Abstract
Our aim was to demonstrate that biofilm formation in a clinical strain of methicillin-resistant Staphylococcus aureus (MRSA) can be enhanced by environment exposure in an endotracheal tube (ETT) and to determine how it is affected by systemic treatment and atmospheric conditions. Second, we aimed to assess biofilm production dynamics after extubation. We prospectively analyzed 70 ETT samples obtained from pigs randomized to be untreated (controls, n = 20), or treated with vancomycin (n = 32) or linezolid (n = 18). A clinical MRSA strain (MRSA-in) was inoculated in pigs to create a pneumonia model, before treating with antibiotics. Tracheally intubated pigs with MRSA severe pneumonia, were mechanically ventilated for 69 ± 16 hours. All MRSA isolates retrieved from ETTs (ETT-MRSA) were tested for their in vitro biofilm production by microtiter plate assay. In vitro biofilm production of MRSA isolates was sequentially studied over the next 8 days post-extubation to assess biofilm capability dynamics over time. All experiments were performed under ambient air (O2) or ambient air supplemented with 5% CO2. We collected 52 ETT-MRSA isolates (placebo N = 19, linezolid N = 11, and vancomycin N = 22) that were clonally identical to the MRSA-in. Among the ETT-MRSA isolates, biofilm production more than doubled after extubation in 40% and 50% under 5% CO2 and O2, respectively. Systemic antibiotic treatment during intubation did not affect this outcome. Under both atmospheric conditions, biofilm production for MRSA-in was at least doubled for 9 ETT-MRSA isolates, and assessment of these showed that biofilm production decreased progressively over a 4-day period after extubation. In conclusion, a weak biofilm producer MRSA strain significantly enhances its biofilm production within an ETT, but it is influenced by the ETT environment rather than by the systemic treatment used during intubation or by the atmospheric conditions used for bacterial growth.
Introduction
Intubation with an endotracheal tube (ETT) is a routine procedure that is applied to 40%–50% of patients admitted to intensive care units (ICUs)1. However, when covered by secretions or cell debris, microorganisms from the stomach or oropharynx2 can rapidly colonize the ETT by directly or indirectly adhering to its surface3,4. The attachment of these microorganisms to the ETT surface changes gene expression to allow the bacteria to grow in a sessile mode that promotes biofilm formation5. Several studies have found a relationship between ETT biofilms and ventilator-associated pneumonia (VAP)6–10, but it is difficult to assess whether ETT colonization results directly from VAP or from concomitant microorganisms in the patients’ airways11. In any case, the ETT biofilm becomes a source of unnecessary pathogens in the critically ill patient.
Staphylococcus aureus is one of the most frequently isolated microorganisms in VAP12, and is especially challenging to treat in its oxacillin-resistant form (methicillin-resistant S. aureus, MRSA). Though decreasing overall, the prevalence of MRSA remains above 25% in many southern and eastern European countries. Therefore, it remains an important public health priority. A major concern of hospital-acquired MRSA infections is its ability to form biofilms on indwelling devices, which in turn, can result in invasive infection, sepsis, morbidity and mortality13. The ability to form a biofilm is considered a virulence factor since the production of surface poly-N-acetylglucosamine is associated with initial stages of colonization and contributes to immune system evasion14, even though microorganisms do not routinely undergo testing for this ability in clinical settings. Unfortunately, however, there are few clinically validated methods for testing biofilms15–17, although some exhibit interesting results18. The microtiter plate method19–21 is extensively used to quantify the in vitro biofilm capability of bacteria, but is limited by the inability to extrapolate those results to in vivo scenarios with confidence. A clinician may therefore reach erroneous conclusions when basing them exclusively on the in vitro method.
We designed our hypothesis based on previous studies demonstrating a thick layer of secretions and biofilm in ETT after several days of mechanical ventilation3,4,6,22,23, and based on the fact that many of the components found within an ETT after mechanical ventilation are well described environmental stress factors that activate the biofilm mode of growth24, such as: exposure to ETT surface, extracellular DNA, cells debris, mucus secretions, sublethal doses of antimicrobials, nutrients shortage, inflammatory response or impaired availability of O2 on some deep layers of respiratory secretions where biofilms are found. We hypothesized that strains with weak in vitro biofilm-producing abilities could have increased ability under in vivo settings. Therefore, based on the assumption that biofilm formation is a reversible trait, we focused on progressive biofilm production on an ETT several days after extubation for a clinical strain of MRSA (MRSA-in) that caused severe pneumonia in a model of tracheally intubated and mechanically ventilated pigs25. Additionally, experiments were performed under ambient air (O2) and under ambient air supplemented with 5% CO2 (5% CO2) to assess the influence of atmospheric conditions on biofilm formation. This reflected the clinical scenario where different proportion of gases can be applied during the mechanical ventilation of intubated patients26. Finally, because linezolid and vancomycin are the two recommended drugs for VAP caused by MRSA27, we assessed the effect of these two systemic treatments—when used during intubation—on the ability to form a biofilm after extubation. Although other factors may also play a role in ETT-biofilm formation we focused on three of them related with the clinical management of ICU patients such as: the endotracheal tube, the systemic antimicrobial treatment and atmospheric conditions that can vary between different ventilatory patterns applied to critical patients.
Results
We assessed 35 ETTs (Mallinckrodt Hi-Lo; Mallinckrodt Medical, Athlone, Ireland) with 7.5 mm internal diameters from 10, 9 and 16 pigs treated with placebo (controls), linezolid and vancomycin, respectively. Fifty-two sessile MRSA isolates (placebo N = 19, linezolid N = 11, vancomycin N = 22) were obtained from these ETTs that were clonally identical to the inoculated MRSA (MRSA-in; data not shown).
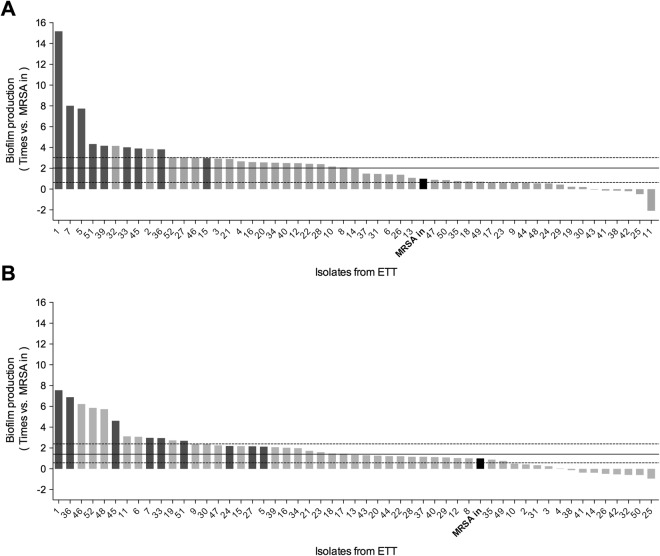
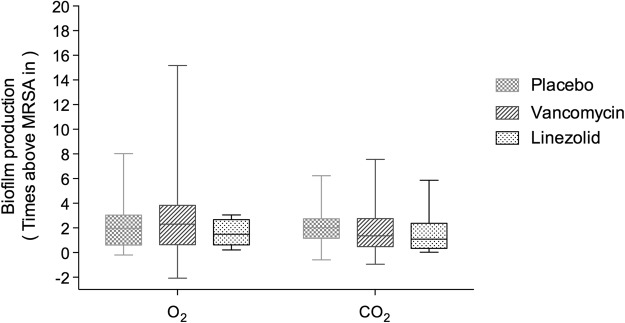
Overall, 61% and 71% of the MRSA isolates from within the ETTs showed a significant increase in biofilm production compared with the MRSA-in under O2 (2.02 [0.63–3.02], p < 0.001) and 5% CO2 (1.40 [0.57–2.39], p = 0.007), respectively (Fig. 1). However, biofilm production was not influenced by systemic treatment with linezolid, vancomycin, or placebo (Fig. 2). In O2 and 5% CO2 MRSA-in biofilm production doubled in 50% and 40% of ETT-MRSA isolates respectively. Of these, 9 MRSA isolates doubled biofilm production under both O2 and 5% CO2 conditions, and these were selected to assess progressive in vitro biofilm production for 8 days after extubation.
Figure 1.
Biofilm production of 52 ETT-MRSA isolates compared with the MRSA-in under ambient air or ambient air with 5% CO2. Each bar represents the biofilm production of each ETT-MRSA isolate versus the MRSA-in (black bar). (A) Biofilm production O2 on day 2 after extubation (peak production); 50% of ETT-MRSA isolates increased biofilm production more than double that of MRSA-in. (B) Biofilm production under 5% CO2 on day 1 after extubation (peak production); 40% of ETT-MRSA isolates increased more than twice MRSA-in biofilm production. The highest biofilm producers ETT-MRSA isolates (n = 9), in dark gray, under both O2 and 5% CO2 were selected to undergo the biofilm production dynamics post-extubation. Abbreviations: 5% CO2, ambient air with 5% CO2; O2, ambient air; ETT-MRSA, clinical MRSA isolates from endotracheal tubes; MRSA-in, MRSA inoculated into pigs’ lungs; MRSA, methicillin-resistant Staphylococcus aureus.
Figure 2.
Effect of systemic antibiotic treatment on biofilm production in the 52 ETT-MRSA isolates under O2 or 5% CO2 conditions Median (interquartile range) values for biofilm production of the 52 ETT-MRSA compared with the MRSA-in under O2 (A) and 5% CO2 (B). Time of assessment: day of peak production. Biofilm production was not influenced by systemic treatment with placebo (n = 19), linezolid (n = 11), or vancomycin (n = 22) under either O2 (1.96 [0.61–3.02], 2.30 [0.64–3.83], and 1.49 [0.63–2.67], respectively; p = 0.92) or 5% CO2 (2.02 [1.16–2.34], 1.36 [0.48–2.75], and 1.09 [0.34–2.38], respectively; p = 0.62). Abbreviations: 5% CO2, ambient air with 5% CO2; O2, ambient air; ETT-MRSA, clinical MRSA isolates from endotracheal tubes; MRSA-in, MRSA inoculated into pigs’ lungs; MRSA, methicillin-resistant Staphylococcus aureus.
Progressive biofilm production after extubation
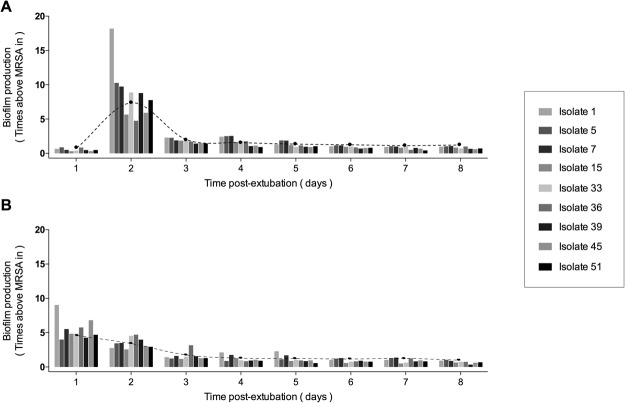
Biofilm production by the nine pre-selected ETT-MRSA isolates showed a significant and progressive decrease after extubation under both O2 and 5% CO2 conditions (Fig. 3). The peak biofilm production was under O2 at day 2 after extubation and was 7.78 times (6.62–9.67 times) that of the MRSA-in. By contrast, biofilm production under 5% CO2 peaked at day 1 after extubation and was only 4.46 times (3.21–7.71 times) that of the MRSA-in. Then, by stratifying time of assessment into ≤ 4 days and ≥ 4 days after extubation, we showed that a time lapse of 4 days was necessary to downregulate biofilm production to a baseline level.
Figure 3.
Biofilm production dynamics after extubation in 9 ETT-MRSA isolates under O2 or 5% CO2 Each color-bar represents the biofilm production of each ETT-MRSA isolate compared with the MRSA-in over days 1–8 after extubation. (A) Biofilm production dynamics under O2. Maximum biofilm production was on day 2. (B) Biofilm production dynamics under CO2. Maximum biofilm production was on day 1. Since the 5% CO2 atmosphere better mimics the atmospheric conditions of mechanical ventilation. When ETT-MRSA are rapidly switched from the ETT environment to O2 alone, they would need a day to adapt their metabolism to the new atmospheric conditions. Black points represent median biofilm production of the 9 isolates each day. Abbreviations: 5% CO2, ambient air with 5% CO2; O2, ambient air; ETT-MRSA, clinical MRSA isolates from endotracheal tubes; MRSA-in, MRSA inoculated into pigs’ lungs; MRSA, methicillin-resistant Staphylococcus aureus.
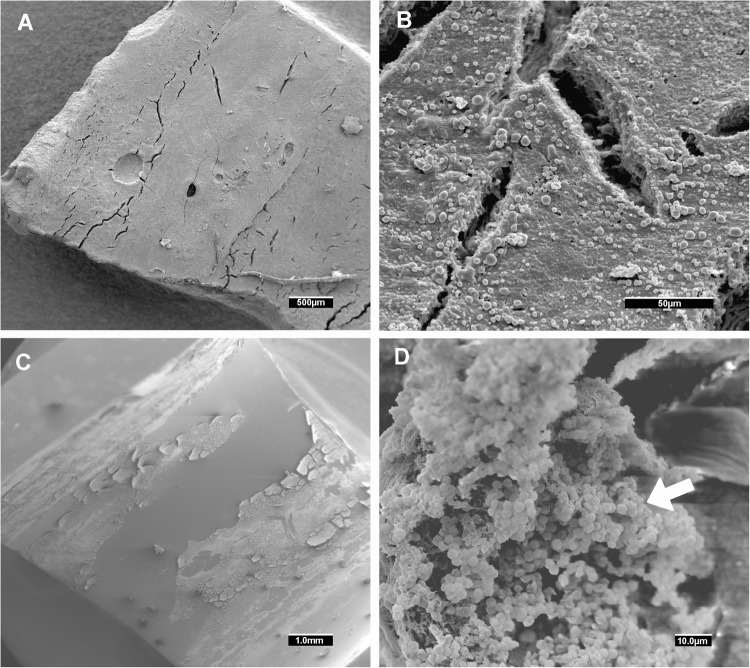
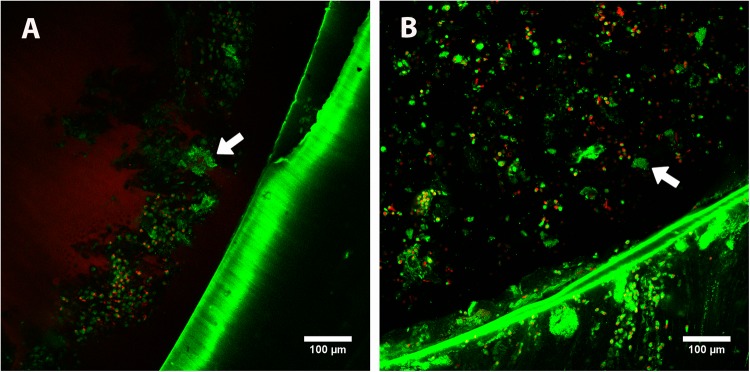
After day 4, biofilm production remained stable at a baseline level in both O2 (1.71 [0.88–3.53] ≤ 4 days vs 0.95 [0.76–1.10] ≥ 4 days, p < 0.001) and 5% CO2 (1.90 [1.08–3.77] ≤ 4 days vs 0.89 [0.68–1.08] ≥ 4 days, p < 0.001). Figures 4 and 5 show the in vivo ETT biofilms under scanning electron microscopy and confocal laser scanning microscopy for three representative ETTs.
Figure 4.
Representative scanning electron microscopy of in vivo MRSA biofilm (A) Isolate 1 showing an in vivo detached biofilm at low magnification. Sometimes the sample processing for scanning electron microscopy released the biofilm cluster from the endotracheal tube surface. At higher magnification (B), cocci morphologies can be distinguished. The pig from which we obtained Isolate 1 was treated with vancomycin. (C) Isolate 45 (from a placebo treated pig) showing an in vivo biofilm attached to the endotracheal tube at low magnification. (D) at higher magnification, a cocci biofilm cluster was found (white arrow). Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus.
Figure 5.
Representative confocal laser scanning microscopy of in vivo MRSA biofilm Biofilm clusters (white arrows) were stained with the LIVE/DEAD BacLight kit (INVITROGEN, Barcelona, Spain). Viable bacteria (stained green by SYTO 9) are visible, but dead bacteria (stained red by propidium iodide) were infrequently detected. The nuclei and cytoplasm of eukaryotic cells from the pig were also stained nonspecifically by propidium iodide and SYTO 9 (large red and green blotches, respectively). (A,B) correspond to the in vivo biofilms of Isolate 39 and Isolate 45 obtained from pigs treated with vancomycin and placebo, respectively. Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus.
Biofilm production under O2 or 5% CO2
Our MRSA strains exhibited similar biofilm production dynamics over time under both O2 and 5% CO2 after extubation. However, peak biofilm production under O2 occurred 2 days after extubation, whereas peak biofilm production under CO2 occurred 1 day after extubation. Our strains exhibited increased biofilm production under O2 compared with 5% CO2, except on day 1, when biofilm production was higher under 5% CO2 than under O2. No differences were found in biofilm growth under O2 and 5% CO2 conditions between days 3, 6, and 7.
Discussion
We demonstrated that endotracheal intubation significantly increased the ability of an MRSA strain to form biofilms in a way not influenced by the systemic treatment received during intubation. This finding is of clinical relevance because a weak in vitro biofilm producer can become a strong in vivo biofilm producer. It was notable that this ability reverted significantly to a baseline level after several days of culture-plate passes, suggesting that the microenvironment within an ETT enhances biofilm formation. Thus, in vitro biofilm phenotype on microtiter plates does not mandatorily speak for the in vivo biofilm phenotype of a particular strain. In addition, we demonstrated how incubation with O2 rather than 5% CO2 can amplify biofilm formation of MRSA, but without affecting the dynamics of biofilm formation after extubation. Finally, our findings impact on using in vitro tests for validating, as far as they don’t exactly mimic in vivo conditions and in this sense our work emphasizes the use of tissue based models28, animal models, or clinical studies for biofilm studies and anti-biofilm technology effectiveness testing for medical devices like ETTs3,29–31.
The dilemma between using in vitro and in vivo models is an important issue when assessing biofilm-associated infections32. For an ICU clinician, it might be relevant to know the behavior of a particular strain within an ETT because of the known association with VAP occurrence7,8,33, but this information is not usually available. Although in vitro studies benefit from high reproducibility given their extremely controlled conditions, they are unable to reproduce the very clinical factors that can affect outcomes. However, in vitro studies using synthetic mucus or gel-entrapped bacteria have shown promising results34–36.
ETT clinical strains behave accordingly to the ETT environment immediately after extubation and a snapshot of the microorganism’s response can be provided based on their in vitro behavior. We demonstrated that a weak biofilm producer in vitro could have a stronger biofilm forming ability within an ETT. This can be attributed to the synergistic effect of respiratory secretions, based on evidence that mucus enhances biofilm production34,37,38, enables colonization39, and disrupts the effect of antiseptics40. However, the reasons why some MRSA isolates maintained or decreased their biofilm production capability or how these differences in biofilm production affected clinical outcomes remains unclear. Although this needs further investigation, variability in mucus secretions, host immune system responses41, sublethal concentration of antimicrobials42, and the underlying clinical conditions per subject will almost certainly have influenced the ETT biofilm formed by each MRSA strain43. Additionally, not only different panel of chemokines and cytokines during S. aureus acute versus biofilm infection models have been previously reported44, but how the proinflammatory response activated by S. aureus can enhance indeed the progress to chronic infection45.
ETT colonization occurs rapidly after intubation2. Although the biofilm mode of growth requires important changes in gene expression46,47, the dynamic changes in expression over time have been poorly investigated for S. aureus41. In the current study, MRSA isolates needed an average of four days to downregulate their biofilm capability to a baseline status, reinforcing the high plasticity of the biofilm mode of growth. Similarly, Fux et al. demonstrated48 that S. aureus reached a steady state biofilm after 4 days in a dynamic flow system. Further studies are needed to elucidate the main genes involved in this process, which could identify new therapeutic targets.
The biofilm mode of growth can be activated by sub-inhibitory concentrations of antimicrobial drugs49,50. For instance, sublethal doses of vancomycin or linezolid have been shown to induce biofilm formation in S. aureus infection42,51. In line with these findings, we previously found that systemic vancomycin therapy was associated with increased biofilm thickness22 and bacterial spread (area) in vivo4, and attributed those results to sub-therapeutic doses of vancomycin achieved in secretions, leading to increased infection severity, mucus production, and ETT biofilm formation. The present results, however, indicate that previous systemic treatment does not affect MRSA biofilm production in vitro after extubation. Thus, these findings suggest that previous exposure to systemic linezolid or vancomycin treatment during intubation did not produce irreversible genetic rearrangements in our model, and therefore did not affect biofilm formation after extubation. New studies to elucidate the impact of sublethal antibiotic doses on biofilm formation in vivo would be of relevance.
Different ventilatory settings and oxygen concentrations are applied to ICU patients during mechanical ventilation26. In our experiments, MRSA biofilm growth after extubation was progressively downregulated over time under both ambient air (O2) and ambient air with 5% CO2 environments. Our results are consistent with those of other authors who used similar methods to show that S. aureus strains showed significantly lower biofilm production when grown in a CO2-rich environment compared with an ambient air environment52. Using a different methodology, Ursic et al. found that MRSA biofilm production increased when grown in CO2-rich environments53. Interestingly, small colony variants of S. aureus have been described to grow in a CO2-dependent manner54. The fact that we did not find such variants, coupled with the different methodologies, may explain why our MRSA isolates exhibited lower biofilm production under 5% CO2.
The effects of atmospheric gases availability on gene expression rearrangement or biofilm composition has been reported previously55,56. Our finding of differences in MRSA response under O2 and 5% CO2 conditions on day 1 after extubation can be attributed to the fact that bacteria need to rearrange their metabolism from the ETT to the in vitro ambient air atmosphere. This metabolic rearrangement seems not to have occurred under 5% CO2 conditions, because the normal in vivo partial pressure of CO2 (35–45 mmHg) closely resembles that found in the in vitro 5% CO2 atmosphere (38 mmHg)26. Further research may elucidate the role of anaerobiosis on ETT-MRSA isolates, which we did not include in the present work. Other authors have demonstrated that anaerobiosis can stimulate ica-specific mRNA expression of some S. aureus species57.
This work has several limitations that deserve to be mentioned. First, we did not assess biofilm formation directly on the ETT-strain, which were frozen and plated before biofilm analysis. However, we previously demonstrated how ETT-MRSA growth and biofilm was not affected by freezing4,6,22,23, and also we performed the same freezing and plating step for all isolates, thereby removing any potential bias. Second, our ETT-MRSA strains were obtained from mechanically ventilated pigs instead of from ICU patients. Nevertheless, the MRSA-in strain used in the animal model was obtained from a patient, and therefore the results from our experimental model are reproducible and can be useful for future studies of clinical isolates from ICU patients.
In conclusion, our work shows how the biofilm-producing ability of a MRSA strain is influenced by the ETT environment in vivo rather than by systemic antibiotic treatment during intubation or atmospheric conditions during bacterial growth. In addition, this biofilm upregulation is reversible: with longer exposure to in vitro conditions the ability to form a biofilm decreased, and eventually returned to the baseline level.
Materials and Methods
Subjects
Pigs received an intrabronchial challenge with 75 mL of 106 colonies forming units (CFU)/mL of a pathogenic Panton–Valentine leukocidin negative clinical strain of MRSA (MRSA-in), agr II, and ST 125 type, susceptible to vancomycin and linezolid. Following clinical pneumonia diagnosis, animals were randomized to be treated with vancomycin (15 mg/kg every 12 hrs intravenously), linezolid (10 mg/kg every 12 hrs intravenously), or 0.9% saline (placebo/controls) and mechanically ventilated for 69 ± 16 hours, as previously reported22,58. The MRSA-in and the MRSA isolates retrieved from ETT microbiological cultures were stored at −80 °C until analysis.
MRSA genotyping
The relatedness of all the MRSA isolates was assessed by pulsed-field gel electrophoresis (PFGE), following previously published methods59,60. Briefly, a washed bacterial suspension was mixed with 1.8% agarose (FMC BioProducts, USA) at 50 °C and allowed to solidify into plug molds for 10 min at 4 °C. Chromosomal DNA was prepared over several incubating and washing steps, using ESP (0.5 M EDTA, 1% sarkosyl), lysostaphin 400 µg/mL (SIGMA-ALDRICH, Spain), and proteinase K (ROCHE diagnostics S.L., Spain). DNA fragments generated by Sma I (SIGMA-ALDRICH, Spain) were separated by PFGE, using a CHEF-DRII apparatus (BIO-RAD, Richmond, CA, USA). The pulse times were increased linearly over 20 hrs from 5 s to 40 s at 200 V. The MRSA-in strain was used as a molecular size DNA marker, because the aim was to demonstrate that the ETT-MRSA isolates and the MRSA-in exhibited the same PFGE patterns. Following electrophoresis, the gel was stained with SYBR Safe (THERMO FISHER scientific, Spain) and photographed. To analyze the PFGE patterns, we used Bio-Rad software (Diversity Database, BIO-RAD, Richmond, CA, USA).
Microtiter plate assay
The in vitro ability of all isolated MRSA strains to form biofilm was assessed by microtiter plate assay of Christensen G. slightly modified as follows19. The MRSA-in and all ETT-MRSA isolates were first cultured overnight at 37 °C with agitation on tryptic soy broth media (TSB; SIGMA-ALDRICH, Spain); After this, each isolate was diluted 1/50 in 200 μL TSB with 0.25% glucose in a micro well of a 96 flat bottom microtiter plate (polystyrene, sterile; SIGMA-ALDRICH, Spain) and incubated, without shaking, overnight at 37 °C. Then, the medium was removed, 200 μL of 0.1% safranin (SIGMA-ALDRICH, Spain) were added to stain the biofilm over 1 min. Then, the saturated dye and non-adherent bacteria were removed by rinsing with 200 μL phosphate-buffered saline, three times. The optical density of biofilm was measured in a Synergy 2 Multimode Microplate Reader (BIOTEK Instruments, Inc., USA) at a wavelength of 490 nm. For each MRSA isolate and condition tested we performed four independent experiments, with three intra-assay replicates each.
Assessment of biofilm capability over time
MRSA isolates were first unfrozen and cultured on blood agar plates. Then, we plated up to eight different passes over 8 days after extubation to allow gene expression to adapt from the ETT to the culture plates. In so doing, we aimed to determine if their ability to form a biofilm was reversed to baseline level after several in vitro plate-cultures post-extubation. This was performed separately under O2 and 5% CO2 conditions. Although we assessed biofilm capability for all MRSA isolates over the first two days, we only included the higher biofilm producers for all 8 days.
Microscopy image acquisition
To assess the biofilm clusters in vivo, we performed scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) as previously reported4,22. CLSM and SEM images were obtained with a Leica TCS SP5 laser scanning confocal system (LEICA Microsystems Heidelberg GmbH, Manheim, Germany) equipped with a DMI6000 inverted microscope and a scanning electron microscope DSM 940 A (ZEISS, Oberkochen, Germany), respectively.
Statistical analyses
Data are reported as the median (interquartile range, IQR) or as mean ± SD, and were tested for normal distribution using the Shapiro–Wilk test. Qualitative or categorical variables were compared between groups with the Mann–Whitney test. Paired variables were assessed using the non-parametric Wilcoxon signed ranks test and independent samples were assessed using the non-parametric Kruskal–Wallis test. The Bonferroni correction was used for all post-hoc comparisons. To determine the relationship between quantitative variables, the Spearman rank-order correlation coefficient was used. A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS, Version 21 software (IBM SPSS statistics, 21, Chicago, IL, USA).
Ethical approval
The institutional review board and animal ethics committee approved all included studies. The project license number that covered the animal experiments were the following: Ethical Board of animal experimentation of the University of Barcelona (code: 296/09) and Ethical Board of the Hospital Clinic of Barcelona (code: 2009/5409). Animals were managed according to the National Institutes of Health guidelines for the Use and Care of Animals61.
Acknowledgements
We thank the following for their valuable contributions: Albert Gabarrús for his assistance with the statistical analyses, Josep Mª Sierra and Mar Solé for their assistance with the PFGE. We also thank Maria Calvo, Anna Bosch, and Elisenda Coll of the Confocal Microscopy Unit, Scientific and Technical Services, University of Barcelona, for their assistance in image acquisition. This manuscript is dedicated to Núria Cortadellas, who died in May 2017, for her valuable assistance in Scanning Electron Microscopy image acquisition.
Author Contributions
L.F.B. and A.T. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, methodology and supervision: L.F.B., J.V. and A.T.; Investigation: L.F.B., S.B., A.M., L.M., G.L.B., M.R. and F.M.; analysis and interpretation of data: L.F.B., S.B. and J.V.; statistical analysis: S.B., L.F.B.; Project Administration: L.F.B., S.B.; Writing – Original Draft Preparation: L.F.B.; Writing – Review & Editing: L.F.B., J.V., M.F. and A.T.; All authors read and approved the manuscript.
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laia Fernández-Barat and Soumaya Ben-Aicha contributed equally to this work.
Contributor Information
Laia Fernández-Barat, Email: lfernan1@clinic.cat.
Antoni Torres, Email: atorres@clinic.cat.
References
- 1.Esperatti M, et al. Nosocomial Pneumonia in the Intensive Care Unit Acquired during Mechanical Ventilation or Not. Am. J. Respir. Crit Care Med. 2010;182:1533–1539. doi: 10.1164/rccm.201001-0094OC. [DOI] [PubMed] [Google Scholar]
- 2.Perkins SD, Woeltje KF, Angenent LT. Endotracheal tube biofilm inoculation of oral flora and subsequent colonization of opportunistic pathogens. Int. J. Med. Microbiol. 2010;300:503–511. doi: 10.1016/j.ijmm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Barat L, Torres A. Biofilms in ventilator-associated pneumonia. Future. Microbiol. 2016;11:1599–1610. doi: 10.2217/fmb-2016-0040. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Barat L, et al. Direct analysis of bacterial viability in endotracheal tube biofilm from a pig model of methicillin-resistant Staphylococcus aureus pneumonia following antimicrobial therapy. FEMS Immunol. Med. Microbiol. 2012;65:309–317. doi: 10.1111/j.1574-695X.2012.00961.x. [DOI] [PubMed] [Google Scholar]
- 5.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;21:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 6.Li BG, et al. Endotracheal tube biofilm translocation in the lateral Trendelenburg position. Crit Care. 2015;19:59. doi: 10.1186/s13054-015-0785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil-Perotin S, et al. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: a state of concept. Crit Care. 2012;16:R93. doi: 10.1186/cc11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adair CG, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25:1072–1076. doi: 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- 9.Feldman C, et al. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur Respir J. 1999;13:546–551. doi: 10.1183/09031936.99.13354699. [DOI] [PubMed] [Google Scholar]
- 10.Inglis TJ, Millar MR, Jones JG, Robinson DA. Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol. 1989;27:2014–2018. doi: 10.1128/jcm.27.9.2014-2018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres A, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152:137–141. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control. Annual Epidemiological Report. http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?Li st=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1292 (2014).
- 13.Silva-Santana G, Lenzi-Almeida KC, Lopes VGS, guiar-Alves F. Biofilm formation in catheter-related infections by Panton-Valentine leukocidin-producing Staphylococcus aureus. Int Microbiol. 2016;19:199–207. doi: 10.2436/20.1501.01.278. [DOI] [PubMed] [Google Scholar]
- 14.Kropec A, et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 2005;73:6868–6876. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantanella F, Valenti P, Natalizi T, Passeri D, Berlutti F. Analytical techniques to study microbial biofilm on abiotic surfaces: pros and cons of the main techniques currently in use. Ann. Ig. 2013;25:31–42. doi: 10.7416/ai.2013.1904. [DOI] [PubMed] [Google Scholar]
- 16.Hassan A, et al. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz. J Infect. Dis. 2011;15:305–311. doi: 10.1016/S1413-8670(11)70197-0. [DOI] [PubMed] [Google Scholar]
- 17.Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol. Methods. 2008;72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Olivares E, et al. The BioFilm Ring Test: a Rapid Method for Routine Analysis of Pseudomonas aeruginosa Biofilm Formation Kinetics. J Clin. Microbiol. 2016;54:657–661. doi: 10.1128/JCM.02938-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen GD, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin. Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkhatib WF, Khairalla AS, Ashour HM. Evaluation of different microtiter plate-based methods for the quantitative assessment of Staphylococcus aureus biofilms. Future. Microbiol. 2014;9:725–735. doi: 10.2217/fmb.14.33. [DOI] [PubMed] [Google Scholar]
- 21.Knobloch JK, Horstkotte MA, Rohde H, Mack D. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med Microbiol. Immunol. 2002;191:101–106. doi: 10.1007/s00430-002-0124-3. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Barat L, et al. Linezolid limits burden of methicillin-resistant Staphylococcus aureus in biofilm of tracheal tubes. Crit Care Med. 2012;40:2385–2389. doi: 10.1097/CCM.0b013e31825332fc. [DOI] [PubMed] [Google Scholar]
- 23.Aguilera XE, et al. Tracheal tube biofilm removal through a novel closed-suctioning system: an experimental study. Br. J. Anaesth. 2015;115:775–783. doi: 10.1093/bja/aev340. [DOI] [PubMed] [Google Scholar]
- 24.Bui LM, Turnidge JD, Kidd SP. The induction of Staphylococcus aureus biofilm formation or Small Colony Variants is a strain-specific response to host-generated chemical stresses. Microbes. Infect. 2015;17:77–82. doi: 10.1016/j.micinf.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Olondris P, et al. An experimental model of pneumonia induced by methicillin-resistent Staphylococcus aureus in ventilated piglets. Eur Respir J. 2010;36:901–906. doi: 10.1183/09031936.00176709. [DOI] [PubMed] [Google Scholar]
- 26. Tobin,M.J. Principles and practice of mechanical ventilation (The McGraw-Hill Companies, Inc., New York, 2013).
- 27.American Thoracic Society & Infectious Diseases Society of America Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am J Respir Crit Care Med171, 388–416 (2005). [DOI] [PubMed]
- 28.Wang Y, Leng V, Patel V, Phillips KS. Injections through skin colonized with Staphylococcus aureus biofilm introduce contamination despite standard antimicrobial preparation procedures. Sci. Rep. 2017;7:45070. doi: 10.1038/srep45070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardes JM, Gray D, Wilson A. Effect of the endOclear((R)) Device on Biofilm in Endotracheal Tubes. Surg. Infect. (Larchmt.) 2017;18:293–298. doi: 10.1089/sur.2016.052. [DOI] [PubMed] [Google Scholar]
- 30.Berra L, et al. A clinical assessment of the Mucus Shaver: a device to keep the endotracheal tube free from secretions. Crit Care Med. 2012;40:119–124. doi: 10.1097/CCM.0b013e31822e9fe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinciroli R, et al. Endotracheal Tubes Cleaned With a Novel Mechanism for Secretion Removal: A Randomized Controlled Clinical Study. Respir. Care. 2016;61:1431–1439. doi: 10.4187/respcare.04363. [DOI] [PubMed] [Google Scholar]
- 32.Lebeaux D, Chauhan A, Rendueles O, Beloin C. From in vitro to in vivo Models of Bacterial Biofilm-Related Infections. Pathogens. 2013;2:288–356. doi: 10.3390/pathogens2020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danin PE, et al. Description and microbiology of endotracheal tube biofilm in mechanically ventilated subjects. Respir. Care. 2015;60:21–29. doi: 10.4187/respcare.02722. [DOI] [PubMed] [Google Scholar]
- 34.Haley CL, Colmer-Hamood JA, Hamood AN. Characterization of biofilm-like structures formed by Pseudomonas aeruginosa in a synthetic mucus medium. BMC. Microbiol. 2012;12:181. doi: 10.1186/1471-2180-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sriramulu DD, Lunsdorf H, Lam JS, Romling U. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2005;54:667–676. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- 36.Pabst B, Pitts B, Lauchnor E, Stewart PS. Gel-Entrapped Staphylococcus aureus Bacteria as Models of Biofilm Infection Exhibit Growth in Dense Aggregates, Oxygen Limitation, Antibiotic Tolerance, and Heterogeneous Gene Expression. Antimicrob. Agents Chemother. 2016;60:6294–6301. doi: 10.1128/AAC.01336-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landry RM, An D, Hupp JT, Singh PK, Parsek MR. Mucin-Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Mol. Microbiol. 2006;59:142–151. doi: 10.1111/j.1365-2958.2005.04941.x. [DOI] [PubMed] [Google Scholar]
- 38.Worlitzsch D, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 2002;109:317–325. doi: 10.1172/JCI0213870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gries DM, Pultz NJ, Donskey CJ. Growth in cecal mucus facilitates colonization of the mouse intestinal tract by methicillin-resistant Staphylococcus aureus. J Infect. Dis. 2005;192:1621–1627. doi: 10.1086/491737. [DOI] [PubMed] [Google Scholar]
- 40.Ansorg RA, Azem T, Fabry WH, Rath PM. Influence of mucin on the activity of the antiseptic Lavasept against Staphylococcus aureus. Chemotherapy. 2002;48:129–133. doi: 10.1159/000064917. [DOI] [PubMed] [Google Scholar]
- 41.Scherr TD, et al. Global transcriptome analysis of Staphylococcus aureus biofilms in response to innate immune cells. Infect. Immun. 2013;81:4363–4376. doi: 10.1128/IAI.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu CY, et al. Vancomycin promotes the bacterial autolysis, release of extracellular DNA, and biofilm formation in vancomycin-non-susceptible Staphylococcus aureus. FEMS Immunol. Med Microbiol. 2011;63:236–247. doi: 10.1111/j.1574-695X.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 43.Jaffe A, Bush A. Anti-inflammatory effects of macrolides in lung disease. Pediatr. Pulmonol. 2001;31:464–473. doi: 10.1002/ppul.1076. [DOI] [PubMed] [Google Scholar]
- 44.Brady RA, Mocca CP, Plaut RD, Takeda K, Burns DL. Comparison of the immune response during acute and chronic Staphylococcus aureus infection. PLoS. One. 2018;13:e0195342. doi: 10.1371/journal.pone.0195342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prabhakara R, et al. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect. Immun. 2011;79:5010–5018. doi: 10.1128/IAI.05571-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohde H, et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials. 2007;28:1711–1720. doi: 10.1016/j.biomaterials.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 47.Delaune A, et al. The WalKR system controls major staphylococcal virulence genes and is involved in triggering the host inflammatory response. Infect. Immun. 2012;80:3438–3453. doi: 10.1128/IAI.00195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fux CA, Wilson S, Stoodley P. Detachment characteristics and oxacillin resistance of Staphyloccocus aureus biofilm emboli in an in vitro catheter infection model. J. Bacteriol. 2004;186:4486–4491. doi: 10.1128/JB.186.14.4486-4491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman LR, et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 50.Mesak LR, Miao V, Davies J. Effects of subinhibitory concentrations of antibiotics on SOS and DNA repair gene expression in Staphylococcus aureus. Antimicrob. Agents Chemother. 2008;52:3394–3397. doi: 10.1128/AAC.01599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrer MD, et al. Effect of antibiotics on biofilm inhibition and induction measured by real-time cell analysis. J Appl. Microbiol. 2017;122:640–650. doi: 10.1111/jam.13368. [DOI] [PubMed] [Google Scholar]
- 52.Stepanovic S, Djukic N, Djordjevic V, Djukic S. Influence of the incubation atmosphere on the production of biofilm by staphylococci. Clin. Microbiol. Infect. 2003;9:955–958. doi: 10.1046/j.1469-0691.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- 53.Ursic V, Tomic V, Kosnik M. Effect of different incubation atmospheres on the production of biofilm in methicillin-resistant Staphylococcus aureus (MRSA) grown in nutrient-limited medium. Curr. Microbiol. 2008;57:386–390. doi: 10.1007/s00284-008-9211-z. [DOI] [PubMed] [Google Scholar]
- 54.Gomez-Gonzalez C, et al. Clinical and molecular characteristics of infections with CO2-dependent small-colony variants of Staphylococcus aureus. J Clin. Microbiol. 2010;48:2878–2884. doi: 10.1128/JCM.00520-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez-Barat L, et al. Phenotypic shift in Pseudomonas aeruginosa populations from cystic fibrosis lungs after 2-week antipseudomonal treatment. J Cyst. Fibros. 2017;16:222–229. doi: 10.1016/j.jcf.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Asai K, et al. Effect of incubation atmosphere on the production and composition of staphylococcal biofilms. J Infect. Chemother. 2015;21:55–61. doi: 10.1016/j.jiac.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Cramton SE, Ulrich M, Gotz F, Doring G. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 2001;69:4079–4085. doi: 10.1128/IAI.69.6.4079-4085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez-Olondris, P. et al. Efficacy of linezolid compared to vancomycin in an experimental model of pneumonia induced by methicillin-resistant Staphylococcus aureus in ventilated pigs. Crit Care Med (2011). [DOI] [PubMed]
- 59.Sierra JM, Marco F, Ruiz J, Jimenez de Anta MT, Vila J. Correlation between the activity of different fluoroquinolones and the presence of mechanisms of quinolone resistance in epidemiologically related and unrelated strains of methicillin-susceptible and -resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2002;8:781–790. doi: 10.1046/j.1469-0691.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- 60.Gautom RK. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J Clin. Microbiol. 1997;35:2977–2980. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Research Council. Guide for the Care and Use of Laboratory Animals: Eight Edition. Washington, DC: The National Academies Press. 10.17226/12910 (2011).