Abstract
Background
To evaluate the safety and efficacy of a novel radioactive bare metal stent (RBMS) compared with a conventional bare metal stent (CBMS) in patients with inoperable malignant airway obstruction.
Methods
This prospective study was approved by the Institutional Ethics Committee, and informed consent was obtained from each participant. Patients with malignant airway obstruction who had dyspnea were randomly assigned to receive RBMS or CBMS placement. The primary endpoint was stenosis grade, while the secondary endpoints were technical success, overall survival, and complications. A p value of <0·05 was considered statistically significant.
Results
Between September 2013 and July 2015, 66 patients with inoperable malignant airway obstruction received stent placement fluoroscopically (33 in either group). The median follow-up time was 154 days (range, 15–335 days). The baseline stenosis was immediately relieved in both groups after stent placement, and the stenosis grades in the RBMS group were significantly lower than that in the CBMS group since the second month (p < 0·05). The technical success rates of stent placement were 100% in both groups. The median survival in the RBMS group was significantly longer than that in the CBMS group (170 days vs. 123 days, p < 0·05). There was no significant difference in the incidence of complications between the two groups (p < 0·05).
Conclusions
The placement of RBMS in patients with inoperable malignant airway obstruction is feasible and safe, and it significantly reduces restenosis and improves overall survival compared with the placement of CBMS.
Keywords: Malignant airway obstruction, Stent, Intraluminal brachytherapy, Restenosis
Abbreviations: MAO, malignant airway obstruction; 125I, iodine 125; RBMS, radioactive bare metal stent; CBMS, conventional bare metal stent; CAO, central airway obstruction; TPS, treatment planning system; EBRT, external beam radiotherapy; LDR, low dose rate; CTCAE, Common Terminology Criteria for Adverse Events; MPD, matched peripheral dose; 192Ir, Iridium-192
Graphical Abstract
Highlights
-
•
The baseline stenosis was immediately relieved in both groups after stent placement.
-
•
The stenosis grades in the RBMS group were significantly lower than that in the CBMS group since the second month.
-
•
The technical success rates of stent placement were 100% in both groups.
-
•
The median survival in the RBMS group was significantly longer than that in the CBMS group.
-
•
There was no significant difference of the incidence of complications between the two groups.
Malignant airway obstruction (MAO) is a serious situation of 20-30% cases of lung cancer, resulting in dyspnea, decreased functional status, and asphyxiation risk. Conventional stent placement provides immediate palliation of dyspnea. However, stent restenosis occurs during the follow up. A novel radioactive bare metal stent (RBMS) loaded with 125I seed was developed for preventing such restenosis. Our study showed that placement of RBMS in patients with inoperable malignant airway obstruction is feasible and safe, and it significantly reduces the restenosis and improves overall survival compared with placement of conventional stent.
1. Introduction
Malignant airway obstruction (MAO) is a serious situation in 20–30% cases of lung cancer, resulting in dyspnea, decreased functional status, and asphyxiation risk [1]. In addition, metastases to the lungs from other malignancies, including esophageal, thyroid, breast, colon, and renal cell cancers, commonly result in MAO [2, 3]. While occasionally surgery may be an option for the long-term management of MAO, most cases are considered inoperable due to the late-stage diagnosis, poor tolerance, and high incidence of perioperative mortality [4]. For patients with inoperable MAO, various treatment modalities, including laser therapy, contact electrocautery, argon plasma coagulation, cryotherapy, photodynamic therapy, brachytherapy, etc., are employed to recanalize the obstruction [3, 5].
Covered or uncovered stent placement has been regarded as a safe and effective technique for the immediate palliation of dyspnea [6]. However, stent restenosis occurs in 5–45% of cases as a result of neoplastic infiltration through the meshes into the lumen and tumor overgrowth at the ends of the stent [[7], [8], [9]]. Conventional external beam radiotherapy (EBRT) after airway stenting provides an option for such restenosis with borderline survival benefits [10, 11]. Unfortunately, 37% of patients failed to complete radiation therapy because of intolerable complications, and more than one-third of patients succumbed to tumor growth-related asphyxia [10]. Based on the successful clinical application of a radioactive stent in patients with esophageal carcinoma or malignant biliary obstruction [[12], [13], [14]], the tracheal stent loaded with 125I seeds was developed for preventing restenosis of the stent. The feasibility and safety of inserting such a radioactive stent into the airway have been demonstrated in the healthy beagle dog models [15]. In this prospective study, we aim to evaluate the safety and efficacy of this radioactive bare metal stent (RBMS) versus conventional bare metal stent (CBMS) in patients with inoperable MAO.
2. Materials & Methods
2.1. Study Design and Participants
This single-center, randomized, controlled study was approved by the Institutional Review Board of our hospital. Informed consent was obtained from all patients and their families. The inclusion criteria and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
|
| |
| |
| |
| |
| |
| Exclusion criteria |
|
| |
| |
|
The patients were randomly allocated, with a 1:1 ratio, into the RBMS or CBMS group. The randomization sequence was computer-generated using a block randomization method (given a block size of four). The coded treatment assignments were stored at a special coordinating center in sealed, consecutively numbered, opaque envelopes, which would be unsealed by contacting the coordinating center until the participant enrollment. With the radiologists being the only exception, all patients, research nurses, and statisticians performing the analyses in this study were masked to the results of randomization.
2.2. Sample Size
Based on the median survival time of 90–181 days after treatment with CBMS placement in the previous studies [7, 8, 16], we hypothesized that the median survival time of the patients in the CBMS group would be approximately 3 months. While the median survival time of EBRT combined with stent placement was approximately 5–8 months [10, 17, 18], we hypothesized that the expected median survival in the RBMS group would be approximately 7 months according to our previous experience. With an alpha level of 0·05 (one-sided test), the smallest calculated sample size was 24 patients per group. When the censored cases were considered, 33 patients per group would be necessary to detect significant differences.
Fibro-bronchoscopy was performed in all patients 3 days after stent insertion to evaluate the status of the expansion and position of the stent. The telephone follow-ups were performed by a research nurse on a monthly basis, and the patients were asked to return for laboratory and imaging examinations every month or whenever dyspnea recurred. Each visit included the evaluation of symptoms and signs related to airway obstruction, laboratory values (white blood cell count and immunoglobulin), and a computed tomography (CT) scan. A single-photon emission computed tomography (SPECT) scan was performed in patients with RBMS in the first and third months after stent placement.
2.3. Endpoints and Definitions
The primary endpoint was stenosis grade, and the secondary endpoints included technical success, overall survival (OS), and complications and side effects related to stent placement. Stenosis grade was classified as 5 grades on the cross-sectional CT imaging area: grade 0 = non-appreciable stenosis; grade 1, 2, 3, and 4 = 25, 50, 75, and 90% decrease in area, respectively; grade 5 = complete obstruction [19]. Re-stenting was performed if the grade of restenosis was higher than 3. Technical success was defined as successful placement of the stent across the stricture with appropriate positioning of the stent and full expansion. OS was defined as the time from stent placement to death or the last follow-up. Complications or side effects were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4·02). Possible radiation related complications included neutropenia, decrease in IgA, IgG, and IgM, and leakage of radioactive seeds. Neutropenia was defined as a total white blood cell count <4000/mm3 in the plasma. The leakage of radioactive seeds was defined as the detection of non-target radioactive source verified by radiography and/or SPECT.
2.4. Procedure
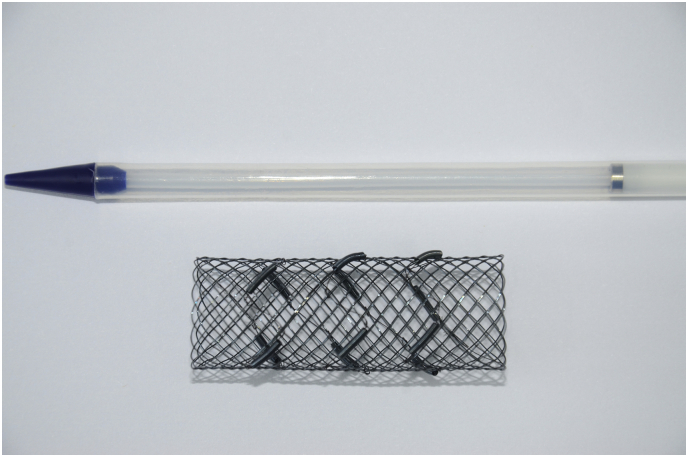
Before the procedure, blood biochemistry, performance status, clinical signs, and imaging were evaluated in all patients. Pre-procedure fibro-bronchoscopy was performed to assess the stenosis of the airway in patients with stable vital signs. Both RBMS and CBMS were provided by MTN Nanjing MicroInvasive Medical (Nanjing, China). The stent was “L”, “I”, or “Y” shaped with the following dimensions: the tracheal part with 18 mm in diameter and 50 mm in length, the right bronchial limb with 14 mm in diameter and 20 mm in length, and the left bronchial limb with 12 mm in diameter and 30 mm in length. The sheaths (6·0 mm in length × 1·3 mm in diameter) containing 125I radioactive seeds were attached to the outer surface of the stent. (Shanghai Xinke Medical Company Co., Ltd., Shanghai, China; Fig. 1). The 125I seed has a half-life of 59·4 days with an effective irradiating distance of 17 mm. The principal photon emissions are 27·4–31·5 keV X-ray and 35·5 keV γ-ray. 125I seeds with an activity of 0·8 mCi were administered to the RBMS group. The number, distribution, and dose of the seeds were determined according to the dedicated treatment planning system (TPS, Qilin Co., Ltd., Peking, China).
Fig. 1.
Photograph of a radioactive bare metallic stent loaded with 125I seeds.
The procedures of RBMS placement were performed by experienced interventional radiologists (J.H.G., G.J.T., and G.Y.Z., with, 27, 30, and 19 years of experience in interventional radiology, respectively) under fluoroscopic guidance in a C-arm angiographic unit (Innova 3100; GE Healthcare, Waukesha, WI). Under general anesthesia, the stent catheter coaxial with a 0·035-in. guidewire (Boston Scientific Corporation, Natick, MA) was orally placed over the stricture of lesion through the tracheal cannula, and then the stent loaded with radioactive seeds was delivered. After the stent was released in the target position under the guidance of radiopaque marker of the stent, the stent delivery catheter was immediately withdrawn. The placement of CBMS was conducted in the same manner except for the preloading of 125I seeds into the sheaths.
After stent placement, aerosol inhalation of budesonide (4000 iu, SPH No.1 Biochemical & Pharmaceutical Co., Ltd., Shanghai, China), dexamethasone (10 mg, Aodong Medicine Industry Group Co., Ltd., Shanghai, China), and ipratropine (500 μg, SCS Boehringer Ingelheim Comm. V, Brussels, Belgium) were intermittently administered in all patients within the first week.
All managements related to the safety of RBMS met the criteria recommended by the International Commission on Radiological Protection [20] and were reviewed by an independent radiation safety supervision group. The radiologists who performed the procedures were required to wear lead bib, lead gloves, lead glasses, and lead aprons. The patients with RBMS were kept in a single room for 7 days before discharge, and the accompanying family members were asked to stay at least one meter away from the patient.
2.5. Statistical Analysis
All the 66 patients at randomization were included into the Intention to treat analysis in this study. Categorical variables are described as frequencies and percentages. Continuous data were expressed as mean ± standard deviation (SD) or median [95% confidence interval (CI)]. Sex, tumor type, Eastern Cooperative Oncology Group (ECOG) performance status score, location of disease, stenosis grade, and dyspnea score were analyzed using the Chi-square test. The age and laboratory values prior to the stent treatment were analyzed using independent samples t-test. The laboratory results and ECOG scores pre- and post-procedure in the same group were compared using the Wilcoxon signed-rank test, while the difference pre- and post-procedure between the two groups were compared using the Mann-Whitney test. The stent placement related side effects and complications between the two groups were analyzed using the Fisher exact-test. The cubic spline function was used for comparing the stenosis grade after stent placement. The Kaplan-Meier method and log-rank test were used for the evaluation of survival time. A p value of <0·05 indicates a significant difference. The statistical analysis was performed using a computer software (SPSS, version 19·0, IBM Corp, Armonk, New York).
3. Results
3.1. Patients and Procedures
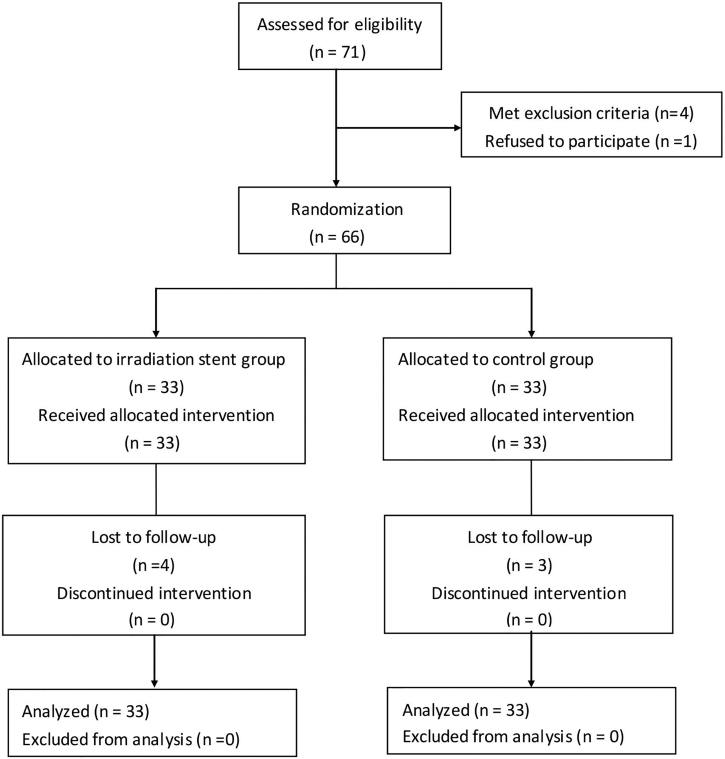
Between September 2013 and July 2015, 66 patients with inoperable histologically- diagnosed MAO with dyspnea were equally and randomly assigned to the RBMS or CBMS group. The initial stent placement procedure was successful in all 66 patients. Seven patients lost to follow-up (4 in the RBMS group versus 3 in the CBMS group; Fig. 2). The baseline characteristics of all patients were listed in Table 2. There was no significant difference in any item between the two groups.
Fig. 2.
Flow diagram.
Table 2.
Baseline demographic and disease characteristics of randomized patients.
| Characteristics | CBMS group (n = 33) | RBMS group (n = 33) | P value |
|---|---|---|---|
| Age (yr)a | 62·06 ± 6·98 | 61·58 ± 6·40 | 0·770 |
| Sex | 0·415 | ||
| Male | 25 | 22 | |
| Female | 8 | 11 | |
| Tumor type | 0·789 | ||
| Lung cancer | 24 | 22 | |
| Esophageal cancer | 9 | 11 | |
| ECOG | 0·523 | ||
| 2 | 7 | 9 | |
| 3 | 20 | 21 | |
| 4 | 6 | 3 | |
| Location(s) of disease | 0·422 | ||
| M or R or L | 13 | 11 | |
| M + R or M + L | 11 | 8 | |
| M + L + R | 9 | 14 | |
| Length of stenosis | 28·58 ± 6·77 | 27·61 ± 5·80 | 0·261 |
| Stenosis grade | 0·671 | ||
| 2 | 3 | 4 | |
| 3 | 20 | 15 | |
| 4 | 6 | 8 | |
| 5 | 4 | 6 | |
| Dyspnea score | 0·513 | ||
| 2 | 19 | 17 | |
| 3 | 7 | 11 | |
| 4 | 7 | 5 | |
| Distant metastasis | 0·375 | ||
| Yes | 5 | 7 | |
| No | 28 | 26 | |
| Laboratory valuesa | |||
| White blood cell (×109/L) | 8·12 ± 4·33 | 8·80 ± 3·95 | 0·356 |
| Immunoglobulin A | 2·79 ± 1·16 | 2·67 ± 1·04 | 0·657 |
| Immunoglobulin G | 11·81 ± 3·74 | 11·50 ± 3·26 | 0·847 |
| Immunoglobulin M | 1·49 ± 0·70 | 1·52 ± 0·69 | 0·985 |
Abbreviation: CBMS = Conventional Bare Metal Stent; RBMS = Radioactive Bare Metal Stent; ECOG = Eastern Cooperative Oncology Group. M = Main; L = Left; R = Right
Note: Data are mean ± standard deviation.
Unless indicated, chi square test was used.
Independent samples t-test was used.
The matched peripheral dose (MPD) at the reference point (10 mm from the seed surface) was approximately 35–45 Gy, calculated using a computerized TPS. The scores of ECOG performance status decreased significantly in both groups 3 days after stenting, with no difference detected between two groups (Table 3). There were no significant changes in the white blood cell counts or immunological tests (IgA, IgG, and IgM) before and after stent placement in either group or in the variance between the two groups (Table 3). Atelectasis was completely resolved in 5 patients (83·33%) and partially resolved in 1 patient (16·67%) in the RBMS group, as compared to 6 patients (85·71%) and 1 patient (14·29%) in the CBMS group, respectively.
Table 3.
Various values of pre- and post-stent placement.
| Characteristics | CBMS group (n = 33) | RBMS group (n = 33) | P value |
|---|---|---|---|
| White blood cell (×109/L) | 0·920a | ||
| Before | 8·80 ± 3·95 | 8·12 ± 4·32 | |
| After | 9·22 ± 3·71 | 8·45 ± 4·37 | |
| P value | 0·188b | 0·191b | |
| Immunoglobulin A | 0·660a | ||
| Before | 2·66 ± 1·04 | 2·79 ± 1·16 | |
| After | 2·67 ± 0·89 | 2·83 ± 1·12 | |
| P value | 0·746b | 0·926b | |
| Immunoglobulin G | 0·668a | ||
| Before | 11·51 ± 3·26 | 11·81 ± 3·74 | |
| After | 12·25 ± 3·50 | 12·01 ± 3·19 | |
| P value | 0·382b | 0·723b | |
| Immunoglobulin M | 0·501a | ||
| Before | 1·52 ± 0·69 | 1·49 ± 0·70 | |
| After | 1·60 ± 0·72 | 1·55 ± 0·64 | |
| P value | 0·160b | 0·386b | |
| ECOG | |||
| Before | 2·97 ± 0·64 | 2·82 ± 0·58 | 0·912a |
| After | 2·55 ± 0·94 | 2·39 ± 1·00 | |
| P value | 0·004b | 0·010b |
Abbreviation: CBMS = Conventional Bare Metal Stent; RBMS = Radioactive Bare Metal Stent; ECOG = Eastern Cooperative Oncology Group
Data are mean ± standard deviation.
Difference in the data before and after the procedure in the same group (Wilcoxon Signed Rank test).
Difference in the variance (pre-procedure subtracted post-procedure) between the two groups (Mann-Whitney test).
3.2. Primary Endpoint
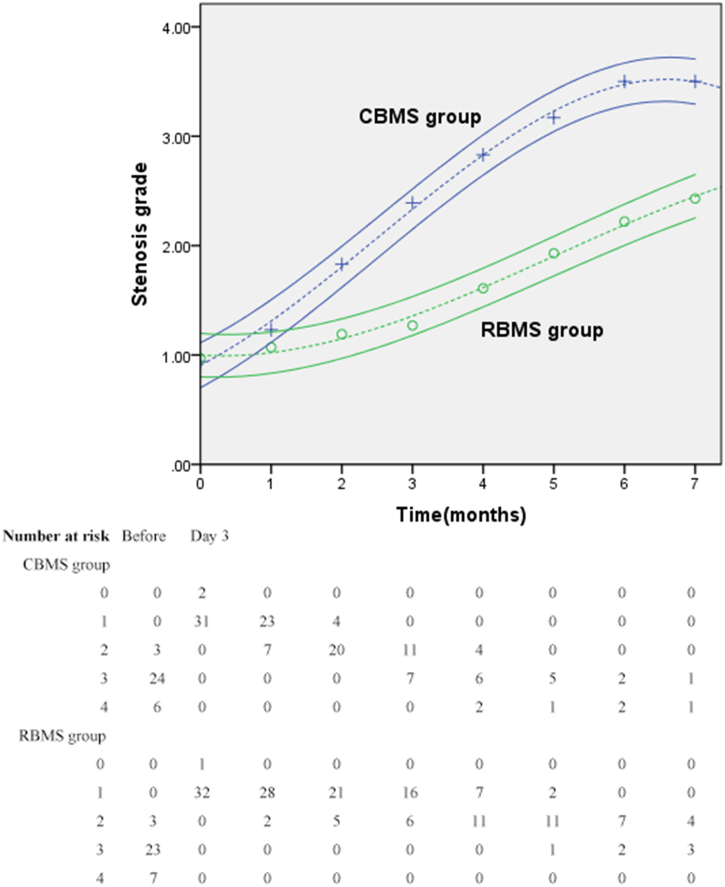
The baseline stenosis of all patients in both groups was immediately relived following the stent placement and then increased gradually as per each follow-up. Follow-up fibro-bronchoscopy 3 days after stent placement showed that all stents expanded fully without stent migration. The mean stenosis grade of the RBMS group was 0·94 three days after stent placement versus 3·48 before the procedure, and that of the CBMS group was 0·97 versus 3·33. No significant differences between two groups were observed on the third day after stent placement (p = 0·558). One month after stent placement, the stenosis grades increased in both groups with non-significant difference (p = 0·073). Since the second month to the last follow-up, the difference in stenosis score between two groups turned out to be significant at each visit (p2–5 < 0·001, p6 = 0·006, p7 = 0·036; Fig. 3).
Fig. 3.
Stenosis grades after stent insertion.
Graphs show mean stenosis grade 95% CI. Higher scores represent increased stenosis.
Stent restenosis was observed in 21·2% (7/33) of patients in the RBMS group and 45·45% (15/33) in the CBMS group (p = 0·037). Two additional conventional bare stents (one stent per patient) were placed over the initial stents when restenosis occurred in two patients with RBMS (one on Day 172 and the other one on Day 203 after stent placement). While 6 stents (one stent per patient) were placed in the CBMS group due to restenosis at a median time of 149 days (range, 113–182 days) after stent placement. Re-intervention was not attempted in 14 patients (5 in the RBMS group and 9 in the CBMS group) because of poor systemic conditions (n = 6), expected short life-span (n = 2), patients' refusal (n = 4), or other reasons (n = 2) although restenosis was demonstrated.
3.3. Secondary Endpoints
The technical success rate of stent placement was 100% in both groups. After general anesthesia, median procedure times (from initial insertion to complete withdrawal of stent catheter) were 30 and 28 s in the RBMS and CBMS groups, respectively (p = 0·471). Post-procedure SPECT examination in the RBMS group did not show radioactive concentration outside the target position of the airway. Absorbed dose of radiation monitoring during the operation was approximately 12,892–19,155 mGycm2. None of the 125I seeds was lost from the stent during the delivery or deployment process.
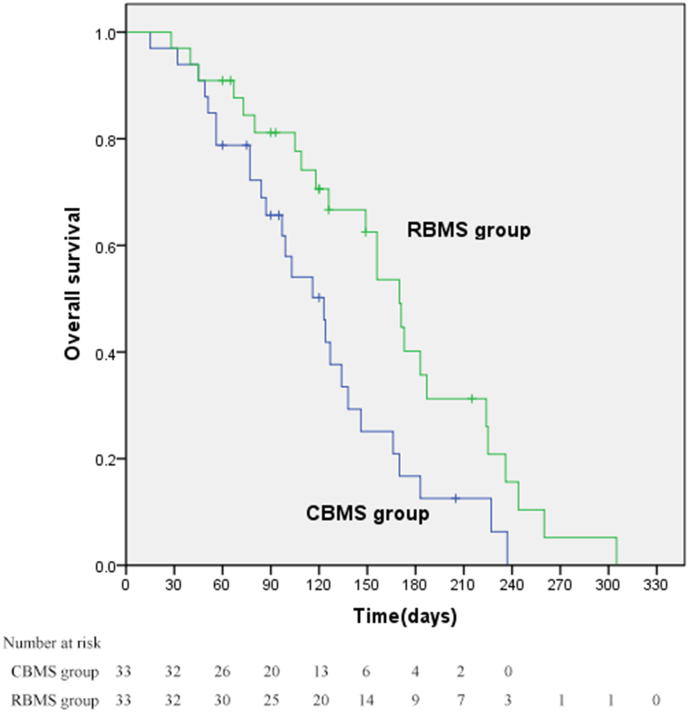
Fifty (25 per group) of the 59 patients died in a median follow-up period of 154 days (range, 15–335 days). The 30-day mortality was 3·33% (1/33) in the RBMS group versus 3·33% (1/33) in the CBMS group (p = 0·999). The median OS time in the RBMS group was 170 days (95% CI: 146, 194), significantly longer than 123 days (95% CI: 94, 152) in the CBMS group (p = 0·015; Fig. 4). There was no difference in terms of causes of death, with hemoptysis in 2 and 3 patients, pulmonary infection in 3 and 3 patients, and cachexia or multiple organ failure in 20 and 19 patients, respectively, in the RBMS and CBMS group (Fig. 5).
Fig. 4.
Kaplan-Meier overall survival curve.
The median overall survival was 170 days (95% CI: 146, 194) in the RBMS group and 123 days (95% CI: 94, 152) in the CBMS group (p = 0·015).
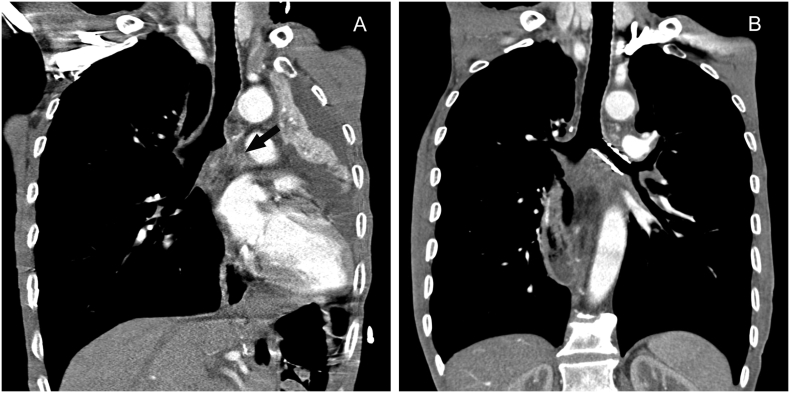
Fig. 5.
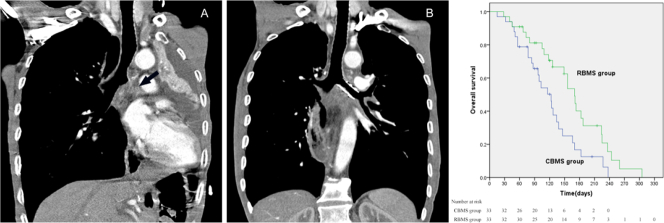
An esophageal cancer patient complicated by mediastinal lymph node metastasis, atelectasis, and pleural effusion.
5A: CT scan showed that the left bronchus had been involved and completely obstructed (black arrow). 5B: Follow-up CT one month post operation showed the left lung re-expanded, and pleural effusion was absorbed completely.
The rate of overall complications was 69·70% in the RBMS group versus 66·67% in the CBMS group (p = 0·999). The overall incidence of grade 3 or 4 complications was also comparable (24.24% v 27.27%; p = 0·999). Chest pain, fistula formation, pulmonary infection, and hemoptysis were observed in 8, 1, 4, and 9 patients in the RBMS group, respectively, whereas 9, 2, 5, and 7 were detected in the CBMS group, respectively (p > 0·05) (Table 4).
Table 4.
Complications after stent placement.
| Complications | CBMS group (n = 33) |
RBMS group (n = 33) |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All events | CTCAE Grade |
All events | CTCAE Grade |
||||||
| 1 | 2 | ≥3 | 1 | 2 | ≥3 | ||||
| Chest pain | 9 | 6 | 3 | 0 | 8 | 6 | 2 | 0 | 0·999 |
| Fistula formation | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0·999 |
| Pneumonia | 5 | 0 | 1 | 4 | 4 | 0 | 1 | 3 | 0·999 |
| Stent migration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | n.d. |
| Hemoptysis | 7 | 1 | 3 | 3 | 9 | 2 | 2 | 5 | 0·775 |
| Total | 23 | 7 | 8 | 8 | 22 | 8 | 5 | 9 | 0·999 |
Abbreviation: CBMS = Conventional Bare Metal Stent; RBMS = Radioactive Bare Metal Stent; CTCAE = Common Terminology Criteria for Adverse Events
Note: Data are number.
Fisher exact test was used to compare all events between two groups.
n.d, Not done.
4. Discussion
MAO usually results from late-stage malignancies such as lung cancer, esophageal carcinoma, and malignant lymph nodules. The long-term survival of patients with MAO is dismal. Systemic chemotherapy is a standard therapy for late-stage lung cancer and esophageal carcinoma, but this treatment is hardly tolerable to some patients with poor performance status and does not achieve immediate recanalization of the air passage [21]. Although EBRT has been recommended as a preferred choice for the palliative treatment of inoperable MAO, the effects are often delayed and unpredictable. Moreover, EBRT may aggravate airway obstruction related to early tumor edema [10]. Optionally, the expandable metallic stent placement served as a palliative therapy with the advantage of rapid relief of MAO [6]. Unfortunately, stent restenosis has a high rate up to 24% within 3 months due to the ingrowth of the initial malignancies [7]. Moreover, placement with a conventional SEMS offers limited survival benefits in the absence of locoregional therapy for endobronchial lesions. Recently, a novel stent loaded with 125I seeds was developed and exhibited promising outcomes in treating esophageal cancer, malignant biliary tract obstruction, and portal vein tumor thrombosis caused by hepatocellular carcinoma [[12], [13], [14], 22, 23]. Theoretically, this new radioactive stent may offer the advantages of both rapidly achieving lumen patency and enabling continuous brachytherapy to control the malignancies. To our best knowledge, this is the first attempt of the combination of 125I seeds brachytherapy and stent placement in treating inoperable MAO.
Brachytherapy can be delivered as low-dose-rate (LDR) therapies, in which <1 Gy per hour is delivered by radioactive sources that are left permanently in place, or as high-dose-rate (HDR) therapies, in which the radiation is delivered over several courses [24]. Iridium-192 (192Ir) is the most common isotope used for HDR brachytherapy for airway malignancies in the previous reports, with common dose fractionation regimens ranging from 4 treatments of 5 Gy each to 2 treatments of 15 Gy each [25]. However, 125I seeds were employed as the brachytherapy source loaded on the airway stent in this study, with the administrated dose of 35–45 Gy. 125I seeds may provide advances such as continuous LDR brachytherapy and a buildup of radiation damage by synchronizing tumor cells to radiosensitive G2-M phase [26]. Besides, as a LDR source, it is understandable that 125I allows efficient repair of sublethal radiation damage in normal tissue [27]. In addition, the easy preparation and relatively low energy (27·4–31·5 keV) of the emitted X-rays ensured reduced normal tissue exposure and safety for radiologists during the radioactive stent preparation and implantation. In the present study, the technical success rate of the RBMS groups achieved 100% during a short operation time. Neither 125I seed loss nor stent migration occurred during the delivery. All radioactive stents were fully expanded within 3 days after placement. The absorbed dose during the operation was relatively low and easily accepted. These results may be owing to the reasonable choice of isotope, sophisticated design of assembling, and easy controlled delivery system by an experienced interventional radiologist.
In the previous studies on esophageal cancer using the radioactive stent, the results showed that the radioactive stent allows a longer relief of dysphagia compared to the conventional stent in both a single and multiple institutional randomized controlled studies [12, 14]. Furthermore, the novel stent loaded with 125I seeds dedicated to biliary tract was developed and showed longer patency in both the single and multicenter institutional randomized controlled studies compared to a conventional stent in malignant biliary obstruction [13, 23]. In the present study, although the mean stenosis grade decreased in both groups immediately after stent placement, the stenosis grades increased gradually in both groups mainly due to the tumor infiltration and low-dose rate nature of 125I seed. Meanwhile, from the second month to the last follow-up, the difference in stenosis score between two groups turned out to be significant at each visit. This phenomenon may be attributed to the continuous increase of accumulated dose of 125I seeds in the RBMS group. These results reached the design goal of reducing restenosis of the stent by inhibiting tumor growth with 125I seeds brachytherapy.
Although the previous trials show longer survival with the radioactive stent in esophageal cancer and malignant biliary obstruction [[12], [13], [14], 23], it is uncertain whether it can prolong the OS in MAO caused by various malignancies. Conventional EBRT following stent insertion was previously reported in patients with inoperable MAO, with a modest median OS of 3·44 months (95% CI: 1·1, 5·8) [10]. As an alternative choice, endobronchial HDR brachytherapy with 192Ir was delivered to treat inoperable MAO with considerable symptom alleviation, good tumor control, and improved OS [25]. In this study, the OS in the RBMS group was significantly longer than that in the CBMS group and also seemed longer than that previously reported using CBMS or HDR brachytherapy in the literature [3, [28], [29], [30]]. The survival benefit may be attributed to the inhibition of tumor growth by the brachytherapy.
Previous studies indicated that the decrease in white blood cell counts and total immunoglobulins (IgA, IgG, and IgM) may be related to the long-term LDR radiation [31]. In this study, Neither white blood cell counts nor IgA, IgG, and IgM levels showed significant changes between pre- and post-procedures. This finding appeared to be associated with diverse types of tissue reaction which may result from different dosimetric properties with various isotopes and irradiation techniques [32]. SPECT examination following the stent placement disproved any 125I seed leakage outside the location of the stent, which indicates a well-formed mechanical design of the radioactive stent. Airway infection after stenting varies from 5·9% to 10% in the previous literature [8, 18, 33]. The various data reflect the undefined diagnostic criteria because of the pre-existed respiratory infection before stent placement in many MAO patients. In the present study, there was no difference in the occurrence of respiratory tract infection between the RBMS and CBMS groups, indicating that the radioactive stent does not increase the risk of infection. The incidences of grade ≥ 3 and total complications between the two groups were comparable in this study, suggesting that the combination of brachytherapy and stenting did not increase the incidence of treatment-related complications.
This study has several limitations. Firstly, due to the various experience of the interventional radiologists who performed the procedures and various morphological characteristics of the malignancies, the precise localization of the radioactive seeds as programmed by the preoperative plan of the TPS is sometimes challenging. Secondly, the tumor response was not assessed using any radiological measurement (e.g. RECIST criteria) for the stenting caused compression of the tumor. Thirdly, a personalized protocol on radiation dose prescription was not adopted, although the etiologies of MAO varied from patient to patient in the present study. Fortunately, the dose used in this study seems to be satisfactory in compromising treatment effects and tolerance. Nevertheless, it is warranted to have studies focusing on the radiation dose of 125I seeds in MAO.
In conclusion, the placement of RBMS in patients with inoperable MAO is feasible and safe. It significantly reduces the incidence of restenosis and improves the overall survival compared with CBMS.
Funding Sources
This study is funded by the National High-tech Research Foundation of China (863 project # 2012AA022701), the National Basic Research Program of China (973 Program # 2013CB733800, 2013733803), the Jiangsu Provincial Special Program of Medical Science (BL2013029), the National Scientific and Technical Achievement Translation Foundation ([2012]258), and the National Natural Science Foundation of China (81471762).
Conflicts of Interest
We declare no competing interests.
Author Contributions
JHG and GJT conceived the study. JHG, YW, and GJT participated in the study design. CW and JL participated in the statistical design. GYZ, HDZ, and LC conducted the study, including acquisition, analysis, and interpretation of data. CW and JL completed the statistical analysis and data interpretation. YW and JL drafted the manuscript. All authors critically reviewed, edited, and approved the manuscript and made the decision to submit for publication. All authors assume responsibility for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
Acknowledgments
Acknowledgements
The authors thank De-Rong Leng and Chun-Jun Liu from Nanjing Micro-Tech Co Ltd., Nanjing, China, for their efforts on the design of this tent. The authors also thank Xin Huang, MS, for his assistance in the protocol review and statistical analyses.
Role of the Funding Source
The funder had no role in study design, data collection, data analysis, data interpretation, or in the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Ernst A., Feller-Kopman D., Becker H.D., Mehta A.C. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278–1297. doi: 10.1164/rccm.200210-1181SO. [DOI] [PubMed] [Google Scholar]
- 2.Ernst A., Simoff M., Ost D., Goldman Y., Herth F.J.F. Prospective risk-adjusted morbidity and mortality outcome analysis after therapeutic bronchoscopic procedures: results of a multi-institutional outcomes database. Chest. 2008;134(3):514–519. doi: 10.1378/chest.08-0580. [DOI] [PubMed] [Google Scholar]
- 3.Ost D.E., Ernst A., Grosu H.B., Lei X., Diaz-Mendoza J., Slade M. Complications following therapeutic bronchoscopy for malignant central airway obstruction: results of the AQuIRE registry. Chest. 2015;148(2):450–471. doi: 10.1378/chest.14-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mudambi L., Miller R., Eapen G.A. Malignant central airway obstruction. J. Thorac. Dis. 2017;9(Suppl. 10) doi: 10.21037/jtd.2017.07.27. (S1087-S110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ost D.E., Ernst A., Grosu H.B., Lei X., Diaz-Mendoza J., Slade M. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147(5):1282–1298. doi: 10.1378/chest.14-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitag L., Gordes M., Zarogoulidis P., Darwiche K., Franzen D., Funke F. Towards individualized tracheobronchial stents: technical, practical and legal considerations. Respiration. 2017;94(5):442–456. doi: 10.1159/000479164. [DOI] [PubMed] [Google Scholar]
- 7.Miyazawa T., Yamakido M., Ikeda S., Furukawa K., Takiguchi Y., Tada H. Implantation of ultraflex nitinol stents in malignant tracheobronchial stenoses. Chest. 2000;118(4):959–965. doi: 10.1378/chest.118.4.959. [DOI] [PubMed] [Google Scholar]
- 8.Breitenbucher A., Chhajed P.N., Brutsche M.H., Mordasini C., Schilter D., Tamm M. Long-term follow-up and survival after Ultraflex stent insertion in the management of complex malignant airway stenoses. Respiration. 2008;75(4):443–449. doi: 10.1159/000119053. [DOI] [PubMed] [Google Scholar]
- 9.Profili S., Manca A., Feo C.F., Padua G., Ortu R., Canalis G.C. Palliative airway stenting performed under radiological guidance and local anesthesia. Cardiovasc Intervent Radiol. 2007;30(1):74–78. doi: 10.1007/s00270-006-0027-6. [DOI] [PubMed] [Google Scholar]
- 10.Rochet N., Hauswald H., Schmaus M., Hensley F., Huber P., Eberhardt R. Safety and efficacy of thoracic external beam radiotherapy after airway stenting in malignant airway obstruction. Int J Radiat Oncol Biol Phys. 2012;83(1):e129–e135. doi: 10.1016/j.ijrobp.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 11.Saji H., Furukawa K., Tsutsui H., Tsuboi M., Ichinose S., Usuda J. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg. 2010;11(4):425–428. doi: 10.1510/icvts.2010.238196. [DOI] [PubMed] [Google Scholar]
- 12.Guo J.H., Teng G.J., Zhu G.Y., He S.C., Fang W., Deng G. Self-expandable esophageal stent loaded with 125I seeds: initial experience in patients with advanced esophageal cancer. Radiology. 2008;247(2):574–581. doi: 10.1148/radiol.2472070999. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H.D., Guo J.H., Zhu G.Y., He S.C., Fang W., Deng G. A novel biliary stent loaded with (125)I seeds in patients with malignant biliary obstruction: preliminary results versus a conventional biliary stent. J Hepatol. 2012;56(5):1104–1111. doi: 10.1016/j.jhep.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H.D., Guo J.H., Mao A.W., Lv W.F., Ji J.S., Wang W.H. Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncol. 2014;15(6):612–619. doi: 10.1016/S1470-2045(14)70131-7. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Guo J.H., Zhu G.Y., Zhu H.D., Chen L., Lu J. A novel self-expandable, radioactive airway stent loaded with (125)I seeds: a feasibility and safety study in healthy beagle dog. Cardiovasc Intervent Radiol. 2017;40(7):1086–1093. doi: 10.1007/s00270-017-1639-8. [DOI] [PubMed] [Google Scholar]
- 16.Dutau H., Toutblanc B., Lamb C., Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951–958. doi: 10.1378/chest.126.3.951. [DOI] [PubMed] [Google Scholar]
- 17.Park J.Y., Shin J.H., Song H.Y., Yi S.Y., Kim J.H. Airway complications after covered stent placement for malignant esophageal stricture: special reference to radiation therapy. AJR Am J Roentgenol. 2012;198(2):453–459. doi: 10.2214/AJR.10.5780. [DOI] [PubMed] [Google Scholar]
- 18.Yerushalmi R., Fenig E., Shitrit D., Bendayan D., Sulkes A., Flex D. Endobronchial stent for malignant airway obstructions. Isr Med Assoc J. 2006;8(9):615–617. [PubMed] [Google Scholar]
- 19.Freitag L., Ernst A., Unger M., Kovitz K., Marquette C.H. A proposed classification system of central airway stenosis. Eur Respir J. 2007;30(1):7–12. doi: 10.1183/09031936.00132804. [DOI] [PubMed] [Google Scholar]
- 20.Vano E., Rosenstein M., Liniecki J., Rehani M.M., Martin C.J., Vetter R.J. ICRP Publication 113. Education and training in radiological protection for diagnostic and interventional procedures. Ann ICRP. 2009;39(5):7–68. doi: 10.1016/j.icrp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Celikoglu F., Celikoglu S.I., Goldberg E.P. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer. 2008;61(1):1–12. doi: 10.1016/j.lungcan.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Lu J., Guo J.H., Zhu H.D., Zhu G.Y., Chen L., Teng G.J. Safety and efficacy of irradiation stent placement for malignant portal vein thrombus combined with transarterial chemoembolization for hepatocellular carcinoma: a single-center experience. J Vasc Interv Radiol. 2017;28(6):786–794. doi: 10.1016/j.jvir.2017.02.014. [e3] [DOI] [PubMed] [Google Scholar]
- 23.Zhu H.D., Guo J.H., Huang M., Ji J.S., Xu H., Lu J. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: a multicentre trial. J Hepatol. 2018;68(5):970–977. doi: 10.1016/j.jhep.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Klopp A.H., Eapen G.A., Komaki R.R. Endobronchial brachytherapy: an effective option for palliation of malignant bronchial obstruction. Clin Lung Cancer. 2006;8(3):203–207. doi: 10.3816/CLC.2006.n.048. [DOI] [PubMed] [Google Scholar]
- 25.Escobar-Sacristan J.A., Granda-Orive J.I., Gutierrez Jimenez T., Delgado J.M., Rodero Banos A., Saez Valls R. Endobronchial brachytherapy in the treatment of malignant lung tumours. Eur Respir J. 2004;24(3):348–352. doi: 10.1183/09031936.04.00114902. [DOI] [PubMed] [Google Scholar]
- 26.Qu A., Wang H., Li J., Wang J., Liu J., Hou Y. Biological effects of (125)i seeds radiation on A549 lung cancer cells: G2/M arrest and enhanced cell death. Cancer Invest. 2014;32(6):209–217. doi: 10.3109/07357907.2014.905585. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z., Nath R. Biologically effective dose (BED) for interstitial seed implants containing a mixture of radionuclides with different half-lives. Int J Radiat Oncol Biol Phys. 2003;55(3):825–834. doi: 10.1016/s0360-3016(02)04282-7. [DOI] [PubMed] [Google Scholar]
- 28.Gorden J.A., Ernst A. Endoscopic management of central airway obstruction. Semin Thorac Cardiovasc Surg. 2009;21(3):263–273. doi: 10.1053/j.semtcvs.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Saad C.P., Murthy S., Krizmanich G., Mehta A.C. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124(5):1993–1999. doi: 10.1378/chest.124.5.1993. [DOI] [PubMed] [Google Scholar]
- 30.Donovan E., Timotin E., Farrell T., Donde B., Puksa S., Sur R. Endobronchial brachytherapy for metastasis from extrapulmonary malignancies as an effective treatment for palliation of symptoms. Brachytherapy. 2017;16(3):630–638. doi: 10.1016/j.brachy.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Serhatlioglu S., Ogur E., Ozan A.T., Gursu F., Godekmerdan A., Ayar A. Biochemical and immunological effects of ionizing radiation in radiology staff members. Tani Girisim Radyol. 2004;10(2):97–102. [PubMed] [Google Scholar]
- 32.Guo J.H., Teng G.J., Zhu G.Y., He S.C., Deng G., He J. Self-expandable stent loaded with 125I seeds: feasibility and safety in a rabbit model. Eur J Radiol. 2007;61(2):356–361. doi: 10.1016/j.ejrad.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Chung F.T., Chen H.C., Chou C.L., Yu C.T., Kuo C.H., Kuo H.P. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg. 2011;6:46. doi: 10.1186/1749-8090-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]