Abstract
Purpose
To report the case of a 57 years old woman who showed a macular ganglion cell complex (GCC), that is a combination of ganglion cell layer and inner plexiform layer, and peripapillary Retinal Nerve Fiber Layer (pRNFL) thickness reduction in association with left homonymous hemianopia subsequent to surgical excision of an arteriovenous malformation in the cerebral right occipital lobe 37 years before.
Observations
One patient with left homonymous hemianopia due to surgical excision of an arteriovenous malformation in the right cerebral occipital lobe came to our attention for transient blurred vision.
Measurement of the GCC and pRNFL thickness was performed using spectral domain optical coherence tomography (SDOCT; Cirrus HD-OCT model 400). Visual field (VF) defects were assessed using Humphrey field analyzer using the central 30-2 Swedish Interactive Threshold Algorithm (SITA) program with appropriate trial lenses (Humphrey Field Analyzer II, Carl Zeiss Meditech, Inc, Dublin, California).
The average pRNFL thickness was bilaterally reduced, showing a symmetry value of 39%. The patients showed a significant GCC thinning in the projecting sector of the retina mapping to the brain lesion. Corresponding VF defects were found.
Conclusions and importance
These findings show SDOCT potentials in the field of neuro-ophthalmology, supporting the usefulness of GCC thickness as a possible imaging marker before and after brain surgery, and, possibly, in the diagnosis of neurodegenerative conditions.
Keywords: GCC, pRNFL, Hemianopia, OCT, Neuro-ophthalmology, Arteriovenous malformation, Retrograde transneural degeneration, Neurodegenerative diseases
1. Introduction
Several cases of retrograde transneuronal degeneration of retinal ganglion cells (RGCs) subsequent to damage of the striate cortex, V1, are reported in monkeys and in humans.1, 2, 3
The retinal nerve fiber layer (RNFL) is composed of the axons of RGCs; most of these axons project to the lateral geniculate nucleus (LGN) and reach the primary visual cortex. Accordingly, patients with optic nerve, optic chiasm, optic tract, or LGN damage develop retrograde RGCs atrophy.2
Here we discuss a case of macular ganglion cell complex (GCC) and peripapillary RNFL (pRNFL) alterations in association with left homonymous hemianopia subsequent to iatrogenic post-geniculate visual pathway damage occurred 37 years before.
2. Case report
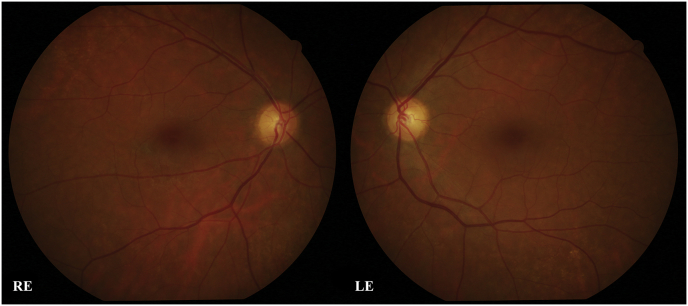
In this paper, we discuss the case of a 57 years old woman who presented to our attention following two episodes of transient blurred vision previously diagnosed as consequences of a posterior vitreous detachment. At the first visit, when questioned, the patient did not report any previous systemic and ocular disease or surgery. Family history was negative for ocular diseases. Best corrected visual acuity for near and far was 0.0 logMar in both eyes. No afferent pupillary defect was found. Intraocular pressure was 12 mmHg in right and 13 mmHg in the left eye, corneal thickness was in the normal range. The anterior segment was normal in both eyes. Fundus oculi examination revealed a bilateral posterior vitreous detachment. Optic nerve head (ONH) was slightly more cupped in the left eye (LE) than the right eye (RE) and no swelling or haemorrhages of the ONH could be found bilaterally. No ophthalmoscopic alterations of the macular area were present (Fig. 1).
Fig. 1.
Fundus oculi photographs of the right eye (RE) and left eye (LE).
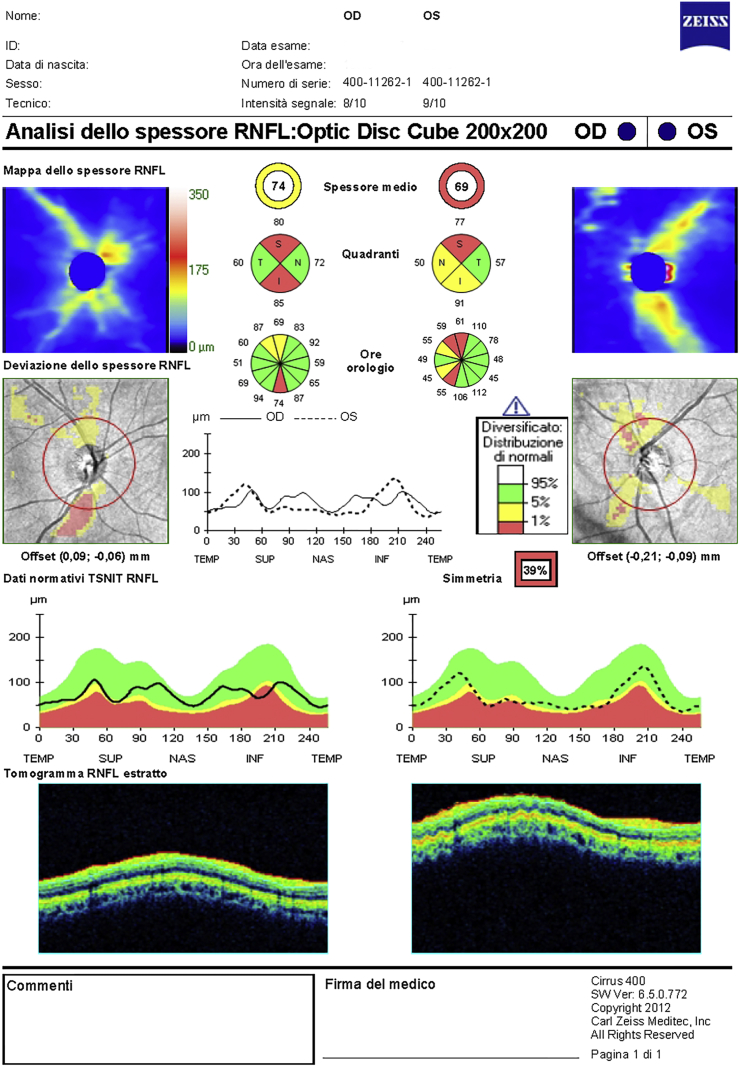
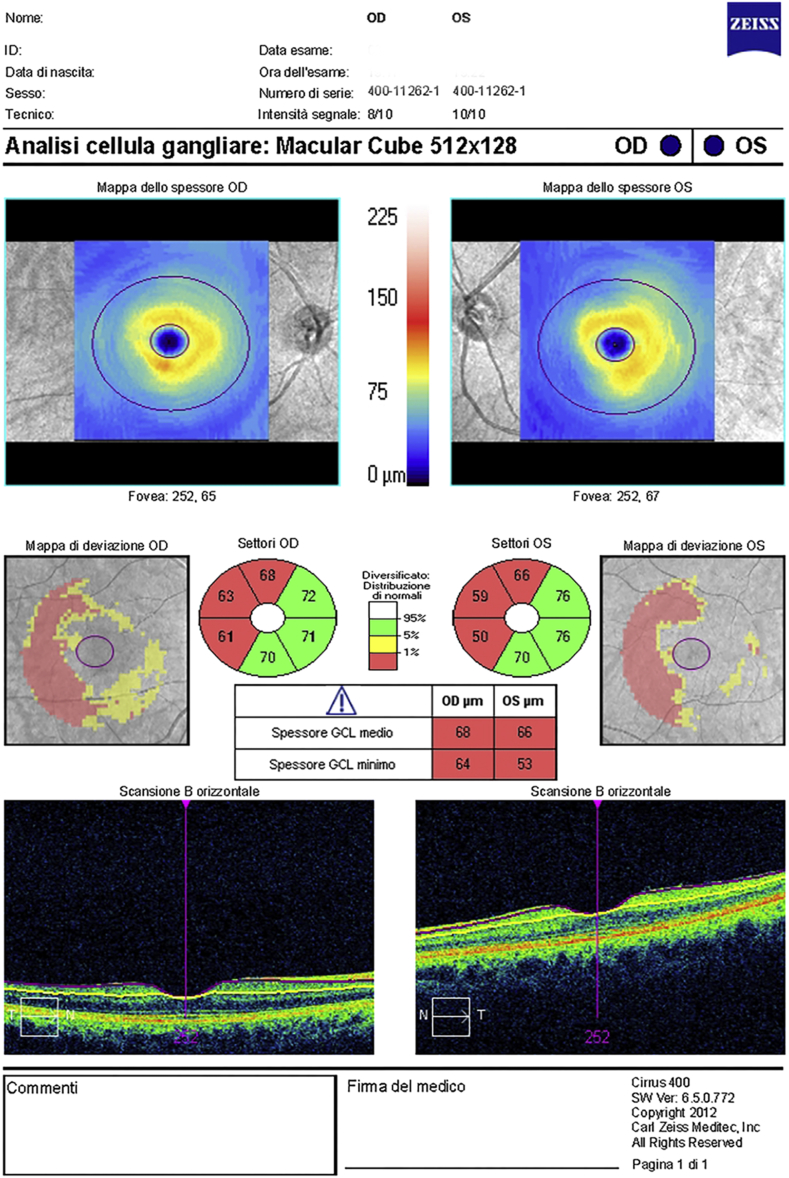
Optical coherence tomography (OCT) of the macular area and of pRNFL was obtained. The Cirrus HD-OCT model 400 (Carl Zeiss Meditec, Dublin, CA, software version 6.5.0.772) instrument was used to examine optic disc (Optic Disc Cube 200 × 200 protocol) and the macula (Macular Cube 512 × 128 protocol) scans to obtain GCC and pRNFL measurements. The spectral domain OCT (SDOCT) imaging technology is characterized by a scan rate of 27,000 A-scans/second, an A-scan depth of 2.0 mm (in tissue) an axial resolution of 5 μm (in tissue) and a transverse resolution of 15 μm (in tissue). The Optic Disc Cube protocol included the pRNFL, and the Macular Cube protocol included the ganglion cell analysis (GCA). The OCT examination revealed altered pRNFL thickness in the superior and inferior sectors of the RE. In the LE pRNFL thickness resulted altered in the superior and borderline in the nasal and inferior sectors. pRNFL thickness symmetry was 39% (Fig. 2). The GCA reported a significant GCC thinning in both eyes, which suggested the presence of a hemianopia (Fig. 3).
Fig. 2.
Retinal Nerve Fiber Layer Thickness Analysis report.
Fig. 3.
Ganglion Cells Analysis report.
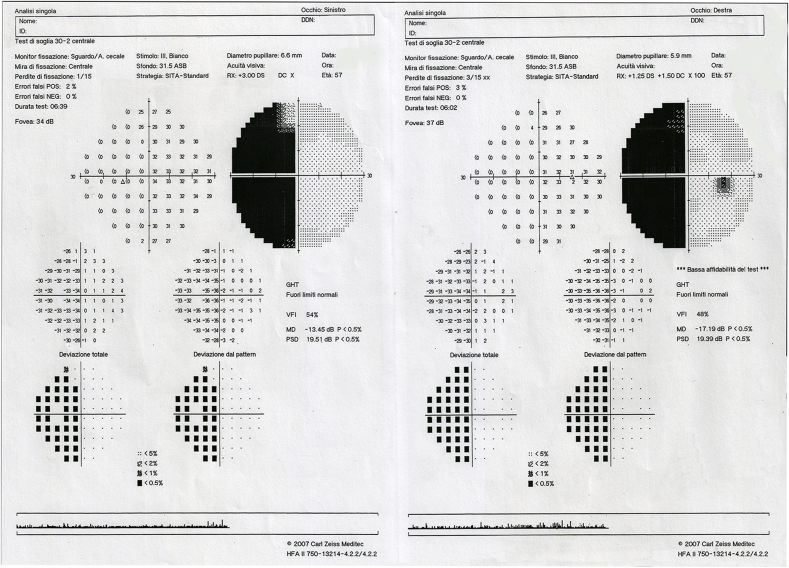
After the exam, the OCT results were reported to the patient explaining that they were compatible with the presence of hemianopic visual field (VF) alterations. At this point emerged that the patient previously had a surgical excision of an arteriovenous malformation in the right cerebral occipital lobe and that, after surgery, visual symptoms compatible with left homonymous hemianopia started. The homonymous hemianopia was subsequently confirmed performing a VF test using the central 30-2 Swedish Interactive Threshold Algorithm (SITA) program with appropriate trial lenses (Humphrey Field Analyzer II, Carl Zeiss Meditech, Inc, Dublin, California). (Fig. 4).
Fig. 4.
Humphrey visual field results of the right eye (destra) and left eye (sinistra).
Interestingly, the areas of OCT thinning corresponded to the areas of deficit on VF.
However, given the longstanding nature of the disease, patient's visual symptoms of transient blurred vision would not be expected to be due to her homonymous field defect. Conceivably, as the symptoms improved with hydration and specific vitamin/mineral supplements, they were correlated to the posterior vitreous detachment.
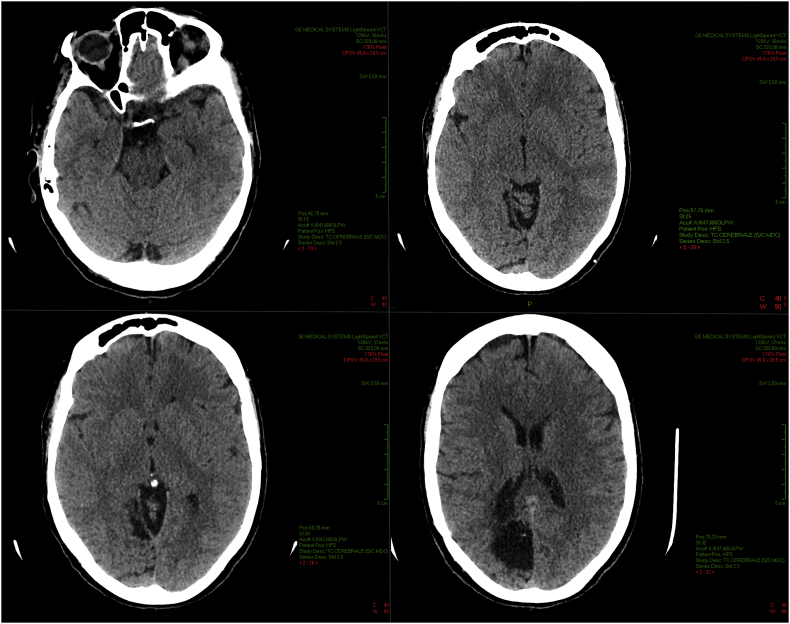
To confirm positioning of the surgical excision or possible recurrence of the arteriovenous malformation, and due to the presence of surgical sutures made of unknown metallic materials at the bone level, a CT scan of the brain was performed (Fig. 5).
Fig. 5.
Brain imaging scans showing excision positioning at different levels.
3. Discussion
OCT has become one of the most important instruments in ophthalmic practice. The SDOCT technology offers high quality images yielding reproducible and reliable measurements of the pRNFL and macular GCC thickness.2 Therefore, GCC examination has become one of the most interesting tools from an ophthalmic, but also a neuro-ophthalmic, point of view.2
Retrograde trans neuronal degeneration of RGCs subsequent to destruction of the striate cortex, V1, has been proven in monkeys and recently in humans.1, 2, 3
OCT allows to evaluate the integrity of the afferent visual pathway and, compared with perimetry, is faster, more reproducible, precise and less dependent on patient responses.1 Sometimes, in some patients with brain damage, assessment of the VF may be difficult, or test results may be unreliable or too subtle.4 In these cases, evaluation of the pattern of RGC loss using GCC or pRNFL may be a useful diagnostic approach. In this regard, macular GCC thickness measurements have been reported to provide more valuable information than pRNFL thickness for detecting the loss of RGCs in patients with retrograde degeneration of the optic nerve fibers,5 and in patients with homonymous hemianopia due to posterior cerebral artery stroke.6 Nevertheless, there are few studies correlating macular GCC thickness with VF defects induced by cortical lesions.
In this case report, the effect of iatrogenic brain damage on RGCs by trans-synaptic retrograde degeneration (TRD) is documented using the GCA, which is an indirect measure of both the GCL and the IPL of the retina and is a more specific measure of inner retinal thinning. Interestingly, GCA showed significantly reduced GCC thickness in the affected hemiretinas as compared with the unaffected ones. This supports the theory that retrograde neuronal degeneration can occur in the visual pathway and maintains the topographic distribution of the GCC as projected to the visual cortex.7, 8, 9 TRD of GCL has been proposed as one of the mechanisms contributing to permanent disability after visual pathway damage.10, 11, 12 Increasing evidence suggested retinal damage following TRD.13 Permanent VF impairment has been shown to correlate with atrophy of the GCL in glaucoma, chiasm tumours, posterior visual pathway lesions, and, reasonably, in traumatic brain injury (TBI). However, only few studies reported features of TRD following post-surgical or post-TBI.8,10,13, 14, 15 TBI is among the most common causes of death and disability in young people. In addition to the acute risk of morbidity, TBI is associated with a number of chronic neurological and neuropsychiatric sequelae including neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease.16 Due to capacity of the initial traumatic event to induce biochemical changes in nervous tissue, secondary injury in TBI typically occurs following the traumatic event.16,17 Interestingly, some of these characteristic changes, such as oxidative stress and excitotoxicity, have also been observed in the pathophysiology of neurodegenerative diseases. Thus, suggests a potential etiopathological link between TBI and neurodegenerative diseases.16, 17, 18 Due to the high prevalence of TBI and neurodegenerative diseases, the development of new strategies for early diagnosis is imperative. In this regard, it is possible to speculate that degenerative changes, such as those observed in the patient with a surgical resection, may be extendable to acquired degenerative disorders of the brain. Consequently, studying patterns of ganglion cell layer-inner plexiform layer change might be an easy to use potential tool that may help us understand or detect manifestations of these conditions.
4. Conclusions
This case supports the hypothesis that RGCs TRD may occur after brain lesions inducing GCC thinning and consequent functional alterations. Therefore, it supports the use of GCC thickness, in addition to VF, to evaluate TRD in the visual pathway after brain injuries, or before and after a neuro surgical procedure. Moreover, due to potential pathological mechanistic link between TBI and neurodegenerative diseases it is possible to speculate that GCC evaluation might also help us to understand or detect manifestations of neurodegenerative disease. However, studies on larger samples and prospective design are required to confirm usefulness of GCC thickness evaluation of RGCs degenerations in these fields of application.
Patient consent
Patient gave written informed consent.
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures: AM, MC, CN, RM.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2018.07.009.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cowey A., Alexander I., Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain. 2011 Jul;134(Pt 7):2149–2157. doi: 10.1093/brain/awr125. [DOI] [PubMed] [Google Scholar]
- 2.Rebolleda G., Diez-Alvarez L., Casado A. OCT: new perspectives in neuro-ophthalmology. Saudi J Ophthalmol. 2015 Jan-Mar;29(1):9–25. doi: 10.1016/j.sjopt.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinkin M. Trans-synaptic retrograde degeneration in the human visual system: slow, silent, and real. Curr Neurol Neurosci Rep. 2017 Feb;17(2):16. doi: 10.1007/s11910-017-0725-2. [DOI] [PubMed] [Google Scholar]
- 4.Shon K., Sung K.R. Assessment of macular ganglion cell loss patterns in neurologic lesions that mimic glaucoma. Kor J Ophthalmol. 2014 Aug;28(4):314–322. doi: 10.3341/kjo.2014.28.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin H.Y., Park H.Y., Choi J.A., Park C.K. Macular ganglion cell-inner plexiform layer thinning in patients with visual field defect that respects the vertical meridian. Graefes Arch Clin Exp Ophthalmol. 2014 Sep;252(9):1501–1507. doi: 10.1007/s00417-014-2706-3. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita T., Miki A., Goto K. Retinal ganglion cell atrophy in homonymous hemianopia due to acquired occipital lesions observed using Cirrus high-definition-OCT. J Ophthalmol. 2016;2016:2394957. doi: 10.1155/2016/2394957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herro Angela M., Lam Byron L. Retrograde degeneration of retinal ganglion cells in homonymous hemianopsia. Clin Ophthalmol. 2015;9:1057–1064. doi: 10.2147/OPTH.S81749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller J., Sánchez-Dalmau B.F., Villoslada P. Lesions in the posterior visual pathway promote trans-synaptic degeneration of retinal ganglion cells. PLoS One. 2014 May;9(5) doi: 10.1371/journal.pone.0097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jindahra P., Petrie A., Plant G.T. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012 Feb;135(Pt 2):534–541. doi: 10.1093/brain/awr324. [DOI] [PubMed] [Google Scholar]
- 10.Keller J., Sánchez-Dalmau B.F., Villoslada P. Lesions in the posterior visual pathway promote trans-synaptic degeneration of retinal ganglion loss. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nucci C., Martucci A., Cesareo M. Brain involvement in glaucoma: advanced neuroimaging for understanding and monitoring a new target for therapy. Curr Opin Pharmacol. 2013 Feb;13(1):128–133. doi: 10.1016/j.coph.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Nucci C., Martucci A., Cesareo M. Links among glaucoma, neurodegenerative, and vascular diseases of the central nervous system. Prog Brain Res. 2015;221:49–65. doi: 10.1016/bs.pbr.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Mancino R., Cesareo M., Martucci A. Neurodegenerative process linking the eye and the brain. Curr Med Chem. 2018 Mar 6 doi: 10.2174/0929867325666180307114332. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Cesareo M., Martucci A., Ciuffoletti E. Association between Alzheimer's disease and glaucoma: a study based on heidelberg retinal tomography and frequency doubling technology perimetry. Front Neurosci. 2015;9:479. doi: 10.3389/fnins.2015.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancino R., Martucci A., Cesareo M. Glaucoma and alzheimer disease: a single age-related neurodegenerative disease of the brain. Curr Neuropharmacol. 2017 Dec 6 doi: 10.2174/1570159X16666171206144045. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Haces M., Tang J., Acosta G., Fernandez J., Shi R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl Neurodegener. 2017;6:20. doi: 10.1186/s40035-017-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeKosky S.T., Asken B.M. Injury cascades in TBI-related neurodegeneration. Brain Inj. 2017;31(9):1177–1182. doi: 10.1080/02699052.2017.1312528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esopenko C., Levine B. Aging, neurodegenerative disease, and traumatic brain injury: the role of neuroimaging. J Neurotrauma. 2015 Feb;32(4):209–220. doi: 10.1089/neu.2014.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.