Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA), typified by the pulse-field type USA300, is an emerging endemic pathogen that is spreading rapidly among healthy people. CA-MRSA causes skin and soft tissue infections, life-threatening necrotizing pneumonia and sepsis, and is remarkably resistant to many antibiotics. Here we show that engineered liposomes composed of naturally occurring sphingomyelin were able to sequester cytolytic toxins secreted by USA300 and prevent necrosis of human erythrocytes, peripheral blood mononuclear cells and bronchial epithelial cells. Mass spectrometric analysis revealed the capture by liposomes of phenol-soluble modulins, α-hemolysin and other toxins. Sphingomyelin liposomes prevented hemolysis induced by pure phenol-soluble modulin-α3, one of the main cytolytic components in the USA300 secretome. In contrast, sphingomyelin liposomes harboring a high cholesterol content (66 mol/%) were unable to protect human cells from phenol-soluble modulin-α3-induced lysis, however these liposomes efficiently sequestered the potent staphylococcal toxin α-hemolysin. In a murine cutaneous abscess model, a single dose of either type of liposomes was sufficient to significantly decrease tissue dermonecrosis. Our results provide further insights into the promising potential of tailored liposomal therapy in the battle against infectious diseases.
Keywords: Liposomes, Anti-toxin therapy, CA-MRSA, USA300, Skin and soft tissue infections, Phenol-soluble modulins, α-Hemolysin, Dermonecrosis
Highlights
-
•
Sphingomyelin liposomes protected human cells from necrosis induced by CA-MRSA supernatants.
-
•
Sphingomyelin liposomes sequestered purified phenol-soluble modulin α3.
-
•
Cholesterol-containing liposomes bound α-hemolysin.
-
•
Both types of liposomes, given therapeutically, attenuated dermonecrosis in a CA-MRSA murine cutaneous abscess model.
Spreading endemically among society, community-associated methicillin resistant S. aureus (CA-MRSA) predominantly causes skin and soft tissue infections but is also associated with life-threatening diseases like sepsis. The bacterial toxins phenol-soluble modulins and α-hemolysin have been demonstrated to contribute substantially to pathogenesis and success of CA-MRSA. Here we report the efficacy of liposomes composed of naturally occurring lipids in binding and neutralizing these potent toxins, thereby protecting human cells in vitro. In a murine cutaneous abscess model, liposomal treatment reduced CA-MRSA-mediated dermonecrosis. Liposomes thus represent a potential adjunctive therapy to abscess drainage and antibiotic treatment during staphylococcal abscess infections.
1. Introduction
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is an emerging public health threat, especially the predominant, most aggressive USA300 type [1]. Resistant to many conventional antibiotics, it rapidly spreads outside the health care setting and infects even young, healthy individuals with no previous hospital exposure [2]. Infections caused by USA300 mainly manifest as purulent skin and soft tissue infections (SSTIs) [1, 2] or life-threatening diseases such as necrotizing pneumonia and sepsis [1, 2]. CA-MRSA expresses an arsenal of cytotoxic virulence factors influencing the complex interplay between the pathogen and the host's immune system [[3], [4], [5]].
A major virulence-associated feature of S. aureus is its capacity to kill host cells, mediated by secreted toxins such as α-hemolysin, leukocidins and phenol-soluble modulins (PSMs) [5]. α-Hemolysin and PSMs have receptor-independent, cytolytic activities if present at high levels [5, 6]. When expressed at lower concentrations, these toxins exert additional pro-inflammatory and/or cytotoxic properties by binding to specific receptors [[7], [8], [9]].
For example, α-hemolysin, plays an important role during staphylococcal pathogenesis, e.g. in SSTIs [[10], [11], [12]], mouse lung infection models [13, 14], and sepsis [15]. It is secreted as soluble monomers, binds to and activates the host cell receptor, A Disintegrin And Metalloprotease 10 (ADAM10) [9]. ADAM10 is present on human epithelial, endothelial and myeloid cells but is absent on human erythrocytes [16]. Receptor binding elicits powerful host inflammatory responses and the formation of pore structures in the plasma membrane of host cells that ultimately lead to the destruction of membrane integrity [16, 17].
PSMs are small (2–5 kDa), α-helical, amphipathic peptides exhibiting multiple functions in Staphylococcus pathogenesis. α-Type PSMs (PSM-α1, PSM-α2, PSM-α3, PSM-α4, δ-toxin) are ~20–30 amino acids and more cytolytic than the ~45 amino acid β-types (PSM-β1, PSM-β2). PSM-α3 possesses the most potent killing activity among all PSMs [6]. A receptor-independent mode of membrane insertion enables PSMs to kill multiple eukaryotic cell types, including endothelial, epithelial cells and myeloid cells (e.g., erythrocytes, monocytes, and neutrophils) [4, 6]. PSMs also induce receptor-dependent host inflammatory responses [8, 18, 19] and exhibit synergy with other staphylococcal toxins such as α-hemolysin and the Panton-Valentin leukocidin [PVL [11, 20]], boosting their pathogenic impact. Furthermore, PSMs are involved in biofilm formation, a stress-induced bacterial life-style that confers heightened adaptive antimicrobial resistance [4, 21]. The expression of the psmα and psmβ genes is strongly enhanced at high bacterial cell densities [6, 22]. MRSA isolates from SSTIs, especially USA300, produce significantly higher levels of PSMs than other S. aureus stains [23].
Since the above-mentioned toxins harm host cells and contribute to pathogenicity, sequestration and neutralization of one or more of these virulence factors represents a promising therapeutic approach for attenuating disease severity. Recently, we reported that a liposomal-based toxin-sequestration therapy protected host cells and attenuated bacterial virulence in vitro and in vivo [24]. Engineered liposomes composed of cholesterol and sphingomyelin (Ch:Sm, 66 mol/% cholesterol) efficiently scavenged a plethora of virulence factors, including cholesterol-dependent cytolysins, phospholipase C and staphylococcal α-hemolysin by mimicking plasma membrane lipid raft-like microdomains that are the preferred target sites for many bacterial toxins [24, 25]. As a single therapy, Ch:Sm liposomes provided only partial protection against staphylococcal and pneumococcal supernatants in vitro and in mouse infection models. However their combination with sphingomyelin-only (Sm) liposomes was fully protective, indicating that Sm liposomes neutralized as-yet unidentified virulence factors distinct from those neutralized by Ch:Sm liposomes [24]. The mixture of both liposome types under the trade name CAL02 is currently being tested in a clinical trial against severe pneumococcal pneumonia (ClinicalTrials.gov Identifier: NCT02583373).
Here we demonstrate that Sm, but not Ch:Sm liposomes bound and neutralized hemolytic virulence factors present in USA300 supernatants and protected human red blood cells (RBCs), peripheral blood mononuclear cells (PBMCs) and bronchial epithelial 16HBE140- (HBE) cells from rapid cell lysis. Mass spectrometric analysis of bacterial proteins bound by the Sm liposomes revealed α-type PSMs as an interacting target. Sm liposomes, but not Ch:Sm liposomes decreased hemolysis induced by purified recombinant PSM-α3. In contrast to Sm liposomes, Ch:Sm liposomes efficiently bound α-hemolysin. Furthermore, both types of liposomes attenuated CA-MRSA virulence by significantly reducing dermonecrosis in a murine cutaneous abscess model.
2. Materials and Methods
2.1. Liposomes
Unilamellar cholesterol:sphingomyelin (Ch:Sm, 66 mol/% cholesterol, 40 mg/ml, diameter 130 nm) and sphingomyelin (Sm, 40 mg/ml, diameter 60 nm) liposomes in sodium Tyrode's buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, 10 mM glucose, 10 mM HEPES; pH = 7.4) were provided by Lascco (Geneva, Switzerland, product name CAL02).
2.2. Bacterial strains and supernatants
The MRSA USA300 pulse field type isolate LAC (USA300) was kindly provided by Michael Otto (National Institute of Health, Bethesda, MD) and bioluminescent LAC USA300 was kindly provided by Scott Stibitz (Food and Drug Administration, Silver Spring, MD). Bacteria were cultured in Tryptic Soy Broth (TSB, Becton Dickinson). Overnight cultures were diluted in fresh TSB to an optical density OD600 of 0.1 and incubated at 37 °C under shaking conditions for 22 h. Bacteria were pelleted (5000 ×g, 10 min) and the resulting supernatants were filter sterilized (pore size 0.2 μm, Nalgene).
If indicated, bacterial supernatants were high-speed centrifuged (100,000 ×g) at 4 °C for 1 h. The resulting supernatants were treated with liposomes or sodium Tyrode's buffer (vehicle) for 5 min. Subsequently, liposomes were pelleted (100,000 ×g) at 4 °C for 1 h. The resulting liposome-free supernatants were used for the cytotoxicity assay in Fig. 1e. The liposome/toxin or vehicle/toxin pellets were applied to SDS-PAGE or mass spectrometric analysis.
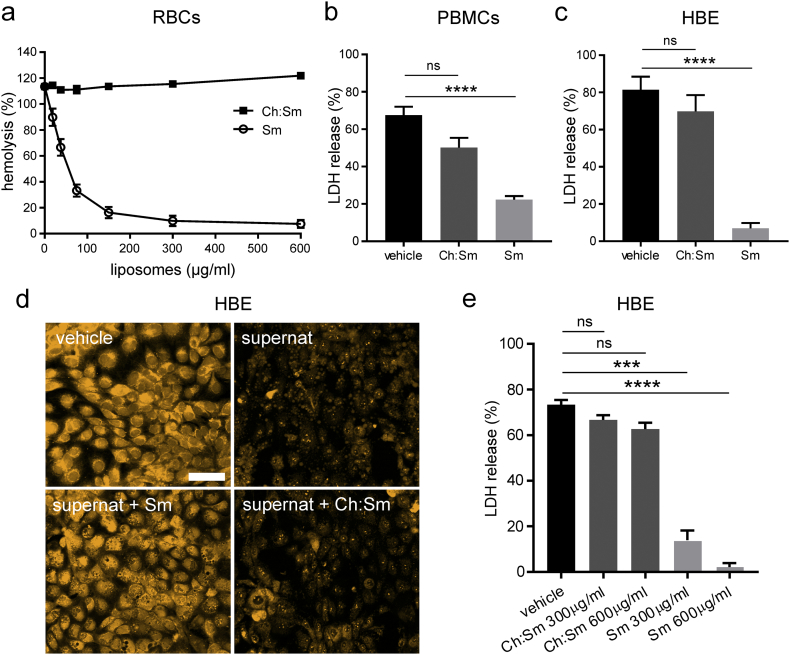
Fig. 1.
Liposomal treatment decreased necrosis of human cells induced by the secretome of S. aureus USA300.
(a) Human red blood cells (RBCs) were incubated with increasing concentrations of sphingomyelin (Sm) and cholesterol:sphingomyelin (Ch:Sm) liposomes (≥3 bacterial filtrates were tested per condition, RBCs were isolated from ≥3 different donors).
(b-d) Human cells were incubated with sodium Tyrode's buffer (vehicle), bacterial supernatants and Sm or Ch:Sm liposomes (both 300 μg/ml, or as indicated) for 1 h.
(b, c, e) The release of lactate dehydrogenase (LDH) of challenged human cells was determined.
(b) PBMCs were isolated from 5 different donors and 6 bacterial filtrates were tested per condition (one-way ANOVA Dunn's multiple comparisons test, ∗∗∗∗p < .0001, ns = not significant).
(c) Human bronchial epithelial 16HBE14o- cells (HBE) were incubated with 6 bacterial filtrates per condition (one-way ANOVA Dunn's multiple comparisons test, ∗∗∗∗p < .0001, ns = not significant).
(d) Laser scanning micrographs of HBE cells stained with CellTracker Orange. HBE cells were treated with sodium Tyrode's buffer (vehicle), bacterial supernatant (supernat), supernat and Sm liposomes (supernat+Sm), and supernatant with Ch:Sm liposomes (supernat+Ch:Sm) (representative images from 3 bacterial filtrates per condition). Scale bar = 50 μm.
(e) USA300 supernatants (50 μl) pre-incubated with Sm, Ch:Sm liposomes (300 and 600 μg/ml), or vehicle and subsequently depleted of liposomes, were applied to human bronchial epithelial HBE cells for 1 h (≥3 bacterial filtrates were tested per condition, one-way ANOVA Dunn's multiple comparisons test, ∗∗∗∗p < .0001, ∗∗∗p < .001, ns = not significant).
Error bars (a-c, e), mean ± s.e.m.
2.3. Human cells
Peripheral blood mononuclear cells (PBMCs) and red blood cells (RBCs) were isolated from the blood of healthy, consenting human volunteers (following the University of British Columbia ethics guidelines). Blood was collected in sodium heparin anticoagulant collection tubes (BD Biosciences), diluted in phosphate buffered saline (PBS, ThermoFisher/Gibco) and layered onto Lymphoprep density gradient medium (STEMCELL Technologies). After centrifugation (500 ×g for 20 min) the buffy coat was transferred to a new tube, washed three times with PBS and resuspended in RPMI-1640 Medium (+25 mM HEPES, +l-Glutamine, GE Healthcare) supplemented with 10% fetal bovine serum (FBS, ThermoFisher/Gibco). PBMCs were seeded at density of 100,000 cells and rested.
RBCs were collected from the bottom of the density gradient, washed three times with PBS and stored for a maximum of 4 weeks in Alsever's solution (Sigma Aldrich).
The human bronchial epithelial cell line 16HBE14o- (HBE, RRID:CVCL_0112) was kindly provided by Dr. D. Gruenert (University of California San Francisco). HBE cells were maintained in MEM medium (ThermoFisher/Gibco) supplemented with 10% FBS, 2 mM l-glutamine (ThermoFisher/Gibco) and 1% penicillin/streptomycin (ThermoFisher/Gibco) at 37 °C in 5% CO2. Cells were dissociated with 0.25% trypsin-EDTA (ThermoFisher/Gibco) at 80–90% confluency.
2.4. Hemolysis assay
Prior to use, RBCs were washed three times with TSB (1000 × g, 10 min). Serial dilutions of liposomes were incubated with bacterial supernatants (50 μl) and 2% RBCs in a 200 μl reaction volume with TSB in microtiter plates (Falcon). The hemolytic activity of purified staphylococcal PSM-α3 peptide (IBT Bioservices, Cat# 1401–004) was assessed in PBS. Triton X-100 (2% v/v, Sigma–Aldrich)-treated RBCs served as a positive control and TSB-treated RBCs as a negative control. After incubation for 1 h at 37 °C, RBCs were pelleted (1000 × g, 10 min) and the hemoglobin content in the supernatant was measured at OD450 (reference 630 nm) using a microplate reader. Relative hemolysis (%) was calculated as (ΔODsample − ΔODnegative control)/(ΔODpositive control − ΔODnegative control) × 100.
2.5. Cytotoxicity assays
Cytotoxicity assays were performed in cell-culture treated microtiter plates (Costar). 100,000 PBMCs were seeded directly after their isolation in RPMI +10% FBS (100 μl) and rested for 1 h. 40,000 HBE cells were seeded two days prior treatment in MEM + 10% FBS and grown to confluency. Shortly before treatment the medium was replaced with MEM + 1% FBS (100 μl). Bacterial supernatants (12.5 μl PBMCs, 50 μl HBE cells) and liposomes (300 μg/ml) or sodium Tyrode's buffer (vehicle) were added (total reaction volumes: 150 μl PBMCs, 200 μl HBE cells). Triton X-100 (2% v/v, Sigma–Aldrich)-treated PBMCs or HBE cells served as a positive control and TSB-treated PBMCs or HBE cells as a negative control. After incubation for 1 h at 37 °C, PBMCs or HBE cells were centrifuged (500 ×g, 5 min) and the lactate dehydrogenase (LDH) content in the supernatant was assessed with the Cytotoxicity Detection KitPlus (Roche) according to the manufacturer's instructions. Relative LDH release (%) was calculated by (ΔODsample − ΔODnegative control)/(ΔODpositive control − ΔODnegative control) × 100.
2.6. Confocal microscopy
Confluent HBE cells in cell-culture treated glass bottom microtiter plates (Greiner bio-one) were stained with the CellTracker Orange CMTMR dye (5 μM, excitation 548/emission 576, ThermoFisher Scientific, Cat# C2927) in serum-free MEM for 30 min. The medium was replaced with 100 μl MEM without phenol red (ThermoFisher/Gibco) and cells were treated with USA300 supernatants and liposomes (300 μg/ml) or sodium Tyrode's buffer (vehicle) for 1 h. Imaging was performed with a ZEISS LSM 800 confocal microscope equipped with an incubation system at 37 °C and analyzed with the ZEISS ZEN software 2.3 blue edition.
2.7. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining
Samples were resuspended in 2 × SDS-loading buffer (65.8 mM Tris-HCL pH = 6.8, 26.3% (w/v) glycerol, 2.1% SDS, 0.01% bromophenol blue, 355 mM 2-mercaptoethanol) and were boiled at 95 °C for 5 min. Pellets, bacterial supernatants and a protein standard (Precision Plus Protein Dual Colour Standard, Bio-rad) were applied to SDS-polyacrylamide gels (12% Mini-PROTEAN TGX stain-free precast gels, Bio-rad) and ran in SDS-running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS) at 150 V for ~30 min. Protein silver staining was performed as described [26]. In brief, the gel was soaked in 50% methanol overnight, washed in deionized water and agitated for 10 min in staining reagent (1.4 ml ammonium hydroxide, 21.0 ml of 0.36% NaOH, 4.0 ml of 20% w/v AgNO3, increased to 100 ml with deionized water). After washing the gel in deionized water, it was incubated in developer solution (2.5 ml citric acid (1% w/v), 0.25 ml formaldehyde (38% v/v), increased to 250 ml with deionized water). When the gel achieved the desired state of staining, it was soaked in 50% methanol/10% acetic acid. Bands were visualized with an imaging system (ChemiDoc Touch Imaging System, Bio-rad).
2.8. Western blotting
All steps were performed at room temperature. SDS-gels were transferred to a PVDF membrane (Immun-Blot PVDF membrane, Bio-rad) in Towbin buffer (25 mM TRIS, 192 mM Glycine, 20% methanol, pH = 8.6) at 30 V overnight. The membrane was blocked in blocking buffer (PBS, 0.3% Tween-20, 3% bovine serum albumin) for 1 h and incubated with 1 μg/ml of a monoclonal mouse anti-alpha-hemolysin antibody (8B7 N-terminal, ab190467, Abcam) diluted in washing buffer (PBS, 0.3% Tween-20, 1% bovine serum albumin) for 2 h. After washing the membrane three times for 5 min with washing buffer, it was incubated with an ECL mouse IgG, HRP-linked whole antibody from sheep (NXA931, Amersham, RRID:AB_772209) at a dilution of 1 to 5000 in washing buffer for 1 h. The membrane was washed three times with washing buffer and the Clarity Western ECL detection Kit (Bio-rad) was used according to the manufacturer's instructions. Bands were visualized with an imaging system. Black and white values were inverted for data presentation.
2.9. Mass spectrometry
In gel digestion and mass spectrometric analysis of Sm liposome/toxin pellets (35 μl/sample) were performed by the Proteomics Core Facility of the University of British Columbia (Vancouver, BC, Canada). The in gel digestion procedure was performed as described by Shevchenko et al. (1996) [27]. In brief, the protein lanes were excised from SDS-PAGE, chopped into small pieces, washed with 50% digestion buffer (50 mM NH4HCO3) and 50% EtOH, dehydrated with absolute EtOH. Gel pieces were incubated with dithiothreitol (10 mM) at 56 °C for 45 min and with iodoacetamide (55 mM) for 30 min at room temperature. After washing with digestion buffer, gel pieces were dehydrated and remaining EtOH was removed by vacuum centrifugation. Samples were incubated in trypsin solution (12.5 ng/μL) at 37 °C overnight. Neat acetic acid was added, samples were vortexed and the liquid was collected. Three gel extractions were performed with the following extraction solutions: [1] 0.5% AcOH, [2] 30% MeCN, 0.5% AcOH, and [3] 100% MeCN. All liquids were combined and the organic portion was removed by vacuum centrifugation. Following digestion, the samples were desalted on C18 STAGE tips [28] eluted with 80% acetonitrile, dried and suspended in 3% acetonitrile +0.1% formic acid. Approximately 5 μg of protein was loaded onto an Agilent 6550 QToF mass spectrometer, through and Agilent 1200 capillary HPLC connected by a 2.1 mm × 250 mm POROShell C18 column. The QToF was run in AutoMS/MS mode, at 2 spectra/s for MS and 3 spectra/s for MS/MS scans. LC-MS/MS data was processed using MaxQuant 1.5.3.30 using default values for Agilent QToF data (including 1% FDR), against the Uniprot Staphylococcus aureus USA300 (UP000001939) database.
2.10. Murine cutaneous infection model
The mouse skin infection model was performed as described previously [29]. In brief, the backs of 6-week old female CD-1 mice (Charles River Laboratories, Wilmington, MA) were shaved and depilated. USA300 and bioluminescent USA300 were grown to OD600 of 1 in TSB, washed twice with PBS and resuspended to a final concentration of 5 × 107 colony forming units (CFU)/50 μl. Bacteria (50 μl) were injected subcutaneously to the right flank of the back. Sm or Ch:Sm liposomes (50 μl of 40 mg/ml, ~80 mg/kg) were applied via intra-abscess injection after 1 h. After 72 h dermonecrosis was measured using a caliper and skin abscesses were excised and homogenized for CFU quantification (non-luminescent USA300). Bioluminescent USA300 LAC bacteria were used to track the disease progress (abscess size and bacterial burden) in real-time. Mice were anesthetized with isoflurane and imaged using the Lumina in vivo Imaging System (IVIS) (Perkin Elmer, Waltham MA) up to 10 days post-infection. Luminescence counts were determined using Living Image® Software, and reported as region of interest (ROI) values. Abscess size measurements were read up to 10 days using a caliper. All animal experiments were performed in accordance with The Canadian Council on Animal Care (CCAC) guidelines and were approved by the University of British Columbia Animal Care Committee.
2.11. Quantification and statistical analysis
Statistical significance was analyzed by Graph Pad Prism 7. All details (statistical test, number of experiments, and definition of significance) are provided in the corresponding figure legends.
3. Results
3.1. Sphingomyelin liposomes protected human cells from necrosis induced by the S. aureus USA300 secretome
Initially we tested the efficacy of Sm and Ch:Sm liposomes in preventing lysis of human red blood cells (RBCs) induced by supernatants of USA300 grown for 22 h. Hemolysis, as measured by the leakage of hemoglobin from ruptured erythrocytes, was reduced by >90% if Sm liposomes were added at concentrations of ≥300 μg/ml (Fig. 1a). Interestingly, the Ch:Sm liposomes did not decrease hemolysis at the highest concentration tested (600 μg/ml) although the same concentration of the sphingomyelin lipid in Sm liposomes (200 μg/ml) led to a reduction of >80%. Addition of Sm, but not Ch:Sm liposomes (both at 300 μg/ml), significantly diminished necrosis, induced by USA300 supernatants, of human peripheral blood mononuclear cells (PBMCs) (Fig. 1b) and HBE cells (Fig. 1c). Laser scanning micrographs of HBE cells stained with CellTracker Orange, a cell-permeable dye that is intracellularly converted to fluorescent membrane-impermeant products, illustrated the destructive effect of USA300 supernatants leading to disassembly of the cells into necrotic blebs, and an overall decline in the fluorescent signal (Fig. 1d). Addition of Ch:Sm liposomes slightly attenuated cell fragmentation, whereas Sm liposomes prevented fatal necrosis, as indicated by the retention of the cell shape and intracellular fluorescent products (Fig. 1d). We further addressed whether cytolytic virulence factors became liposome-bound and thus might be removed with the liposomes. Virulence factors in bacterial supernatants were neutralized by liposomal treatment and the liposomes were subsequently removed by centrifugation. This revealed that the cell cytolytic factors in the supernatants were sequestered by Sm, but not by Ch:Sm liposomes (Fig. 1e).
3.2. Sphingomyelin liposomes neutralized α-type phenol-soluble modulins; cholesterol-containing liposomes bound α-hemolysin
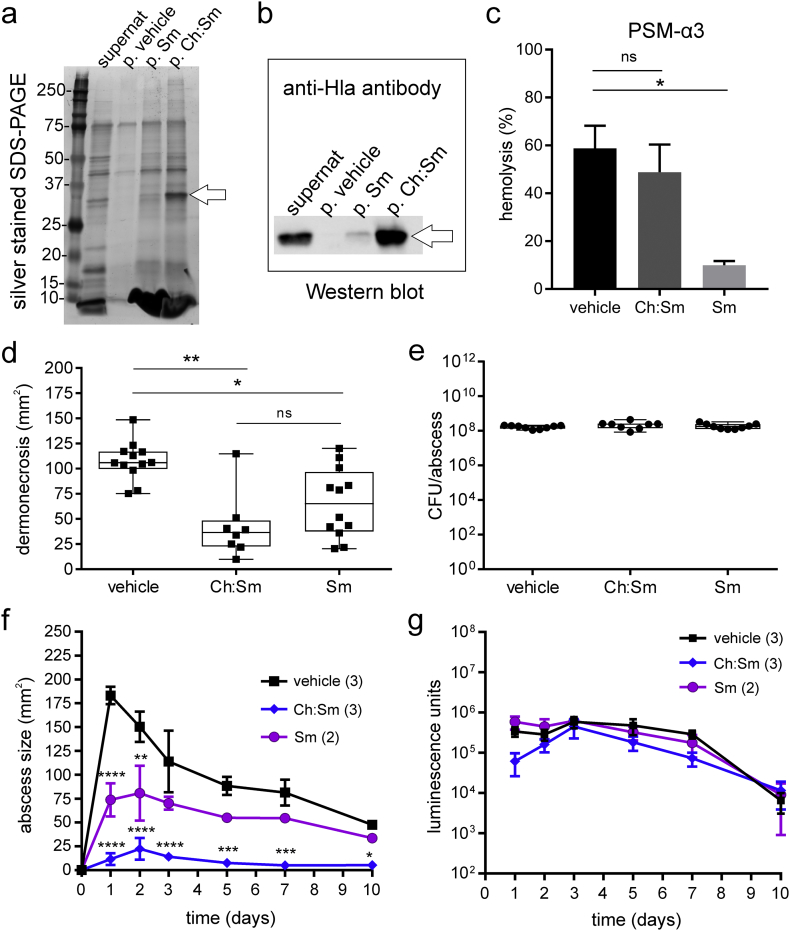
To identify secreted bacterial virulence factors that were bound to the liposome pellets, proteins were separated by SDS-PAGE (Fig. 2a). Protein bands in the liposome-free control (p. vehicle) indicated the presence of a considerable non-specific protein background. However, a prominent band appeared in the Ch:Sm liposome pellet at ~35 kDa (Fig. 2a, lane p; Ch:Sm, band marked with an arrow). Immunoblotting with an anti-α-hemolysin antibody confirmed the identity of this band demonstrating that Ch:Sm liposomes efficiently sequestered α-hemolysin (36 kDa) from USA300 supernatants, while Sm liposomes sequestered lower amounts and the vehicle pellet showed no signal (Fig. 2b; see Supplementary Fig. 1 for the complete Western Blot). Mass spectrometric analysis of the Sm pellet was performed to verify the presence of virulence factors and led to the identification of 159 proteins (Supplementary Table 1) commonly found in 3 independent bacterial supernatant preparations. As expected, proteomic analysis of the Sm/protein pellets reflected most of the proteins secreted by USA300. In addition to α-hemolysin (Hla), the bi-component leukocidin PVL (LukS-PV, LukF-PV), γ-hemolysin components A (HlgA) and B (HlgB), PSM-α1, 3, and 4, and δ-hemolysin (Hld) were also present in the Sm/protein pellets (Table 1). Since PSMs, especially PSM-α3, are the main cytolytic components of USA300 supernatants [6], and can interact with PC liposomes [30, 31], we tested whether engineered liposomes sequestered purified PSM-α3. PSM-α3 (50 μg/ml) led to 60% hemolysis of human RBCs. Sm but not Ch:Sm liposomes (300 μg/ml) significantly diminished PSM-α3-induced hemolysis (Fig. 2c).
Fig. 2.
Liposomes neutralized major S. aureus USA300 virulence factors and attenuated dermonecrosis in a murine cutaneous abscess model.
(a) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of the USA300 supernatant (supernat) and pellets (p.) of vehicle, sphingomyelin (Sm) and cholesterol:sphingomyelin (Ch:Sm) liposome-treated supernatant revealed a strong protein band in p. Ch:Sm (25–37 kDa) indicated by an arrow (representative data of ≥3 bacterial supernatants per condition).
(b) Western blotting with an anti-α-hemolysin antibody (anti-Hla antibody) identified the ~35 kDa protein band of p. Ch:Sm (arrow) as α-hemolysin (representative data of ≥2 bacterial supernatants per condition).
(c) Human red blood cells were treated with purified phenol-soluble modulin α3 (PSM-α3, 50 μg/ml) and Tyrode's buffer (vehicle), Sm or by Ch:Sm liposomes (both liposomes 300 μg/ml) (n ≥ 3, two independent experiments, ∗p < .05). Error bars, mean ± s.e.m.
(d-e) Sm or Ch:Sm liposomes (80 mg/kg) or vehicle were applied 1 h after the injection of 5 × 107 CFU/injection USA300 in the back of CD-1 mice (vehicle and Sm treatment: n ≥ 9, four independent experiments; Ch:Sm treatment n = 8, two independent experiments, box and whiskers plot). (d) Dermonecrosis (Kruskal-Wallis and Dunn's multiple comparisons test, ∗∗p < .01, ∗p < .05, ns = not significant) and (e) CFU/abscess were evaluated 72 h after infection.
(f-g) Sm or Ch:Sm liposomes (80 mg/kg) or vehicle were applied 1 h after the injection of 5 × 107 CFU/injection bioluminescent USA300 in the back of CD-1 mice and the disease progress was monitored up to 10 days post-infection (one experiment, number of mice in brackets, error bars, mean ± s.e.m.). (f) Abscess size measurements (two-way ANOVA, Dunnett's multiple comparisons test, compared to vehicle, ∗∗∗∗p < .0001, ∗∗∗p < 0.001, ∗∗p < .01, ∗p < .05). (g) Bacterial loads indicated by luminescence counts.
Table 1.
Selection of virulence-associated S. aureus USA300 proteins found in the sphingomyelin liposome/toxin pellets identified by mass spectrometry.
| Protein | Gene Name Locus Tag |
Size (kDa) | Human host cell targets |
|---|---|---|---|
| α-hemolysin |
hla SAUSA300_1058 |
36 | Cholesterol and sphingomyelin enriched microdomains [24]; A Disintegrin And Metalloprotease 10 (ADAM10) [9] |
| γ-hemolysin component A |
hlgA SAUSA300_2365 |
35 | Chemokine receptors [40, 41] |
| γ-hemolysin component B |
hlgB SAUSA300_2367 |
37 | Chemokine receptors [40, 41] |
| Panton-Valentine leukocidin, LukS-PV |
lukS-PV SAUSA300_1382 |
35 | Complement receptors [41, 42] |
| Panton-Valentine leukocidin, LukF-PV |
lukF-PV SAUSA300_1381 |
37 | Complement receptors [41, 42] |
| δ-hemolysin |
hld SAUSA300_1988 |
5 | Receptor-independent binding to the plasma membrane [6]; formyl peptide receptor 2 (FPR2/ALX) [8] |
| Phenol-soluble modulin α1 peptide, PSM-α1 |
psmA1 SAUSA300_0424.4 |
2 | Receptor-independent binding to the plasma membrane [6]; formyl peptide receptor 2 (FPR2/ALX) [8] |
| Phenol-soluble modulin α3 peptide, PSM-α3 |
psmA3 SAUSA300_0424.2 |
3 | Receptor-independent binding to the plasma membrane [6]; formyl peptide receptor 2 (FPR2/ALX) [8] |
| Phenol-soluble modulin α4 peptide, PSM-α4 |
psmA4 SAUSA300_0424.1 |
2 | Receptor-independent binding to the plasma membrane [6]; formyl peptide receptor 2 (FPR2/ALX) [8] |
The full list of proteins commonly found in three bacterial supernatant preparations is given in Supplementary Table 1.
3.3. Liposomes reduced dermonecrosis in a murine USA300 abscess model
Since USA300 skin infection studies in rodents have demonstrated the importance of α-type PSM toxins [6, 12] and α-hemolysin [11, 32] in tissue necrosis, we investigated whether Sm and Ch:Sm liposomes could be used therapeutically to target α-type PSMs and α-hemolysin of USA300 in a murine cutaneous abscess model.
Subcutaneously injected Sm or Ch:Sm liposomes (80 mg/kg) did not induce dermonecrosis, redness or any other sign of inflammation. Intriguingly, a single dose of Sm or Ch:Sm liposomes (80 mg/kg) applied subcutaneously 1 h after infection significantly decreased USA300-induced tissue necrosis measured 72 h post infection (Fig. 2d). Liposomal treatment did not reduce the bacterial load (Fig. 2e). Abscess sizes and bacterial burden measurements of abscesses induced by bioluminescent USA300 up to 10 days post-infection and are shown in Fig. 2f and g, respectively. These results showed that Sm liposomes caused moderate but significant decreases at days 1 and 2, while a single dose of Ch:Sm liposomes caused very substantial to complete abrogation of tissue necrosis at all measured time points up to day 10. In contrast there was no significant change in bioburden on any day, but rather a steady decline in concentrations over the 10-day experiment.
4. Discussion
PSMs and α-hemolysin are critical determinants of CA-MRSA virulence [4, 6, 12, 16, 19, 20, 23]. Studies investigating the effect of PSMs in animal models of SSTIs have shown that dermonecrosis caused by a ∆psmα mutant was highly attenuated compared to the wildtype strain, whereas the deletion of β-type PSMs had no effect and the knockout of δ-toxin showed only a modest reduction of virulence [4, 6, 12]. During SSTIs, PSMs cause the massive recruitment of neutrophils and contribute to tissue damage [6, 18, 20]. Furthermore, PSMs have been shown to influence the expression of virulence-promoting α-hemolysin [11], a toxin that is also involved in abscess formation [11, 32].
Here, we demonstrated that PSM-α3 was bound and neutralized by Sm liposomes in vitro (Fig. 2c) and that dermonecrosis was attenuated by Sm liposome treatment in a cutaneous abscess mouse model (Fig. 2d, f). Therefore we suggest that Sm liposomes sequester α-type PSMs and protect host cells from deleterious PSM effects.
Surewaard et al. (2012) showed that the lytic and pro-inflammatory activities of PSMs were blocked in a serum environment. They identified lipoproteins as PSM scavengers and reasoned that PSMs preferentially bound the lipid rather than protein components. Thus PSM-α1, PSM-α2, PSM-α3, PSM-α4 and δ-toxin were efficiently sequestered by high density lipoprotein (HDL) isolated from S. aureus MW2 human blood cultures. They concluded that PSMs mainly contributed to pathogenesis by acting intracellularly after bacterial uptake by neutrophils rather than as secreted toxins [33]. To our knowledge, no data regarding in vivo PSM concentrations during skin infection or other USA300 animal models exist. However in SSTIs it is likely that localized bacterial densities and consequent quorum sensing result in sufficiently high local PSM concentrations to promote receptor-dependent pro-inflammatory responses [8, 18, 19], the potentiation of other virulence factors [11, 20] and liberation of bacterial cytoplasmic protein stores including other toxins [22, 34].
In contrast to Sm liposomes, Ch:Sm liposomes efficiently sequestered α-hemolysin [24] (Fig. 2b) but did not target PSM-α3 (Fig. 2c) or other factors responsible for prompt host cell lysis (Fig. 1). Previous studies demonstrated that adding ≥50 mol/% cholesterol concentrations into dipalmitoyl-phosphatidylcholine (DPPC) liposomes led to increased membrane rigidity and negatively affected but did not completely block the vesicle-lysing activity of PSMs [31]. This may explain the poor efficacy of the 66 mol/% cholesterol-containing Ch:Sm liposomes in protecting host cells from PSM-mediated lysis. However, Ch:Sm liposomes were very effective in reducing tissue necrosis in the murine abscess model (Fig. 2d, f).
We point out that the high efficacy of Sm liposomes in neutralizing PSM-α3 and Ch:Sm liposomes in sequestering α-hemolysin in vitro does not exclude the possibility of binding of other staphylococcal or host proteins to the liposomes during the infection. Moreover, the fact that liposomes do not exclusively bind one toxin type (Supplementary Table 1) confers on them even greater therapeutic power due to broad spectrum protection of host cells.
Blocking PSMs or α-hemolysin in vivo is a desired strategy to attenuate overall virulence. To date several approaches exist affecting staphylococcal PSM expression (8, 29, 35). Recently we demonstrated that the cationic synthetic peptide DJK-5 targeted the bacterial stringent stress response in the USA300 murine cutaneous abscess model, leading to a significant reduction of the lesion size as well as the bacterial burden. In vitro, the expression of stringently regulated PSMs was suppressed at sub-lytic DJK-5 concentrations [29]. This constitutes an indirect approach to target PSM expression. Other studies investigated the potential of blocking bacterial PSM export systems [35] or the human formyl peptide receptor 2 (FPR2/ALX) that senses PSMs and controls PSM-induced inflammatory processes [8]. Although monoclonal antibodies against α-hemolysin have been shown to be protective in a CA-MRSA lung infection model [36], to date no PSM antibodies have been developed, probably because of the high amino acid sequence diversity and overlapping functions of PSMs [37].
In contrast to therapeutic strategies intervening with the expression of staphylococcal virulence-associated genes, we demonstrated here a liposome-based strategy to sequester the most potent S. aureus toxins. The neutralization of α-type PSMs by Sm liposomes and α-hemolysin by Ch:Sm liposomes could beneficially influence the course of disease during localized infections such as necrotizing pneumonia or SSTIs. Liposomal therapy is not strain specific and exerts no known selective pressure on pathogens. To date, incision and drainage followed, in severe cases, by antibiotic therapy are the recommended treatment for cutaneous abscesses [38]. Nevertheless, in killing bacteria, antibiotic treatment can cause massive liberation of host cell-damaging and pro-inflammatory bacterial agents [39]. In contrast, the neutralization of virulence-promoting factors disarms bacteria, prevents tissue damage and supports the immune system [24]. The targeting and neutralization of PSMs and α-hemolysin by liposomes may be a valuable supportive therapy for the treatment of SSTIs. After incision and drainage of abscesses, liposomes added to topical creams or adjunctive to antibiotics may have toxin sequestration and health-promoting effects.
Conflict of Interest
E.B.B. and A.D. are inventors on the patent of tailored liposomes for the treatment of bacterial infections (CA 2875470 A1).
Funding Sources
The authors acknowledge funding from the Canadian Institutes for Health Research FDN-154287. H.W. received an Early Postdoc Mobility fellowship from the Swiss National Science Foundation under Award Number P2BEP3_165401, D.P. received a Cystic Fibrosis Canada postdoctoral fellowship, and R.E.W.H. holds a Canada Research Chair in Health and Genomics and a UBC Killam Professorship.
Author Contributions
H.W. and R.E.W.H. designed and interpreted experiments and wrote the manuscript. H.W. and L.T.L. performed all in vitro experiments. S.C.M. performed all mouse experiments. A.D. and E.B.B. contributed to the initial findings. D.P., A.D., and E.B.B. contributed to interpretation of the experiments and editing of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.06.016.
Appendix A. Supplementary data
Supplementary material
References
- 1.Carrel M., Perencevich E.N., David M.Z. USA300 methicillin-resistant Staphylococcus aureus, United States, 2000-2013. Emerg Infect Dis. 2015;21(11):1973–1980. doi: 10.3201/eid2111.150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David M.Z., Daum R.S. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi S.D., Malachowa N., Deleo F.R. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185(6):1518–1527. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung G.Y., Joo H.S., Chatterjee S.S., Otto M. Phenol-soluble modulins—critical determinants of staphylococcal virulence. FEMS Microbiol Rev. 2014;38(4):698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenesch F., Lina G., Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R., Braughton K.R., Kretschmer D., Bach T.H.L., Queck S.Y., Li M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13(12):1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 7.Craven R.R., Gao X., Allen I.C., Gris D., Bubeck Wardenburg J., Mcelvania-Tekippe E. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One. 2009;4(10) doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kretschmer D., Gleske A.K., Rautenberg M., Wang R., Koberle M., Bohn E. Human Formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7(6):463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilke G.A., Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A. 2010;107(30):13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tkaczyk C., Hamilton M.M., Datta V., Yang X.P., Hilliard J.J., Stephens G.L. Staphylococcus aureus alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis model. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berube B.J., Sampedro G.R., Otto M., Wardenburg J.B. The psm alpha locus regulates production of Staphylococcus aureus alpha-toxin during infection. Infect Immun. 2014;82(8):3350–3358. doi: 10.1128/IAI.00089-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi S.D., Malachowa N., Whitney A.R., Braughton K.R., Gardner D.J., Long D. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204(6):937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kebaier C., Chamberland R.R., Allen I.C., Gao X., Broglie P.M., Hall J.D. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis. 2012;205(5):807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bubeck Wardenburg J., Patel R.J., Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75(2):1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powers M.E., Becker R.E., Sailer A., Turner J.R., Bubeck Wardenburg J. Synergistic action of Staphylococcus aureus alpha-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. Cell Host Microbe. 2015;17(6):775–787. doi: 10.1016/j.chom.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berube B.J., Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5(6):1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L., Hobaugh M.R., Shustak C., Cheley S., Bayley H., Gouaux J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274(5294):1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa S., Matsumoto M., Katayama Y., Oguma R., Wakabayashi S., Nygaard T. Staphylococcus aureus Virulent PSM alpha Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe. 2017;22(5):667. doi: 10.1016/j.chom.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syed A.K., Reed T.J., Clark K.L., Boles B.R., Kahlenberg J.M. Staphlyococcus aureus phenol-soluble modulins stimulate the release of proinflammatory cytokines from keratinocytes and are required for induction of skin inflammation. Infect Immun. 2015;83(9):3428–3437. doi: 10.1128/IAI.00401-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hongo I., Baba T., Oishi K., Morimoto Y., Ito T., Hiramatsu K. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-valentine leukocidin. J Infect Dis. 2009;200(5):715–723. doi: 10.1086/605332. [DOI] [PubMed] [Google Scholar]
- 21.Dastgheyb S.S., Villaruz A.E., Le K.Y., Tan V.Y., Duong A.C., Chatterjee S.S. Role of phenol-soluble modulins in formation of Staphylococcus aureus biofilms in synovial fluid. Infect Immun. 2015;83(7):2966–2975. doi: 10.1128/IAI.00394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Queck S.Y., Jameson-Lee M., Villaruz A.E., Bach T.H., Khan B.A., Sturdevant D.E. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32(1):150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berlon N.R., Qi R., Sharma-Kuinkel B.K., Joo H.S., Park L.P., George D. Clinical MRSA isolates from skin and soft tissue infections show increased in vitro production of phenol soluble modulins. J Infect. 2015;71(4):447–457. doi: 10.1016/j.jinf.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry B.D., Neill D.R., Becker K.A., Gore S., Bricio-Moreno L., Ziobro R. Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat Biotechnol. 2015;33(1):81–88. doi: 10.1038/nbt.3037. [DOI] [PubMed] [Google Scholar]
- 25.Fivaz M., Abrami L., van der Goot F.G. Pathogens, toxins, and lipid rafts. Protoplasma. 2000;212(1–2):8–14. [Google Scholar]
- 26.Wray W., Boulikas T., Wray V.P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 27.Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68(5):850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 28.Rappsilber J., Mann M., Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2(8):1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 29.Mansour S.C., Pletzer D., de la Fuente-Nunez C., Kim P., Cheung G.Y.C., Joo H.S. Bacterial abscess formation is controlled by the stringent stress response and can be targeted therapeutically. EBioMedicine. 2016;12:219–226. doi: 10.1016/j.ebiom.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duong A.C., Cheung G.Y., Otto M. Interaction of phenol-soluble modulins with phosphatidylcholine vesicles. Pathogens. 2012;1(1):3–11. doi: 10.3390/pathogens1010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laabei M., Jamieson W.D., Yang Y., van den Elsen J., Jenkins A.T. Investigating the lytic activity and structural properties of Staphylococcus aureus phenol soluble modulin (PSM) peptide toxins. Biochim Biophys Acta. 2014;1838(12):3153–3161. doi: 10.1016/j.bbamem.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy A.D., Bubeck Wardenburg J., Gardner D.J., Long D., Whitney A.R., Braughton K.R. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202(7):1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surewaard B.G., Nijland R., Spaan A.N., Kruijtzer J.A., de Haas C.J., van Strijp J.A. Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog. 2012;8(3) doi: 10.1371/journal.ppat.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebner P., Luqman A., Reichert S., Hauf K., Popella P., Forchhammer K. Non-classical protein excretion is boosted by PSM alpha-induced cell leakage. Cell Rep. 2017;20(6):1278–1286. doi: 10.1016/j.celrep.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatterjee S.S., Joo H.S., Duong A.C., Dieringer T.D., Tan V.Y., Song Y. Essential Staphylococcus aureus toxin export system. Nat Med. 2013;19(3):364–367. doi: 10.1038/nm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ragle B.E., Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun. 2009;77(7):2712–2718. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung G.Y.C., Otto M. The potential use of toxin antibodies as a strategy for controlling acute Staphylococcus aureus infections. Expert Opin Ther Tar. 2012;16(6):601–612. doi: 10.1517/14728222.2012.682573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer A.J., Talan D.A. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047. doi: 10.1056/NEJMra1212788. [DOI] [PubMed] [Google Scholar]
- 39.Nau R., Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev. 2002;15(1):95–110. doi: 10.1128/CMR.15.1.95-110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spaan A.N., Vrieling M., Wallet P., Badiou C., Reyes-Robles T., Ohneck E.A. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun. 2014;5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaan A.N., van Strijp J.A.G., Torres V.J. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017;15(7):435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spaan A.N., Henry T., van Rooijen W.J., Perret M., Badiou C., Aerts P.C. The staphylococcal toxin Panton-valentine Leukocidin targets human C5a receptors. Cell Host Microbe. 2013;13(5):584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material