Abstract
Immunotherapeutic agents have demonstrated encouraging signs of clinical utility in non-Hodgkin lymphoma. The goal of this study is to analyze the immune characteristics of Chinese patients with diffuse large B-cell lymphoma (DLBCL) to inform the development of immunotherapies in this patient population. Tumor samples from 211 DLBCL patients were analyzed for cell of origin (COO) and immune characteristics using the NanoString platform as well as MYC protein expression through immunohistochemistry. Lower incidence of the germinal center B-cell (GCB) subtype (93/211, 44.1%) was observed in this cohort. Compared to the GCB subtype, the activated B-cell (ABC) subtype was associated with significantly increased expression of multiple pro-inflammatory gene signatures and decreased expression of anti-inflammatory gene signatures. Instead of affecting the pro-inflammatory genes, MYC protein overexpression showed a negative correlation with the expression of T-cell receptor (TCR) and T regulatory genes as well as the OX40 gene. Regardless of COO, higher PD-L1 or IDO1 gene expression correlated with increased expression of T effector and Interferon-γ gene signatures while the expression of multiple oncogenes including ACTR3B, ERBB2, AKT2 and SMARCD1 was down-regulated. Our findings may thus be helpful in guiding further development of immunotherapies for the different subsets of Chinese DLBCL patients.
Keywords: DLBCL, Cell of origin, Immune characteristics, Immunotherapy
Highlights
-
•
The ABC subtype has higher pro-inflammatory gene expression than the GCB subtype.
-
•
MYC protein expression correlates with lower TCR and T regulatory gene expression.
-
•
Higher PD-L1 or IDO1 gene expression indicates more inflammatory phenotypes.
The revolutionary efficacy of immunotherapies in some solid tumors has prompted much enthusiasm for applying such treatments in lymphomas including diffuse large B-cell lymphoma (DLBCL). To help inform and guide the drug development efforts in this field, it is important to gain a deep understanding of immune characteristics of DLBCL patients. Currently such data are lacking, especially in Chinese patients. NanoString is a high-throughput, easy-to-use platform that allows accurate quantification of the mRNA expression of hundreds of genes simultaneously. In addition, there is a commercialized DLBCL subtying assay, Lymph2Cx, available on this platform. Therefore, we used mainly the NanoString platform to analyze tumor samples from 211 Chinese DLBCL patients to understand their immune characteristics.
1. Introduction
Cancer immunotherapies have proven efficacious in multiple solid tumors. In particular, agents targeting the programmed death 1 (PD1) pathway have been applied to treat melanoma, lung cancer, renal cell carcinoma, head and neck cancer and bladder cancer [[1], [2], [3], [4], [5], [6], [7]]. Recent clinical results showed that such agents also have utilities in classical Hodgkin lymphoma. In a Phase II study that used the PD1 antibody Nivolumab to treat relapsed/refractory Hodgkin lymphoma after failure with autologous stem cell transplantation and brentuximab vedotin, the ORR was 66.3% with complete remission at 8.8% [8]. Similar response rates were reported for another PD1 antibody Pembrolizumab [9]. In non-Hodgkin lymphoma (NHL), however, the clinical efficacy of immunotherapeutic agents has not yet been demonstrated. In a phase I study of Nivolumab, the response rates were only 40% and 36% for 10 patients with diffuse large B-cell lymphoma (DLBCL) and 11 patients with follicular lymphoma, respectively [10]. Another immunotherapy target is indoleamine 2,3-dioxygenase-1 (IDO1) which is an immunosuppressive enzyme that catalyzes the degradation of tryptophan to N-formyl-kynurenine [11]. IDO1 is over-expressed in multiple tumor types, including acute myeloid leukemia [12], and seems to be an independent prognostic factor for inferior survival [[13], [14], [15], [16], [17], [18], [19], [20]]. IDO1 inhibitors are generally ineffective as mono-therapies for cancer [21], but have shown promising signs of efficacy in phase I/II clinical trials in combinations with anti-PD1/PD-L1 agents for non-small cell lung cancer [22], renal cell carcinoma [23], squamous cell carcinoma of the head and neck [24], advanced urothelial carcinoma [25] and triple-negative breast cancer [26]. OX40 has also emerged as a promising target for cancer immunotherapies. Coded by the TNFRSF4 gene, OX40 is a member of the TNFR super-family [27] and is expressed by CD4+ and CD8+ T cells during antigen-specific priming [[28], [29], [30], [31]]. Promising signs of clinical efficacy and acceptable toxicity have been observed in a Phase 1 trial using an OX40 agonist monotherapy for patients with multiple solid tumors [32]. For stronger efficacy, anti-OX40 treatment is now also being tested in combinations with various chemo-, radio-, targeted and other immuno-therapies [33].
DLBCL represents the most common subtype of NHL and is heterogeneous in clinical, immunophenotypic and genetic features. Using gene expression profiling, patients were assigned to three subgroups of different cell of origin (COO): germinal center B-cell (GCB) group, activated B-cell (ABC) group, and unclassified group [34, 35]. These subgroups have distinct biology, pathogenesis and response to standard immunochemotherapies such as rituximab combined with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) [[35], [36], [37]]. In addition, the MYC oncogene is known to play an important role in the development and clinical characteristics of DLBCL [38]. MYC protein is overexpressed (≥40% tumor cell staining) in about 30% of DLBCL patients and has been found to be an unfavorable prognostic factor [39, 40].
In terms of immune characteristics associated with clinical response to immunotherapies, data from solid tumor studies showed that high PD-L1 expression [5, 41], pre-existing CD8 + T cell infiltration [42], and/or high T effector gene signatures [41] predict better response to anti-PD/PD-L1 therapies. More recently, micro-satellite instability and high tumor mutation burden were found to correlate with better efficacy of this type of therapies [[43], [44], [45], [46], [47]]. Such data on DLBCL are much more limited, with only preliminary results suggesting that viral infection (especially by EBV) and T-cell/histocyte-rich large cell lymphoma histology seemed to be associated with PD-L1 overexpression [48]. Even fewer data are available for anti-IDO1 and anti-OX40 treatments. In the present study, COO-related genes and 805 immunity-related genes were assessed using the NanoString platform in a large cohort of Chinese patients with previously untreated DLBCL. Their correlation with the aforementioned existing molecular subtypes was then examined to see whether different DLBCL subtypes have distinct immunological characteristics and to examine the gene expression of important potential immunotherapy targets including PD-L1, IDO1 and OX40. The results may help formulate biomarker plans in prospective clinical trials for identifying the patient subsets that will respond to anti-PD1/PD-L1, anti-IDO1 and/or anti-OX40 treatment as well as other cancer immunotherapies.
2. Materials and Methods
2.1. Patients
From July 2013 to March 2016, 211 patients with de novo consecutive DLBCL were enrolled in this study. All the patients received R-CHOP-based regimens. The patients were selected according to the following criteria: histologically confirmed DLBCL according to the World Health Organization classification with complete clinical data and sufficient tissue for the analysis described below. Patients with primary mediastinal B-cell lymphoma were not included in this study.
This study was approved by the Institutional Review Board of Shanghai Rui Jin Hospital with informed consent obtained in accordance with the Declaration of Helsinki.
2.2. Immunohistochemistry (IHC) for MYC Expression and Hans Classification of COO
Hematoxylin-eosin-stained slides from all DLBCL patients underwent a centralized review to record tumor percentage by pathologists. All samples for IHC were provided as 4 μm formalin-fixed, paraffin-embedded (FFPE) sections.
IHC evaluation of MYC expression was performed using an anti c-MYC (Y69) rabbit monoclonal primary antibody (Ventana, Tucson, AZ, USA) on the BenchMark Ultra platform (Ventana, Tucson, AZ, USA) according to the manufacture's protocol. The primary antibody was incubated at 36 °C for 32 min and the chromogen was then revealed by OptiView DAB Detection Kit (Ventana, Tucson, AZ, USA). A cutoff value of 40% tumor cell staining was established from analysis of receiver-operating characteristic curves to achieve maximum specificity and sensitivity as described previously [49], dividing patients into MYC-High (≥40%) and MYC-Low (<40%) group.
According to the Hans classification, each patient was assigned as GCB or non-GCB using CD10, BCL6 and MUM1 immunohistochemistry staining. Thirty percent was considered positive cutoff with the antibody. Cases with discordant results were evaluated by two pathologists to reach consensus.
2.3. Nanostring Assay for COO
Total RNA was extracted from FFPE or fresh-frozen tumor tissue samples using the RNeasy MiniKit (QIAGEN, Hilden, Germany) according to the manufacturer's protocol. After qualification with Nanodrop (Thermo Scientific, Wilmington, DE, USA) and Qubit RNA Assay Kit (Life Technologies, Carlsbad, CA, USA), 200 ng of RNA was used for immune gene expression analysis.
RNA samples were subjected to the Lymph2Cx gene expression assay (NanoString Technologies, Seattle, WA, USA) according to the manufacturer's protocol. NanoString raw data were analyzed using the RUO version of the NanoString Lymphoma Subtyping Test (LST) algorithm [50] to determine the COO molecular subtype of each sample.
2.4. NanoString Assay for Immune Gene Expression
RNA samples were also used to evaluate the expression of 805 immunity-related genes on the NanoString platform (NanoString Technologies, Seattle, WA, USA). Probes and 200 ng total RNA were hybridized overnight at 65 °C according to the manufacturer's protocol. A NanoString nCounter Digital Analyzer was used to count the digital barcodes representing the number of transcripts. The raw counts data were normalized as the following before statistical analysis: first, the raw counts data were converted to log2 data; expression levels of each sample were adjusted by subtracting the median expression of its positive controls; after the positive control adjustment, the median and median absolute deviation of the background median values of all samples were used to define a background threshold; all samples with median below the background threshold were excluded from further analysis; for each sample, the median of its correlations with all other samples was calculated; any sample whose median of correlations is below the cut-off value (defined as three standard deviations from the mean of all samples' medians) was excluded from further analysis as outliers.
2.5. Differential Gene Expression Analysis
Gene expression difference is assessed according to COO subtypes, MYC protein expression, PD-L1 mRNA expression or IDO1 mRNA expression.
For COO subtypes, Kruskal-Wallis rank sum test was used to perform the differential gene expression analysis. Benjamini-Hochberg procedure by controlling the false discovery rate was used to adjust the p-values for multiple comparisons. Genes with adjusted p-value < 0.05 are selected as having distinct expression pattern between different COO subtypes. 163 selected genes are clustered into 4 groups according to consensus clustering method [51]. The clustering was implemented via the ConsensusClusterPlus R package. For MYC protein expression, PD-L1 mRNA expression or IDO1 mRNA expression, a linear model was used to perform differential expression analysis. It was implemented via the Bioconductor limma package. Benjamini-Hochberg procedure by controlling the false discovery rate was used to adjust the p-value for multiple comparisons. Adjusted p-value and logFC (FC: fold change) were used to select genes. For MYC protein expression, 90 genes with adjusted p-value < 0.1 and absolute value of logFC ≥ 0.5 were selected as differentially expressed ones. For PD-L1 mRNA expression and IDO1 mRNA expression (each using its median as the cut-off), 223 and 51 genes with adjusted p-value < 0.05 and absolute value of logFC ≥ 0.5 were selected as the differentially expressed ones, respectively.
2.6. Signaling Pathway Analysis
Signaling pathway analysis was carried out using the Ingenuity Pathway Analysis software (QIAGEN, Hilden, Germany). The top 10 differentially activated pathways between the GCB and the ABC subtypes were shown.
2.7. Gene Signature Expression Analysis
Gene signature expression was quantified by averaging the normalized NanoString data for all the genes included in each signature. P-value was then calculated using Mann-Whitney U test. If there were three groups, namely “GCB”, “ABC” and “Unclassified, in the analysis, the p-value was calculated for the correlation between the “GCB” and the “ABC” groups.
2.8. Survival Analysis
The progression-free survival (PFS) time was measured from the date of diagnosis to the first occurrence of progression, relapse or death while in the study. The overall survival (OS) time was measured from date of diagnosis to the date of death. Survival analysis was performed using the Kaplan-Meier method and the corresponding p-values were calculated using the log-rank test. The p-values reported are for descriptive purpose only. All statistical procedures were performed with the SPSS Version 20.0 statistical software package (IBM, New York, NY, USA) or GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Patient Demographics and Clinical Characteristics
The main clinicopathological characteristics of the 211 DLBCL patients are summarized in Table 1. The median age was 57 years old, ranging from 16 to 79 years old. The male:female ratio was 1.32:1. More than half of the patients were Ann Arbor stage I-II (56.9%) and at low risk according to the IPI score (51.7%). All the patients received R-CHOP-based regimens.
Table 1.
Demographic and clinical characteristics of study cohorts.
| Characteristics | TOTAL (N = 211) % | ||
|---|---|---|---|
| Age, years | Median (range) | 57 (16–79) | |
| >60 years | 83 | 39.3 | |
| Gender | Male | 120 | 56.9 |
| Female | 91 | 43.1 | |
| Ann Arbor stage | I/II | 120 | 56.9 |
| III/IV | 91 | 43.1 | |
| LDH | Normal | 123 | 58.3 |
| >ULN | 88 | 41.7 | |
| ECOG | 0–1 | 183 | 86.7 |
| 2–4 | 28 | 13.3 | |
| Extranodal sites | 0–1 | 155 | 73.5 |
| ≧2 | 56 | 26.5 | |
| IPI score | Low (0–1) | 109 | 51.7 |
| Intermediate (2–3) | 83 | 39.3 | |
| High (4–5) | 19 | 9.0 | |
Abbreviations: ECOG PS, Eastern Cooperate Oncology Group Performance Status; IPI: International Prognostic Index; LDH, Lactate Dehydrogenase; ULN, Upper Level of Normal
3.2. COO Profile
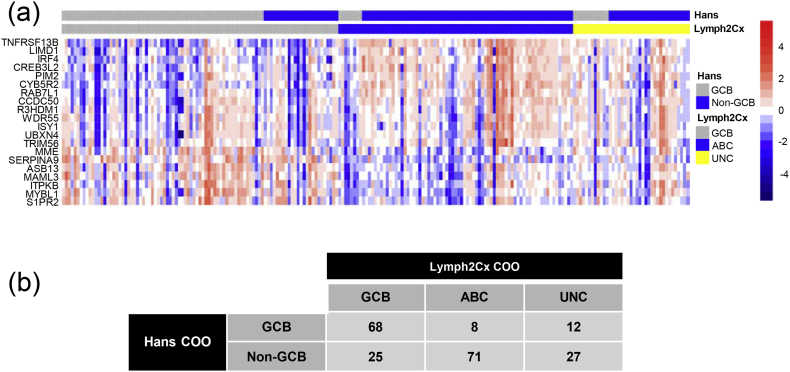
Using the Lymph2Cx assay, 93 (44.1%), 79 (37.4%) and 39 (18.5%) of the 211 samples were classified as the GCB subtype, the ABC subtype and the Unclassified subtype, respectively. These samples were also analyzed with the Hans IHC algorithm for COO [52] and the results of these two methods are shown in Fig. 1a and b. 25 out of 93 (26.9%) NanoString-GCB samples were classified as Non-GCB by the Hans assay and 8 out of 79 (10.1%) NanoString-ABC samples were classified as GCB by the Hans assay. The overall concordance was 78.7%. The median follow-up time was 32.7 months (0.3–58.0 months). The 2-year PFS was 79.9% for GCB patients and 67.4% for ABC patients (Log-rank 5.215, p = 0.0224). The 2-year OS was 88.1% for GCB patients and 79.2% for ABC patients (Log-rank 3.928, p = 0.0475).
Fig. 1.
Cell-of-origin (COO) subtyping and the correlation with progression-free survival. (a) COO assignment and the expression of the 20 genes included in the Lymph2Cx assay for the 211 patient samples in this study. Names of the 20 genes are shown at the left of the heatmap. The COO assignment from the Hans IHC algorithm is shown at the top of this panel for a comparison. (b) Numerical comparison of the Lymph2Cx and the Hans COO assignments.
3.3. COO and Immune Characteristics
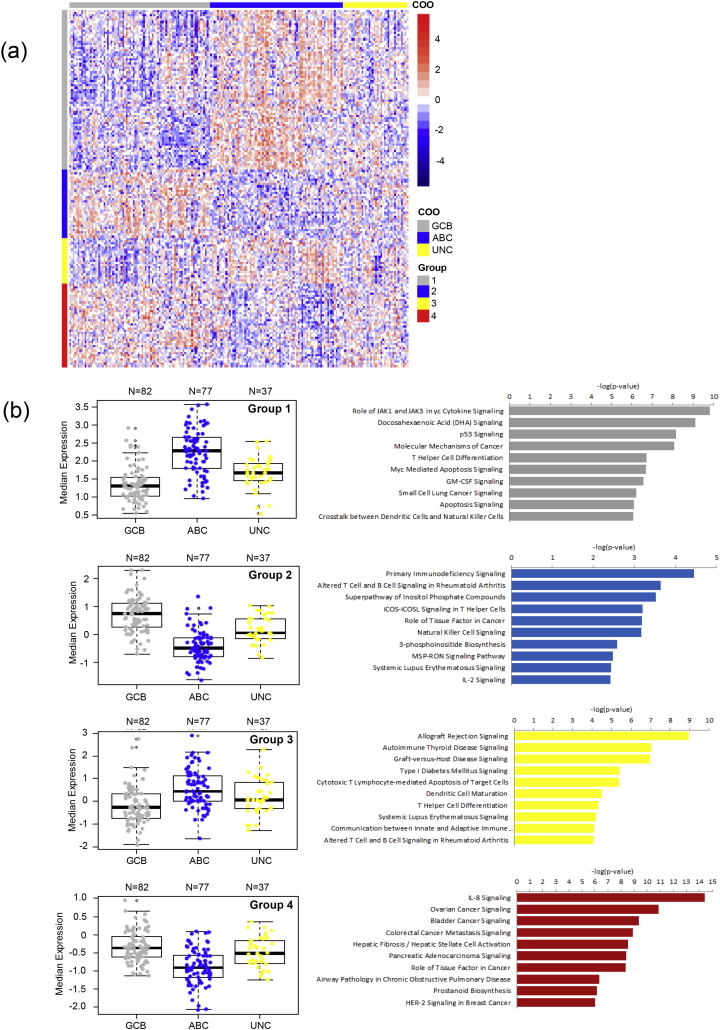
To study the immune characteristics of different COO subtypes, we conducted supervised clustering of NanoString gene expression data from the 805 immunity-related genes. 196 samples passed quality control and had both COO and immune gene expression results. Analysis of these samples resolved 4 groups of a total of 163 genes with distinct patterns of expression between the GCB, ABC and Unclassified groups (Fig. 2a; p < 0.001 for each group). Cell signaling pathway analysis showed that Allograft Rejection Signaling, Autoimmune Thyroid Disease Signaling, JAK1 and JAK3 in Cytokine Signaling, T Helper Cell Differentiation and MYC Mediated Apoptosis Signaling were among the top up-regulated pathways in the ABC subtypes while Primary Immunodeficiency Signaling, IL-8 Signaling, Ovarian Cancer Signaling, Bladder Cancer Signaling, Colorectal Cancer Metastasis Signaling and Pancreatic Adenocarcinoma Signaling were among the top up-regulated pathways in the GCB subtype (Fig. 2b).
Fig. 2.
Gene expression pattern and signaling pathway analysis according to the Lymph2Cx COO assignment. (a) The expression of 163 genes in our 805-gene NanoString panel by 196 of the 211 samples in this study. The expression of these 163 genes was significantly different between the ABC, GCB and Unclassified subgroups. The genes were further clustered into 4 groups (shown by the color bars at the left of the heatmap). The Lymph2Cx COO assignments are shown by the color bar at the top of the heatmap. (b) Median gene expression values (left; each data point represents one sample) and pathway analysis results (right) for the 4 gene groups identified in panel A. Labels at the left of the horizontal bars show the top 10 most differentially up-regulated signaling pathways by the COO subtype with higher expression of that gene group.
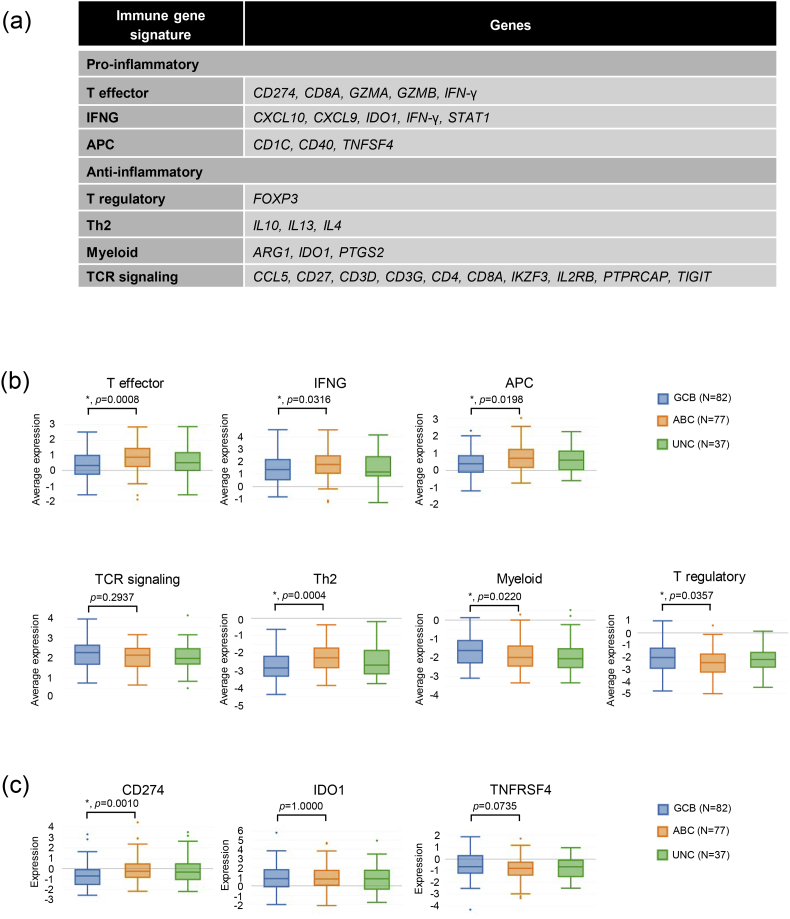
We then quantified the expression of well-established pro-inflammatory gene signatures, including T effector, Interferon-γ (IFNG) and antigen presenting cells (APC), as well as anti-inflammatory gene signatures, including T cell receptor signaling (TCR), T regulatory, Th2, and Myeloid in the different COO subtypes (Fig. 3a). As shown in Fig. 3b, the ABC subtype presented with significantly increased expression of all three pro-inflammatory signatures and decreased expression of the T regulatory and Myeloid signatures. Please see the detailed results in Supplementary Fig. 3a. Further examination of individual gene expression revealed that within the pro-inflammatory signatures, IFNG, CD274 (which codes for PD-L1 protein), GZMB, CXCL9 and CXCL10 were expressed at higher levels by the ABC subtype than by the GCB subtype (Supplementary Fig. 1a–c). For the anti-inflammatory genes, FOXP3, PTGS2, CD27, CD3G were expressed at higher levels by the GCB subtype (Supplementary Fig. 1d, f and g). On the contrary, the anti-inflammatory Th2 signature (Fig. 3b) and within it, the IL10 gene (Supplementary Fig. 1e), were overexpressed by the ABC subtype, which has been reported previously [53].
Fig. 3.
COO and immune characteristics. (a) List of genes included in the pro-inflammatory or the anti-inflammatory gene signatures used in our analysis. (b) Expression of immune gene signatures. P values are calculated between the GCB and the ABC subgroups. “*” indicates p < 0.05. (c) Expression of selected potential immunotherapy targets. Note that CD274 codes for PD-L1 and TNFRSF4 codes for OX40. P values are calculated between the GCB and the ABC subgroups. “*” indicates p < 0.05.
In addition, we checked the association between COO and the mRNA expression of potential immunotherapeutic targets including PD-L1, IDO1, and OX40. Our results showed that the ABC subtype had significantly higher expression of PD-L1 but not the other two (Fig. 3c).
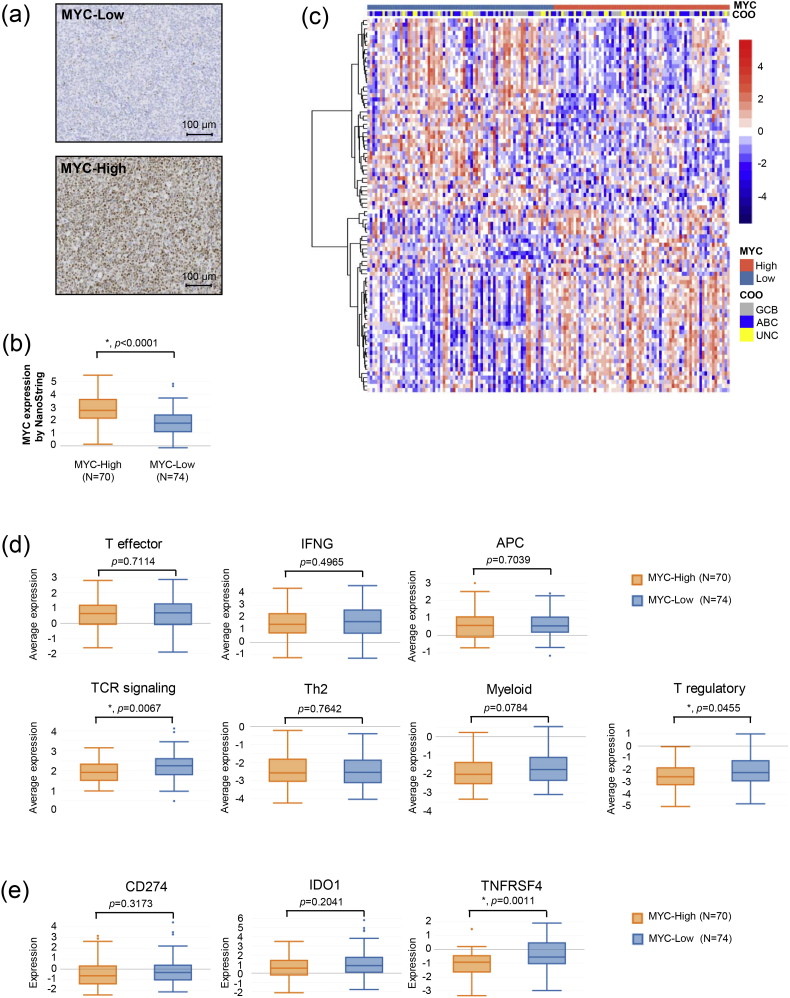
3.4. MYC Protein Expression and Immune Characteristics
MYC protein expression (see Fig. 4a for typical staining patterns) showed a strong correlation with MYC mRNA expression (Fig. 4b). We then examined the correlation between MYC protein expression and immune gene expression. MYC status (MYC-High or MYC-Low) led to a clear clustering of 90 differentially expressed genes in our 805-gene panel (Fig. 4c). Immune gene signature analysis revealed that while there was no statistical difference in the expression of pro-inflammatory gene signatures, MYC-High patients showed significantly decreased expression of TCR signaling and T regulatory genes (Fig. 4d), thereby reflecting a less anti-inflammatory microenvironment. Please see the detailed results in Supplementary Fig. 3b. Checking individual genes in these two signatures, we found that FOXP3, CD4, CD3G, CD3D and TIGIT genes had significantly lower expression in the MYC-High subgroup (Supplementary Fig. 2a–g).
Fig. 4.
MYC protein expression and immune characteristics. (a) Representative images of MYC IHC staining. (b) MYC gene expression by samples of high and low MYC IHC staining. “*” indicates p < 0.05. (c) The expression of 90 genes in our 805-gene NanoString panel by 144 of the 211 samples in this study. The expression of these 90 genes was significantly different between MYC-Low and MYC-High subgroups. The MYC protein expression status and Lymph2Cx COO assignments are shown by the color bars at the top of the heatmap. (d) Expression of immune gene signatures. “*” indicates p < 0.05. (e) Expression of selected potential immunotherapy targets. Note that CD274 codes for PD-L1 and TNFRSF4 codes for OX40. “*” indicates p < 0.05.
As to immunotherapeutic targets, we found that MYC-High patients had significantly decreased levels of OX40 expression as compared to MYC-Low patients while the expression of PD-L1 and IDO1 showed no statistically significant correlation with MYC status (Fig. 4e).
3.5. PD-L1 mRNA Expression and Immune Characteristics
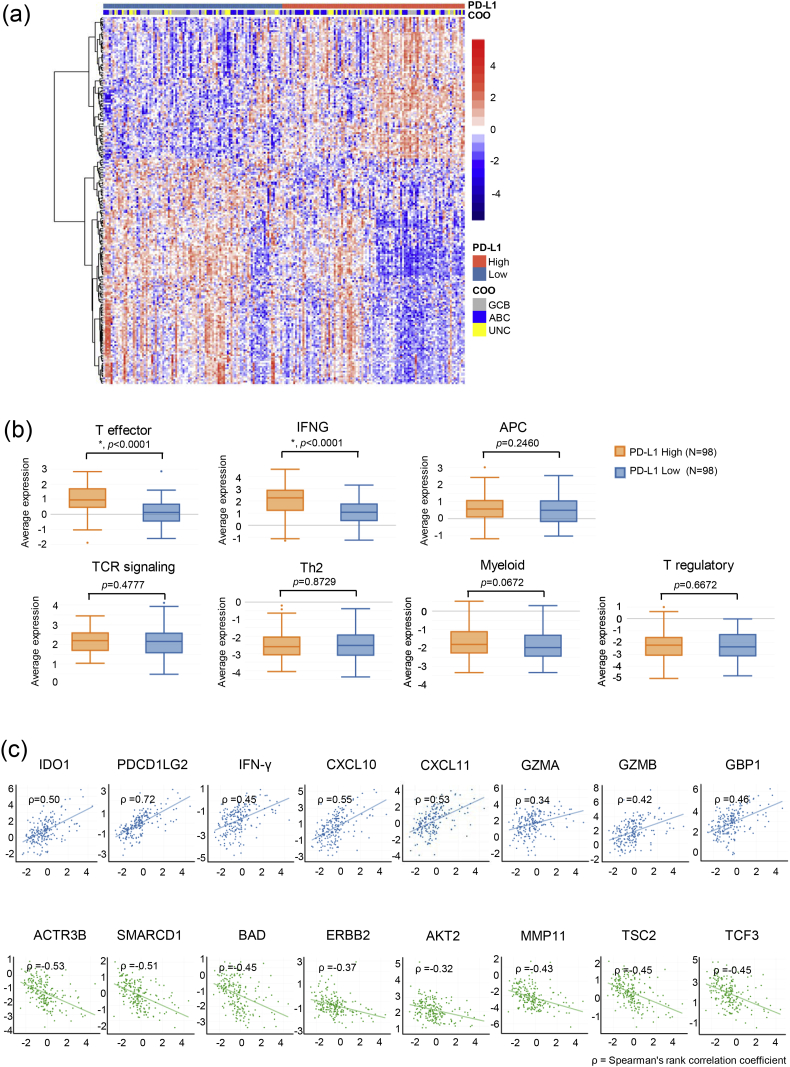
PD-L1 protein expression currently serves as the predictive [7] or complementary [54] biomarker for several anti-PD1/PD-L1 drugs. Due to the lack of PD-L1 IHC assays for DLBCL that had clinically meaningful cut-offs, we studied the correlation between PD-L1 mRNA expression and immune characteristics. PD-L1 status (with the median as the cut-off) led to a clustering of 223 differentially expressed genes in our 805-gene panel (Fig. 5a). Samples with higher PD-L1 expression showed significantly stronger expression of T effector and IFNG gene signatures while the expression of the other gene signatures was comparable to that of samples with lower PD-L1 expression (Fig. 5b). Please see the detailed results in Supplementary Fig. 3c. In addition, we found that PD-L1 expression level had a positive near-linear correlation with the expression of pro-inflammatory genes including IDO1, PDCD1LG2, IFNG, CXCL10, CXCL11, GZMA, GZMB and GBP1 (Fig. 5c). Interestingly, this correlation became negative when we looked at selected oncogenes including ACTR3B, SMARCD1, BAD, ERBB2, AKT2, MMP11, TSC2 and TCF3 (Fig. 5c).
Fig. 5.
PD-L1 mRNA expression and immune characteristics. (a) The expression of 223 genes in our 805-gene NanoString panel by 196 of the 211 samples in this study. The expression of these 223 genes was significantly different between PD-L1 High and PD-L1 Low (with the median as the cut-off) subgroups. The PD-L1 mRNA expression status and Lymph2Cx COO assignments are shown by the color bars at the top of the heatmap. (b) Expression of immune gene signatures. “*” indicates p < 0.05. (c) The correlations between the mRNA expression of PD-L1 (X axis of all plots) and selected immune-related genes (Y axis of plots in the first row) or solid tumor-related oncogenes (Y axis of plots in the second row). ρ is Spearman's rank correlation coefficient.
3.6. IDO1 mRNA Expression and Immune Characteristics
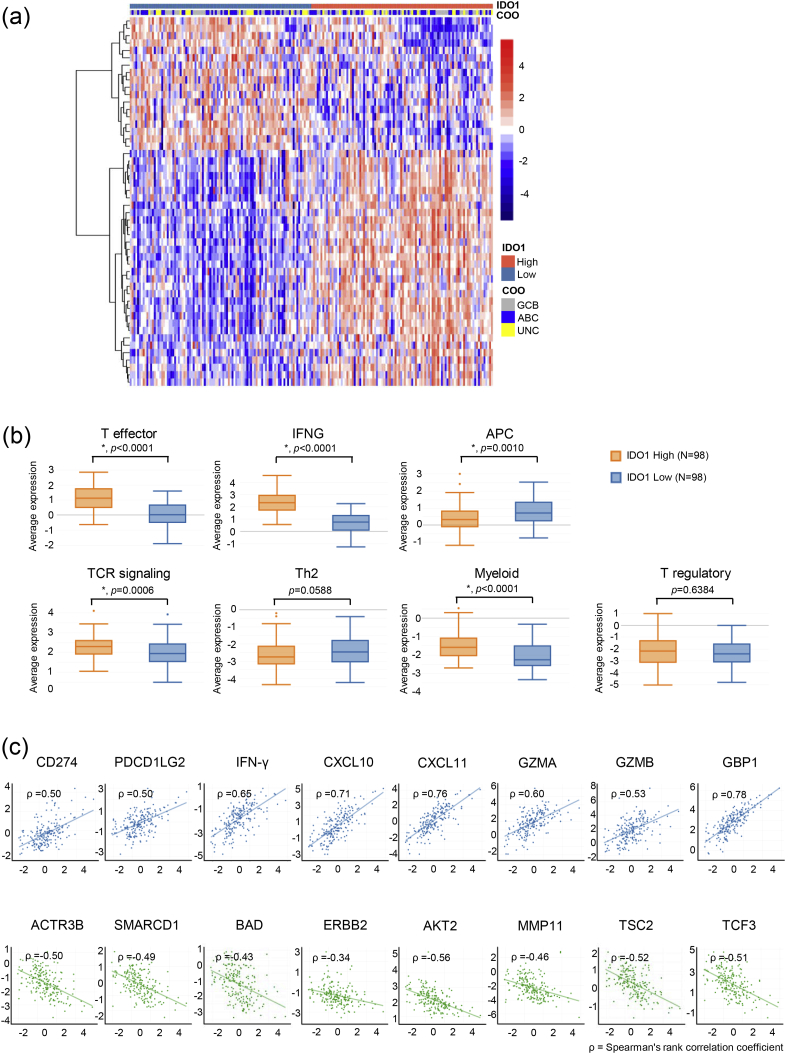
We next examined the correlation between IDO1 expression and immune characteristics of DLBCL patients. The analysis was similar to what was done on PD-L1 mRNA expression and the results are shown in Fig. 6. We found that IDO1 status (with the median as the cut-off) led to a clear clustering of 51 most differentially expressed genes in our 805-gene panel (Fig. 6a) and that higher IDO1 expression was associated with stronger expression of T effector, IFNG, TCR signaling and Myeloid gene signatures as well as weaker expression of the APC gene signature (Fig. 6b). Please see the detailed results in Supplementary Fig. 3d. Similar to what we observed in Fig. 5c, IDO1 mRNA expression also had positive near-linear correlation with pro-inflammatory genes and negative near-linear correlation with selected oncogenes (Fig. 6c).
Fig. 6.
IDO1 mRNA expression and immune characteristics. (a) The expression of 51 genes in our 805-gene NanoString panel by 196 of the 211 samples in this study. The expression of these 51 genes was significantly different between IDO1 High and IDO1 Low (with the median as the cut-off) subgroups. The IDO1 mRNA expression status and Lymph2Cx COO assignments are shown by the color bars at the top of the heatmap. (b) Expression of immune gene signatures. “*” indicates p < 0.05. (c) The correlations between the mRNA expression of IDO1 (X axis of all plots) and selected immune-related genes (Y axis of plots in the first row) or solid tumor-related oncogenes (Y axis of plots in the second row). ρ is Spearman's rank correlation coefficient.
4. Discussion
The efficacy of immunotherapies in solid tumors has prompted much enthusiasm for applying such treatments in lymphomas including DLBCL. To help inform and guide the drug development efforts in this field, it is important to gain a deep understanding of the immune characteristics of DLBCL patients and in particular, the expression of promising potential drug targets including PD-L1, IDO1 and OX40.
Using the NanoString-based Lymph2Cx assay, we divided a cohort of 211 Chinese DLBCL patients into the GCB, ABC, and Unclassified subtypes. To our knowledge, this is the first report on the application of this assay in a Chinese DLBCL cohort. The fraction of the ABC subtype was comparable to that in Western patients (37.4% vs. 32%) while that of the GCB subtype was much lower (44.1% vs. 56%) and that of the Unclassified subtype was higher (18.5% vs. 11%) than in Western patients. This leads to the question of whether the Unclassified subtype in Chinese DLBCL patients should be further studied to see if there are subsets with different clinical and/or molecular characteristics.
With a customized NanoString panel covering 805 immunity-related genes, we then studied the pathway activation and the immune characteristics of different COO subtypes. In terms of pathway activation, the ABC subtype seemed to have more active immune activities whereas the GCB subtype appeared to be driven more by oncogenes. As for immune characteristics, the ABC subtype seemed to have a more inflammatory microenvironment with significantly increased expression of typical pro-inflammatory gene signatures than the GCB subtype and this was coupled with decreased expression of anti-inflammatory gene signatures. There have been relatively limited reports on the immune characteristics of DLBCL. Monti et al. employed whole genome arrays to examine the transcriptional profile of DLBCL through multiple clustering methods [55]. They found that there was a host-response subset that had increased expression of T/natural killer cell receptor and activation pathway components, complement cascade members, macrophage/dendritic cell markers, and inflammatory mediators. However, the fraction of host-response samples in the ABC subtype was not higher than in the GCB subtype in their study. It should be noted that COO was not assessed with the Lymph2Cx assay in their study as in this one. In addition to a more inflammatory immune characteristic, we found that the ABC subtype had significantly higher expression of PD-L1 than the GCB subtype. In non-small cell lung cancer, higher expression of a pro-inflammatory gene signature that includes IFNG, CD274 and GZMB correlated with better response to anti-PD-L1 treatment [41]. More broadly, it has been suggested that regardless of the anatomic location, solid tumors with “pre-existing immunity” (represented by abundance of tumor-infiltrating lymphocytes, dense functional CD8+ T-cell infiltration reflected by increased IFNG signaling, expression of checkpoint markers, including PD-L1, and high mutational burden) will have better response to anti-PD1/PD-L1 treatment [56]. This points to the possibility that patients of the ABC subtype may have better response to the same type of treatment, a hypothesis that is probably worth verifying in prospective clinical trials.
In solid tumors, MYC has been found to promote tumorigenesis via the activation of CD47 and PD-L1 genes and its inactivation enhanced anti-tumor immune response [57]. In our study, however, neither protein expression nor gene expression of MYC showed statistically significant correlation with that of CD274 which codes for the PD-L1 protein (Supplementary Fig. 2h). Moreover, MYC-High samples showed a less anti-inflammatory phenotype, which is contrary to the findings of the aforementioned study. It is not clear whether this is due to the difference in disease biology between solid tumors and DLBCL. As to promising cancer immunotherapy targets, higher MYC protein expression seemed to correlate with OX40 down-regulation, indicating that such patients might be more sensitive to OX40 agonist treatment [33].
PD-L1 and IDO1 mRNA expression showed not only a strongly positive correlation with the expression of the T effector and the IFNG gene signatures, but also a positive near-linear correlation with each other. This provides the preliminary rationale for combining anti-PD1/PD-L1 and anti-IDO1 agents in treating DLBCL, a strategy that has shown promises in the treatment of solid tumors [[22], [23], [24], [25], [26]]. In addition, while it may not be surprising to see positive near-linear correlation between PD-L1 or IDO1 and pro-inflammatory genes including PDCD1LG2, IFNG, CXCL10, CXCL11, GZMA, GZMB and GBP1, it was intriguing to see that both genes showed negative near-linear correlation with multiple oncogenes including ACTR3B, SMARCD1, BAD, ERBB2, AKT2 and BAD. Pivotal trials of anti-PD1/PD-L1 agents in non-small cell lung cancer have reported that the efficacy of such treatment is much more inferior in EGFR-mutant patients than in EGFR-wild type ones [54, 58]. Our results point to the possibility that maybe tumors mainly driven by prominent oncogenes have a less active immune microenvironment and thus worse response to cancer immunotherapies. In summary, we analyzed tumor samples from 211 Chinese DLBCL patients to understand their immune characteristics and the implication for the development of cancer immunotherapies for this malignancy. We found that the ABC subtype has a significantly more pro-inflammatory immune microenvironment as well as higher expression of PD-L1 mRNA. MYC-High patients showed a less anti-inflammatory phenotype and might be sensitive to OX40 agonist treatment. PD-L1 and IDO1 mRNA expression correlated positively with each other and with pro-inflammatory genes, but was negatively associated with the expression of multiple oncogenes. Such patients may be sensitive to combination therapies of anti-PD1/PD-L1 and anti-IDO1 agents. It should be emphasized that DLBCL is very different from solid tumors in both the underlying biology and the clinical characteristics. Therefore, the correlation between solid tumors' immune characteristics or target gene expression and their response to immunotherapies may not apply to DLBCL. Prospective clinical trials will be needed to test all the hypotheses generated from this study.
The following are the supplementary data related to this article.
Correlation between COO and the expression of individual immune genes. (a–g) Correlation between COO and the expression of individual immune genes associated with T effector, IFNG, APC, T regulatory, Th2, Myeloid and TCR signaling, respectively.
Correlation between MYC protein expression and the expression of individual immune genes. (a–g) Correlation between MYC protein expression and the expression of individual immune genes associated with T effector, IFNG, APC, T regulatory, Th2, Myeloid and TCR signaling, respectively. (h) Correlation between MYC gene expression and CD274 gene expression.
Detailed results of the correlations between immune gene signature expression and, respectively COO, MYC protein expression, PD-L1 mRNA expression and IDO1 mRNA expression. (a) The medium and the standard deviation of immune gene signature expression in the GCB, ABC and UNC subgroups. P values are calculated between the GCB and the ABC subgroups. (b–d) The medium and the standard deviation of immune gene signature expression in the MYC-High and MYC-Low subgroups (b), the PD-L1 High and PD-L1 Low subgroups (c) and the IDO1 High and the IDO1 Low subgroups (d).
Conflicts of Interest
Chun Sun, Xu Cao, Hang-jun Dai, Shan Lu, Jian-jun Guo, Shi-jing Fu, Yu-xia Liu, Su-chun Li, Meng Chen, Ron McCord, Jeff Venstrom, Edith Szafer-Glusman, Elizabeth Punnoose, Astrid Kiermaier and Gang Cheng are employees of F. Hoffmann-La Roche/Genentech Inc. All other authors declare no conflict of interest.
Funding
This study was supported, in part, by research funding from the National Natural Science Foundationn of China (81325003, 81520108003, 81670716 and 81201863), the Shanghai Commission of Science and Technology (14430723400, 14140903100 and 16JC1405800), Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152206 and 20152208), Multicenter Clinical Research Project by Shanghai Jiao Tong University School of Medicine (DLY201601), Clinical Research Plan of SHDC (16CR2017A), Chang Jiang Scholars Program, Innovation Fund Projects of Shanghai Jiao Tong University (BXJ201607), Collaborative Innovation Center of Systems Biomedicine, the Samuel Waxman Cancer Research Foundation, and by Genentech Inc., a member of the Roche Group.
Authors' Contributions
Wei-li Zhao, Gang Cheng, Ron McCord and Jeff Venstrom designed the research; Peng-peng Xu, Chun Sun, Xu Cao, Xia Zhao, Jian-jun Guo and Shi-jing Fu acquired data; Gang Cheng, Wei-li Zhao, Xia Zhao, Chun Sun, Xu Cao, Peng-peng Xu, Hang-jun Dai, Shan Lu, Yu-xia Liu, Su-chun Li and Meng Chen analyzed the data and made the figures; Gang Cheng, Wei-li Zhao, Xia Zhao, Chun Sun, Xu Cao, Peng-peng Xu, Jian-jun Guo and Hang-jun Dai drafted the manuscript; all authors reviewed the manuscript critically for important intellectual content.
Acknowledgements
We thank Ventana Medical Systems, Inc. (Tucson, AZ, USA) for making the MYC IHC assay available for this study.
Contributor Information
Gang Cheng, Email: gcheng00@hotmail.com.
Wei-li Zhao, Email: zhao.weili@yahoo.com, zwl10909@rjh.com.cn.
References
- 1.Motzer R.J., Escudier B., Mcdermott D.F., George S., Hammers H.J., Srinivas S. Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Bauml J., Seiwert T.Y., Pfister D.G., Worden F., Liu S.V., Gilbert J. Pembrolizumab for platinum- and Cetuximab-refractory head and neck Cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rittmeyer Achim, Barlesi Fabrice, Waterkamp Daniel, Park Keunchil, Ciardiello Fortunato, von Pawel Joachim. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E. Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reck M., Rodriguez-Abreu D., Robinson A.G., Hui R., Csoszi T., Fulop A. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 8.Younes A., Santoro A., Shipp M., Zinzani P.L., Timmerman J.M., Ansell S. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moskowitz C.H., Zinzani P.L., Michelle A., Fanale, Armand P., Johnson N.A. Pembrolizumab in relapsed/refractory classical hodgkin lymphoma: Primary end point analysis of the phase 2 keynote-087 study. ASH Annu Meet Abstr. 2016;1107 [Google Scholar]
- 10.Lesokhin A.M., Ansell S.M., Armand P., Scott E.C., Halwani A., Gutierrez M. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 12.Corm S., Berthon C., Imbenotte M., Biggio V., Lhermitte M., Dupont C. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients' sera by HPLC and is inducible by IFN-gamma. Leuk Res. 2009;33:490–494. doi: 10.1016/j.leukres.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Astigiano S., Morandi B., Costa R., Mastracci L., D'Agostino A., Ratto G.B. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia. 2005;7:390–396. doi: 10.1593/neo.04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Haj-Ayed A., Moussa A., Ghedira R., Gabbouj S., Miled S., Bouzid N. Prognostic value of indoleamine 2,3-dioxygenase activity and expression in nasopharyngeal carcinoma. Immunol Lett. 2016;169:23–32. doi: 10.1016/j.imlet.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Brandacher G., Perathoner A., Ladurner R., Schneeberger S., Obrist P., Winkler C. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 16.Hara T., Matsumoto T., Shibata Y., Nakamura N., Nakamura H., Ninomiya S. Prognostic value of the combination of serum l-kynurenine level and indoleamine 2,3-dioxygenase mRNA expression in acute myeloid leukemia. Leuk Lymphoma. 2016;57:2208–2211. doi: 10.3109/10428194.2015.1128541. [DOI] [PubMed] [Google Scholar]
- 17.Ino K., Yoshida N., Kajiyama H., Shibata K., Yamamoto E., Kidokoro K. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006;95:1555–1561. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T., Shima T., Saeki A., Hidaka T., Nakashima A., Takikawa O. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98:874–881. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto A., Nikaido T., Ochiai K., Takakura S., Saito M., Aoki Y. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 20.Witkiewicz A., Williams T.K., Cozzitorto J., Durkan B., Showalter S.L., Yeo C.J. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg. 2008;206:849–854. doi: 10.1016/j.jamcollsurg.2007.12.014. [discussion 854-846] [DOI] [PubMed] [Google Scholar]
- 21.Beatty G.L., O'Dwyer P.J., Clark J., Shi J.G., Bowman K.J., Scherle P.A. First-in-human phase I study of the oral inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res. 2017;23:3269–3276. doi: 10.1158/1078-0432.CCR-16-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangadhar T.C., Schneider B.J., Bauer T.M., Wasser J.S., Spira A.I., Patel S.P. Efficacy and safety of epacadostat plus pembrolizumab treatment of NSCLC: preliminary phase I/II results of ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:9014. [Google Scholar]
- 23.Lara P., Bauer T.M., Hamid O., Smith D.C., Gajewski T., Gangadhar T.C. Epacadostat plus pembrolizumab in patients with advanced RCC: preliminary phase I/II results from ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:4515. [Google Scholar]
- 24.Hamid O., Bauer T.M., Spira A.I., Olszanski A.J., Patel S.P., Wasser J.S. Epacadostat plus pembrolizumab in patients with SCCHN: preliminary phase I/II results from ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:6010. [Google Scholar]
- 25.Smith D.C., Gajewski T., Hamid O., Wasser J.S., Olszanski A.J., Patel S.P. Epacadostat plus pembrolizumab in patients with advanced urothelial carcinoma: preliminary phase I/II results of ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:4503. [Google Scholar]
- 26.Spira A.I., Hamid O., Bauer T.M., Borges V.F., Wasser J.S., Smith D.C. Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: phase I/II ECHO-202 study. J Clin Oncol. 2017;35:1103. [Google Scholar]
- 27.Mallett S., Fossum S., Barclay A.N. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes—a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Shamkhani A., Birkeland M.L., Puklavec M., Brown M.H., James W., Barclay A.N. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur J Immunol. 1996;26:1695–1699. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- 29.Bansal-Pakala P., Halteman B.S., Cheng M.H., Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 30.Baum P.R., Gayle R.B., 3rd, Ramsdell F., Srinivasan S., Sorensen R.A., Watson M.L. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane P. Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th)1 and Th2 cells. J Exp Med. 2000;191:201–206. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curti B.D., Kovacsovics-Bankowski M., Morris N., Walker E., Chisholm L., Floyd K. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73:7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linch S.N., Mcnamara M.J., Redmond W.L. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alizadeh A.A., Eisen M.B., Davis R.E., Ma C., Lossos I.S., Rosenwald A. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 35.Lenz G., Wright G., Dave S.S., Xiao W., Powell J., Zhao H. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenwald A., Wright G., Chan W.C., Connors J.M., Campo E., Fisher R.I. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 37.Shaffer A.L., 3rd, Young R.M., Staudt L.M. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ott G., Rosenwald A., Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Hematology Am Soc Hematol Educ Program. 2013;2013:575–583. doi: 10.1182/asheducation-2013.1.575. [DOI] [PubMed] [Google Scholar]
- 39.Horn H., Ziepert M., Becher C., Barth T.F., Bernd H.W., Feller A.C. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 40.Johnson N.A., Slack G.W., Savage K.J., Connors J.M., Ben-Neriah S., Rogic S. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fehrenbacher L., Spira A., Ballinger M., Kowanetz M., Vansteenkiste J., Mazieres J. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 42.Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carbone D.P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M. First-line Nivolumab in stage IV or recurrent non-small-cell lung Cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overman M.J., Mcdermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodman A., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V.A. Comprehensive genomic profiling to identify tumor mutational burden (TMB) as an independent predictor of response to immunotherapy in diverse cancers. J Clin Oncol. 2017;35 doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen B.J., Chapuy B., Ouyang J., Sun H.H., Roemer M.G., Xu M.L. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visco C., Li Y., Xu-Monette Z.Y., Miranda R.N., Green T.M., Li Y. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the international DLBCL rituximab-CHOP consortium program study. Leukemia. 2012;26:2103–2113. doi: 10.1038/leu.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallden B., Ferree S., Ravi H., Dowidar N., Hood T., Danaher P. Development of the molecular diagnostic (MDx) DLBCL lymphoma subtyping test (LST) on the nCounter analysis system. J Clin Oncol. 2015;33 [Google Scholar]
- 51.Monti S., Tamayo P., Mesirov J., Golub T. Consensus clustering: a resampling-based method for class discovery and visualization of gene expression microarray data. Mach Learn. 2003;52:91–118. [Google Scholar]
- 52.Hans C.P., Weisenburger D.D., Greiner T.C., Gascoyne R.D., Delabie J., Ott G. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 53.Beguelin W., Sawh S., Chambwe N., Chan F.C., Jiang Y., Choo J.W. IL10 receptor is a novel therapeutic target in DLBCLs. Leukemia. 2015;29:1684–1694. doi: 10.1038/leu.2015.57. [DOI] [PubMed] [Google Scholar]
- 54.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monti S., Savage K.J., Kutok J.L., Feuerhake F., Kurtin P., Mihm M. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 56.Hegde P.S., Karanikas V., Evers S. The where, the when, and the how of immune monitoring for Cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22:1865–1874. doi: 10.1158/1078-0432.CCR-15-1507. [DOI] [PubMed] [Google Scholar]
- 57.Casey S.C., Tong L., Li Y., Do R., Walz S., Fitzgerald K.N. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herbst R.S., Baas P., Kim D.W., Felip E., Perez-Gracia J.L., Han J.Y. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between COO and the expression of individual immune genes. (a–g) Correlation between COO and the expression of individual immune genes associated with T effector, IFNG, APC, T regulatory, Th2, Myeloid and TCR signaling, respectively.
Correlation between MYC protein expression and the expression of individual immune genes. (a–g) Correlation between MYC protein expression and the expression of individual immune genes associated with T effector, IFNG, APC, T regulatory, Th2, Myeloid and TCR signaling, respectively. (h) Correlation between MYC gene expression and CD274 gene expression.
Detailed results of the correlations between immune gene signature expression and, respectively COO, MYC protein expression, PD-L1 mRNA expression and IDO1 mRNA expression. (a) The medium and the standard deviation of immune gene signature expression in the GCB, ABC and UNC subgroups. P values are calculated between the GCB and the ABC subgroups. (b–d) The medium and the standard deviation of immune gene signature expression in the MYC-High and MYC-Low subgroups (b), the PD-L1 High and PD-L1 Low subgroups (c) and the IDO1 High and the IDO1 Low subgroups (d).