Abstract
In schistosomiasis mansoni, parasite eggs precipitate an intrahepatic granulomatous and fibrosing inflammatory process, which is mediated by, and dependent on, MHC class II-restricted CD4 T helper (Th) lymphocytes specific for schistosome egg antigens (SEA). In the mouse model of the disease, CBA mice develop large granulomas, whereas in C57BL/6 (BL/6) mice these granulomas are significantly smaller. To further investigate how the prevailing cytokine environment influences the development of the egg-induced immunopathology, we immunized the low-pathology BL/6 mice with SEA in complete Freund's adjuvant (CFA) once before, and once again during, the course of a 7-week infection. This immunization caused a pronounced Th1 shift in the SEA-specific CD4 T cell response, which was detected in the mesenteric lymph nodes (MLNs) and spleens, as well as in the granulomatous lesions themselves. The immunized mice displayed a dramatic enhancement of hepatic egg-induced immunopathology manifested by a marked increase in granuloma size and parenchymal inflammation, leading to early death. Control mice immunized with equivalent amounts of SEA or CFA alone displayed the smaller hepatic lesions in a Th2-dominant environment typically seen in the unimmunized BL/6 mice. Analysis of granuloma and MLN lymphocytes from the SEA/CFA-immunized mice revealed that the proportion of CD4 T cells was unchanged in comparison with the control BL/6 groups and remained significantly lower than that seen in the normally high-pathology CBA strain. These results suggest that the shift toward Th1-type cytokine production by a numerically stable population of CD4 T cells correlates with severe exacerbation of immunopathology in schistosomiasis.
Oviposition within mesenteric veins followed by embolization into the hepatic microvasculature precipitates circumoval granulomatous inflammation, the main pathological event associated with infection with the helminth parasite Schistosoma mansoni. However, the consequences of this event in terms of magnitude of ensuing disease varies considerably both in humans as well as in an experimental murine model of infection. In humans, severe “hepatosplenic” schistosomiasis is characterized by liver fibrosis, portal hypertension, and gastrointestinal hemorrhage often resulting in death. In contrast, on the opposite end of the spectrum, “intestinal” schistosomiasis is the milder, chronic, and often symptomless form of the disease (1). In the mouse model, the CBA and C3H strains develop significantly larger granulomas than the C57BL/6 strain (2, 3).
There is now general agreement that the development of granulomas depends on MHC class II-restricted, egg antigen-specific CD4 T helper (Th) lymphocytes, as these lesions fail to materialize in athymic mice (4) or mice lacking αβ-expressing T cells, MHC class II molecules, or recombination-activating gene 1 (Rag-1) (5, 6). However, the regulatory factors that control the severity of disease in each individual patient or mouse strain, now largely attributed to genetically determined host factors (7, 8), still remain poorly understood.
One particular issue that has been difficult to settle is the role of the Th1 vs. Th2 subsets of CD4 T cells in egg granuloma formation. Our laboratory's early findings demonstrating that cloned specific Th1-type lymphocytes are capable of mediating granuloma formation (9) seemed consistent with the assumption that the development of these lesions depends on the proinflammatory CD4 Th1-type cells producing IFN-γ and IL-2. However, the detection of anti-inflammatory IL-4 and IL-5 (Th2-type) cytokine production in response to schistosomal egg antigens (SEA) during peak granuloma formation after 8 weeks of infection, suggested, instead, a critical pathogenic role for the Th2 cells (10). This concept was later extended with the proposition that Th1 cells inhibit pathology, and, consequently, that the stimulation of the Th1 phenotype may serve to improve and prevent clinical disease (11, 12). The use of gene knockout mouse models failed to solve this discrepancy as there were reports variously maintaining that Th2 cells are (13), or are not (14), required for granuloma formation, and that both IFN-γ and IL-4 positively or negatively influence, or are essentially irrelevant for, the development of these lesions (15–19).
A link between increased egg-induced immunopathology and a Th1-dominated environment became apparent after the kinetic analysis of cytokine production by SEA-stimulated mesenteric lymph node (MLN) cells. These studies showed that in the low-pathology BL/6 (H-2b) mice an initial brief Th1-type response is promptly replaced by a sustained Th2-type response (20), whereas in the high-pathology C3H (H-2k) mice the Th1 response (IFN-γ and IL-2) persists alongside the Th2 response (IL-4 and IL-10) (21). To further explore these observations, we have now immunized schistosome-infected, low-pathology, and Th2-biased BL/6 mice with SEA in complete Freund's adjuvant (CFA). This treatment precipitated a striking reversal in cytokine production toward a Th1-dominant profile, which was detected in the MLNs, spleens, and granulomatous lesions themselves. Most importantly, these mice displayed a dramatic increase in their hepatic egg-induced immunopathology, which surpassed the one displayed spontaneously by the high-pathology strain CBA (H-2k).
Materials and Methods
Mice, Infection, and Immunizations.
Female C57BL/6 (BL/6) and CBA/J (CBA) mice, 6–8 weeks old, were purchased from The Jackson Laboratory and maintained in the Animal Facility at Tufts University School of Medicine. Mice were infected by i.p. injection with 80 cercariae of S. mansoni (Puerto Rico strain), which were obtained from infected Biomphalaria glabrata snails, provided to us by the Biomedical Research Institute (Rockville, MD), through National Institutes of Health/National Institute of Allergy and Infectious Diseases Contract N01-AI-55270. Five days before infection, some BL/6 mice were immunized by s.c. injection at four different sites (back and base of tail) with 50 μg of SEA, either emulsified in CFA, or solubilized in PBS. SEA were purchased from the Biomedical Research Institute and prepared as described (22). Additional mice were similarly immunized with an emulsion of PBS in CFA. Four weeks after infection, the variously immunized mice received a second immunization at contralateral sites, identical to the one they had received before.
Cell Preparations.
Livers, MLN, and spleens were removed aseptically from 7-week infected mice. Granulomas from 6–10 pooled livers per group were isolated by homogenization in a Waring blender followed by 1 g sedimentation and extensive washing. Cells in granulomas were freed after enzymatic digestion with 1 mg/ml of collagenase type H, from Clostridium histolyticum (Sigma). Single-cell suspensions from pooled MLNs and spleens were prepared by teasing the tissues in complete RPMI medium (9). Erythrocytes were lysed with Tris ammonium chloride buffer (pH 7.2; Sigma) for 15 min on ice. Cells that excluded trypan blue were resuspended at desired concentrations. CD4 T cells were purified from MLNs by negative selection as described (21). The resulting cell preparations were >94% CD4+ T cells as determined by flow cytometry.
Cell Culture Supernatants and Cytokine Determinations.
Bulk cell suspensions (5 × 106 cells per ml) from pooled hepatic granulomas, MLNs, and spleens, or purified CD4 T cells from MLNs (1 × 106 cells per ml) plus normal irradiated syngeneic splenic antigen-presenting cells (4 × 106 cells per ml), were incubated in the presence or absence of 20 μg/ml of SEA for 24, 36, and 48 h. At these times, the culture supernatants were removed, filtered, and stored at −36°C until analysis. The cytokines IFN-γ, IL-2, tumor necrosis factor (TNF)α, IL-12 (p40), IL-4, IL-5, and IL-10, which were present in the supernatants, were measured by ELISA using antibodies, standard cytokines, and protocols obtained from PharMingen; IL-13 was determined with antibodies, standard cytokine, and protocol obtained from R&D Systems.
Flow Cytometric Analysis.
Granuloma, MLNs, and spleen cells at a concentration of 2 × 106 cells per ml were cultured with or without 20 μg/ml of SEA. After 36 h of culture, the cells were harvested and washed twice in fluorescence-activated cell sorting (FACS) buffer [PBS containing 0.1% BSA (Sigma) and 0.01% NaN3] and incubated at 4°C for 15 min. in FACS blocking buffer (FACS buffer containing 0.3 mg/ml rat IgG; Sigma) for the purpose of inhibiting nonspecific antibody binding. Aliquots of 106 cells were then incubated for 30 min at 4°C with phycoerythrin and cyanine 5 (Cy-Chrome) tandem-conjugated anti-CD3 (clone 145–2C11) and phycoerythrin (PE)-conjugated anti-CD4 (clone GK1.5), or Cy-Chrome-conjugated anti-CD3 and PE-conjugated anti-CD8 (clone 53–6.7) monoclonal antibodies (PharMingen), optimally diluted in FACS blocking buffer. Labeled cells were washed twice with FACS buffer, fixed in 1% paraformaldehyde, and acquired on a FACScalibur flow cytometer using CELLQUEST software version 3.2.1 (Becton Dickinson). Unstained cells and cells stained with irrelevant isotype-matched antibodies were included as controls to assess the amount of nonspecific staining. Data were analyzed by using WINLIST 3D 4.0 software. Lymphocyte-rich areas, comprising 8–15% of the granuloma cells and 80–95% of the MLN and spleen cells, were defined and gated based on forward light scatter and side light scatter characteristics of the cells (“lymphocyte gate”).
Histopathology and Morphometric Analysis.
Liver, lung, and intestine samples were fixed in 10% buffered formalin and processed for routine histopathologic analysis. Sections (5 μm) stained with hematoxylin and eosin were examined for qualitative and quantitative changes. The extent of hepatic granulomatous inflammation around schistosome eggs was measured by computer-assisted morphometric analysis using ImagePro Plus (Media Cybernetics, Silver Spring, MD) software. The lesions were assessed on coded slides by an observer unaware of the experimental setting. To reflect more accurately the true shape and dimension of the granulomas, only those with a visible central egg were counted. A minimum of 11 granulomas per liver section, meeting these criteria, was studied.
Statistical Analysis.
Student's t test and the χ2 test were used to determine the statistical significance of the differences between experimental and control groups. Results differing with a P < 0.05 were considered significant. Each individual experiment was conducted with mouse groups composed of 6–10 mice.
Results
Immunization with SEA/CFA Causes Severe Exacerbation of Egg-Induced Hepatic Immunopathology and Increased Mortality.
Groups of BL/6 mice were immunized with SEA/CFA, SEA, and CFA with the intent of studying the effect of these immunizations on the immune response and immunopathology elicited during infection with S. mansoni. Unexpectedly, in every experiment, some of the mice died early during the course of their sixth week of infection (Table 1). Most of the dead mice belonged to the SEA/CFA-immunized group. Their livers revealed severe granulomatous and interstitial inflammation, often with parenchymal necrosis. There was marked intestinal dilatation with mucosal hemorrhage and necrosis. The lungs had dense pulmonary inflammatory cell infiltrates and a high presence of schistosome eggs, a likely consequence of portal-systemic shunts.
Table 1.
Mortality by 7 weeks after infection
| Mouse group | Mortality* | |
|---|---|---|
| BL/6 | SEA/CFA-immunized | 12/33† |
| SEA-immunized | 0/18 | |
| CFA-immunized | 2/35 | |
| control | 0/24 | |
| CBA | control | 0/20 |
Dead mice/infected mice in four pooled experiments.
Significantly different from controls (P < 0.01).
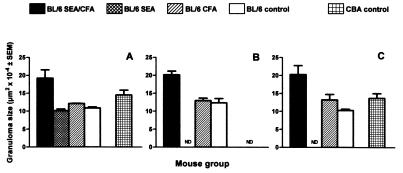
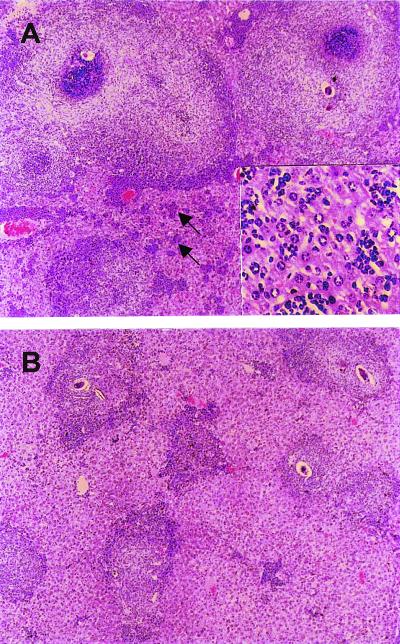
The livers in the remaining SEA/CFA-immunized BL/6 mice completing a 7-week schistosome infection disclosed a striking increase in granuloma size in comparison with the control-immunized and unimmunized BL/6 mice. As shown in Figs. 1 and 2, granulomas in the SEA/CFA-immunized group were often double in size of those in the control groups, even exceeding the normally larger granulomas characteristic of the high-pathology CBA strain. By comparison, the granulomas in the SEA- or CFA-immunized mice did not differ significantly from the unimmunized BL/6 controls. The granulomas from the SEA/CFA-immunized mice were composed of a mixed inflammatory cell population that typically included eosinophils, neutrophils, macrophages (some with parasite-derived heme pigment), and lymphocytes, with an expanded, poorly collagenized extracellular matrix that surrounded the increased cell infiltrates but did not appear to be qualitatively different from controls. Of additional significance were the numerous small, mixed inflammatory cell foci (Fig. 2) dispersed throughout the hepatic parenchyma. These interstitial infiltrates were significantly less conspicuous or nonexisting in all control groups.
Figure 1.
Size of hepatic egg granulomas. Granulomas on stained liver sections were measured by computer-assisted morphometric analysis. Results represent means of granuloma areas ± SEM from three independent experiments. Granulomas from SEA/CFA-immunized BL/6 mice were significantly larger than granulomas from control-immunized or unimmunized BL/6 mice (P < 0.01). ND, not done.
Figure 2.
Histology of hepatic egg granulomas. (A) Large granulomas and severe interstitial inflammation (arrows) in the SEA/CFA-immunized BL/6 mice. (Inset) A higher magnification of the inflammatory cell aggregates. (B) Smaller granulomas without significant interstitial inflammation in the control BL/6 mice. (Original magnification, ×40; Inset, ×200.)
Immunization with SEA/CFA Induces a High Th1-Type, Low Th2-Type Cytokine Environment in Hepatic Egg Granulomas, MLNs, and Spleens.
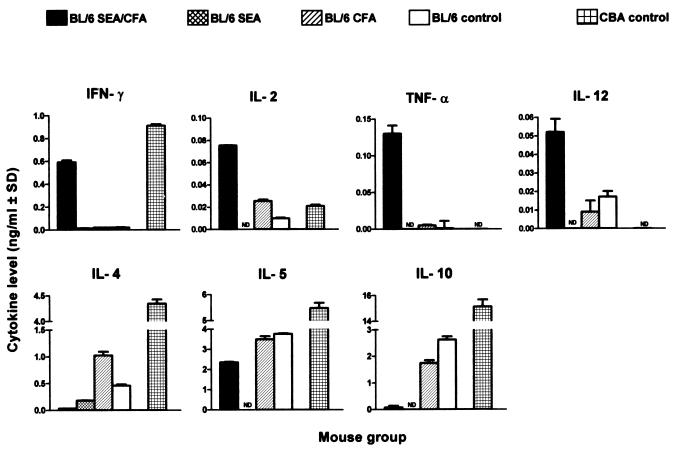
Schistosome egg granuloma formation is mediated by CD4 T cells, but the type of cytokines produced by these cells after antigenic stimulation can vary considerably during the course of the infection and also in accordance with individual mouse strains. An undisturbed 7-week schistosome infection in the BL/6 mouse is characterized by a Th2-dominant environment (20). To ascertain the prevailing cytokine environment conducive to the observed pronounced increase in immunopathology after immunization with SEA/CFA, we studied the Th1-type cytokines IFN-γ, IL-2, TNF-α, and IL-12, and the Th2-type cytokines IL-4, IL-5, IL-10, and IL-13, produced by lymphoid cells from granulomas, MLNs, and spleens.
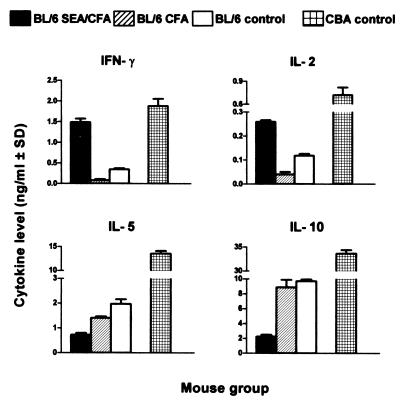
The cytokines produced by SEA-stimulated granuloma cells from infected, SEA/CFA-immunized mice showed a striking polarization toward the Th1 type. Thus, IFN-γ, IL-2, TNF-α, and IL-12 levels were markedly elevated in comparison with those observed in all of the BL/6 control groups, with IFN-γ reaching the high levels usually seen in the high-pathology CBA strain, in which a Th1 response normally persists alongside a Th2 response (21) (Fig. 3). On the other hand, the Th2-type cytokines IL-4, IL-5, and IL-10 produced by the SEA/CFA-immunized mice were significantly lower than in the control BL/6 and CBA mice. Interestingly, in the case of IL-12, there also was a considerable production of cytokine in unstimulated cultures from the SEA/CFA-immunized BL/6 mice (0.050 ± 0.003 ng/ml) that was significantly higher than that produced by the unimmunized BL/6 controls (0.031 ± 0.003 ng/ml; P < 0.01).
Figure 3.
Cytokine production by unfractionated granuloma cells. Pooled granuloma cells from each group were incubated in the presence of 20 μg/ml of SEA. Cytokines in 36-h culture supernatants were measured by ELISA, and levels were expressed as means of triplicate determinations ± SD. IFN-γ, IL-2, TNF-α, and IL-12 levels are significantly higher, whereas IL-4, IL-5, and IL-10 levels were significantly lower, in SEA/CFA-immunized mice than in all BL/6 controls (both P < 0.01). In all figures, background levels from unstimulated cultures were subtracted and results were representative of three independent experiments. ND, not done.
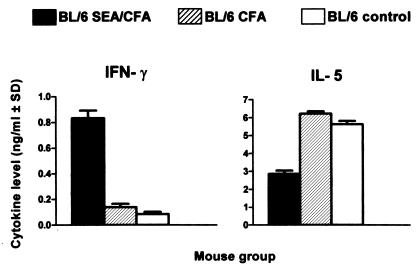
Analysis of cytokine production by SEA-stimulated, unfractionated MLN cells also disclosed a strong Th1 presence in the SEA/CFA-immunized group that was similar to that seen in CBA mice; in contrast, the same cells produced less IL-4, IL-5, IL-10, and IL-13 (Fig. 4). Cytokine production by cells from mice immunized with SEA and CFA alone was not significantly different from the unimmunized BL/6 controls. IL-12 was higher in SEA/CFA-immunized mice than in BL/6 control groups, but these responses were independent of SEA stimulation (data not shown). There also was a sharp increase in IFN-γ production by purified SEA-stimulated CD4 T cells from the SEA/CFA-immunized mice, whereas their production of IL-5 was significantly decreased with respect to the controls (Fig. 5). These findings suggest that the altered cytokine production in the SEA/CFA-immunized mice is of CD4 T cell origin.
Figure 4.
Cytokine production by unfractionated MLN cells. Pooled MLN cells from each group were incubated in the presence of 20 μg/ml of SEA. IL-2 in culture supernatants was measured at 24 h; the other cytokines were measured at 48 h. Data are expressed as in Fig. 3. IFN-γ and IL-2 levels were significantly higher (P < 0.01), whereas IL-4, IL-5, IL-10, and IL-13 levels were significantly lower (P < 0.05) in SEA/CFA-immunized mice than in all BL/6 controls.
Figure 5.
Cytokine production by purified CD4 T cells from MLNs. Pooled CD4 T cells from MLNs were cultured with irradiated normal splenic antigen-presenting cells and 20 μg/ml of SEA. IFN-γ and IL-5 were measured in 48-h culture supernatants. Data are expressed as in Fig. 3. IFN-γ levels were significantly higher and IL-5 levels were significantly lower in SEA/CFA-immunized mice than in the controls (both P < 0.01).
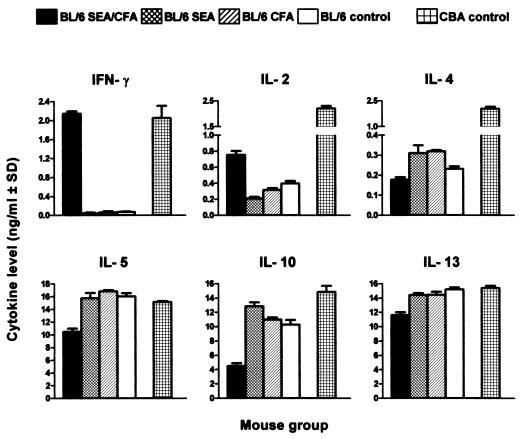
Lastly, the same striking change in cytokine production in the SEA/CFA-immunized BL/6 mice was also found in the spleen, where there was a sharp increase in IFN-γ and IL-2, together with a marked decrease in IL-5 and IL-10, in comparison with the BL/6 controls (Fig. 6). In the CBA controls, all cytokines were typically elevated.
Figure 6.
Cytokine production by unfractionated spleen cells. Pooled spleen cells from each group were incubated in the presence of 20 μg/ml of SEA. IL-2 in culture supernatants was measured at 24 h; the other cytokines were measured at 48 h. Data are expressed as in Fig. 3. IFN-γ and IL-2 levels were significantly higher, and IL-5 and IL-10 levels were significantly lower in SEA/CFA-immunized mice than in all BL/6 controls (both P < 0.01).
In SEA/CFA-Immunized Mice, the Proportion of CD4 T Cells Among the Granuloma Lymphocytes Is Unchanged, Whereas the CD8 T Cells Decrease with Respect to Controls.
Because the cytokines produced in response to stimulation with SEA are largely of T cell origin, we more closely examined the main T cell subpopulations for possible quantitative or qualitative changes that may correlate with the pronounced differences in pathology and cytokine secretion. These CD3-expressing T cells comprise the CD4 T cells most directly associated with mediating the egg-induced immunopathology in schistosomiasis (6, 23), and the CD8 T cells, which have frequently been proposed as regulatory cells (24–26). Analysis of SEA-stimulated lymphocyte populations within the granulomas, surprisingly, revealed no significant difference in the proportion of CD4 T cells between the SEA/CFA-immunized mice and the control-immunized or unimmunized BL/6 mice (Table 2). Unimmunized control CBA mice, however, displayed significantly more CD4 T cells. In contrast, the proportion of granuloma CD8 T cells was significantly lower in the SEA/CFA-immunized BL/6 mice than in the CFA-immunized or the unimmunized controls; CD8 T cells were also significantly lower in the CBA mice.
Table 2.
Percentage of lymphocyte subsets within lymphocyte gate
| Mouse group | Granuloma
|
Mesenteric lymph node
|
Spleen
|
||||
|---|---|---|---|---|---|---|---|
| CD3+CD4+ | CD3+CD8+ | CD3+CD4+ | CD3+CD8+ | CD3+CD4+ | CD3+CD8+ | ||
| BL/6 | SEA/CFA-immunized | 25.64 ± 3.30 | 7.87 ± 0.21* | 32.52 ± 6.23 | 38.50 ± 8.27 | 13.25 ± 0.56 | 13.78 ± 0.30 |
| SEA-immunized | 21.26 ± 1.43 | ND | 38.18 ± 1.97 | ND | ND | ND | |
| CFA-immunized | 27.61 ± 6.72 | 16.28 ± 0.74 | 34.86 ± 7.80 | 33.56 ± 0.90 | 10.99 ± 1.58 | 12.60 ± 1.17 | |
| control | 24.22 ± 3.40 | 19.31 ± 2.61 | 35.37 ± 7.22 | 36.66 ± 5.73 | 14.74 ± 0.67 | 17.21 ± 0.13 | |
| CBA | control | 42.53 ± 5.62* | 5.80 ± 2.14* | 53.82 ± 1.00* | 28.14 ± 7.12 | 18.63 ± 3.99 | 18.53 ± 1.40 |
Pooled hepatic granuloma, MLNs and spleen cells were cultured in the presence of 20 μg/ml SEA for 36 h and stained with anti-CD3 and anti-CD4 or anti-CD3 and anti-CD8 monoclonal antibodies. The proportion of lymphocytes within each lymphocyte gate was >85% for granuloma cells, >95% for MLN cells, and >90% for spleen cells. ND, not done.
Significantly different from BL/6 control (P < 0.05).
Regarding MLNs, there was no significant difference in CD4 T cells from experimental and control BL/6 groups, whereas these cells were again significantly higher in the CBA mice (Table 2). Moreover, CD8 T cells were lower in CBA than in all tested BL/6 mice, but the difference was not statistically significant. Lastly, there were no differences in CD4 or CD8 T cells among the splenic lymphocytes in any of the mouse groups (Table 2). Interestingly, the addition of SEA to the cell cultures was irrelevant, as the proportion of CD4 and CD8 T cells in unstimulated populations was not significantly different from those shown in Table 2 (data not shown).
Discussion
The work presented in this paper shows that immunization of schistosome-infected low-pathology mice of the BL/6 strain with SEA/CFA is conducive to a sharp Th2 to Th1 shift of the cytokine environment, which clearly correlates with a marked exacerbation of the immunopathology in these mice. A significant proportion of the SEA/CFA-immunized mice reproducibly died before completion of 7 weeks of infection. All SEA/CFA-immunized mice displayed an increased hepatic pathology, both in terms of perioval granuloma formation as well as in interstitial hepatocellular inflammation. There was a most dramatic increase in measured granuloma areas, which, if extrapolated to theoretical spheres, amounts to a triplication in average granuloma volume. There also was a striking intralobular mono- and polynuclear inflammatory cell infiltration independent of the granulomas. This marked increase in pathology was not seen in any of the control-immunized or unimmunized BL/6 mice and frequently surpassed the normally elevated pathology characteristically seen in schistosome-infected CBA mice. Of note is that the exacerbated disease in the SEA/CFA-immunized BL/6 mice developed over a relatively short time, considering that after 7 weeks, an unperturbed murine schistosome infection has not yet reached its maximal intensity.
The enhanced immunopathology observed in the SEA/CFA-immunized BL/6 mice clearly correlated with a generalized Th1-biased cytokine production. The Th1-type cytokines were produced by SEA-stimulated cells from granulomas, MLNs, and spleens and sharply contrasted with the Th2-type cytokines elicited in the SEA- or CFA-immunized controls. A similarly contrasting cytokine pattern between experimental and control mouse groups was obtained with purified, SEA-stimulated CD4 T cells, suggesting that these antigen-sensitive cells are responsible for the cytokine secretion.
There were no significant differences in the proportion of CD4 T cells present in the granuloma, MLN and splenic lymphocyte populations from the SEA/CFA-immunized and control BL/6 mice. This finding suggests that the marked difference in the magnitude of the immunopathology is associated with a reversal in cytokine secretion by a numerically unchanged population of SEA-specific CD4 T cells. A lack of difference between lymphoproliferative responses to SEA of the experimental and control BL/6 groups supports this contention (data not shown). However, there was a significant reduction in the proportion of CD8 T cells in the granulomas of SEA/CFA-immunized mice, which could not be attributed to a disproportionate increase in CD4 T cells. It is uncertain whether the reduction of these CD8 T cells influenced CD4 T cell cytokine secretion or granuloma formation in this experimental setting, but the observation is consistent with a regulatory function ascribed to CD8 T cells (24–26). Moreover, preliminary data suggest that natural killer and natural killer T cells are similarly reduced in the granuloma and spleen cell populations from SEA/CFA-immunized mice (data not shown).
The markedly enhanced immunopathology as a consequence of SEA/CFA immunization in BL/6 mice revealed some interesting differences with the naturally pronounced pathology characteristic of the CBA strain. In the SEA/CFA-immunized BL/6 mice, Th1 cytokines variously replaced the normally dominant Th2 phenotype at 7 weeks of infection, whereas CBA (and other H-2k) mice typically display and maintain a much higher mixed cytokine production of both the Th1 and Th2 types (21). Moreover, the exacerbated pathology in the SEA/CFA-immunized BL/6 mice is not associated with a change in the proportion of CD4 T cells, whereas in CBA mice these cells represent almost twice the amount in the granuloma lymphocyte population.
The CBA mice and the SEA/CFA-immunized BL/6 mice thus exemplify two instances of severe immunopathology in experimental schistosomiasis. The first one seems to be based on a genetically determined elevated number of activated CD4 T cells, whereas the second one is a product of immunization with specific antigen in combination with a strong adjuvant, but not of either one of these alone, leading to a reversal in cytokine production by CD4 T cells. T cell populations are most capable of shifts in cytokine polarization, although the factors that drive a given type of cytokine expression/secretion by CD4 T cells are not precisely understood and are likely to be multifold (27–29). The polarized T cell populations are also likely to induce and interact with antigen-presenting cells exhibiting distinct forms of activation and properties (30, 31). Regardless, both of these high-pathology models share in common the striking, more or less dominant, Th1 component.
Taken together, our findings indicate that a Th1-dominant environment is compatible with, if not directly linked to, the development of severe disease and death. This notion disagrees with a previous study in which a regimen involving i.p. injection of schistosome eggs and IL-12 similarly lead to an increase in Th1 cytokines, but had a beneficial effect on the egg-induced pathology (12). Intriguingly, in these mice, a substantial amelioration of hepatic pathology was also achieved with the injection of eggs alone (even though eggs tend to promote Th2 responses; refs. 10 and 32), and the role of IL-12, per se, was not appraised. There is no obvious explanation for this discrepancy in pathology, inasmuch as the same strain of mice (BL/6) was used. Moreover, our infected animals immunized with SEA/CFA were similarly exposed to copious parasite egg production in context with a significant increase in (endogenous) IL-12. Interestingly, results from another study consonant with our findings indicate that the development of an IL-12-dependent Th1 response was directly linked with severe liver disease and death in BL/6 mice coinfected with S. mansoni and Toxoplasma gondii; this study also raised the possibility of a role for IL-12 in tissue damage during schistosomiasis (33).
Although inconsistent with the notion that Th1 cells inhibit pathology in schistosomiasis, our findings do not negate the possible development of egg granulomas in a Th2-dominant environment. In fact, Th1- and Th2-dominant backgrounds appear to be associated with particular forms of egg-induced immunopathology. In a Th1-dominant milieu, less well circumscribed egg granulomas are usually accompanied by pronounced changes in the surrounding hepatic parenchyma characterized by inflammation infiltrating the lobules with possible hepatocellular damage and necrosis; this condition can result in early death (34, 35). In contrast, a Th2 milieu is conducive to tighter, more fibrotic granulomas (36, 37) with little surrounding hepatocellular injury, and a more prolonged survival. This concept is in agreement with recent studies in human schistosomiasis, in which a Th1-polarized environment has been found in the severe hepatosplenic form (38), whereas anti-inflammatory Th2 cytokines are dominant in the milder intestinal form of the disease (39, 40). Among these Th2 cytokines, IL-10 has been shown to regulate SEA-specific T cell function in vitro and to down-modulate schistosome pathology in vivo in the murine model (41–43); it also has been strongly linked to reduced granuloma formation (44) and the development of mild intestinal schistosomiasis in humans (39, 45, 46).
In conclusion, this paper describes a form of specific immunization of schistosome-infected low-pathology mice conducive to marked exacerbation of disease and death, in context with a clear-cut Th1 shift in cytokine environment. These findings imply that strategies to promote a Th2 phenotype could result in overall amelioration of the immunopathology associated with severe schistosomiasis.
Acknowledgments
We thank Dr. Geoffrey Sunshine for critically reading the manuscript. This work was supported by U.S. Public Health Service Grant RO1–18919 and by the United Nations Development Program/World Bank World Health Organization Special Program for Research and Training in Tropical Diseases.
Abbreviations
- SEA
schistosomal egg antigens
- MLN
mesenteric lymph node
- Th
T helper
- CFA
complete Freund's adjuvant
- TNF-α
tumor necrosis factor α
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bica I, Hamer D H, Stadecker M J. Infect Dis Clin North Am. 2000;14:583–604. doi: 10.1016/s0891-5520(05)70122-7. , viii. [DOI] [PubMed] [Google Scholar]
- 2.Fanning M, Peters P, Davis R, Kazura J, Mahmoud A. J Infect Dis. 1981;144:148–153. doi: 10.1093/infdis/144.2.148. [DOI] [PubMed] [Google Scholar]
- 3.Cheever A, Duvall R, Hallack T, Jr, Minker R, Malley J, Malley K. Am J Trop Med Hyg. 1987;37:85–97. doi: 10.4269/ajtmh.1987.37.85. [DOI] [PubMed] [Google Scholar]
- 4.Phillips S M, DiConza J J, Gold J A, Reid W A. J Immunol. 1977;118:594–599. [PubMed] [Google Scholar]
- 5.Iacomini J, Ricklan D, Stadecker M. Eur J Immunol. 1995;25:884–888. doi: 10.1002/eji.1830250404. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez H J, Wang Y, Tzellas N, Stadecker M J. Eur J Immunol. 1997;27:1170–1176. doi: 10.1002/eji.1830270518. [DOI] [PubMed] [Google Scholar]
- 7.Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbach J, Dessein A J. Nat Genet. 1996;14:181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 8.Secor W E, del Corral H, dos Reis M G, Ramos E A, Zimon A E, Matos E P, Reis E A, do Carmo T M, Hirayama K, David R A, et al. J Infect Dis. 1996;174:1131–1135. doi: 10.1093/infdis/174.5.1131. [DOI] [PubMed] [Google Scholar]
- 9.Chikunguwo S, Kanazawa T, Dayal Y, Stadecker M. J Immunol. 1991;147:3921–3925. [PubMed] [Google Scholar]
- 10.Pearce E, Caspar P, Grzych J, Lewis F, Sher A. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wynn T A, Eltoum I, Oswald I P, Cheever A W, Sher A. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wynn T A, Cheever A W, Jankovic D, Poindexter R W, Caspar P, Lewis F A, Sher A. Nature (London) 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan M H, Whitfield J R, Boros D L, Grusby M J. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- 14.Hernandez H J, Wang Y, Stadecker M J. J Immunol. 1997;158:4832–4837. [PubMed] [Google Scholar]
- 15.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 16.Pearce E J, Cheever A, Leonard S, Covalesky M, Fernandez-Botran R, Kohler G, Kopf M. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 17.Rezende S A, Oliveira V R, Silva A M, Alves J B, Goes A M, Reis L F. Infect Immun. 1997;65:3457–3461. doi: 10.1128/iai.65.8.3457-3461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn T A, Jankovic D, Hieny S, Zioncheck K, Jardieu P, Cheever A W, Sher A. J Immunol. 1995;154:3999–4009. [PubMed] [Google Scholar]
- 19.Akhiani A A, Lycke N, Nilsson L A, Olling S, Ouchterlony O. Scand J Immunol. 1996;43:257–262. doi: 10.1046/j.1365-3083.1996.d01-33.x. [DOI] [PubMed] [Google Scholar]
- 20.Stadecker M J, Hernandez H J. Parasite Immunol. 1998;20:217–221. doi: 10.1046/j.1365-3024.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez H J, Edson C M, Harn D A, Ianelli C J, Stadecker M J. Exp Parasitol. 1998;90:122–130. doi: 10.1006/expr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 22.Boros D, Warren K. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew R C, Boros D L. Infect Immun. 1986;54:820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boros D L. Ann NY Acad Sci. 1986;465:313–323. doi: 10.1111/j.1749-6632.1986.tb18507.x. [DOI] [PubMed] [Google Scholar]
- 25.Chensue S W, Warmington K S, Hershey S D, Terebuh P D, Othman M, Kunkel S L. J Immunol. 1993;151:1391–1400. [PubMed] [Google Scholar]
- 26.Pedras-Vasconcelos J A, Pearce E J. J Immunol. 1996;157:3046–3053. [PubMed] [Google Scholar]
- 27.Murphy K M, Ouyang W, Farrar J D, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy T L. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 28.Constant S L, Bottomly K. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 29.Kelso A. Springer Semin Immunopathol. 1999;21:231–248. doi: 10.1007/BF00812255. [DOI] [PubMed] [Google Scholar]
- 30.Goerdt S, Orfanos C E. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 31.Stadecker M J. Pathobiology. 1999;67:269–272. doi: 10.1159/000028108. [DOI] [PubMed] [Google Scholar]
- 32.Vella A T, Pearce E J. J Immunol. 1992;148:2283–2290. [PubMed] [Google Scholar]
- 33.Araujo M I, Bliss S K, Suzuki Y, Alcaraz A, Denkers E Y, Pearce E J. Infect Immun. 2001;69:1454–1462. doi: 10.1128/IAI.69.3.1454-1462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunet L R, Finkelman F D, Cheever A W, Kopf M A, Pearce E J. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 35.Hernandez H J, Sharpe A H, Stadecker M J. J Immunol. 1999;162:2884–2889. [PubMed] [Google Scholar]
- 36.Chiaramonte M G, Donaldson D D, Cheever A W, Wynn T A. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallon P G, Richardson E J, McKenzie G J, McKenzie A N. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 38.Mwatha J K, Kimani G, Kamau T, Mbugua G G, Ouma J H, Mumo J, Fulford A J, Jones F M, Butterworth A E, Roberts M B, Dunne D W. J Immunol. 1998;160:1992–1999. [PubMed] [Google Scholar]
- 39.Malaquias L C, Falcao P L, Silveira A M, Gazzinelli G, Prata A, Coffman R L, Pizziolo V, Souza C P, Colley D G, Correa-Oliveira R. Scand J Immunol. 1997;46:393–398. doi: 10.1046/j.1365-3083.1997.d01-136.x. [DOI] [PubMed] [Google Scholar]
- 40.Araujo M I, de Jesus A R, Bacellar O, Sabin E, Pearce E, Carvalho E M. Eur J Immunol. 1996;26:1399–1403. doi: 10.1002/eji.1830260633. [DOI] [PubMed] [Google Scholar]
- 41.Flores Villanueva P O, Reiser H, Stadecker M J. J Immunol. 1994;153:5190–5199. [PubMed] [Google Scholar]
- 42.Flores-Villanueva P O, Zheng X X, Strom T B, Stadecker M J. J Immunol. 1996;156:3315–3320. [PubMed] [Google Scholar]
- 43.Bosshardt S C, Freeman G L, Jr, Secor W E, Colley D G. Parasite Immunol. 1997;19:347–353. doi: 10.1046/j.1365-3024.1997.d01-224.x. [DOI] [PubMed] [Google Scholar]
- 44.Falcao P L, Malaquias L C, Martins-Filho O A, Silveira A M, Passos V M, Prata A, Gazzinelli G, Coffman R L, Correa-Oliveira R. Parasite Immunol. 1998;20:447–454. doi: 10.1046/j.1365-3024.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 45.Correa-Oliveira R, Malaquias L C, Falcao P L, Viana I R, Bahia-Oliveira L M, Silveira A M, Fraga L A, Prata A, Coffman R L, Lambertucci J R, et al. Braz J Med Biol Res. 1998;31:171–177. doi: 10.1590/s0100-879x1998000100024. [DOI] [PubMed] [Google Scholar]
- 46.Montenegro S M, Miranda P, Mahanty S, Abath F G, Teixeira K M, Coutinho E M, Brinkman J, Goncalves I, Domingues L A, Domingues A L, Sher A, Wynn T A. J Infect Dis. 1999;179:1502–1514. doi: 10.1086/314748. [DOI] [PubMed] [Google Scholar]