Abstract
Background
In previous retrospective studies, we identified the 50 most influential clinical predictors of cardiovascular outcomes in patients with heart failure (HF). The present study aimed to use the novel limitless-arity multiple-testing procedure to filter these 50 clinical factors and thus yield combinations of no more than four factors that could potentially predict the onset of cardiovascular events. A Kaplan–Meier analysis was used to investigate the importance of the combinations.
Methods
In a multi-centre observational trial, we prospectively enrolled 213 patients with HF who were hospitalized because of exacerbation, discharged according to HF treatment guidelines and observed to monitor cardiovascular events. After the observation period, we stratified patients according to whether they experienced cardiovascular events (rehospitalisation or cardiovascular death).
Findings
Among 77,562 combinations of fewer than five clinical parameters, we identified 151 combinations that could potentially explain the occurrence of cardiovascular events. Of these, 145 combinations included the use of inotropic agents, whereas the remaining 6 included the use of diuretics without bradycardia or tachycardia, suggesting that the high probability of cardiovascular events is exclusively determined by these two clinical factors. Importantly, Kaplan–Meier curves demonstrated that the use of inotropes or of diuretics without bradycardia or tachycardia were independent predictors of a markedly worse cardiovascular prognosis.
Interpretation
Patients treated with either inotropic agents or diuretics without bradycardia or tachycardia were at a higher risk of cardiovascular events. The uses of these drugs, regardless of heart rate, are the strongest clinical predictors of cardiovascular events in patients with HF.
Keywords: Heart failure, Data mining, Cardiovascular events, Combinational factors, Inotropic agents, Diuretics
Research in Context.
Evidence before this study
Many lines of evidence from the observational or randomised clinical studies have identified the important clinical factors for the prediction of the cardiovascular events by multivariate analyses of observationally collected or randomised controlled data in patients with heart failure (HF), however, there have been no data analyses using many clinical parameters related or unrelated to the pathophysiology of HF patients to seek to the strongest clinical factors by data-mining methods. Here, one of the novel data mining methods of limitless-arity multiple-testing procedure (LANP) could identify the strongest clinical factors to predict the cardiovascular events among all combinations of the clinical factors in HF patients.
We employed 167 HF patients who were admitted between November 2007 and October 2009 and followed to monitor the incidence of cardiovascular events until December 2014 to narrow down 50 important clinical parameters to predict cardiovascular events, and we generated a new cohort of 213 HF patients who received contemporary treatment in the context of a multi-centre trial, and prospectively evaluated the combination that could best predict cardiovascular outcomes between May 2013 and March 2015 and followed these patients until the end of April 2016.
Added Value of This Study
Using the LANP method for the patients with HF, we found that the patients treated with either inotropic agents or diuretics without bradycardia or tachycardia were at a higher risk of cardiovascular events, which are novel finding on the top of the conventional knowledge of the current HF treatment strategy.
Implications of all the Available Evidence
The cardiologists are usually interested in the symptoms of the patients, results of biomarkers of HF such as plasma BNP levels, laboratory data of echocardiograms and the effectiveness and side-effects of the drugs for HF when they examine the HF patients. On the top of the ordinary knowledge or guidelines of treatment of HF, the present finding cautions that the cardiologists should focus on the present use of inotropic agents or the use of diuretics without either bradycardia or tachycardia as the strongest predictors of an increased risk of cardiovascular events in patients with HF, when cardiologists treat such patients. Such analyses using the big data of HF patients would notify the unexpected parameters to predict the occurrence of the cardiovascular events such as re-hospitalisation.
1. Introduction
Globally, cardiovascular disease has placed a significant burden both on individual patients and national economies [1, 2]. Despite the availability of effective medical treatments, heart failure (HF) remains a major cause of increased morbidity and mortality [[3], [4], [5]]. Notably, hospitalisation for a pathophysiologic exacerbation of HF can increase the severity of this condition, thus activating a vicious cycle that leads to cardiovascular death. Therefore, it is very important to identify the strongest clinical predictors of cardiovascular events followed by hospitalisation among patients with HF. Comorbidity (hypertension or renal dysfunction), the presence of anaemia or cardiomegaly, age and sex have been suggested as major determinants of hospitalisation or cardiac death among patients with HF [6]. However, the interactions between these comorbidities are complex, and the strongest clinical influences on the risk of a cardiovascular event remain unclear. In previous studies, several biomarkers, including blood levels of brain natriuretic peptide (BNP) [7], C-reactive protein [8] and albumin [9], have been measured in patients with HF with the aim of determining the severity and probability of cardiovascular events. Additionally, various drugs, such as angiotensin-converting inhibitors [10], diuretics [11] and inotropic agents [12], have been administered to patients with the intent to improve the pathophysiology of HF. Still, it remains difficult to determine the most important clinical predictors of cardiovascular events and to apply this knowledge to patients with HF in a clinical setting.
The existing limitations can be partially attributed to the use of different hypotheses and the lack of comprehensive or systematic investigations among the various studies. Accordingly, it is important to use a comprehensive method to determine the most essential parameters or combinations of parameters predictive of cardiovascular events in a cohort of patients with HF. As the combination of clinical parameters A + B + C may have synergistic effects on cardiovascular events even if A, B or C alone has no effect, the ability of every combination of clinical parameters to predict the occurrence of cardiovascular events should be tested. To overcome the difficulties associated with such testing in patients with HF, we have implemented recent, novel advances in statistical testing that will allow us to analyse all significant combinations of clinical parameters without any limits via the limitless-arity multiple testing procedure (LAMP) [13].
In this study, we evaluated the effects of combinations of clinical parameters on the incidence of cardiovascular events among patients with HF. First, we narrowed down all the combinations to those that could best explain the occurrence of the cardiovascular events. Second, we identified two combinations of clinical parameters, the use of inotropes or the use of diuretics without bradycardia or tachycardia, which correlated with the highest probability of cardiovascular event incidence among patients with HF.
2. Methods
2.1. Ethics Statement
This study was approved by the National Cerebral and Cardiovascular Centre Research Ethics Committee, which waived the requirement to obtain informed consent from the 167 subjects according to the Japanese Clinical Research Guideline because of the retrospective observational design. Instead, we made a public announcement on both the Internet homepage of our institution and the bulletin boards in our outpatient and inpatient clinics to comply with the Japanese Clinical Research Guideline and a request of the Ethics Committee.
For the analysis, we created a specified database of anonymised data in the Department of HF at our institution and analysed the anonymous data. Additionally, we obtained written informed consent from the 213 subjects included in the prospective observational study after receiving approval from the Research Ethics Committees at the National Cerebral and Cardiovascular Centre, Hokkaido University and Kyushu University.
2.2. Protocols for the First and Second Screenings
We filtered the clinical parameters to identify those most important with regard to the incidence of cardiovascular events in patients with HF. Initially, we obtained data of 402 clinical parameters in 151 patients with acute decompensated heart failure (ADHF) and used these data to derive an equation with which to determine the probability of cardiovascular events (hospitalisation or death due to HF) [14]. In this step, we narrowed the list to 251 clinical parameters. Next, after data cleaning, we added 16 patients to the cohort from the previous study to yield a total of 167 patients with ADHF who were admitted between November 2007 and October 2009 and followed to monitor the incidence of cardiovascular events until December 2014. HF diagnoses were confirmed by an expert team of cardiologists using the Framingham criteria. Finally, we selected the 50 most influential candidates from among the 251 parameters identified in previous studies (Table 1) [14, 15].
Table 1.
The clinical parameters in patients with heart failure, and the differences in the clinical parameters with or without cardiovascular events.
| Clinical factors | |
|---|---|
| Age, (years) | 72 (60–79) |
| Gender, male/female | 98/69 |
| NYHA class (II/III/IV) at admission | 52/54/61 |
| Heart rate at admission (beats/min) | 81 (69–104) |
| Leg edema | 91 (54) |
| Etiology of HF | |
| Cardiomyopathy | 56 (34) |
| Hypertensive heart disease | 25 (15) |
| Ischemic heart disease | 16 (10) |
| Valvular heart disease | 47 (28) |
| Comorbidity | |
| Hypertension | 81 (49) |
| Hyperlipidemia | 47 (28) |
| Chronic Af | 67 (40) |
| Cerebrovascular disease | 31 (19) |
| Obstructive pulmonary disease | 10 (6) |
| CRT | 35 (20) |
| ICD | 35 (20) |
| Pacemaker | 14 (8) |
| Number of family members in the same household | 1 (1, 2) |
| Albumin at admission, (g/dl) | 3.7 (3.4–4.0) |
| CRP at admission, (mg/dl) | 0.3 (0.1–0.9) |
| WBC at admission, (/μl) | 6500 (5000–8850) |
| AST at discharge, (U/l) | 25.0 (20.5–21.5) |
| BUN at discharge, (mg/dl) | 21.0 (16–30.8) |
| Uric acid at discharge, (mg/dl) | 7.0 (5.7–8.4) |
| CRP at discharge, (mg/dl) | 0.18 (0.04–0.53) |
| BNP at discharge, (pg/ml) | 191 (102–413) |
| %FS at admission, (%) | 19 (11–29) |
| LVDs at admission, (mm) | 48 (36–57) |
| %FS at discharge, (%) | 20 (13−31) |
| IVST at discharge, (mm) | 9 (8–11) |
| AR grade (≥II) at discharge | 21 (13) |
| MR grade (≥II) at discharge | 48 (29) |
| TR grade (≥II) at discharge | 43 (26) |
| Oral medications at discharge | |
| ACE inhibitor | 80 (48) |
| Anti-allergic | 12 (7) |
| Anti-inflammatory drug | 5 (3) |
| Antiplatelet | 45 (27) |
| Antithyroid drug | 2 (1) |
| Beta-blockers | 109 (65) |
| Bronchodilator | 7 (4) |
| Choleretic drug | 10 (6) |
| Digitalis | 48 (29) |
| Diuretics | 151 (90) |
| Inotropic agent | 22 (13) |
| Intestinal disease drug | 4 (2) |
| Lipid-lowering drug | 37 (22) |
| Proton pump inhibitor | 60 (36) |
| Purgative | 49 (29) |
| Sedative-hypnotic (benzodiazepin) | 36 (22) |
| Vitamins | 14 (8) |
Data are given as the Median (interquartile range) or n (%). ACE inhibitor, angiotensin-converting enzyme inhibitor; ADHF, acute decompensated heart failure; Af, atrial fibrillation; AR, aortic regurgitation; BNP, B-type natriuretic peptide; BUN, Blood urea nitrogen; CRT, cardiac resynchronization therapy; CRP, C-reactive protein; FS, fractional shortening; ICD, Implantable Cardioverter Defibrillator; VST, interventricular septum thickness; LVDs, Left ventricular end-systolic dimension MR, mitral regurgitation; NYHA, New York Heart Association; TR, tricuspid regurgitation.
In the present study, we generated a new cohort of HF patients who received contemporary treatment in the context of a multi-centre trial and prospectively evaluated the combination that could best predict cardiovascular outcomes. For this purpose, we enrolled 213 patients with ADHF who were admitted to three different hospitals in Japan—National Cerebral and Cardiovascular Centre (n = 114), Hokkaido University (n = 80) and Kyushu University (n = 19)—between May 2013 and March 2015 and followed these patients until the end of April 2016. All patients underwent a careful history-taking process, physical examinations, laboratory testing, chest X-rays, electrocardiograms and complete Doppler echocardiographic studies. An expert team of cardiologists in charge of the HF department determined the timing of patient discharge, which was recommended when the patient presented with a stable blood pressure and improved renal function due to an optimal treatment according to international guidelines, as well as none of the following: signs of decompensation such as a New York Heart Association functional class <3, rales and galloping rhythm. Rehospitalisation of HF patients was defined as hospitalisation of an enrolled patient for decompensated HF, and cardiovascular death was defined as death attributed to a worsening of HF. The primary endpoint was a cardiovascular event: either rehospitalisation or death due to a worsening of HF, whichever occurred first. Among the 50 clinical parameters, we determined the left ventricular dimensions at diastole and systole from the calculated of percent fractional shortening. As we included additional parameters related to the etiology of HF, such as cardiomyopathy (Table 2), the LAMP analysis actually included 54 clinical parameters at the time of hospitalisation or discharge in HF patients.
Table 2.
The clinical parameters in patients with heart failure, and the differences in the clinical parameters with or without cardiovascular events.
| Clinical factors | Without (n = 114) | With (n = 99) |
|---|---|---|
| Age, (years) | 72 (60–79) | 70 (60–79) |
| Gender, male/female | 71/43 | 64/35 |
| NYHA class (II/III/IV) at admission | 34/55/25 | 13/53/33 |
| Heart rate at admission (beats/min) | 86 (69–102) | 75 (69–87) |
| Leg edema | 65 (57) | 71 (62) |
| Etiology of HF | ||
| Cardiomyopathy | 34 (30) | 42 (37) |
| Hypertensive heart disease | 23 (20) | 6 (5) |
| Ischemic heart disease | 12 (11) | 14 (12) |
| Valvular heart disease | 23 (20) | 24 (21) |
| Others | 22 (19) | 13 (11) |
| Comorbidity | ||
| Hypertension | 64 (56) | 44 (39) |
| Hyperlipidemia | 40 (35) | 33 (29) |
| Chronic Af | 50 (44) | 54 (47) |
| Cerebrovascular disease | 7 (6) | 7 (6) |
| Obstructive pulmonary disease | 5 (4) | 1 (1) |
| CRT | 8 (7) | 16 (14) |
| ICD | 11 (10) | 20 (18) |
| Pacemaker | 18 (16) | 13 (11) |
| Number of family members in the same household | 1 (1, 2) | 1 (1) |
| Albumin at admission, (g/dl) | 3.8 (3.5–4.1) | 3.8 (3.5–4.1) |
| CRP at admission, (mg/dl) | 0.4 (0.1–1.2) | 0.4 (0.15–1.05) |
| WBC at admission, (/μl) | 5300 (4100–6369) | 5100 (4200–6700) |
| AST at discharge, (U/l) | 20 (18–28) | 25 (20−32) |
| BUN at discharge, (mg/dl) | 22 (18–28) | 27 (20.5–44) |
| Uric acid at discharge, (mg/dl) | 6.4 (5.3–7.6) | 6.8 (5.3–8.1) |
| CRP at discharge, (mg/dl) | 0.1 (0.1–0.4) | 0.2 (0.1–0.7) |
| BNP at discharge, (pg/ml) | 196 (117–407) | 294 (165–534) |
| %FS at admission | 18.8 (10.1–29.1) | 17.2 (9.7–32.1) |
| LVDd at admission | 58 (49–65) | 58 (48–67) |
| LVDs at admission, (mm) | 47 (34–57) | 47 (32–58) |
| %FS at discharge, (%) | 21.8 (10.5–31.5) | 19 (10−32) |
| LVDd at discharge | 57 (49–63) | 59 (48–68) |
| LVDs at discharge | 45 (33–54) | 47 (32–60) |
| IVST at discharge, (mm) | 10 (8–11) | 10 (8–11) |
| AR grade (≥II) at discharge | 13 (11) | 13 (11) |
| MR grade (≥II) at discharge | 45 (39) | 48 (42) |
| TR grade (≥II) at discharge | 24 (21) | 35 (31) |
| Oral medications at discharge | ||
| ACE inhibitor | 66 (58) | 45 (39) |
| Anti-allergic | 3 (3) | 5 (4) |
| Anti-inflammatory drug | 25 (22) | 23 (20) |
| Antiplatelet | 17 (15) | 10 (9) |
| Antithyroid drug | 1 (1) | 2 (2) |
| Beta-blockers | 88 (77) | 73 (64) |
| Broncodilator | 0 (0) | 2 (2) |
| Choleretic drug | 4 (4) | 7 (6) |
| Digitalis | 16 (14) | 26 (23) |
| Diuretics | 89 (78) | 92 (81) |
| Inotropic agent | 4 (4) | 32 (28) |
| Intestinal disease drug | 5 (4) | 14 (12) |
| Lipid-lowering drug | 44 (39) | 35 (31) |
| Proton pump inhibitor | 62 (54) | 57 (50) |
| Purgative | 28 (25) | 35 (31) |
| Sedative-hypnotic (benzodiazepin) | 6 (5) | 6 (5) |
| Vitamins | 3 | 4 (4) |
Data are given as the Median (interquartile range) or n (%). ACE inhibitor, angiotensin-converting enzyme inhibitor; ADHF, acute decompensated heart failure; Af, atrial fibrillation; AR, aortic regurgitation; BNP, B-type natriuretic peptide; BUN, Blood urea nitrogen; CRT, cardiac resynchronization therapy; CRP, C-reactive protein; FS, fractional shortening; ICD, Implantable Cardioverter Defibrillator; VST, interventricular septum thickness; LVDs, Left ventricular end-systolic dimension MR, mitral regurgitation; NYHA, New York Heart Association; TR, tricuspid regurgitation.
2.3. Analytic Procedures for the Third Screening
All data related to the events prior to discharge were evaluated in our investigation of the known or unknown factors that contribute to cardiovascular events and are listed in Table 1. We used the novel LAMP to our data-mining initiative to identify both single factors and combinations of factors that would significantly affect the occurrence of cardiovascular events [13]. In our analysis, a patient with HF was represented by both individual clinical factors and the class labels of groups with or without cardiovascular events, and the set of the patients was used to form a data table in which each row represented a patient. This data table D comprises N rows, each of which consists of M factors and a positive or negative class label of an object. Accordingly, LAMP uses Fisher's exact test to draw conclusions from a complete set of statistically significant hypotheses regarding a class label. Here, the hypothesis is based on a combination of a class label and a condition defined as a subset of the M factors in D. As the condition of the uncovered significant hypothesis may include any number of factors from 1 to M, the term ‘limitless-arity’ has been used to describe this method. Accordingly, LAMP applies a highly efficient search algorithm to quickly and completely derive significant hypotheses from 2M candidates.
If k is the number of all hypotheses for which the conditions exceed or remain equal to σ objects in D (σ < N), the relationship between k and σ, k = kD(σ) depends on D but is always anti-monotonic because fewer hypothesis conditions remain true at a higher frequency in D. Although the formula of kD(σ) is not analytically determined, LAMP includes a mining algorithm used to efficiently derive all k hypothesis conditions under a given σ. The Bonferroni correction, which sets a boundary for the family-wise error rate of the false negative in the multiple tests at <1 significance level α by correcting the level to α/kD(σ), can be used as a standard multiple testing procedure for the k hypotheses. Note that this level is monotonic to σ, as kD(σ) is anti-monotonic. If we use a very small set value of σ for a complete search of the significant hypotheses, α/kD(σ) is extremely small because kD(σ) approaches 2M. In this scenario, almost no hypotheses will be accepted as significant. Conversely, if the set value of σ and, consequently, α/kD(σ) is too large, kD(σ) will be very small and some significant hypothesis conditions will be missed. To overcome this limitation, LAMP uses the fact that any hypothesis with a frequency less than σ will not have a p value less than the following level.
Here, np is the number of the objects with positive class labels in D (np < N). Accordingly, any hypothesis with a frequency less than σ will not be accepted if f(σ) > α/kD(σ). Because f(σ) is anti-monotonic for σ and α/kD(σ) is monotonic, LAMP selects σ⁎ to balance f(σ⁎) and α/kD(σ⁎). The selected value of σ⁎ yields the smallest number of candidate hypotheses without applying the tests or missing any significant hypotheses.
For practical reasons, we were interested in a hypothesis that would hold true for at least 19 patients. As all hypotheses involving more than four factors failed to meet this criterion, we limited our LAMP-based search of the hypotheses to a maximum of four factors. This limitation further reduced the number kD(σ⁎) of the candidate hypotheses and increased the level α/kD(σ⁎) in LAMP. After we obtained all significant hypotheses regarding single clinical factors or combinations of factors, we excluded each hypothesis for which the condition was a superset of the conditions from other simpler hypotheses, as the significance of the former would be trivial in comparison with the significance of the latter. Once we had narrowed down all single or combination clinical parameters to single or combinational clinical factors, we used a Kaplan–Meier analysis to test whether these clinical factors could predict cardiovascular events among the enrolled patients.
3. Results
Table 1 lists the patients' clinical characteristics, whereas Table 2 stratifies the characteristics of patients who did and did not experience cardiovascular events. We next performed a LAMP analysis that maintained the family-wise error rate below the required significance level by calibrating the Bonferroni factor to examine the significant combinations of these 54 clinical parameters and thus characterised the cardiovascular outcomes. In our analysis of 77,562 combinations with no >4 clinical parameters, we identified 151 combinations involving 54 parameters that predicted the occurrence of cardiovascular events (Table 3). Among these 151 combinations, 145 included the use of inotropic agents as a factor, which was also found to significantly correlate with the occurrence of cardiovascular events as a single factor (Rank 1 in Table 3). Therefore, we pooled all ranks that included the use of inotropic agents (Category 1 in Table 4). Of the remaining six combinations (Category 2 in Table 4), all included the use of diuretics without either bradycardia or tachycardia as a factor. We defined either tachycardia and bradycardia as heart rate >100/min or <50/min. As none of the combinations excluded both of these factors (Table 4), this suggests that the use of inotropic agents or the use of diuretics without either bradycardia or tachycardia may be the most essential clinical factors predictive of the likelihood of cardiovascular events in patients with HF.
Table 3.
The combinations of clinical parameters to predict the occurrence of the cardiovascular events.
| Rank | The combination of clinical parameters | Adjusted p-value | |||
|---|---|---|---|---|---|
| 1 | The use of inotropic agents | 0.00071 | |||
| 2 | The use of diuretics | The use of inotropic agents | 0.00071 | ||
| 3 | The use of diuretics | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.00071 | |
| 4 | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.00071 | ||
| 5 | The use of diuretics | In nyha class iii or ivat admission | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | 0.00237 | |
| 6 | The use of diuretics | In nyha class iii or ivat admission | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.00237 |
| 7 | The use of diuretics | In nyha class iii or ivat admission | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | Living with family members in the same household | 0.00262 |
| 8 | The use of inotropic agents | In nyha class iii or ivat admission | 0.00383 | ||
| 9 | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | In nyha class iii or ivat admission | 0.00383 | |
| 10 | The use of diuretics | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | In nyha class iii or ivat admission | 0.00383 |
| 11 | The use of diuretics | The use of inotropic agents | In nyha class iii or ivat admission | 0.00383 | |
| 12 | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | 0.00383 | ||
| 13 | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.00383 | |
| 14 | The use of diuretics | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.00383 |
| 15 | The use of diuretics | The use of inotropic agents | The abnormal value of lvds (34 mm <) at admission | 0.00383 | |
| 16 | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | 0.00383 | ||
| 17 | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.00383 | |
| 18 | The use of diuretics | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.00383 |
| 19 | The use of diuretics | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | 0.00383 | |
| 20 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | 0.00383 | ||
| 21 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.00383 | |
| 22 | The use of diuretics | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.00383 |
| 23 | The use of diuretics | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | 0.00383 | |
| 24 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | 0.00871 | ||
| 25 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvds (34 mm <) at admission | 0.00871 | |
| 26 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of BNP (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.00871 |
| 27 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The use of diuretics | The abnormal value of lvds (34 mm <) at admission | 0.00871 |
| 28 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of BNP (18.4 pg/ml <) at discharge | 0.00871 | |
| 29 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The use of diuretics | The abnormal value of BNP (18.4 pg/ml <) at discharge | 0.00871 |
| 30 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The use of diuretics | 0.00871 | |
| 31 | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.00871 | |
| 32 | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.00871 |
| 33 | The use of diuretics | The use of inotropic agents | The abnormal value of lvds (34 mm <) at admission | The abnormal value of lvds (34 mm <) at discharge | 0.00871 |
| 34 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.00871 | |
| 35 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.00871 |
| 36 | The use of diuretics | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.00871 |
| 37 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of %FS (<30%) at discharge | 0.00871 | |
| 38 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of %FS (<30%) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.00871 |
| 39 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of %FS (<30%) at discharge | The abnormal value of BNP (18.4 pg/ml <) at discharge | 0.00871 |
| 40 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of %FS (<30%) at discharge | The use of diuretics | 0.00871 |
| 41 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.00871 | |
| 42 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.00871 |
| 43 | The use of diuretics | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.00871 |
| 44 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | 0.00871 | ||
| 45 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.00871 | |
| 46 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.00871 |
| 47 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | 0.00871 | |
| 48 | The use of diuretics | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | 0.01388 | ||
| 49 | The use of inotropic agents | With leg edema | 0.01857 | ||
| 50 | The use of inotropic agents | With leg edema | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.01857 | |
| 51 | The use of diuretics | The use of inotropic agents | With leg edema | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.01857 |
| 52 | The use of diuretics | The use of inotropic agents | With leg edema | 0.01857 | |
| 53 | The use of diuretics | The abnormal value of bnp (18.4 pg/ml <) at discharge | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | 0.01873 | |
| 54 | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | 0.0196 | ||
| 55 | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.0196 | |
| 56 | The use of diuretics | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.0196 |
| 57 | The use of diuretics | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | 0.0196 | |
| 58 | The use of inotropic agents | In nyha class iii or ivat admission | The abnormal value of lvds (34 mm <) at admission | 0.0196 | |
| 59 | The use of inotropic agents | In nyha class iii or ivat admission | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.0196 |
| 60 | The use of diuretics | The use of inotropic agents | In nyha class iii or ivat admission | The abnormal value of lvds (34 mm <) at admission | 0.0196 |
| 61 | The use of inotropic agents | In nyha class iii or ivat admission | The abnormal value of lvds (34 mm <) at discharge | 0.0196 | |
| 62 | The use of inotropic agents | In nyha class iii or ivat admission | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 63 | The use of diuretics | The use of inotropic agents | In nyha class iii or ivat admission | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 64 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | 0.0196 | |
| 65 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvds (34 mm <) at admission | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 66 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of BNP (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 67 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The use of diuretics | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 68 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | In nyha class iii or ivat admission | 0.0196 | |
| 69 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | In nyha class iii or ivat admission | 0.0196 |
| 70 | The use of diuretics | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | In nyha class iii or ivat admission | 0.0196 |
| 71 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvds (34 mm <) at admission | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 72 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of %FS (<30%) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 73 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.0196 | |
| 74 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.0196 |
| 75 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.0196 |
| 76 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 | |
| 77 | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 78 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 79 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 80 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | 0.0196 | ||
| 81 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.0196 | |
| 82 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 83 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.0196 |
| 84 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.0196 |
| 85 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.0196 |
| 86 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 | |
| 87 | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 88 | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 89 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.0196 |
| 90 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.0196 | |
| 91 | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.0196 |
| 92 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.0196 |
| 93 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.0196 | |
| 94 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.0196 |
| 95 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | 0.0196 | |
| 96 | The use of inotropic agents | With leg edema | In nyha class iii or ivat admission | 0.04253 | |
| 97 | The use of inotropic agents | With leg edema | The abnormal value of bnp (18.4 pg/ml <) at discharge | In nyha class iii or ivat admission | 0.04253 |
| 98 | The use of diuretics | The use of inotropic agents | With leg edema | In nyha class iii or ivat admission | 0.04253 |
| 99 | The use of inotropic agents | Living with family members in the same household | 0.04356 | ||
| 100 | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | Living with family members in the same household | 0.04356 | |
| 101 | The use of diuretics | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | Living with family members in the same household | 0.04356 |
| 102 | The use of diuretics | The use of inotropic agents | Living with family members in the same household | 0.04356 | |
| 103 | The use of inotropic agents | The use of beta-blockers | 0.04356 | ||
| 104 | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | The use of beta-blockers | 0.04356 | |
| 105 | The use of diuretics | The use of inotropic agents | The abnormal value of bnp (18.4 pg/ml <) at discharge | The use of beta-blockers | 0.04356 |
| 106 | The use of diuretics | The use of inotropic agents | The use of beta-blockers | 0.04356 | |
| 107 | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of lvds (34 mm <) at admission | 0.04356 | |
| 108 | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.04356 |
| 109 | The use of diuretics | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of lvds (34 mm <) at admission | 0.04356 |
| 110 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | In NYHA class III or ivat admission | 0.04356 | |
| 111 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvds (34 mm <) at admission | In NYHA class III or ivat admission | 0.04356 |
| 112 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of BNP (18.4 pg/ml <) at discharge | In NYHA class III or ivat admission | 0.04356 |
| 113 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The use of diuretics | In NYHA class III or ivat admission | 0.04356 |
| 114 | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of lvds (34 mm <) at discharge | 0.04356 | |
| 115 | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of bnp (18.4 pg/ml <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.04356 |
| 116 | The use of diuretics | The use of inotropic agents | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of lvds (34 mm <) at discharge | 0.04356 |
| 117 | The use of inotropic agents | The abnormal value of lvds (34 mm <) at discharge | In nyha class iii or ivat admission | The abnormal value of lvds (34 mm <) at discharge | 0.04356 |
| 118 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | 0.04356 | |
| 119 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.04356 |
| 120 | The use of diuretics | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | 0.04356 |
| 121 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | In nyha class iii or ivat admission | The abnormal value of lvds (34 mm <) at admission | 0.04356 |
| 122 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of %FS (<30%) at discharge | In NYHA class III or ivat admission | 0.04356 |
| 123 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | In nyha class iii or ivat admission | The abnormal value of lvds (34 mm <) at discharge | 0.04356 |
| 124 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | In nyha class iii or ivat admission | 0.04356 | |
| 125 | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | In nyha class iii or ivat admission | 0.04356 |
| 126 | The use of diuretics | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | In nyha class iii or ivat admission | 0.04356 |
| 127 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 | |
| 128 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.04356 |
| 129 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of BNP (18.4 pg/ml <) at discharge | 0.04356 |
| 130 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The use of diuretics | 0.04356 |
| 131 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.04356 |
| 132 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 | |
| 133 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.04356 |
| 134 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of %FS (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 |
| 135 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.04356 |
| 136 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.04356 |
| 137 | The use of diuretics | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 |
| 138 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 | |
| 139 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.04356 |
| 140 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.04356 |
| 141 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 |
| 142 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of BNP (18.4 pg/ml <) at discharge | 0.04356 |
| 143 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of lvdd (52 mm <) at discharge | The use of diuretics | 0.04356 |
| 144 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 | |
| 145 | The abnormal value of %FS (<30%) at admission | The use of inotropic agents | The abnormal value of %FS (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 |
| 146 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at admission | 0.04356 |
| 147 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvds (34 mm <) at discharge | 0.04356 |
| 148 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of lvdd (52 mm <) at discharge | 0.04356 |
| 149 | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of lvdd (52 mm <) at discharge | The abnormal value of bnp (18.4 pg/ml <) at discharge | 0.04356 |
| 150 | The use of diuretics | The use of inotropic agents | The abnormal value of %fs (<30%) at discharge | The abnormal value of LVDd (52 mm <) at discharge | 0.04356 |
| 151 | The use of diuretics | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | Living with family members in the same household | 0.04969 | |
Table 4.
Summary of the results of LAMP procedure.
| Category | The combination of clinical parameters | Number of the combination of clinical parameters | |||
|---|---|---|---|---|---|
| 1 | The use of inotropic agents | 145 | |||
| 2 | The use of diuretics | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | In NYHA class III or IVat admission | 1 | |
| The use of diuretics | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | In NYHA class III or IVat admission | The abnormal value of BNP (18.4 pg/ml <) at discharge | 1 | |
| The use of diuretics | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | In NYHA class III or IVat admission | Living with family members in the same household | 1 | |
| The use of diuretics | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | 1 | |||
| The use of diuretics | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | The abnormal value of BNP (18.4 pg/ml <) at discharge | 1 | ||
| The use of diuretics | Without either tachycardia (100 bpm <) or bradycardia(<50 bpm) | Living with family members in the same household | 1 | ||
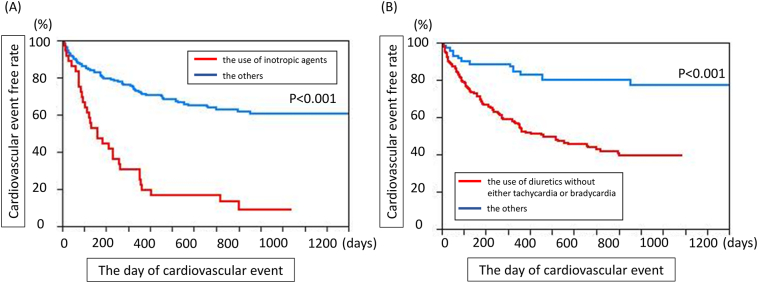
Finally, we conducted a Kaplan–Meier analysis of these two clinical factors to determine whether they could accurately predict the occurrence of cardiovascular events in this patient population. Notably, both the use of inotropic agents and the use of diuretics without either tachycardia or bradycardia were strong and significant predictors of the occurrence of cardiovascular events among patients with HF (Fig. 1).
Fig. 1.
Kaplan-Meier curves for the cardiovascular events using the use of inotropic agents (A) and the use of diuretics without either bradycardia or tachycardia (B) in the HF patients.
We further tested whether the approved treatment of HF such as angiotensin inhibitors (ACE-Is) is also found to be effective in the present cohort of the HF patients, and we found that ACE-Is seem to be effective in the prevention of cardiovascular events despite statistically insignificant levels of p = 0.08 (Fig. 2), indicating that the conventional and approved treatment strategies of HF patients seem to be effective in the present cohort. We further suggested that the use of pimobendan or the use of diuretics without either bradycardia or tachycardia more potently affects the severity of HF than ACE-Is, and may blunt the cardioprotective effects of ACE-Is.
Fig. 2.
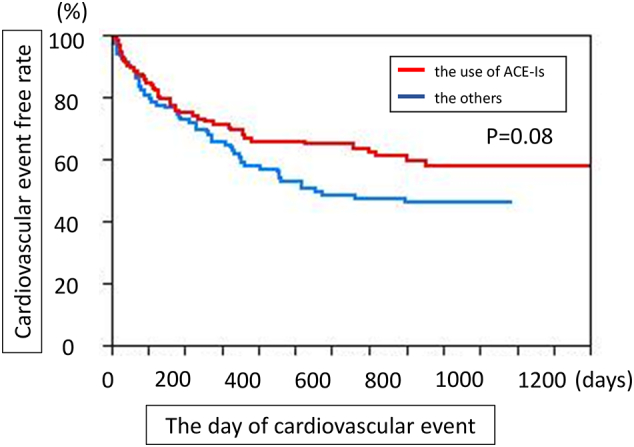
Kaplan-Meier curves for the cardiovascular events with and without the use of angiotensin converting enzymes (ACE-Is), the conventional and effective treatment of HF in the HF patients.
4. Discussion
The effects of the present investigation are twofold. First, this study provides new pathophysiological evidence of the potential risk factors indicative of more severe HF; second, this research proposes a novel big data analysis strategy based on the new data-mining method, LAMP.
4.1. Ultimate Clinical Factors Affecting the Occurrence of Cardiovascular Outcomes
The present study has shown that either the use of inotropic agents or the use of diuretics without either bradycardia or tachycardia is a strong predictor of cardiovascular outcomes in patients with HF. Regarding the former factor, pimobendan was exclusively used in the present study because we considered digitalis to be an independent drug class rather than an inotropic agent. Indeed, a previous study found that although digoxin did not reduce the overall mortality, it reduced the rate of hospitalisation both overall and for worsening HF [16]. In the present study, the use of digitalis was not found to significantly reduce the incidence of cardiovascular events. By contrast, pimobendan was previously reported to improve the exercise capacity in patients with HF, although it was also associated with a 1.8-fold higher hazard of death [12]. Although pimobendan is often used for weaning from intravenous inotropic agents (e.g. PDE III inhibitors) [17], the present study suggests that this drug should not be administered to patients with HF. Furthermore, patients with HF who are already treated with pimobendan should be monitored carefully, given the high probability of the occurrence of the cardiovascular events.
As noted above, the use of diuretics also increased the risk of cardiovascular events among patients without either tachycardia or bradycardia. Consistent with our findings, a previous report described the difficulty of using diuretics to improve cardiovascular outcomes [18], and another study reported that vasodilators were superior to diuretics in terms of improved oxygen saturation and pulmonary ventilation [19]. In the present study, furosemide was the most frequently administered diuretic. However, furosemide may have the following detrimental effects: [1] exacerbation of renal dysfunction, [2] hyponatraemia and [3] activation of the renin–angiotensin and sympathetic nerve systems, which may worsen the clinical outcomes [20, 21]. These findings indicate that although diuretics may reduce symptoms, they do not improve cardiovascular outcomes [22].
Intriguingly, the second term specified diuretics ‘without either bradycardia or tachycardia’ as predictive of the occurrence of cardiovascular outcomes, leading us to wonder how the heart rate is involved; we were unable to determine an exact answer for this issue. Possibly, treatment with diuretics activates the sympathetic nervous system and, consequently, heart rate. Accordingly, the condition of diuretics without tachycardia may encompass patients in whom the sympathetic nerve system is exhausted even in the presence of diuretics (i.e. patients with more severe HF). Regardless of the underlying mechanism, we should focus on the present use of inotropic agents or the use of diuretics without either bradycardia or tachycardia as the strongest predictors of an increased risk of cardiovascular events in patients with HF.
4.2. Novel Mathematical Evaluation Protocol and Data-Centric Medicine
The present study has proposed the expediency of big data mining based on the LAMP [13] with the intent to identify unexpected single or combinational factors predictive of cardiovascular events. Briefly, data-mining methods are used to examine all possible combinations of all clinical parameters that might affect cardiovascular outcomes [23, 24]. This approach allowed us to employ and test both single and combinations of clinical parameters that might not appear to be directly linked to cardiovascular events. By contrast, a multivariate analysis evaluates the effects of each parameter on the clinical outcomes but cannot determine the effects of combinational factors. As noted above, LAMP minimises false negatives by calibrating the Bonferroni factor, maintains statistical power under multiple comparisons and provides the significant p values for each factor against the outcomes. Still, the factors identified using LAMP should be confirmed using ordinary statistical methods. In this study, we observed significantly different ratios of patients with and without cardiovascular events after dichotomising the patients according to each single or combinational factor (Fig. 1). Finally, these data-mining methods can be used in medical fields wherein cause–effect relationships are difficult to identify [25]. As for the required number of the data to be collected, there is no upper or lower limitation, however, when the data number is small, we cannot obtain the large number of the combination of the factors to explain the objective function.
4.3. Limitations of the Present Study
This study had a couple of noteworthy limitations. First, the study included a relatively small sample of patients. However, we achieved high levels of significance when we applied the use of inotropic agents or the use of diuretics without either bradycardia or tachycardia to determine the presence or absence of cardiovascular events, which suggests that the results in the present study are reliable. Additionally, our results were based on data from three Japanese hospitals that specialised in the treatment of HF. The results of the multicentre clinical trials are superior to those of the single center trails because the results of the multicetre clinical trials are more comprehensive. Interestingly, these three hospitals are Hokkaido University located in the north of Japan, National Cerebral and Cardiovascular Center at the center of Japan and Kyushu University at the southern part, which may guarantee the applicability of the present finding throughout Japan. One may argue that the present results may not be valid in other countries; however, as long as the pathophysiology and treatment strategy of HF are common worldwide, the present results should be valid to provide the future occurrence of cardiovascular events in other countries.
Second, we enrolled the moderate severity of the patients with HF in the present study, and the present results may not be applicable for very severe HF patients.
Third, it would be possible that the medications are given to sicker patients, and that the use of such medications may naturally predict the occurrence of the cardiovascular events. However, among measured many clinical parameters such as the BNP levels or used many drugs in HF patients, we found the use of pimobendan or the use of diuretics under the certain circumstance of heart rate only predicts cardiovascular events. What the present study suggest is that the patients treated with pimobendan or diuretics are very easily re-hospitalized due to the worsening of HF. Indeed, since pimobendan provided a 1.8-fold higher hazard of death in HF patients, we need to be careful to treat the HF patients with pimobendan. Although we cannot deny the possibility that pimobendan is used the severe HF patients, we are cautioned that we try not to use pimobendan for the HF patients.
Fourth, the use of beta-blockers or ACE-Is was not included among the strongest clinical parameters in the present study, although ACE-Is have some impacts on the prevention of cardiovascular events (Fig. 2). Although this finding might be expected to reduce the accuracy of the present study, both drugs are considered standard therapies for HF and are administered to many patients. Therefore, they no longer have a significant effect on the clinical outcomes. The other possibility is that the use of pimobendan or diuretics may confound the cardioprotective HF drugs such as ACE-Is in the cohort study, not in the randomised studies.
Taken together, these lines of evidence and consideration suggest that either the use of inotropic agents or the use of diuretics without either bradycardia or tachycardia culminated from the examination of all combination of the important clinical parameters is the strongest in predicting cardiovascular events in the HF patients in the contemporary era.
5. Conclusion
In conclusion, this analysis, which was based on the novel big data-mining technique, LAMP, identified the use of inotropic agents or the use of diuretics without either bradycardia or tachycardia as the most deleterious clinical parameters affecting patients receiving standard therapies for HF.
Acknowledgments
Acknowledgement
None.
Funding Sources
This research was funded by Grants-in-Aid from the Ministry of Health, Labour and Welfare of Japan; Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and Grants-in-Aid from the Japan Agency for Medical Research and Development (JP17ek0210080). These funding sources did not play any role in the study design, data colletion, data anaylsis, interpretation, writing of reports or decision to submit the paper for the publication in the present study.
Declaration of Interests
Nothing to disclose for Hiroki Fukuda, Kazuhiro Shindo, Mari Sakamoto, Tomomi Ide, Shintaro Kinugawa, Arata Fukushima, Akira Ishii, Shin Ito, and Takashi Washio. Hiroyuki Tsutsui reports personal fees from Astellas, personal fees from Ohtsuka, personal fees from Takeda, personal fees from Daiichi-Sankyo, personal fees from Tanabe-Mitsubishi, personal fees from Nippon Boehringer Ingelheim, personal fees from Novartis, personal fees from Bayer, personal fees from Bristol Myers Squibb, outside the submitted work. Masafumi Kitakaze reports grants from Japanese government, during the conduct of the study; grants from Japanese government, grants from Japan Heart Foundation, grants from Japan Cardiovascular Research Foundation, grants and personal fees from Asteras, personal fees from Daiichi-sankyo, grants and personal fees from Pfizer, grants and personal fees from Ono, personal fees from Bayer, grants from Novartis, grants and personal fees from Tanabe-mitubishi, personal fees from Kowa, personal fees from MSD, grants from Nihon Kohden, personal fees from Shionogi, personal fees from Astrazeneca, grants and personal fees from Astra Zeneca, personal fees from Taisho-Toyama, personal fees from Toyama-Kagaku, grants and personal fees from Kureha, personal fees from Toaeiyo, outside the submitted work.
Authors Contributions
The authors contribution are as follows; Study concept and design: Takashi Washio, Masafumi Kitakaze; Data collection, Mari Sakamoto, Tomomi Ide, Shintaro Kinugawa, Arata Fukushima, Hiroyuki Tsutsui; Data analysis: Hiroki Fukuda, Akira Ishii, Shin Ito; Figures and Tables: Hiroki Fukuda, Kazuhiro Shindo; Writing: Masafumi Kitakaze.
References
- 1.Braunwald E., Bristow M.R. Congestive heart failure: Fifty years of progress. Circulation. 2000;102:Iv14–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosy A.P., Fonarow G.C., Butler J. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Thom T., Haase N., Rosamond W. Heart disease and stroke statistics—2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 4.Jessup M., Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 5.Levy D., Kenchaiah S., Larson M.G. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 6.McMurray J.J., Adamopoulos S., Anker S.D. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33 doi: 10.1093/eurheartj/ehs104. (1787-847) [DOI] [PubMed] [Google Scholar]
- 7.Fukuda H., Suwa H., Nakano A. Non-linear equation using plasma brain natriuretic peptide levels to predict cardiovascular outcomes in patients with heart failure. Sci Rep. 2016;6 doi: 10.1038/srep37073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalogeropoulos A., Georgiopoulou V., Psaty B.M. Inflammatory markers and incident heart failure risk in older adults: The health ABC (health, aging, and body composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson C.E., Solomon S.D., Gerstein H.C. Albuminuria in chronic heart failure: Prevalence and prognostic importance. Lancet. 2009;374:543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 10.Garg R., Yusuf S., Bussmann W.D. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA. 1995;273:1450–1456. [PubMed] [Google Scholar]
- 11.Felker G.M., Lee K.L., Bull D.A. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lubsen J., Just H., Hjalmarsson A.C. Effect of pimobendan on exercise capacity in patients with heart failure: Main results from the pimobendan in Congestive Heart Failure (PICO) trial. Heart (British Cardiac Society) 1996;76:223–231. doi: 10.1136/hrt.76.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terada A., Okada-Hatakeyama M., Tsuda K., Sese J. Statistical significance of combinatorial regulations. Proc Natl Acad Sci U S A. 2013;110:12996–13001. doi: 10.1073/pnas.1302233110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida A., Asakura M., Asanuma H. Derivation of a mathematical expression for predicting the time to cardiac events in patients with heart failure: A retrospective clinical study. Hypertens. Res. 2013;36:450–456. doi: 10.1038/hr.2012.200. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto M., Fukuda H., Kim J. The impact of creating mathematical formula to predict cardiovascular events in patients with heart failure. Sci Rep. 2018;8:3986. doi: 10.1038/s41598-018-22347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The effect of digoxin on mortality and morbidity in patients with heart failure. The N. Engl. J. Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 17.Endoh M., Hori M. Acute heart failure: Inotropic agents and their clinical uses. Expert Opin Pharmacother. 2006;7:2179–2202. doi: 10.1517/14656566.7.16.2179. [DOI] [PubMed] [Google Scholar]
- 18.Shah M.R., Stevenson L.W. Searching for evidence: Refractory questions in advanced heart failure. J Card Fail. 2004;10:210–218. doi: 10.1016/j.cardfail.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Cotter G., Metzkor E., Kaluski E. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351:389–393. doi: 10.1016/S0140-6736(97)08417-1. [DOI] [PubMed] [Google Scholar]
- 20.Bayliss J., Norell M., Canepa-Anson R., Sutton G., Poole-Wilson P. Untreated heart failure: Clinical and neuroendocrine effects of introducing diuretics. Br Heart J. 1987;57:17–22. doi: 10.1136/hrt.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen J.S., DiBona G.F. Reflex control of renal sympathetic nerve activity during furosemide diuresis in rats. Am J Physiol. 1994;266:R537–R545. doi: 10.1152/ajpregu.1994.266.2.R537. [DOI] [PubMed] [Google Scholar]
- 22.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62 doi: 10.1016/j.jacc.2013.05.019. (e147-239) [DOI] [PubMed] [Google Scholar]
- 23.Podgorelec V., Kokol P., Stiglic B., Rozman I. Decision trees: An overview and their use in medicine. J Med Syst. 2002;26:445–463. doi: 10.1023/a:1016409317640. [DOI] [PubMed] [Google Scholar]
- 24.Kim J., Washio T., Yamagishi M. A novel data mining approach to the identification of effective drugs or combinations for targeted endpoints—application to chronic heart failure as a new form of evidence-based medicine. Cardiovascular Drugs Ther. 2004;18:483–489. doi: 10.1007/s10557-004-6226-y. [DOI] [PubMed] [Google Scholar]
- 25.Hey T., Tansley S., Tolle K.M. Microsoft Research Redmond; WA: 2009. The fourth paradigm: Data-intensive scientific discovery. [Google Scholar]