-[N-methyl-N-[14-(2′-epoxyethyl)]amino]- | 22.36 | 18.07 | 164,845.77 | 0.04 | Cholan-24-oic acid, 3,6-bis(acetyloxy)-, methyl ester, (3à,5á,6à)- | 23.22 | 9.27 | 308,515.76 | 0.08 |
| Azafrin | 22.42 | 6.83 | 509,634.27 | 0.14 | Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) | 23.22 | 8.55 | 308,515.76 | 0.08 |
| Pentacyclo[12.3.0.0(1,13).0(2,10).0(5,9)]heptadecan-6-ol-15-one, 5-methyl-13-methoxycarbonyl- | 22.42 | 5.23 | 509,634.27 | 0.14 | Cholestan-26-oic acid, 3,7,12-trihydroxy-, (3à,5á,7à,12à)- | 23.31 | 23.59 | 144,600.45 | 0.04 |
| Stibine, triethyl- | 22.47 | 22.19 | 1,259,213.58 | 0.34 | Quinolin-5(6H)-one, 7,8-dihydro-2-dimethylamino-6-dimethylaminomethylene-7-phenyl- | 23.51 | 6.09 | 233,774.93 | 0.06 |
| Undecanoic acid, 11-bromo-ethylester | 22.47 | 16.99 | 1,259,213.58 | 0.34 | Picrotoxin | 23.51 | 4.91 | 233,774.93 | 0.06 |
| Photodieldrin | 22.64 | 12.92 | 83,344.14 | 0.02 | Androstan-17-one, 3-ethyl-3-hydroxy-, (5à)- | 24.66 | 6.26 | 59,251.95 | 0.02 |
| 1-Heptatriacotanol | 22.64 | 10.91 | 83,344.14 | 0.02 | Androst-5,7-dien-3-ol-17-one | 24.71 | 8.54 | 382,511.96 | 0.10 |
| Triazolam | 24.01 | 11.45 | 89,581.89 | 0.02 | Morphinan-4,5-diol-6-one, 1-bromo- | 24.79 | 4.52 | 174,046.98 | 0.05 |
| Clenbuterol | 24.01 | 9.23 | 89,581.89 | 0.02 | Ursodeoxycholic acid | 24.79 | 3.46 | 174,046.98 | 0.05 |
| Propane-1,1,2,2-tetracarbonitrile, 3-(4-acetyl-2,5-dimethyl-3-furanoyl)- | 24.05 | 13.26 | 157,137.45 | 0.04 | Oxiraneoctanoic acid, 3-octyl-, methyl ester | 24.87 | 12.55 | 203,789.31 | 0.05 |
| 5,8,11,14-Eicosatetraynoic acid | 24.05 | 8.83 | 157,137.45 | 0.04 | Azafrin | 24.87 | 11.09 | 203,789.31 | 0.05 |
| Galactitol, 2,3:4,5-bis-O-(phenylmethylene)- | 22.96 | 32.06 | 241,328.02 | 0.06 | Sumatriptan | 24.93 | 40.27 | 71,681.46 | 0.02 |
| Cholan-24-oic acid, 3,12-bis(acetyloxy)-7-oxo-, methyl ester, (3à,5á,12à)- | 22.96 | 7.43 | 241,328.02 | 0.06 | Dodecanoic acid, hexadecyl ester | 25.98 | 5.11 | 212,740.69 | 0.06 |
| 8,14-Seco-3,19-epoxyandrostane-8,14-dione, 17-acetoxy-3á-methoxy-4,4-dimethyl- | 23.00 | 3.23 | 441,978.85 | 0.12 | Nonanedioic acid, dibutyl ester | 26.06 | 20.75 | 111,213.16 | 0.03 |
| 1,3,2-Dioxaphosphorinane, 2-(3-indolyl)(4-methoxyphenylamino)methyl-, 2-oxide | 26.65 | 8.55 | 1,029,948.09 | 0.28 | 8,14-Seco-3,19-epoxyandrostane-8,14-dione, 17-acetoxy-3á-methoxy-4,4-dimethyl- | 26.06 | 5.21 | 111,213.16 | 0.03 |
| Morphinan-6-one, 7,8-didehydro-4,5à-epoxy-14-hydroxy-3-methoxy-17-methyl-, cyclic ethylene acetal | 26.72 | 6.60 | 260,410.88 | 0.07 | cis-1,2-Bis(aminomethyl)cyclohexane | 26.15 | 8.64 | 1,154,504.76 | 0.31 |
| N-(3-Dimethylamino-2-(4-chlorphenyl)-thioacryloyl)-dimethylformamidin | 26.72 | 6.34 | 260,410.88 | 0.07 | Cyclopentane, chloro- | 26.15 | 3.67 | 1,154,504.76 | 0.31 |
| Ethanethiol, 2-(3-(3-chloro-2-pyridyloxy)propyl)amino-, hydrogen sulfate | 26.96 | 10.47 | 1,838,357.47 | 0.49 | Cyclohexane, 1,1′-dodecylidenebis[4-methyl- | 26.30 | 21.05 | 286,657.53 | 0.08 |
| à-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic butylboronate | 25.68 | 7.47 | 284,387.17 | 0.08 | Cyclohexane, 1,3,5-trimethyl-2-octadecyl- | 26.30 | 4.96 | 286,657.53 | 0.08 |
| 16-Nitrobicyclo[10.4.0]hexadecan-1-ol-13-one | 27.26 | 21.01 | 334,106.57 | 0.09 | Docosanoic acid, 17-oxo-, methyl ester | 26.44 | 7.53 | 928,767.60 | 0.25 |
| Methapyrilene | 27.26 | 4.79 | 334,106.57 | 0.09 | 5-[2-(2-Chloro-6-methyl-phenyl)-vinyl]-3-methyl-4-nitro-isoxazole | 26.44 | 6.66 | 928,767.60 | 0.25 |
| 5,8,11,14-Eicosatetraynoic acid | 27.42 | 6.12 | 1,964,239.46 | 0.53 | There is no signature data to report. | ||||
| Butylaldehyde, 4-benzyloxy-4-[2,2,-dimethyl-4-dioxolanyl]- | 24.18 | 7.23 | 130,833.04 | 0.04 | 9,10-Secocholesta-5,7,10(19)-triene-1,3-diol, 25-[(trimethylsilyl)oxy]-, (3á,5Z,7E)- | 26.65 | 18.78 | 1,029,948.09 | 0.28 |
| Gibberellic acid | 25.88 | 21.01 | 234,012.32 | 0.06 | Linoleic acid ethyl ester | 27.42 | 5.64 | 1,964,239.46 | 0.53 |
| á-D-Galactopyranoside, methyl 2,6-bis-O-(trimethylsilyl)-, cyclic butylboronate | 25.94 | 7.78 | 225,967.69 | 0.06 | Oxiranedodecanoic acid, 3-octyl-, cis- | 27.57 | 8.75 | 294,024.74 | 0.08 |
| Cyclohexane, 1,1′-dodecylidenebis[4-methyl- | 25.98 | 15.25 | 212,740.69 | 0.06 | Retinol | 27.78 | 8.94 | 598,590.48 | 0.16 |
| 2H-Pyran, tetrahydro-2-(12-pentadecynyloxy)- | 27.03 | 8.99 | 1,062,203.87 | 0.29 | 10,13-Octadecadiynoic acid, methyl ester | 27.78 | 8.60 | 598,590.48 | 0.16 |
| 5á-Cholestane-3à,7à,12à,24,25,26-hexol hexa-TMS | 28.84 | 9.34 | 459,133.72 | 0.12 | 2-(5-Cyano-4,4,5-trimethyl-pyrrolidin-2-ylidene)-malonic acid, dimethyl ester | 27.84 | 15.63 | 329,717.56 | 0.09 |
| Withaferin A | 28.84 | 7.89 | 459,133.72 | 0.12 | Heptadecane, 9-hexyl- | 29.39 | 10.50 | 188,333.64 | 0.05 |
| D-Streptamine, O-2-amino-2-deoxy-à-D-glucopyranosyl-(14)-O-[O-2,6-diamino-2,6-dideoxy-á-L-idopyranosyl-(13)-á-D-ribofuranosyl-(15)]-2-deoxy- | 28.96 | 10.05 | 1,102,529.23 | 0.30 | N,N’-Pentamethylenebis[s-3-aminopropyl thiosulfuric acid] | 29.43 | 6.11 | 50,722.74 | 0.01 |
| Curan, 16,17-didehydro-, (20.xi.)- | 29.29 | 9.28 | 1,124,671.49 | 0.30 | Oxytetracycline | 29.43 | 4.93 | 50,722.74 | 0.01 |
| 5,8,11,14-Eicosatetraynoic acid | 29.29 | 4.78 | 1,124,671.49 | 0.30 | Gibberellic acid | 27.99 | 5.99 | 219,057.62 | 0.06 |
| Octadecane, 3-ethyl-5-(2-ethylbutyl)- | 29.39 | 11.88 | 188,333.64 | 0.05 | Morphinan-4,5-epoxy-3,6-di-ol, 6-[7-nitrobenzofurazan-4-yl]amino- | 28.59 | 8.03 | 102,975.61 | 0.03 |
| Androst-4-en-9-thiocyanomethyl-11-ol-3,17-dione | 30.00 | 11.26 | 128,123.12 | 0.03 | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1-carboxaldehyde | 28.59 | 8.03 | 102,975.61 | 0.03 |
| Androst-4-en-11-ol-3,17-dione, 9-thiocyanato- | 30.00 | 9.51 | 128,123.12 | 0.03 | Morphinan-6-one, 7,8-didehydro-4,5à-epoxy-14-hydroxy-3-methoxy-17-methyl-, cyclic ethylene acetal | 29.82 | 12.45 | 436,445.33 | 0.12 |
| DL-Cystine | 31.64 | 6.79 | 253,797.69 | 0.07 | L-Serinamide, 1-methyl-5-oxo-L-prolyl-N,1-dimethyl-L-histidyl-N,1-L-tryptophyl-N,N,N2,O-tetramethyl- | 32.53 | 16.04 | 395,700.81 | 0.11 |
| Pentacosane, 13-phenyl- | 32.15 | 14.25 | 1,946,316.27 | 0.52 | 5,16,20-Pregnatriene-3beta,20-diol diacetate | 32.53 | 7.79 | 395,700.81 | 0.11 |
| 5,8,11,14-Eicosatetraynoic acid | 30.86 | 9.37 | 236,753.27 | 0.06 | N,N’-Pentamethylenebis[s-3-aminopropyl thiosulfuric acid] | 32.62 | 16.66 | 352,442.46 | 0.09 |
| á-D-Galactopyranoside, methyl 2,3-bis-O-(trimethylsilyl)-, cyclic methylboronate | 31.10 | 5.32 | 244,969.67 | 0.07 | Remoxipride | 32.62 | 7.59 | 352,442.46 | 0.09 |
| 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl)methyl ester, cis- | 31.10 | 4.70 | 244,969.67 | 0.07 | 16-Nitrobicyclo[10.4.0]hexadecan-1-ol-13-one | 32.73 | 10.78 | 306,089.94 | 0.08 |
| 1,4,7-Androstatrien-3,17-dione | 31.15 | 12.70 | 264,326.84 | 0.07 | Pregn-4-ene-3,20-dione, 17,21-dihydroxy-, bis(O-methyloxime) | 34.29 | 12.96 | 313,980.28 | 0.08 |
| Androst-5,7-dien-3-ol-17-one, acetate | 31.15 | 12.21 | 264,326.84 | 0.07 | Z,Z-3,15-Octadecadien-1-ol acetate | 34.29 | 5.12 | 313,980.28 | 0.08 |
| 2,7-Diphenyl-1,6-dioxopyridazino[4,5:2′,3′]pyrrolo[4′,5′-d]pyridazine | 31.21 | 9.02 | 75,022.27 | 0.02 | à-D-Galactopyranoside, methyl 2,6-bis-O-(trimethylsilyl)-, cyclic butylboronate | 35.10 | 15.12 | 515,659.92 | 0.14 |
| Octadecanedioic acid | 32.73 | 6.96 | 306,089.94 | 0.08 | á-D-Galactopyranoside, methyl 2,6-bis-O-(trimethylsilyl)-, cyclic butylboronate | 35.10 | 11.28 | 515,659.92 | 0.14 |
| N,N’-Pentamethylenebis[s-3-aminopropyl thiosulfuric acid] | 32.99 | 24.72 | 1,785,383.42 | 0.48 | 6-(2-Nitro-1-phenylethyl)-1,4-dithiaspiro[4.5]decane | 39.66 | 6.90 | 619,045.87 | 0.17 |
| L-Serinamide, 1-methyl-5-oxo-L-prolyl-N,1-dimethyl-L-histidyl-N,1-L-tryptophyl-N,N,N2,O-tetramethyl- | 32.99 | 18.93 | 1,785,383.42 | 0.48 | Podocarpa-1,12-diene-ë14,à-acetic acid, 7-hydroxy-8,13-dimethyl-3-oxo-, ë-lactone | 39.88 | 3.96 | 515,660.54 | 0.14 |
| Benzene, 1,1′-(chloroethenylidene)bis[4-ethyl- | 34.44 | 4.46 | 177,021.32 | 0.05 | Acetamide, N-[2-(acetyloxy)-2-[4-(acetyloxy)phenyl]ethyl]- | 38.47 | 9.78 | 528,379.27 | 0.14 |
| 9-Anthracenepropanoic acid, 10-carboxy-9,10-dihydro-, cis- | 34.44 | 3.24 | 177,021.32 | 0.05 | Oxiraneoctanoic acid, 3-octyl-, cis- | 38.70 | 4.25 | 501,003.03 | 0.13 |
| Allo-cassaic acid methyl ester | 33.81 | 10.20 | 226,266.92 | 0.06 | 2-Octadecenoic acid, methyl ester | 39.05 | 5.79 | 758,454.84 | 0.20 |
| Normorphine | 33.81 | 7.61 | 226,266.92 | 0.06 | 4,6-Androstadien-3á-ol-17-one, acetate | 40.43 | 7.90 | 340,211.09 | 0.09 |
| á-D-Galactopyranoside, methyl 2,3-bis-O-(trimethylsilyl)-, cyclic methylboronate | 35.16 | 6.96 | 360,983.64 | 0.10 | 2,5-Furandione, dihydro-3-octadecyl- | 39.19 | 5.90 | 268,893.08 | 0.07 |
| .psi.,.psi.-Carotene, 3,3′,4,4′-tetradehydro-1,1′,2,2′-tetrahydro-1,1′-dimethoxy-2,2′-dioxo- | 35.16 | 6.69 | 360,983.64 | 0.10 | Glucobrassicin | 39.92 | 6.87 | 322,380.12 | 0.09 |
| Androst-4-en-9-thiocyanomethyl-11-ol-3,17-dione | 35.24 | 4.72 | 705,798.37 | 0.19 | 2-[(Benzo [1,3] dioxole-4-carbonyl)-amino]-3-hydroxy-propionic acid | 40.18 | 10.30 | 822,937.54 | 0.22 |
| Isodihydrohistrionicotoxin 285a | 35.24 | 4.54 | 705,798.37 | 0.19 | Ethyl 5,8,11,14,17-icosapentaenoate | 40.43 | 11.19 | 340,211.09 | 0.09 |
| Picrotoxinin | 35.55 | 15.93 | 964,361.50 | 0.26 | |||||
| Galactopyranoside, methyl 2-acetamido-2-deoxy-3,4,6-tri-O-methyl-, à-D- | 37.76 | 3.38 | 75,953.49 | 0.02 | à-N-Normethadol | 40.55 | 7.46 | 355,655.68 | 0.10 |
| Phorbol 12,13,20-triacetate | 37.86 | 12.51 | 739,177.80 | 0.20 | á Carotene | 38.11 | 3.14 | 202,675.99 | 0.05 |
| 5,6-Dicarbadecaborane(12) | 37.86 | 6.45 | 739,177.80 | 0.20 | L-Serinamide, 1-methyl-5-oxo-L-prolyl-N,1-dimethyl-L-histidyl-N,1-L-tryptophyl-N,N,N2,O-tetramethyl- | 39.73 | 13.54 | 225,351.65 | 0.06 |
| Ajmalan-16-carboxylic acid, 19,20-didehydro-1-demethyl-17-hydroxy-, methyl ester, (2à,17S,19E)- | 39.66 | 8.17 | 619,045.87 | 0.17 | Ethyl 5,8,11,14,17-icosapentaenoate | 39.88 | 11.83 | 515,660.54 | 0.14 |
Fig. 4.
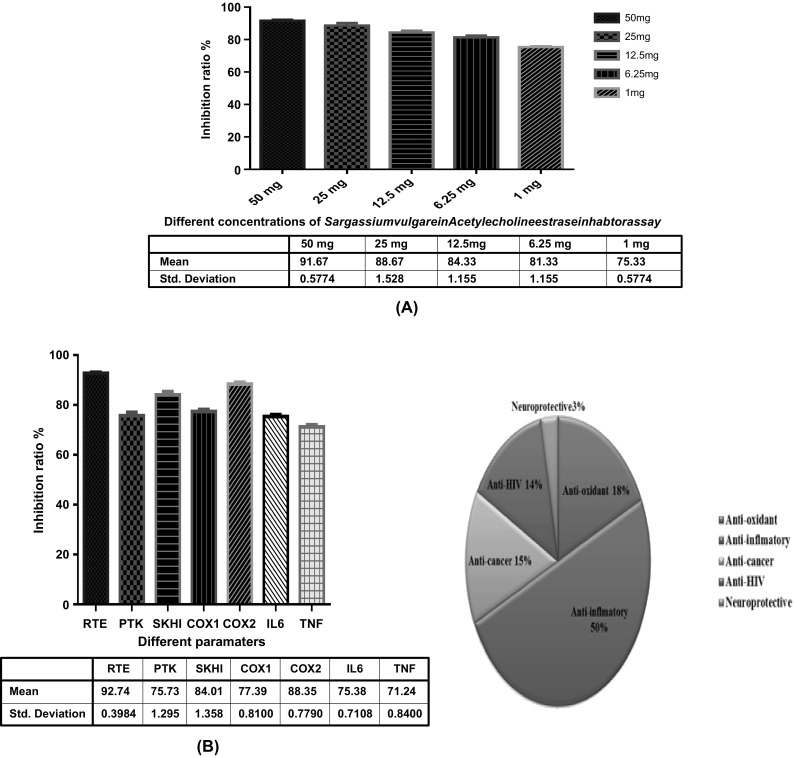
The specialized screening using different screening assay, (A) The Anti-Alzheimer using acetyl cholinesterase inhibition of different marine extracts of Sargassium vulgare extract, (B) The Anti-cancer, anti-inflammatory and anti-HIV of different marine extract of Sargassium vulgare extract
Fig. 5.
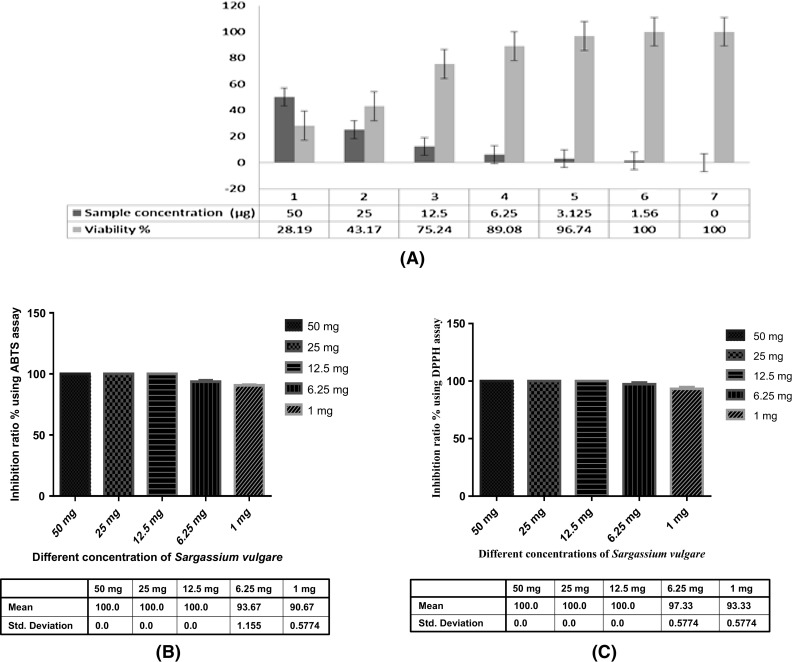
The specialized screening using different screening assay, (A) The cytotoxicity using Hepatocellular carcinoma cells line of Sargassium vulgare extract, (B) The Total antioxidant capacity using DPPH of different marine extracts concentrations of Sargassium vulgare extract, (C) The Total antioxidant capacity using ABTS of different marine extracts concentrations of Sargassium vulgare extract
Biochemical and pharmacological assays
The biochemical and pharmacological bioassay screening assays were performed on different phases including prescreening, primary screening and specialized screening for Sargassium vulgare marine extract.
Broad biochemical drug discovery bioassay
Prescreening: cytotoxicity of marine sargassium
In the present study Sargassium vulgare marine extract was screened against human cancer cell line using the MTT cytotoxicity assay. The cytotoxicity of the marine extract and the inhibitory activity against Hepatocellular carcinoma cells were detected with IC50 = 20.8 µg. The results were presented in Fig. 5(A). The use of marine natural products as therapeutic and cure tools has been started since 1997 with many discoveries [1–7].The results of the present study revealed that marine derived sargassium from the Suez Canal, especially those belong to genus vulgare, are rich source of structurally novel and biologically potent natural products. This is in agreement with recent studies which have shown that many seaweeds extracts from the Egyptian marine environment along the Mediterranean Sea displayed significant cytotoxic and apoptotic activity [5, 6]. The deep-sea anoxic brines of the Red sea are one of the most extreme environments on the earth in comparison to overlying seawater. The anoxic brines are characterized by high salinity (increased from 4% up to 26%), high temperature (up to 70 °C), increased concentration of heavy metals, and decrease in O2 levels [23] Therefore, marine-derived seaweeds bioactive secondary metabolites have the ability to tolerate and survive in these extreme conditions, since they are uniquely equipped with some self-defenses [1–4, 20].
Primary screening assays
Total antioxidant capacity (TAO) of different marine extracts using DPPH and ABTS inhibition assays
The results of total antioxidant capacity at different concentrations of Sargassium vulgare marine extract have been done using DPPH and ABTS inhibition Fig. 5(B), (C). The present study cleared out that the inhibition assay give high inhibition ratio with 93.33 and 90.67% of both DPPH and ABTS, respectively by the marine extract at 1 mg. At concentrations from 50 mg up to 6.25 mg the Sargassium vulgare extract showed complete inhibition (100%). Different kinds of radicals are generated in the normal metabolic activities and sometimes the antioxidant capacity of the body is inadequate to cope with them. Therefore, there is a growing interest on the discovery of natural antioxidants which is more safer than synthetic chemicals because they reduce the risk of developing chronic disease such as cancer [6, 7, 20].
Specialized screening bioassays
The anti-alzheimer activity of different marine extracts using acetyl cholinesterase inhibition assay
Examinations of Sargassium vulgare extract as anti-Alzheimer using acetyl cholinesterase inhibition assay [Fig. 4(A)] cleared out that the inhibition ratio was 76.33% at a concentration of 1 mg of the marine extract. Different concentrations examined (50 mg, 25, 12.5 and 6.25 mg) gave inhibition ratio of 100%. Restoring acetylcholine levels by inhibiting AChE has become the primary treatment for the cognitive deficits of AD. The inhibition of AChE is beneficial not only to the enhancement of cholinergic transmission in the brain, but also to reduce the aggregation of β-amyloid and the formation of the neurotoxic fibrils in AD. In recent decades, researchers have been devoted to developing new AChE inhibitors, especially the so-called “multifunctional AChE inhibitors” with additional efficiency in vascular dementia treatment [24]. There have been plenty of phytochemicals that found to be effective in inhibiting AChE, which mainly consist of alkaloids, cannabinoids, curcumins, stilbenes, and flavonoids. Among them, flavonoids have attracted more and more interest for their high inhibitory activity and low toxicity. Moreover, their diverse activities such as anti-oxidation, inhibition on advanced glycation products, and cardio-cerebrovascular protection give them extra advantages to be the potential multifunctional therapeutic agents for aging related diseases [25]. The highest inhibition ratio of marine sargassium extract as anti-alzheimer is in agreement with previous studies which indicated that marine natural products specifically marine seaweeds produces variety of secondary metabolites and these secondary metabolites serve as source of bioactive compounds for use in human therapies as they thrive in harsh oceanic climate [4–7]. Moreover, Natarajan et al. [26] reported that methanolic extract of Sargassum showed revealed strong inhibition on Cholinesterase activity. Two farnesyl acetone derivatives were isolated from the Korean brown alga Sargassum sagamianum showed moderate acetylcholinesterase and butyrylcholinesterase inhibitory activities. Moreover, two plastoquinones were isolated from Sargassum sagamianum [22].
The results of anti-cancer, anti-viral, anti-inflammatory
The anti-viral effect (anti-HIV) using reverse transcriptase inhibitor assay (RTE)
The activities of Sargassium extracts as anti-HIV using reverse transcriptase inhibitor assay [Fig. 4(B)] showed the highest antiviral activity (92.51%) which is in a good agreement with many previous studies [16]. Seaweeds, especially the genus Gracilaria and sargassium are the most attractive candidate that has the anti-HIV activities [23] because of its stability to achieve high yields and producing commercially valuable extracts. It has been concluded that sessile marine organism (sponge and seaweeds) contain substances capable of potent biological activity which has also been demonstrated against different types of cancer and HIV/AIDS [27]. Two major types of HIV have been identified so far, namely, HIV-1 and HIV-2. HIV-1 is the cause of the worldwide epidemic and is most commonly referred to as HIV. The basic biological processes in the HIV-1 life cycle are now well established. Natural compounds targeting specific steps in this life cycle can be found [28]. According to the different targets, anti-HIV agents are classified under several categories: (1) HIV entry inhibitors, including attachment and fusion inhibitors, and chemokine receptor antagonists [chemokine receptor subtype 5 (CCR5) and CXC-specific receptor subtypes 4 (CXCR4) antagonists] [29]; (2) HIV reverse transcriptase (RT) inhibitors, including nucleotide RT inhibitors and non-nucleotide RT inhibitors; (3) HIV integrase (IN) inhibitors; (4) HIV protease (PR) inhibitors; and (5) other HIV inhibitors, such as maturation inhibitors. Most clinical anti-HIV drugs are HIV RT and PR inhibitors, including the various combinations known as highly active antiretroviral therapy. In the last decade (2002–2011), 132 anti-HIV natural products were obtained from marine organisms. It has been stated that more than half of anti-HIV bioactive marine natural products were derived from marine sponges [1–4].
Anti-cancer effect of Sargassium marine extract
The inhibitory activities of Sargassium marine extract as anticancer using SKH1 inhibition assay [Fig. 4(B)] showed that the highest activity (77.14%) was observed by the Sargassium marine extract. In the last two decades, cancer was the most common cause of increased mortality rate in both genders in the world. Many researchers have developed different anticancer drugs, and subsequently applied in clinical trials [28]. It is important to note that the anticancer results of the present study are matching well with previous results [28]. Recently, many researchers cleared out that seaweeds are important sources of bioactive metabolites for the pharmaceutical industry in drug development. Many of their compounds are used to treat diseases like cancer acquired immune deficiency (AIDS), inflammation, pain, arthritis as well as viral, bacterial and fungal infection [30].
The anti-inflammatory activities using COX 1, Cox2,IL6 and TNF interleukin inhibition assays
The anti-inflammatory activities of Sargassium marine extract using ant-COX 1 and anti-COX 2 assays [Fig. 4(B)] cleared out that the extract of Sargassium vulgare has the highest anti-COX1 and anti-COX2 activity of 77.89 and 88.35%; respectively. On the meantime, the anti-inflammatory activities of Sargassium marine extract against IL6 and TNF [Fig. 4(B)] cleared out that the anti-inflammatory activity using IL-6 inhibition was found to be 76.33%. At the same time, the Sargassium vulgare marine extract indicated an anti-TNF value of 71.24%. Many papers reported that the seaweeds in general and specifically Sargassium extract show impressive potent anti-inflammatory effect and that through different mechanism of action [31]:
Indirect effect through the antioxidant capacity. The sulfated polysaccharide and sulfated polyphenolic show potent antioxidant effect [32].
Through high contents of minerals and metals as sulfur, halide compound such as bromine, iodine and chlorine which are very unique compounds for marine habitat. This finding was confirmed in the present study as represented in Fig. 3.
Inflammatory response is normal process and is consider as one of the body defense action mechanisms to recover the damaged process occur when chemical and physical stimuli produced. Sargassium extract showed direct potent anti-inflammatory activity by suppressing the activation of the NF-κB pathway [33] and through also the effect of different bioactive compounds found in the present study in the Sargassium vulgare marine extract such as Carotenoid, polyphenolic, flavonoids, marine polyunsaturated fatty acids (PUFAs) [34]. Worth mentioning that the mechanism of action as anti-inflammatory in case of marine polyunsaturated fatty acids (PUFAs) is through reducing or substituting the arachidonic acid. Arachidonic acid and Eicosapentaenoic acid is a major intermediate derived from n-6 fatty acids and they competes for the same metabolic pathway. When Eicosapentaenoic acid are consumed in high levels, arachidonic acid levels are reduced and substituted by Eicosapentaenoic acid [35]. Macrophages are activated by different cytokines and stimuli produced by immune cells to induce inflammatory responses. Macrophages secrete the inflammation-mediated cytokines as IL-6 and tumor necrosis factor-α (TNF-α). The formation of these inflammatory mediators’ would cause fatal consequences where arachidonic acid is changed into leukotrienes, thromboxanes, and prostaglandins through the action of cyclooxygenase (COX) and mass generation of nitric oxide (NO). Additionally, nuclear factor-kappa B (NF-κB) is a transcription factor that controls the synthesis of different growth factor chemokines and cytokine. It has been reported that NF-κB is responsible for the synthesizes COX-2, iNOS, and different inflammation-related cytokines [36].
In conclusion, the present study highlighted the highly significance of Sargassium vulgare marine extract collected from Suez Canal as reservoir of new therapeutic and tremendous agents. It revealed the importance of the biodiversity of new chemical structures with biological activities. Today seaweeds are considered to be responsible for the biosynthesis of many of these agents. The present study revealed that seaweeds from the Egyptian marine environment represent a promising source of bioactive compounds resource which needs to be biotechnologically explored.
Acknowledgements
I appreciate the effort for all authors to complete this work and their valuable contributions.
References
- 1.Abdel-Monein, NM, El-Aassar, SA, Shreadah, MA, Nabil-Adam, A. Isolation and identification of Psudomance Sp associated with the marine sponge, Hyrtios aff. Erectus, from the Red sea; Egypt and screening of metabolic pathways NRPs and PKS. Journal of pure & Applied Microbiology. 11(3), 1299–1311, (2017a).
- 2.Abdel-Monein, NM, Shreadah, MA, El-Aassar, SA, Nabil-Adam, A. Protective role of antioxidants capacity of Hyrtios aff. Erectus sponge extract against mixture of persistent organic pollutants (POPs)-induced hepatic toxicity in mice liver: biomarkers and ultrastructural study” Environmental Science and Pollution Research. 1–12, (2017b). DOI 10.10071/s11356-017-9805-8. [DOI] [PubMed]
- 3.Abdel-Monein, NM, Yacout, GA, Aboul-Ela, HM, Shreadah, MA. Hepatoprotective Activity of Chitosan Nanocarriers Loaded With the Ethyl Acetate Extract of A stenotrophomonas sp. Bacteria Associated with the Red Sea Sponge Amphimedon Ochracea In CCl4 Induced Hepatotoxicity in Rats. Advances in Bioscience and Biotechnology (ABB), 8(1), 27–50, (2017c).
- 4.Shreadah, MA, Abdel-Monein, NM, El-Aassar, SA, Nabil-Adam, A. The Ameliorative Role of a Marine Sponge Extract against Mixture of Persistent Organic Pollutants induced Changes in Hematological Parameters in Mice. Expert Opinion Environmental Biology (EOEB), 6(2), (2017). 10.4172/2325-9655.1000143b
- 5.Elkomy, RG, Ibraheem, IB, Shreadah, MA, Mohammed, R. Optimal Conditions for Antimicrobial Activity Production from Two Microalgae Chlorella marina and Nevicula F. delicatul. Journal of Pure and Applied Microbiology, 9(4): 2725–2732, (2015).
- 6.Elkomy RG, Ibraheem IB, Shreadah MA, Mohammed R, Ismael AA. Antibacterial and Antifungal Activity of Three Microalgae Isolated from Egyptian Coast of the Mediterranean Sea”. Journal of Pure and Applied Microbiology. 2015;9(4):2751–2758. [Google Scholar]
- 7.Alghazeer, R, Ibrahim, A, Abdulaziz, A, Abouamer, K (2016). In-vitro antioxidant activity of five selected species of Libyan algae. Int. J. Med. Pharm. Res., 4(1), 1–9, (2016).
- 8.Mohy-Eldin SM, El-Ahwany AMD. Bioactivity and phytochemical constituents of marine Red seaweed (Jania rubens, Corallina mediterranea and Petrocladia capillacea) Journal of Taibah University for Science. 2016;10(4):471–484. doi: 10.1016/j.jtusci.2015.06.004. [DOI] [Google Scholar]
- 9.Taga MS, Miller EE, Pratt DE. Chia seeds as a source of natural lipid antioxidants. Journal of the American Oil Chemists’ Society. 1984;61(5):928–931. doi: 10.1007/BF02542169. [DOI] [Google Scholar]
- 10.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 11.Sun B, Richardo-Da-Silvia JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 1998;46:4267–4274. doi: 10.1021/jf980366j. [DOI] [Google Scholar]
- 12.Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis. 2006;19(6–7):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 13.AOAC. Official Methods analysis of association of offcialanaltical chemists 15th End., Association of official analytical chemists.Washington DC., USA. (1990).
- 14.Singh, G, Kumar, P. Extraction, gas chromatography–mass spectrometry analysis and screening of fruits of Terminalia chebula Retz. for its antimicrobial potential, Pharmacognosy Research,5(3): 162–168. 10.4103/0974-8490.112421, (2013). [DOI] [PMC free article] [PubMed]
- 15.Kosanic M, Rankovic B, Stanojkovic T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J Biol Sci. 2015;22(4):390–397. doi: 10.1016/j.sjbs.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarowicz R, Naczk M, Zadernowski R, Shahidi F. Antioxidant activity of condensed tannins of beach pea, Canola hulls, evening primrose, and faba bean. Journal of Food Lipids. 2000;7:195–205. doi: 10.1111/j.1745-4522.2000.tb00171.x. [DOI] [Google Scholar]
- 17.Chkraborty K, Lipton AP, Paul Raj R, Vijayan KK. Antibacterial labdane diterpenoids of Ulva fasciata Delile from southwestern coast of the Indian Peninsula. Food Chem. 2010;119:1399–1408. doi: 10.1016/j.foodchem.2009.09.019. [DOI] [Google Scholar]
- 18.Moyo SJ, Aboud S, Kasubi M, Lyamuya EF, Maselle SY. Antimicrobial resistance among producers and non-producers of extended spectrum beta- lactamases in urinary isolates at a tertiary Hospital in Tanzania. A Short Report. BMC Research Notes. 2010;3:348. doi: 10.1186/1756-0500-3-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.potential for incorporation into virostatic cocktails Fonteh, PN, Keter, FK. Meyer, D. New bis(thiosemicarbazonate) gold(III) complexes inhibit HIV replication at cytostatic concentrations. J Inorg Biochem. 2011;105:1173–1180. doi: 10.1016/j.jinorgbio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Hegazy MF, Mohamed TA, Elshamy AI, Hassanien AA, Abdel Azimd NS, Shreadah MA, Abdelgawad II, Elkady EM. A New Steroid from the Red Sea Soft Coral Lobophytum Lobophytum. Natural Products Research. 2015;30:340–344. doi: 10.1080/14786419.2015.1046871. [DOI] [PubMed] [Google Scholar]
- 21.Behzad H, Ibarra MA, Mineta K, Gojobori T. Metagenomic studies of the Red Sea. Gene. 2016;576:717–723. doi: 10.1016/j.gene.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Jeeva S, Antonisamy JM, Domettila C, Anantham B, Mahesh M. Preliminary phytochemical studies on some selected seaweeds from Gulf of Mannar India. Asian Pac. J. Trop. Biomed. 2012;2(1):S30–S33. doi: 10.1016/S2221-1691(12)60125-7. [DOI] [Google Scholar]
- 23.Fahmy MA, Abdel Fattah LM, Abdel-Halim AM, Abdel Nabi MA, Abo-El-Khair EM, Ahdy HH, Hemeilly A, Abu El-Soud A, Shreadah MA. Evaluations of the Coastal Water Quality of the Egyptian Red Sea during 2011-2013. J. Environ. Prot. 2016;7(12):1810–1834. doi: 10.4236/jep.2016.712145. [DOI] [Google Scholar]
- 24.Mehdinezhad N, Ghannadi A, Yegdaneh A. Phytochemical and biological evaluation of some Sargassum species from Persian Gulf. Research Pharmaceutical Science. 2016;11(3):243–249. [PMC free article] [PubMed] [Google Scholar]
- 25.Gauthier S, Leuzy A, Rosa-Neto P. How can we improve transfer of outcomes from randomized clinical trials to clinical practice with disease-modifying drugs in Alzheimer’s disease? Neurodegener Dis. 2014;13(2–3):197–199. doi: 10.1159/000353748. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan S, Shanmugiahtheva KP, Kasi PD. Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: seaweeds inhabiting South Indian coastal areas (Hare Island, Gulf of Mannar) Journal of Natural Product Research. 2009;23(4):355–369. doi: 10.1080/14786410802156036. [DOI] [PubMed] [Google Scholar]
- 27.Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Molocular Cancer Therapy. 2005;4(2):333–342. [PubMed] [Google Scholar]
- 28.Lee JC, Hou MF, Huang HW, Chang FR, Yeh CC, Tang JY, Chang HW. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell International. 2013;13:55. doi: 10.1186/1475-2867-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Chen C, Johansson MJ. The pre-mRNA retention and splicing complex controls tRNA maturation by promoting TAN1 expression. Nucleic Acids Research. 2013;41(11):5669–5678. doi: 10.1093/nar/gkt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Almeida CLF, de Falcão H. S., Lima, GR de M, Montenegro, C de A, Lira, NS, de Athayde-Filho, PF, Batista, LM. Bioactivities from Marine Algae of the Genus Gracilaria. International Journal of Molecular. Science. 2011;12(7):4550–4573. doi: 10.3390/ijms12074550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughan VC, Hassing MR, Lewandowski PA. Marine polyunsaturated fatty acids and cancer therapy. British Journal of Cancer. 2013;108(3):486–492. doi: 10.1038/bjc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel S. Therapeutic importance of sulfated polysaccharides from seaweeds: updating the recent findings. 3. Biotech. 2012;2(3):171–185. [Google Scholar]
- 33.Gammone MA, Riccioni G, D’Orazio N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Marine Drugs. 2016;13:6226–6246. doi: 10.3390/md13106226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutration Review, 68:280–289, (2010) [PubMed] [DOI] [PubMed]
- 35.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Molecular Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wojdasiewicz, P, Poniatowski, ŁA,Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediators of Inflammation. (2014), 561459. 10.1155/2014/561459. [DOI] [PMC free article] [PubMed]