Abstract
We developed an fNIRS system for monitoring macaque cerebral motor activity during voluntary movements without head fixation. fNIRS data at 27 channels in 7.5 mm spatial interval were calibrated by simulating light propagation through the macaque cranial tissues. The subject was instructed to repeatedly (75 times) retrieve a food pellet with alternating left or right hands from a food well for each session. We detected significant increases in oxygenated hemoglobin (Hb) and decrease in deoxygenated Hb in the primary motor area (M1) contralateral to the hand used. In more rostral and ventral regions in both hemispheres, the hemodynamic similarly changed regardless of used hand. Direct feeding to the mouth eliminated activity in the hand M1 whereas that at bilateral ventral regions (mouth M1 area) remained. Statistical analyses for the hemodynamics between left/right-hand use revealed the location of each hand M1 in either hemisphere. In these regions, the maximum amplitude and time of the maximum amplitude in the hemodynamic response evoked by food retrieval were highly correlated with the time associated with food retrieval. We could assign each channel to an appropriate functional motor area, providing proof of principle for future studies involving brain damage models in freely moving macaque monkeys.
Introduction
Brain damage, such as that induced by stroke, often causes functional deficits that can recover to varying degrees thereafter. During the recovery process, neurorehabilitation training promotes neuroplastic changes that can lead to functional recovery1–3. However, although numerous clinical trials are currently focused on improving neurological outcomes, the effect of rehabilitative training is largely unclear. In these trials, it is particularly difficult to control for differences in the position, extent, and the elapsed time from injury. Animal model studies in which damage is induced in specific regions of the brain, under controlled conditions, circumvent this issue, enabling more quantitative analyses. In particular, studies of nonhuman primates have the advantage of potentially translating to human patients, because their brains, body structures, and genetic backgrounds are closely similar across primate species4–6. One particularly relevant issue following brain damage relates to hand functionality, which is essential for quality of life as hand dysfunction seriously impairs the ability to perform day-to-day tasks. In some nonhuman primate species, including macaque monkeys, dexterous hand function is as highly developed as in humans7–11, making them an ideal model for the investigation of hand movement following brain damage.
We previously reported that macaque monkeys with induced primary motor area (M1) lesions exhibited recovery of dexterous hand movements after intensive rehabilitative training12. The ability to monitor brain activity is essential to understanding the mechanisms that underlie rehabilitative training-induced functional recovery. We imaged the brains of awake monkeys using H215O positron emission tomography (PET) scans during hand movements and showed that activity in the ventral premotor cortex increased during the recovery period, suggesting that this cortical area contributes to the recovery of motor function after an M1 lesion13. However, PET requires the head to be fixed during scanning, inhibiting natural movement and potentially affecting the results. Moreover, the need to use a radioactive tracer impedes frequent monitoring using PET.
Functional near-infrared spectroscopy (fNIRS) measures the hemodynamic response evoked by functional cerebral activation. Because light travel is limited through tissue, optimization of the location of the source–detector optode pairs allows for high spatial selectivity of fNIRS14. A multichannel array with an appropriate channel interval (15 mm) enables sufficient spatial resolution to discriminate human M1 from other motor-related areas15,16. To adapt this design for a macaque brain requires proper accounting of the smaller size of its head and brain.
Signal contamination of fNIRS measurements is a key concern for accurate quantitative monitoring of the hemodynamic response. In general, fNIRS signal contaminants include experimental errors and systemic physiological fluctuations that can occur in excess of instrumental noise17. Experimental error is typically caused by optode movement due to insufficient fixation. Optodes are generally held to the subject’s head using some fixed holder, and if this holder is unstable, a subject’s movements can alter the optode-scalp gap distance causing artifacts in fNIRS signals. These artifacts exhibit slower baseline fluctuation including drift and level shift after spiky peak18,19. During the study of awake monkeys, subjects may move their heads and/or change postures in association with task execution. Also, as the scalp is intrinsically stretchable and can shift, optode fixation is invariably unstable. In total, movement-induced optode artifacts can cause severe distortions of the shape of hemodynamic responses. Furthermore, blood flow changes in the scalp tissue are a major source of systemic physiological fluctuation-induced fNIRS artifacts20–22. This is because blood flow fluctuates with changes in respiration, blood pressure, posture, and vasomotor reactions in association with certain psychophysiological responses.
In the present study, we developed a new fNIRS system for monitoring macaque cerebral motor activity during voluntary movements without head fixation. We applied our previously developed method for calculating the light propagation in optical models14 to optimize the optodes’ distance and arrangement in the macaque motor-related cortical region. We used the most direct way to address both the need for absolute spatial fixation of optode placement as well as the elimination of systemic physiological fluctuations by directly affixing optodes to the skull surface. On the basis of the spatial and temporal differences in the hemodynamic responses obtained, we were able to assign each measurement channel to an appropriate functional motor area, providing a proof of principle for future studies involving brain damage models in freely moving macaque monkeys. The study was comprised of three parts. First, anatomical data of three macaque monkeys were subjected for optimization of the fNIRS measurement condition. Second, for a subject in the three monkeys, fNIRS optodes were arranged on the skull surface and the fNIRS signal sensitivities was calculated. And third, the fNIRS measurement during behavior experiments was conducted and the data were analyzed. Overview of this study was shown in Supplementary Fig. S1.
Methods
Animal Care and Use Committee approval
The protocol of the present study was approved by the Institutional Animal Care and Use Committee of the National Institute of Advanced Industrial Science and Technology, Japan, and was carried out in accordance with the guidelines within the “Guide for the Care and Use of Laboratory animals” (Eighth ed., National Academy of Sciences).
Optimization of fNIRS optode distance in monkey’s motor activity measurement
The fNIRS optode arrangement was customized for the present monkey study. Because the superficial layers such as the scalp and skull of the monkey head are thinner and the brain is smaller than those of humans, the optimal source–detector distance for measuring fNIRS signals from the monkey brain was presupposed to be smaller. We estimated the source–detector distance optimal for monkey brains through a calculation simulating light propagation in an optical model of the monkey head.
For this simulation, T1 and T2 weighted magnetic resonance (MR) head images of three macaque monkeys including the trained subject were used and optical models of these heads were constructed. Based on simulations over the three individual models, universality of the calculation results were examined. Each model consisted of six optical layers: air, skull, cerebrospinal fluid, gray matter, white matter, and other soft tissues. T1 and T2 weighted MR images present sufficient contrasts among these layers to identify each23. In the MR image of the trained (scalp-incised) subject after forming optode sockets, the skull surface was not clear because the skull tissue and the socket material (acryl resin) contain few protons and thus have little T1 resonance. In this case, the skull surface was presumed to be identical to the boundary between the scalp and skull in the image before forming the sockets (i.e., the image of the intact subject’s head). The position of this boundary in the post-implantation image was identified by matching the before and after brain tissue boundaries with each other. Overlapping was executed through a rigid body transformation (FSL 5.0, Oxford, UK). The boundaries of other tissues and the optode positions were directly identified using the image after forming the sockets. In cases of other two monkeys, six-layered optical models including soft tissue layer were firstly constructed based on MR images of intact heads. Thereafter, for uniforming layered structures in these models with the scalp-incised model, the superficial soft tissue layer of parietal region was replaced by air layer. The coefficients of absorption and reduced scattering in each tissue at 800 nm wavelength were taken from literature24–27. A refractive index of n = 1.40 was used for all tissue layers. Light propagation in the models was calculated using the diffusion equation. The finite element method was conducted to solve the diffusion equation28. In the MR images of each monkey, the center position of the hand knob, known as the hand motor center in the M1 area was identified manually by a researcher well-versed in monkey brain anatomy. The position of the hand knob in the optical model was projected onto the scalp surface. Source–detector pairs separated by 5 to 25 mm (in 5 mm steps) were introduced to the model so that the midpoint of each source–detector pair aligned with the projected point (i.e., the hand knob). Optode pairs aligned both parallel and orthogonal to the central sulcus adjacent to the hand knob were examined. In each condition, the detected light intensity, the mean optical path length29, and spatial sensitivity profile (SSP)30 were calculated. The SSP over all the voxels in the model was obtained through the calculation of the photon measurement density function in each voxel and was normalized by mean optical path length. The photon measurement density function provides the probability of light transit through the voxel in a given source–detector condition and was calculated on the basis of the reciprocity principle14,31. Because the detected light travels through each voxel with different sensitivity, the sum of the SSP of voxels within a certain layer is equivalent to the partial optical path length in that layer. For example, in this calculation, the partial optical path length in the gray matter (Lgray) was calculated as the sum of SSP in the corresponding gray matter. To evaluate the effective signal sensitivity for cerebral motor activity, a regional Lgray in a cubic volume of 5.4 × 5.4 × 5.4 mm3 at the hand knob was also calculated. Further details in the simulation calculation are described elsewhere32.
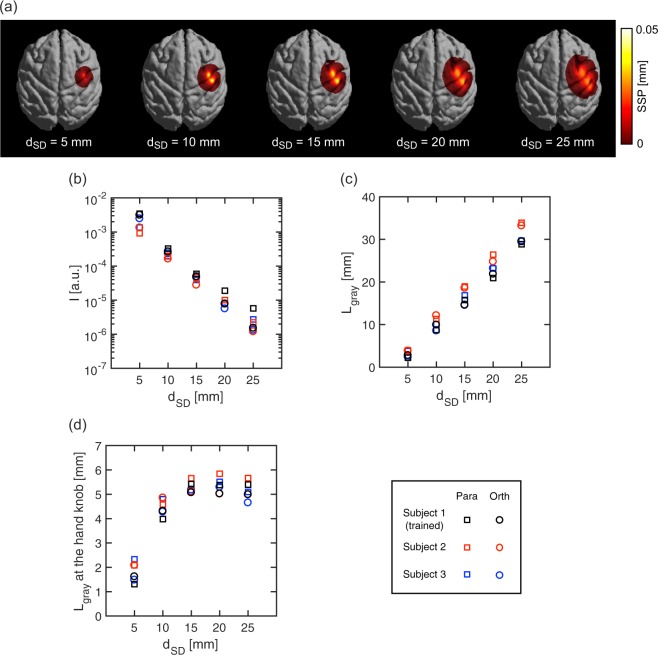
Results of the calculation are shown in Fig. 1. The SSP on the gray matter surface became broader as the source–detector distance (dSD) increased (Fig. 1a), which indicated that shorter distance provided higher spatial resolution but lower detection sensitivity. Similar dependencies of SSP on dSD were observed in all subjects and parallel and orthogonal optode alignment conditions. The predicted detected intensity (I) monotonically decreased with increase in dSD (Fig. 1b) and Lgray monotonically increased (Fig. 1c). However, the regional Lgray at hand knob asymptotically approached to a finite length (Fig. 1d). The predicted values in each calculation were very similar among the cases of subjects and optode alignment conditions. In Fig. 1d, the regional Lgray showed little increase in the range of dSD longer than 15 mm. This indicates that the signal sensitivity for the gray matter in the hand knob region is not gained by increasing dSD more than 15 mm. On the other hand, the Lgray in all over the inter-optode region further increased in this range (Fig. 1c). This indicates the increase in signal contribution from regions other than hand knob, namely, the degradation in spatial resolution of the signal detection in the range more than 20 mm. By taking consideration of these results, we concluded that 15 mm is most appropriate for dSD to detect activity changes in the macaque motor cortex, in which motor representations of the face, hand and arm are located with difference of several millimeters in M133.
Figure 1.
Simulation calculations of light propagation through head tissues of monkey head models (a) Spatial sensitivity profile (SSP) of a macaque monkey (Subject 1) in orthogonal optode alignment for source–detector distances, dSD, of 5, 10, 15, 20, and 25 mm. (b) Detected intensity, I, versus dSD. (c) Partial optical path length in the gray matter layer, Lgray versus dSD. (d) Regional Lgray at the hand knob versus dSD. Optode pairs aligned both parallel (Para) and orthogonal (Orth) to the central sulcus adjacent to the hand knob were examined. Subject 1 is the trained monkey to be measured with fNIRS.
Surgical procedures
One healthy adult Japanese macaque monkey (female; 5.0 kg) without any history of experimentation was used. The positions of M1 and the premotor area (PMA) were determined using stereotaxic coordinates from MR images of the monkey’s brain using a 3.0 T MR imaging (MRI) system (Philips Ingenia 3.0 T, Philips Healthcare, Best, The Netherlands). The anatomical MRI protocols consisted of a T1-weighted turbo field echo sequence (repetition time/echo time, 7.3/3.2 ms; number of excitations, 2; flip angle, 8°; field of view, 134 × 134 mm; matrix, 224 × 224; slice thickness, 0.6 mm; number of slices, 200). Pentobarbital anesthesia was administered at 20 mg/kg, after which the parietal region of scalp was incised and optode sockets were formed on the skull surface with self-curing acrylic resin (UNIFAST II Clear, GC Corporation, Tokyo, Japan). Titanium oxide (KA-30, Titan Kogyo, Ltd., Ube, Japan) was mixed into the resin at a weight ratio of 1:450 to match its optical scattering property to that of the skull. These procedures were done under sterile conditions.
Optode arrangement at parietal region of the monkey head
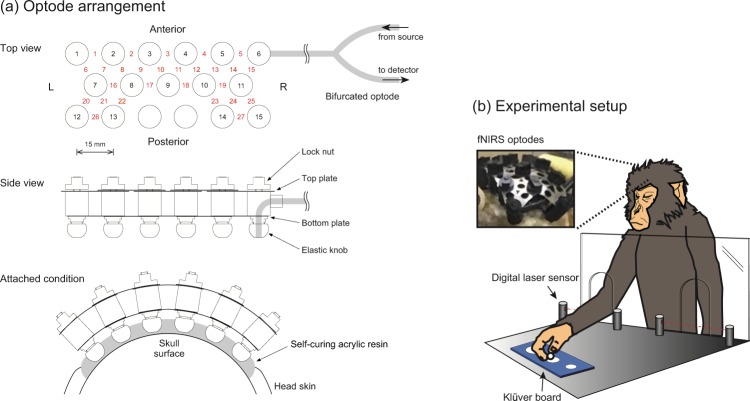
For discriminating the motor representation in M1 with fNIRS signals, the spatial interval between channels in fNIRS should be several millimeters to discriminate the hemodynamic responses at different gyri, whereas the source–detector distance of 15 mm is required for appropriately detecting gray matter responses. To satisfy both conditions, optodes (about 10 mm in diameter) placed conventionally would have to be very densely packed. We therefore adopted a triangular bidirectional optode arrangement, in which the optodes are placed at regular triangle lattice points, and each is used as a source and a detector through temporal switching16. The schematic illustration of the arrangement is shown in Fig. 2a (Top view). As determined above, the source–detector distance was fixed to be 15 mm; thus, the spatial interval among adjacent channels was 7.5 mm. Each optode has bifurcated ends connected to a source or a detector in the fNIRS OMM-3000 system (Shimadzu Corporation, Japan). The bifurcated optodes were custom made of optical fiber bundles (Moritex Corporation, Japan). Using ternary optodes as one source and two detectors, signals from two channels are collected simultaneously, and by completing illumination at all optodes, two measurements for every channel are accomplished.
Figure 2.
(a) Schematic illustration of the triangular bidirectional optode arrangement and the holding system. In the top view, open circles with black-colored numbers indicate optode positions. Red-colored numbers between optodes indicate channel positions. L, and R represent left side and right side of optode arrangements on the subject’s head, respectively. Adjacent optodes were cross-linked by two-layered linkage plates. The lower plates hold the distances between optodes. The upper plates were equipped with elongated holes and nuts. After the optode tips of globular shape were inset into the sockets on the acrylic resin, the optode arrangement was consolidated by locking nuts. (b) Experimental setup for the macaque’s food retrieval task. The macaque monkey retrieved spherical food pellets (5 mm in diameter) from cylindrical wells (20, 11, and 10 mm in diameter) on a Klüver board. A digital laser sensor was installed in front of the Klüver board to detect the initiation and duration of the reach-to-grasp movement.
As shown in Fig. 2a (Side view), fifteen optodes held with a custom holder were fixed into the previously implanted socket wells on the skull surface just before every experimental session. There, adjacent optodes were cross-linked by two-layered linkage plates. The lower plates hold the distances between optodes as 15 mm. The upper plates were equipped with elongated holes and nuts. After the optode tips of globular shape were inset into the sockets, the optode arrangement was consolidated with locking nuts. Switching between illumination and detection in each optode was controlled through software built into the OMM-3000. Twenty-seven channels were available for measurement in the parietal region of the monkey head. Optical absorbance data at wavelengths of 780, 805, and 830 nm for each channel and the digital signal from the Digital Laser Sensor were recorded by the OMM-3000.
We then calculated light propagation in the subject’s head at wavelengths of 780, 805, and 830 nm for this optode arrangement. For constructing an optical model including the optodes’ precise positions, T1 weighted MR images of the subject both before and after implantation of the optode sockets were obtained. After, images were obtained by filling the socket wells with an MR marker (Cu-sulfate solution). On the basis of this optical model, SSP and the optical partial path length at each wavelength at each channel were calculated in the same way as described above.
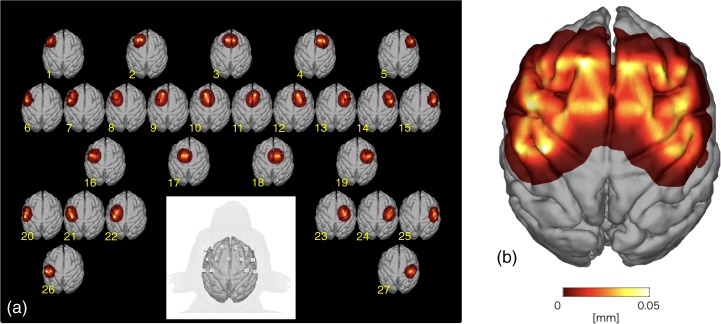
Figure 3a shows the image of subject’s brain with optodes (lower center) and SSPs at the wavelength of 805 nm at 27 channels. In general, the SSPs of adjacent channels overlapped indicating that the optode arrangement offers nearly complete coverage of the underlying gray matter layer. The maximum value of SSPs from all of the channels was calculated (Fig. 3b), allowing for demarcation of areas of higher and lower sensitivities. For appropriately comparing hemodynamic responses among the channels, the fNIRS signals were calibrated using the estimated optical partial path length at each wavelength at each channel.
Figure 3.
Spatial sensitivity profile (SSP) in the subject monkey. (a) SSP of the measurement channels in the subject monkey. (b) The maximum value of each simulated voxel over the SSPs of all channels.
One hundred thirty milliseconds were required to complete illumination at all optodes while recording two measurements for every channel. We used the average of the two measured absorbance data as one sample in a temporal resolution of 130 ms. These absorbance data were calibrated using the optical partial path length estimated through the simulation described above. Baseline drifts in the calibrated absorbance data were removed by a third polynomial fitting. High-frequency noise was filtered using a fourth Butterworth-type filter at 0.7 Hz.
The concentration changes in oxygenated and deoxygenated Hb, ΔHbO and ΔHbR, respectively, were transformed from the calibrated absorbance data at each measurement wavelength on the basis of the modified Beer–Lambert law. The molar absorption coefficients of each Hb species in the translation matrix were taken from a literature34.
Event-related experiment of food retrieval task
The monkey was trained a small-object retrieval task closely resembling that used in our previous studies12,35. In this task, the monkey sat in the primate chair and retrieved a small spherical food pellet (5 mm in diameter) from the Klüver board, which contained cylindrical wells of three different sizes (10, 11, and 20 mm in diameter, 5 mm in depth). The Klüver board was located at the monkey’s waist height and at a sagittal distance of about 300 mm from the monkey’s shoulder. The size of the well was fixed within a daily session, and the monkey retrieved food pellets approximately every 20 s using each hand alternately. One hundred fifty trials (75 for each hand) were performed in each daily session. To observe behavioral changes during the learning of food retrieval, two separate series of food retrieval sessions were executed. In both the session series, a food well of 11 mm in diameter was used for the initial session. Food wells of 20 and 10 mm were separately used for three additional sessions in each session series. Each session series was completed within a week. More than several weeks were left between the two session series.
Two additional sessions were also performed: (1) food retrieval only by left hand in 75 trials in which the subject’s right forelimb was constrained, (2) direct feeding to the mouth by experimenter in 75 trials with both forelimbs constrained. Cross-validation of potential contaminants in the fNIRS signals was conducted more than several weeks before of the start of the testing sessions and was performed using left and right hands alternatively.
The onset and end time points of food retrieval movement by either the left or right hand were detected by a Digital Laser Sensor (LV-11SB with sensor head LV-S72, Keyence, Osaka, Japan) installed in slits within the acrylic resin board located between the primate chair and the Klüver board (Fig. 2b). These retrieval event data were obtained concurrently with the fNIRS signals and were used for event-related signal analyses.
Statistical analysis
As described in the previous section, the onset and end time points of food retrieval movement by the left or right hand were monitored with the Digital Laser Sensor. The signal from the Digital Laser Sensor was received and recorded with the OMM-3000 concurrently with the fNIRS absorbance data. On the basis of this retrieval event data, the times of onset and duration of each retrieval event in each experimental trial were measured and used for the event-related signal analyses described below.
The durations of food retrieval events in 75 trials of each left- and right-hand use in each experimental session were sampled. For normalizing the sample distributions, the Box–Cox transformation with λ = −1 was applied for each session data. The homoscedasticity among the transformed distributions was examined with Bartlett’s test. Differences among the sample averages of different sessions and used hands were compared using analysis of variance (ANOVA) with correction for multiple comparisons using the Tukey–Kramer method.
The fNIRS data in each of the 150 trials (75 right hand, 75 left hand) was time-locked (t = 0) to the onset of food retrieval movement for that trial. Then the values of ΔHbO and ΔHbR for each time point (i.e., a 130 ms period, starting from t = 0 for each trial) were averaged and plotted in Fig. 4 (and in Fig. 5 for additional tasks). For each time point, a paired t-test between the left-hand trials and right-hand trials was also conducted. Figure 6 plots the t-score values for the contrast of left versus right hand movement, for both ΔHbO and ΔHbR, at each time point in each of the eight sessions. When significant amplitude (p < 0.05) in the t-value curves was detected, the maximum amplitude in the ΔHbO and ΔHbR curves and the time elapsed from onset to maximum amplitude was calculated. These calculations at each channel were performed for each experimental session. Maximum amplitude and elapsed time in each session were averaged between channels for each brain hemisphere separately and the Pearson’s product-moment correlation coefficient of them against the time required for food retrieval were calculated.
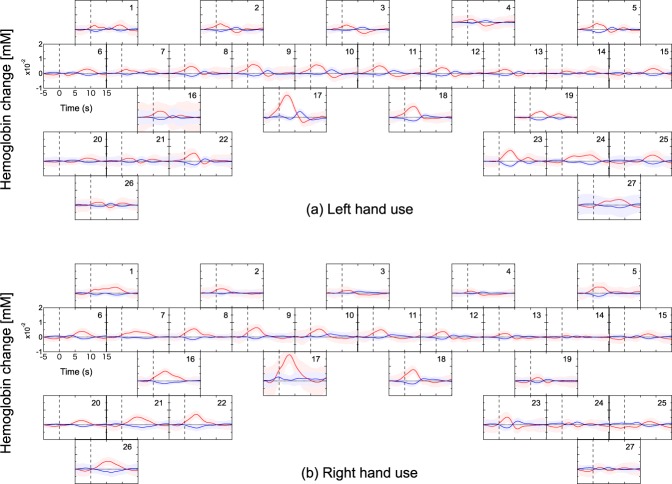
Figure 4.
Representative hemodynamic response during a food retrieval experimental session. (a) Left-hand use and (b) right-hand use. Each frame depicts the block averages and the standard deviations of each channel among for ΔHbO and ΔHbR drawn with red and blue lines and bands, respectively.
Figure 5.
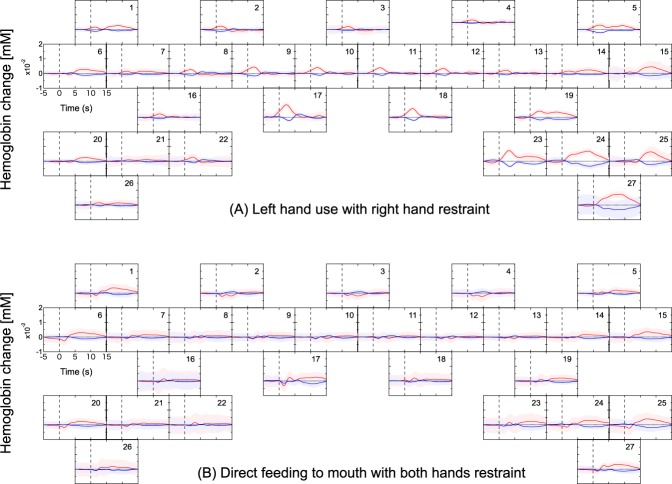
Hemodynamic responses evoked by different restrained conditions. (A) Left hand food retrieval with the right forelimb constrained and (B) direct feeding to mouth with both hands restrained. Each frame depicts the block averages and the standard deviations of each channel among for ΔHbO and ΔHbR drawn with red and blue lines and bands, respectively.
Figure 6.
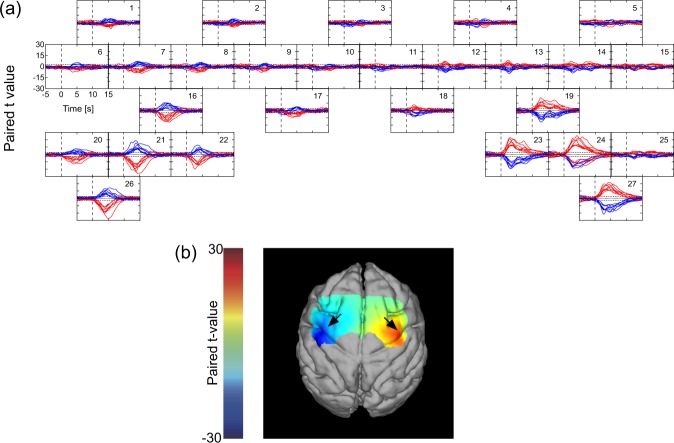
(a) Time course curves of paired t-values of hemodynamic response. The t-values for the paired t-test were calculated from the difference in hemodynamics during trials of left-hand and right-hand use. The t-values of ΔHbO and ΔHbR for each of the eight sessions were drawn with red and blue lines, respectively. The dotted lines in each frame indicate levels of p = 0.05. (b) Color-mapping of t-values onto the subject’s anatomical MRI. The t-values for ΔHbO of the first session using a food well of 20 mm 5 s after task onset are depicted. Arrows indicate the positions of “hand knob” in each hemisphere.
Results
Hemodynamic response evoked by task execution
The representative block averages of ΔHbO and ΔHbR evoked by food retrieval with left-hand and right-hand uses are shown in Fig. 4. In general, the ΔHbO and ΔHbR exhibited opposite directional changes (i.e., an increase in ΔHbO was accompanied with a decrease in ΔHbR, and vice versa). Larger ΔHbO and ΔHbR were recorded from channels in hemispheres contralateral to the hand being used. For example, channels 16, 21, 22, and 26 placed in the left hemisphere recorded the largest changes when the right hand was used. Similarly, channels 19, 23, 24, and 27 located in the right hemisphere indicated markedly larger amplitudes when the left hand was used. In the other channels, similar left/right ΔHbO and ΔHbR amplitudes were observed regardless of which hand was being used.
To further clarify the channels that indicated changes in ΔHbO and ΔHbR that were either dependent or independent of the hand used, additional retrieval tasks were performed in which the right forelimb was restrained during left-handed food retrieval (Fig. 5A). Interestingly, left hand use with right hand restraint caused changes in ΔHbO and ΔHbR to entirely disappear from left hemisphere channels that had previously indicated used-hand-dependent signals, whereas those at the channels of symmetrical positions in right hemisphere remained unchanged versus unrestrained. Channels that indicated used-hand-independent signals, however, exhibited similar ΔHbO and ΔHbR levels to those observed in the corresponding channels during the unrestrained sessions.
To closely examine used-hand-independent signals, a further task was conducted where both forelimbs were constrained and food pellets were fed directly to the subject’s mouth by an experimenter. Feeding via the mouth blunted the appearance of the changes observed in the above identified used-hand-dependent regions as well as rostral regions of both sides (Fig. 5B vs. Figs 4 and 5A). Interestingly, however, the most ventral regions of both hemispheres exhibited changes in ΔHbO and ΔHbR that were similar to those in which a hand was used (Figs 4 and 5A).
Characteristic hemodynamic responses in the motor-related cortex
To statistically evaluate the difference in Hb changes between left- and right-hand uses, paired t-tests were conducted for each Hb species for each channel in each session. Time course curves of t-values for ΔHbO and ΔHbR at each channel are shown in Fig. 6a. Channels that recorded statistically significant amplitudes of t-value in ΔHbO and ΔHbR (p < 0.05) were highly localized to the caudal region of both hemispheres. During right-handed movement, channels in the left hemisphere (Ch 16, 21, 22, and 26) recorded t-values for ΔHbO and ΔHbR that were negative and positive, respectively. Correspondingly, those channels in the right hemisphere (Ch 19, 23, 24, and 27) exhibited opposite directional features. The shape and amplitude of t-values for each Hb species were very nearly inversely correlated with respect to their localizations in the left and right hemispheres. In Fig. 6b, the t-values for ΔHbO from a first session using a 20 mm food well from each channel 5 s after task onset were color-mapped and superimposed upon the anatomical MR image. Foci indicate negative and positive t-values of the hand knobs of the left and right M1, respectively. These representative results were similar to those obtained during other sessions. From these findings, the region at Ch 16, 21, 22, and 26, and the region at Ch 19, 23, 24, and 27 were identified as the hand M1 in the left and right cerebral cortices, respectively.
The hemodynamic responses associated with task execution shown in Figs 4 and 5 generally adhered to specific reproducible patterns. In several instances, hemodynamic responses were detected before the onset of hand movement. This may be due to a time lag of the detection of forelimb by the laser sensor. In other instances, responses were observed after some latency from task onset. These responses were observed in a more ventral region than the hand M1 area (Figs 4 and 5), occurred bilaterally regardless of the side of hand use, and of note, were the only to remain when the subject’s hands were both immobilized and the subject was fed directly (Fig. 5B). One likely explanation is that food retrieval/feeding was always followed by eating, which is associated with bilateral mouth movements. Indeed, a paired t-test indicated that there were no inherent bilateral statistical differences between these regions (Fig. 6a). On the basis of these findings, the bilateral ventral regions at channels 1, 5, 6, 15, 20, and 25 were identified as the mouth M1 area, which is located ventrally to the hand M1 area33.
The channels in a more rostral region than the M1 indicated hemodynamic responses that began before the task onset. These responses were not observed when food was passively eaten after being directly fed (both hands restrained). Also, the responses were distinctively different from those observed in the hand M1 area, indicating no significant laterality associated with the used hand. It is known that bilateral PMAs are activated during forelimb movement regardless of the side of hand actually used36. From these findings, the relatively rostral dorsal region in both hemispheres at channels 8, 9, 10, 11, 12, and 13 were identified as PMA. The channels in the most medial dorsal region (channels 17 and 18) should be assigned as supplementary motor area (SMA).
To quantitatively evaluate different tendencies in block-averaged hemodynamic responses in different regions, the times of maximum amplitude in the hemodynamic responses (representatively shown in Fig. 4) were compared over different regions. The averaged times of maximum amplitude over sessions were 0.71 ± 0.47 s for ΔHbO and 0.55 ± 0.65 s for ΔHbR in PMA, 2.43 ± 0.84 s for ΔHbO and 2.48 ± 1.43 s for ΔHbR in hand M1, and 6.63 ± 0.88 s for ΔHbO and 7.05 ± 0.89 s for ΔHbR in mouth M1. ANOVA with correction for multiple comparisons using the Tukey–Kramer method showed significant differences of p < 0.01 between PMA and hand M1, hand M1 and mouth M1, and mouth M1 and PMA. Difference between ΔHbO and ΔHbR was not significant (p > 0.1). Taken together, the data indicate that using our system, hemodynamic responses characteristic of each motor-related cortex were obtained with high sensitivity and fidelity. The correspondence between the cerebral area and the measurement channels were shown in Table 1.
Table 1.
The correspondence between the cerebral area and the measurement channels.
| Left hemisphere | Right hemisphere | |
|---|---|---|
| PMA | Ch 8, 9, and 10 | Ch 11, 12, and 13 |
| SMA | Ch 17 | Ch 18 |
| Hand M1 | Ch 16, 21, 22, and 26 | Ch 19, 23, 24, and 27 |
| Mouth M1 | Ch 1, 6, and 20 | Ch 5, 15, and 25 |
Comparison of hemodynamics with behavioral measurement
The time required for food retrieval in 75 trials from each session for each hand is shown in Fig. 7a. In both session series, using food wells of 20 and 10 mm, the time required for food retrieval increased once when the size of the food well was changed from the initial size of 11 mm; thereafter, time decreased as training progressed. As expected, time of retrieval was significantly different between the right and left hand (p < 0.01).
Figure 7.
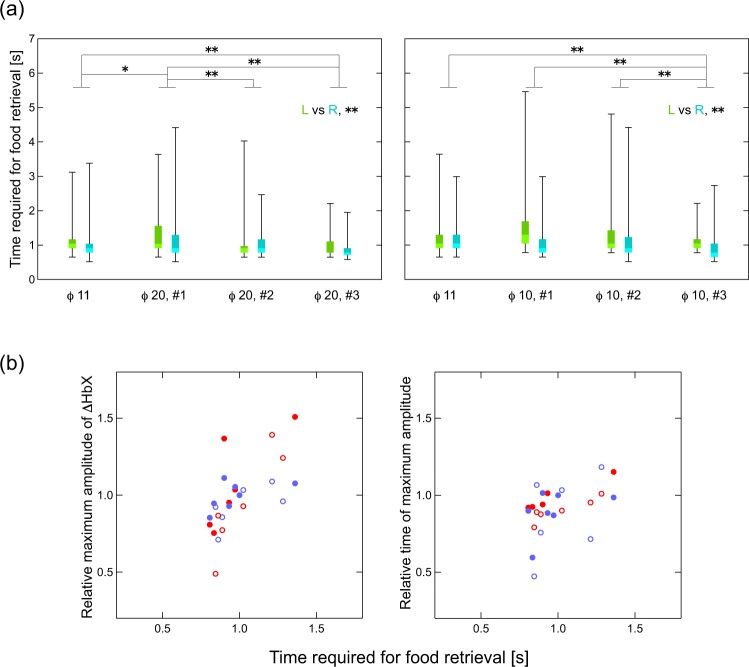
(a) Boxplots of the time required for food retrieval of 75 trials at each session. Left and right frames represent session series using food well sizes of 20 and 10 mm, respectively, each starting with a well size of 11 mm. In each session, plots of trials with left and right hands were colored with green and blue, respectively. The marks * and ** indicate statistical significance of p < 0.05 and p < 0.01, respectively. (b) Correlation between hemodynamic responses and the time required for food retrieval. Left panel, maximum amplitudes in hemodynamic response versus time required for food retrieval; right panel, time of maximum amplitude in hemodynamic response versus time required for food retrievals. Closed red circles indicate ΔHbO in the left hemisphere; closed blue circles indicate ΔHbR in the left hemisphere; open red circles indicate ΔHbO in the right hemisphere; and open blue circles indicate ΔHbR in the right hemisphere.
We hypothesized that differences in retrieval time would be associated with differential activation of the corresponding cerebral region. To examine this possibility, we focused on those channels that were previously associated with hand use (Fig. 4), namely, channels 16, 21, 22, and 26 in the left hemisphere and channels 19, 23, 24, and 27 in the right hemisphere. Maximum amplitudes (either peaks or valleys) in the time course curves of ΔHbO and ΔHbR and the times of the maximum amplitudes were calculated for each session and averaged over the channels in each hemisphere. These averages were plotted against time of food retrieval (normalized to the time of retrieval of the initial session) (Fig. 7b). Pearson’s product-moment correlation coefficients of the time for food retrieval to the maximum amplitudes and the time of maximum amplitudes were calculated for each Hb species and each hemisphere (see Supplementary Table S1). Significantly high positive correlations between the maximum amplitude and the time for retrieval were found for ΔHbO in both hemispheres.
Correlation between ΔHbO and ΔHbR in the cerebral hemodynamic response
The relationship between ΔHbO and ΔHbR in the hemodynamic response to the food retrieval task at each channel over all the sessions were plotted in Supplementary Fig. S2. Regardless of the cases in left (green dots) and right hand use (blue dots), distinctive linear relationships of negative correlations between ΔHbO and ΔHbR were observed in most channels. The Pearson’s product-moment correlation coefficients between ΔHbO and ΔHbR for left and right hand uses at each channel were calculated and denoted with green and blue numbers, respectively. All these values were statistically significant, where even the smallest value at Ch 17 for right hand use indicated a statistical significance of p < 10−19. This consecutive relationship between ΔHbO and ΔHbR over sessions indicate that a certain type of hemodynamics response reproducibly occurred in cortical regions through sessions at different dates ranged over several months.
Discussion
For many years, signal contamination has been one of the major concerns for obtaining functional hemodynamic responses using the fNIRS technique in the human cerebral cortex22,37. A finding that application of pressure on the skin between an optode pair can eliminate signals measured with a conventional fNIRS20 clearly exhibited the existence of signal contamination from blood flow changes in the scalp layer. Removal of contamination from scalp blood flow is crucial for precise fNIRS measurements, including monkey studies. Several studies on the measurement of monkey neural activity using fNIRS have been reported38–43. In these studies, optodes were fixed at the dura39,42,43, at the scalp38,41, or were adhered to the skull40. These types of optode fixations were performed before each measurement, which can lead to infections and can impair positional reproducibility. As such, these methods are problematic for long-term continuous monitoring that is required for studies on neural plasticity in cerebral cortex. In this study, we developed a quickly detachable optode system with high inter-optode density on the skull surface thereby allowing highly reproducible and less-invasive multichannel fNIRS measurement over several months within a single subject.
In both session series, the time required for food retrieval increased when changing the food well size from its initial size. This suggests that picking food from a well of a new size was initially challenging regardless of whether the well size was increased or decreased. Following this initial adjustment phase, retrieval time decreased in all subsequent sessions (Fig. 7a). Presumably, the delayed retrieval time causes alterations in functional activation. Indeed, both maximum amplitude and the time of maximum amplitude of the hemodynamic response in the hand M1 area were positively correlated with the time required for food retrieval (Fig. 7b) and exhibited similar distributions in both hemispheres and both Hb species. This finding confirms that hemodynamic responses between hemispheres and Hb species obey predicable common relationships to neuronal activation.
We also observed that the hemodynamic responses in each motor-related region highly correlated with movement of the relevant body part. Activity was initially detected in the PMA, followed by that in the relevant hand M1 and mouth M1 areas. Several methods of determining causality such as dynamic causal modeling44 and Granger causality analysis45 have been applied to functional blood-oxygen-level-dependent MR images46 and fNIRS data47,48. Application of causality analysis using our fNIRS system may be quite useful in quantitating the relationships of motor cortex activity and movement proficiency.
After the series of experiments in this study, the subject monkey received an artificial cerebral infarct in a unilateral posterior limb of internal capsule using the method we had previously reported49. The subject showed a motor deficit in the contralateral hand of the lesioned hemisphere. After the recovery from the motor deficit through rehabilitative training of about two months, the fNIRS measurement during the food retrieval task was conducted. While the time for food retrieval by the contralateral hand of the lesioned hemisphere recovered to a level of no significant difference from those in the pre-lesioned condition, a part of PMA in the lesioned hemisphere newly increased hemodynamic responses during the retrieval and the statistical interaction between the factors of pre/post-lesion and left/right-hand-use showed significant difference in this region. On the other hand, in the pre-lesion condition even when data were obtained with an interval more than 2 months, such significant difference was not found in any channel (details will be reported elsewhere). We should note that cortical activity changes during recovery from brain damage could be evaluated by using our fNIRS system, which enables long-term reproducible monitoring of functional hemodynamic responses over motor related cerebral cortices.
Because the direct fixation of fNIRS optodes to the skull surface should not be allowed for human study, certain approaches will be required for eliminating artifacts caused by optodes fluctuation. Some commercially available fNIRS systems (NIRSport, NIRx Medical Technologies, USA, etc.) are equipped with improved optode devices of relatively more stable during subject’s motion. For more strict approach, some methods for motion artifact detection17,18 and for motion artifact removal19 are available. Using these improved devices and methods, fNIRS signals containing less motion artifacts will be measured even for human subject in free moving condition.
In order to remove the fNIRS signal contaminations from systemic physiological blood fluctuations in the scalp tissue, several methods have been proposed21,50–54. Especially, the hemodynamic modality separation (HMS)21 realized a real-time removal of contaminants other than the cerebral functional signal55. Based on many previous fNIRS studies, the HMS method theoretically assumed that the changes in oxygenated and deoxygenated Hb in the cerebral functional hemodynamics negatively correlate while those in other hemodynamics positively correlate. However, hemodynamics in the cortical tissue has never directly observed in human and non-human primates. In this study, ΔHbO and ΔHbR opposed one another (Figs 4 and 5), confirming the cerebral hemodynamic response reported by various fNIRS studies using skull-exposed rodents56–59 and human subjects under passive tasks or stimulations60–65. Moreover, the consecutive linear relationships between ΔHbO and ΔHbR were reproduced over several months at almost all channels (see Supplementary Fig. S2). These results confirm the theoretical principle of the HMS method. This finding indicates that contaminants in the conventional fNIRS signal mostly originate from the scalp tissue and that the HMS method effectively removes them even in human subjects. The use of the HMS method should make precise fNIRS measurements of functional hemodynamic responses in humans possible.
Conclusions
In the present study, the hemodynamic responses in the motor-related cerebral region during food retrieval were successfully measured using an optimized fNIRS acquisition system. Because the ability to monitor brain activity was stable over days to months, it will enable future studies that monitor the neuroplastic changes that occur during brain damage recovery.
Electronic supplementary material
Acknowledgements
We are grateful to Mr. T. Takasu and Ms. A. Muramatsu for their excellent care of the animals. This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grants Numbers 16K01489 and Grant-in-Aid for Scientific Research on Innovative Areas “Understanding brain plasticity on body representations to promote their adaptive functions” (Grant Number 17H05917) from MEXT of Japan.
Author Contributions
T.Y., H.K. and N.H. designed the experiments. K.M. and N.H. obtained MRI data. H.K. performed the simulation. T.Y., J.K. and N.H. performed surgical operations, and collected behavioral and fNIRS data of the monkey. T.Y. analyzed fNIRS data. T.Y., H.K. and N.H. wrote the main manuscript text and prepared the figures and tables. All authors reviewed the manuscript.
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30416-7.
References
- 1.Bowden MG, Woodbury ML, Duncan PW. Promoting neuroplasticity and recovery after stroke. Curr. Opin. Neurol. 2013;26:37–42. doi: 10.1097/WCO.0b013e32835c5ba0. [DOI] [PubMed] [Google Scholar]
- 2.Caleo M. Rehabilitation and plasticity following stroke: Insights from rodent models. Neuroscience. 2015;311:180–194. doi: 10.1016/j.neuroscience.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Higo N. Effects of rehabilitative training on recovery of hand motor function: A review of animal studies. Neurosci. Res. 2014;78:9–15. doi: 10.1016/j.neures.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Higo N, et al. SPP1 is expressed in corticospinal neurons of the macaque sensorimotor cortex. J. Comp. Neurol. 2010;518:2633–2644. doi: 10.1002/cne.22356. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T, Murayama S, Takao M, Isa T, Higo N. Expression of secreted phosphoprotein 1 (osteopontin) in human sensorimotor cortex and spinal cord: Changes in patients with amyotrophic lateral sclerosis. Brain Res. 2017;1655:168–175. doi: 10.1016/j.brainres.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto, T. et al. Differential Expression of Secreted Phosphoprotein 1 in the Motor Cortex among Primate Species and during Postnatal Development and Functional Recovery. PLoS One8, e65701 (2013). [DOI] [PMC free article] [PubMed]
- 7.Alstermark B. Lack of Monosynaptic Corticomotoneuronal EPSPs in Rats: Disynaptic EPSPs Mediated Via Reticulospinal Neurons and Polysynaptic EPSPs Via Segmental Interneurons. J. Neurophysiol. 2004;91:1832–1839. doi: 10.1152/jn.00820.2003. [DOI] [PubMed] [Google Scholar]
- 8.Isa, T., Ohki, Y., Alstermark, B., Pettersson, L. & Sasaki, S. Direct and Indirect Cortico-Motoneuronal Pathways and Control of Hand/Arm. Physiology22, 145–152 (2007). [DOI] [PubMed]
- 9.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 10.Courtine G, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuypers HGJM. A New Look at the Organization of the Motor System. Prog. Brain Res. 1982;57:381–403. doi: 10.1016/S0079-6123(08)64138-2. [DOI] [PubMed] [Google Scholar]
- 12.Murata Y, et al. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J. Neurophysiol. 2008;99:773–786. doi: 10.1152/jn.01001.2007. [DOI] [PubMed] [Google Scholar]
- 13.Murata Y, et al. Temporal Plasticity Involved in Recovery from Manual Dexterity Deficit after Motor Cortex Lesion in Macaque Monkeys. J. Neurosci. 2015;35:84–95. doi: 10.1523/JNEUROSCI.1737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi H, Koyama T, Okada E. Effect of probe arrangement on reproducibility of images by near-infrared topography evaluated by a virtual head phantom. Appl. Opt. 2007;46:1658–1668. doi: 10.1364/AO.46.001658. [DOI] [PubMed] [Google Scholar]
- 15.Yamada, T., Umeyama, S. & Matsuda, K. Exploration of cerebral activation using hemodynamic modality separation method in high-density multichannel fNIRS. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 1791–1794 (2013). [DOI] [PubMed]
- 16.Yamada, T., Matsuda, K., Iwano, T. & Umeyama, S. Precise spatial co-registration in simultaneous fNIRS and fMRI measurements using markers coaxially fixable to the optodes. Proc. SPIE8928, 89280S (2014).
- 17.Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009;48:D280–D298. doi: 10.1364/AO.48.00D280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umeyama S, Yamada T. Detection of an unstable and/or a weak probe contact in a multichannel functional near-infrared spectroscopy measurement. J. Biomed. Opt. 2013;18:47003. doi: 10.1117/1.JBO.18.4.047003. [DOI] [PubMed] [Google Scholar]
- 19.Yamada, T., Umeyama, S. & Ohashi, M. Removal of motion artifacts originating from optode fluctuations during functional near-infrared spectroscopy measurements. Biomed. Opt. Express6, 4632–4649 (2015). [DOI] [PMC free article] [PubMed]
- 20.Takahashi T, et al. Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task. Neuroimage. 2011;57:991–1002. doi: 10.1016/j.neuroimage.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Yamada, T., Umeyama, S. & Matsuda, K. Separation of fNIRS Signals into Functional and Systemic Components Based on Differences in Hemodynamic Modalities. PLoS One7, e50271 (2012). [DOI] [PMC free article] [PubMed]
- 22.Scholkmann F, et al. NeuroImage A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage. 2014;85:6–27. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Kurihara K, Kawaguchi H, Obata T, Ito H, Okada E. Magnetic resonance imaging appropriate for construction of subject-specific head models for diffuse optical tomography. Biomed. Opt. Express. 2015;6:3197–3209. doi: 10.1364/BOE.6.003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firbank M, Hiraoka M, Essenpreis M, Delpy DT. Measurement of the optical properties of the skull in the wavelength range 650-950 nm. Phys. Med. Biol. 1993;38:503–510. doi: 10.1088/0031-9155/38/4/002. [DOI] [PubMed] [Google Scholar]
- 25.Okada E, Delpy DT. Near-infrared light propagation in an adult head model II Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl. Opt. 2003;42:2915. doi: 10.1364/AO.42.002915. [DOI] [PubMed] [Google Scholar]
- 26.Simpson, C. R., Kohl, M., Essenpreis, M. & Cope, M. Near-infrared optical properties of ex vivo human skin and subcutaneous tissues measured using the Monte Carlo inversion technique. Phys. Med. Biol. 43, 2465–2478 (1998). [DOI] [PubMed]
- 27.van der Zee P, Essenpreis M, Delpy DT. Optical properties of brain tissue. Proc. SPIE. 1993;1888:454–465. doi: 10.1117/12.154665. [DOI] [Google Scholar]
- 28.Schweiger M, Arridge SR, Hiraoka M, Delpy DT. The finite element method for the propagation of light in scattering media: boundary and source conditions. Med. Phys. 1995;22:1779–1792. doi: 10.1118/1.597634. [DOI] [PubMed] [Google Scholar]
- 29.Arridge SR, Schweiger M. Direct calculation of the moments of the distribution of photon time of flight in tissue with a finite-element method. Appl. Opt. 1995;34:2683–2687. doi: 10.1364/AO.34.002683. [DOI] [PubMed] [Google Scholar]
- 30.Okada E, Firbank M, Delpy DT. The effect of ovelying tissue on the spatial sensitivity profine on near-infrared spectroscopy. Phys. Med. Biol. 1995;40:2093–2108. doi: 10.1088/0031-9155/40/12/007. [DOI] [PubMed] [Google Scholar]
- 31.Arridge SR, Schweiger M. Photon-measurement density functions. Part 2: Finite-element-method calculations. Appl. Opt. 1995;34:8026–8037. doi: 10.1364/AO.34.008026. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi, H., Higo, N., Kato, J., Matsuda, K. & Yamada, T. Functional near infrared spectroscopy for awake monkey to accelerate neurorehabilitation study. Proc. SPIE 10051, 1005117 (2017).
- 33.Higo N, Kunori N, Murata Y. Neural Activity during Voluntary Movements in Each Body Representation of the Intracortical Microstimulation-Derived Map in the Macaque Motor Cortex. PLoS One. 2016;11:1–20. doi: 10.1371/journal.pone.0160720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matcher S, Elwell C, Cooper C, Cope M, Delpy D. Performance comparison of several published tissue near-infrared spectroscopy algorithms. Anal. Biochem. 1995;227:54–68. doi: 10.1006/abio.1995.1252. [DOI] [PubMed] [Google Scholar]
- 35.Sugiyama Y, et al. Effects of early versus late rehabilitative training on manual dexterity after corticospinal tract lesion in macaque monkeys. J. Neurophysiol. 2013;109:2853–2865. doi: 10.1152/jn.00814.2012. [DOI] [PubMed] [Google Scholar]
- 36.Cisek P, Crammond DJ, Kalaska JF. Neural Activity in Primary Motor and Dorsal Premotor Cortex In Reaching Tasks With the Contralateral Versus Ipsilateral Arm. J. Neurophysiol. 2003;89:922–942. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- 37.Obrig, H. & Villringer, A. Beyond the Visible — Imaging the Human Brain With Light. J. Cereb. Blood Flow Metab. 23, 1–18 (2003). [DOI] [PubMed]
- 38.Lee YA, Pollet V, Kato A, Goto Y. Prefrontal cortical activity associated with visual stimulus categorization in non-human primates measured with near-infrared spectroscopy. Behav. Brain Res. 2017;317:327–331. doi: 10.1016/j.bbr.2016.09.068. [DOI] [PubMed] [Google Scholar]
- 39.Zaidi AD, et al. Simultaneous epidural functional near-infrared spectroscopy and cortical electrophysiology as a tool for studying local neurovascular coupling in primates. Neuroimage. 2015;120:394–399. doi: 10.1016/j.neuroimage.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 40.DiStasio, M. M. & Francis, J. T. Use of Frontal Lobe Hemodynamics as Reinforcement Signals to an Adaptive Controller. PLoS One8, e69541 (2013). [DOI] [PMC free article] [PubMed]
- 41.Wakita M, et al. Measurement of neuronal activity in a macaque monkey in response to animate images using near-infrared spectroscopy (NIRS) Front. Behav. Neurosci. 2010;4:1–8. doi: 10.3389/fnbeh.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuster J, et al. Near-infrared spectroscopy (NIRS) in cognitive neuroscience of the primate brain. Neuroimage. 2005;26:215–220. doi: 10.1016/j.neuroimage.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 43.Radhakrishnan H, et al. Fast optical signal not detected in awake behaving monkeys. Neuroimage. 2009;45:410–419. doi: 10.1016/j.neuroimage.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 45.Granger CWJ. Investigating Causal Relations by Econometric Models and Cross-spectral Methods. Econometrica. 1969;37:424–438. doi: 10.2307/1912791. [DOI] [Google Scholar]
- 46.Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014;13:206–216. doi: 10.1016/S1474-4422(13)70264-3. [DOI] [PubMed] [Google Scholar]
- 47.Tak S, Kempny AM, Friston KJ, Leff AP, Penny WD. Dynamic causal modelling for functional near-infrared spectroscopy. Neuroimage. 2015;111:338–349. doi: 10.1016/j.neuroimage.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anwar AR, et al. Effective Connectivity of Cortical Sensorimotor Networks During Finger Movement Tasks: A Simultaneous fNIRS, fMRI, EEG Study. Brain Topogr. 2016;29:645–660. doi: 10.1007/s10548-016-0507-1. [DOI] [PubMed] [Google Scholar]
- 49.Murata, Y. & Higo, N. Development and Characterization of a Macaque Model of Focal Internal Capsular Infarcts. PLoS One 11, e0154752 (2016). [DOI] [PMC free article] [PubMed]
- 50.Saager RB, Berger AJ. Direct characterization and removal of interfering absorption trends in twolayer turbid media. J. Opt. Soc. Am. A. 2005;22:1874–1882. doi: 10.1364/JOSAA.22.001874. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Brown EN, Strangman GE. Adaptive filtering for global interference cancellation and real-time recovery of evoked brain activity: a Monte Carlo simulation study. J. Biomed. Opt. 2007;12:44014. doi: 10.1117/1.2754714. [DOI] [PubMed] [Google Scholar]
- 52.Kohno S, et al. Removal of the skin blood flow artifact in functional near-infrared spectroscopic imaging data through independent component analysis. J. Biomed. Opt. 2007;12:62111. doi: 10.1117/1.2814249. [DOI] [PubMed] [Google Scholar]
- 53.Yamada, T., Umeyama, S. & Matsuda, K. Multidistance probe arrangement to eliminate motion artifacts in fNIRS. Proc. SPIE7174, 717420 (2009). [DOI] [PubMed]
- 54.Funane T, et al. Quantitative evaluation of deep and shallow tissue layers’ contribution to fNIRS signal using multi-distance optodes and independent component analysis. Neuroimage. 2014;85:150–165. doi: 10.1016/j.neuroimage.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 55.Yamada, T., Ohashi, M. & Umeyama, S. Real-time system for extracting and monitoring the cerebral functional component during fNIRS measurements. Proc. SPIE9792, 979219 (2015).
- 56.Huppert TJ, Allen MS, Benav H, Jones PB, Boas DA. A multicompartment vascular model for inferring baseline and functional changes in cerebral oxygen metabolism and arterial dilation. J. Cereb. Blood Flow Metab. 2007;27:1262–1279. doi: 10.1038/sj.jcbfm.9600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage. 2005;27:279–290. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 58.Berwick, J. et al. Hemodynamic Response in the Unanesthetized Rat: Intrinsic Optical Imaging and Spectroscopy of the Barrel Cortex. 22, 670–679 (2002). [DOI] [PubMed]
- 59.Sheth SA, et al. Linear and Nonlinear Relationships between Neuronal Activity, Oxygen. Neuron. 2004;42:347–355. doi: 10.1016/S0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- 60.Jasdzewski G, et al. Differences in the hemodynamic response to event-related motor and visual paradigms as measured by near-infrared spectroscopy. Neuroimage. 2003;20:479–488. doi: 10.1016/S1053-8119(03)00311-2. [DOI] [PubMed] [Google Scholar]
- 61.Tang L, Avison MJ, Gore JC. Nonlinear blood oxygen level-dependent responses for transient activations and deactivations in V1 - insights into the hemodynamic response function with the balloon model. Magn. Reson. Imaging. 2009;27:449–459. doi: 10.1016/j.mri.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McIntosh MA, Shahani U, Boulton RG, McCulloch DL. Absolute quantification of oxygenated hemoglobin within the visual cortex with functional near infrared spectroscopy (fNIRS) Invest. Ophthalmol. Vis. Sci. 2010;51:4856–4860. doi: 10.1167/iovs.09-4940. [DOI] [PubMed] [Google Scholar]
- 63.Zeff, B. W., White, B. R., Dehghani, H., Schlaggar, B. L. & Culver, J. P. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proc. Natl. Acad. Sci. U. S. A. 104 12169–12174 (2007). [DOI] [PMC free article] [PubMed]
- 64.Steinbrink J, et al. Illuminating the BOLD signal: combined fMRI-fNIRS studies. Magn. Reson. Imaging. 2006;24:495–505. doi: 10.1016/j.mri.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 65.Franceschini MA, Joseph DK, Huppert TJ, Diamond SG, Boas DA. Diffuse optical imaging of the whole head. J. Biomed. Opt. 2006;11:54007. doi: 10.1117/1.2363365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.