Abstract
Hypoxia and inflammation are closely intertwined phenomena. Critically ill patients often suffer from systemic inflammatory conditions and concurrently experience short-lived hypoxia. We evaluated the effects of short-term hypoxia on systemic inflammation, and show that it potently attenuates pro-inflammatory cytokine responses during murine endotoxemia. These effects are independent of hypoxia-inducible factors (HIFs), but involve augmented adenosine levels, in turn resulting in an adenosine 2B receptor-mediated post-transcriptional increase of interleukin (IL)-10 production. We translated our findings to humans using the experimental endotoxemia model, where short-term hypoxia resulted in enhanced plasma concentrations of adenosine, augmentation of endotoxin-induced circulating IL-10 levels, and concurrent attenuation of the pro-inflammatory cytokine response. Again, HIFs were shown not to be involved. Taken together, we demonstrate that short-term hypoxia dampens the systemic pro-inflammatory cytokine response through enhanced purinergic signaling in mice and men. These effects may contribute to outcome and provide leads for immunomodulatory treatment strategies for critically ill patients.
Keywords: Adenosine, Adenosine 2B receptor, Cytokines, Hypoxia, Endotoxin
Highlights
-
•
Short-term hypoxia attenuates the systemic pro-inflammatory cytokine response in vivo in both mice and men
-
•
The underlying mechanism involves adenosine 2B receptor-mediated enhanced production of anti-inflammatory interleukin-10
-
•
These effects may contribute to outcome and provide leads for novel treatment strategies for critically ill patients
Inflammation and short bouts of low oxygen levels are frequently encountered phenomena in severely ill patients admitted to the Intensive Care unit. This study investigated the effect of short-term low oxygen levels on the inflammatory response in both mice and humans. The results show that a short period of low levels of oxygen potently dampens inflammation, and identify the underlying mechanisms. These effects of low oxygen levels may contribute to the outcome of severely ill patients. Furthermore, the increased understanding of the underlying mechanisms provided by this study can be used to develop therapies to dampen inflammation in patients.
1. Introduction
Hypoxia and inflammation are two closely linked phenomena that are encountered in many pathological processes; particularly in critical illness such as sepsis and trauma (reviewed in [12, 41]). Inflammation can lead to tissue hypoxia due to both enhanced demand and decreased availability of oxygen, the latter resulting from edema, microthrombi and changes in microcirculation (reviewed in [12]). Conversely, in vitro studies have demonstrated that hypoxia influences the immune response as well, with either pro- or anti-inflammatory effects, depending on the cell type (reviewed in [25]). In vivo data on immunomodulatory effects of hypoxia or hypoxia mimetics in animal models are conflicting. For instance, chronic hypoxia (11–27 days) in mice was reported to result in enhanced TNFα levels upon challenge with endotoxin [2], whereas the hypoxia mimetic DMOG was shown to exert distinct anti-inflammatory effects in endotoxemic mice [19]. A recent study in mice revealed that long-term hypoxia (>12 h) does not relevantly affect the immune response, but increases morbidity and mortality from skin and pulmonary infections [47]. Importantly however, hypoxia is often very short-lived in critically ill patients, as it is quickly corrected by oxygen supplementation and/or mechanical ventilation [31], but the immunologic effects of short-term hypoxia are unknown. Furthermore, no human data on immunomodulatory effects of hypoxia are available. These are nevertheless of clinical relevance, because the majority of critically ill patients suffer from inflammation-related conditions, and putative immunomodulatory effects of short-term hypoxia might therefore contribute to the outcome of these patients.
Hypoxia may modulate the immune response through a group of transcription factors called hypoxia-inducible factors (HIFs) [33]. There are several HIF isoforms, of which primarily HIF-1α has been implicated in regulation of the inflammatory response [33], although HIF-2α was also found to have immunomodulatory properties [23]. Moreover, hypoxia also induces enhanced signaling of the purine nucleoside adenosine [9], which has been shown to exert anti-inflammatory and tissue-protective effects [14]. In addition, adenosine signaling is also augmented by inflammation, through enhanced plasma levels of adenosine and increased expression of adenosine receptors. Therefore, hypoxia and inflammation might have additive or even synergistic effects on adenosine signaling [38].
In the present work, we evaluated the immunologic effects of short-term hypoxia in vivo in mice. Furthermore, we investigated the involvement of HIFs and adenosine signaling in these effects, and translated our findings to the human setting using an in vivo model of systemic inflammation in healthy volunteers.
2. Materials and Methods
2.1. Murine Studies
2.1.1. Animals and Ethics
Experiments were performed at the Experimental Centre at the University of Technology Dresden (Medical Faculty, University Hospital Carl-Gustav Carus), Germany, and the Radboud university in Nijmegen, the Netherlands. Experiments were in accordance with the facility guidelines at the University of Technology Dresden and were approved by the Landesdirektion Dresden, or with the requirements of the Dutch Experiments on Animals Act and the EC Directive 86/609, and approved by the Animal Ethics Committee of the Radboud university. Experiments were performed on male C57BL/6 mice (Charles River Laboratories International, Inc., L'Arbresle Cedex, France). For the experiments depicted in Fig. 3, Vav:cre [44] HIF1f/f [40] and HIF2f/f [18] transgenic mouse lines were obtained from the Jackson Laboratories (Bar Harbor, ME) and crossed in our facility. The obtained mouse lines are respectively: Vav:cre-HIF1αf/f (hematopoietic HIF1f/f) and Vav:cre-HIF2αf/f (hematopoietic HIF2f/f). The degree of HIF1α deficiency in hematopoietic cells of Vav:cre-HIF1αf/f mice versus WT littermates was defined by qPCR on CD45+ bone marrow cells, which revealed >90% reduction of WT HIF1α mRNA compared to WT littermates (Fig. S1). Since HIF2α messenger was scarcely detectable in hematopoietic cells of WT mice, we performed genomic PCRs on DNA isolated from mature white blood cells. In samples displaying Cre-recombinase, HIF2α PCRs revealed virtually full recombination of the floxed-HIF2α (Fig. S1). For the experiments depicted in Fig. 4, Fig. 5, adenosine 2B receptorf/f (provided by Prof. Eltzschig), and B6.129P2-Il10tm1Cgn/J mice ((MGI Cat# 5470153, RRID:MGI:5470153) (IL-10−/−, The Jackson Laboratory, Bar Harbor, ME) and genetically matched controls were used (all C57BL/6 background). Due to the nature of the studies, blinding of the animal researchers was not possible. The laboratory analyses were performed by blinded personnel.
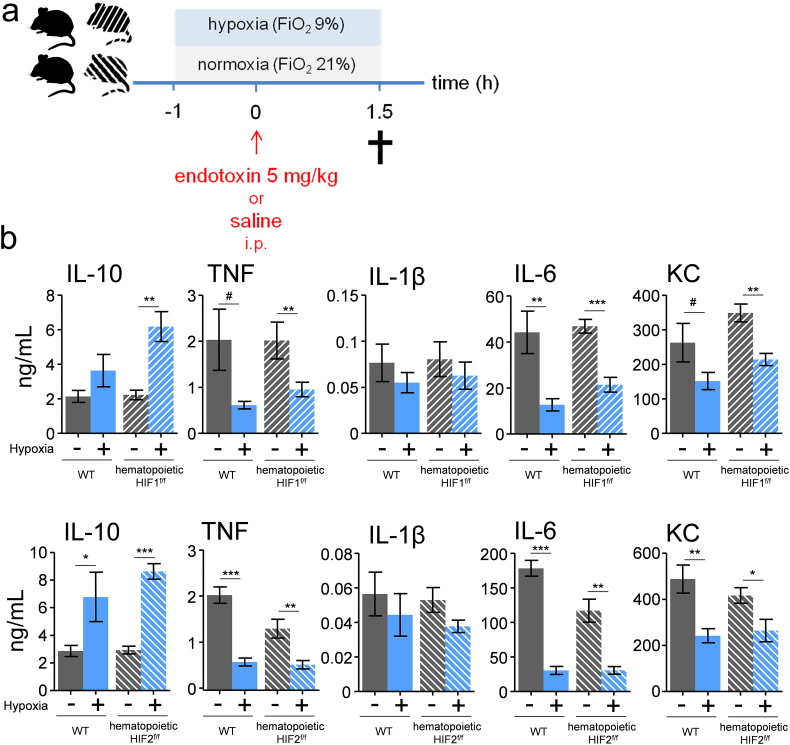
Fig. 3.
The anti-inflammatory effects of short-term hypoxia are not mediated through HIFs.
(a) Experimental setup. Vav:cre HIF-1αf/f,Vav:cre HIF-2αf/f and genetically matched wild type littermates (C57BL/6 background) were exposed to short-term hypoxia and intraperitoneally (i.p.) injected with endotoxin (5 mg/kg). (b) Plasma concentration of IL-10, TNF, IL-1β, IL-6 and KC. n = 6–7 in Vav:cre HIF-1αf/f and wild type groups, n = 6–8 in Vav:cre HIF-2αf/f and wild type groups. Data are expressed as mean (±s.e.m.). *P < .05, **P < .01, ***P < .001 (unpaired Student's t-test).
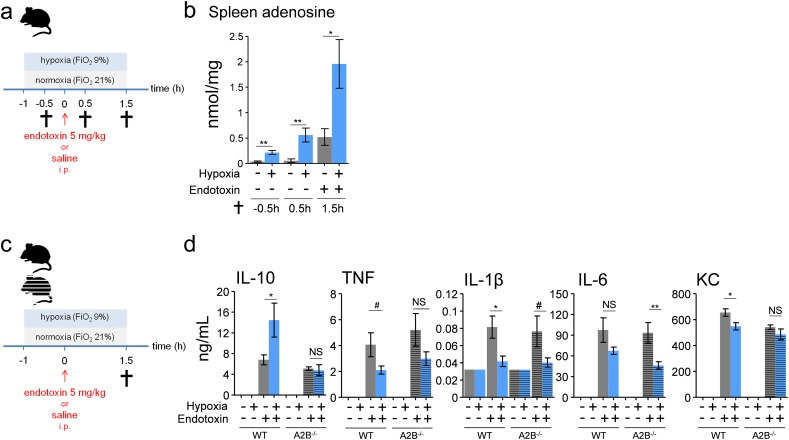
Fig. 4.
The anti-inflammatory effects of short-term hypoxia involve adenosine 2B receptor-dependent augmentation of IL-10 levels.
(a) Experimental setup. C57BL/6 mice were randomized to short-term hypoxia or normoxia and intraperitoneally (i.p.) injected with endotoxin (5 mg/kg) or saline. (b) Splenic tissue concentration of adenosine. n = 6 in saline groups, n = 8 in endotoxin groups. (c) Experimental setup. Adenosine 2B receptor (A2B) genetic deficient and genetically matched control mice (C57BL/6 background) were randomized to short-term hypoxia or normoxia and intraperitoneally injected with endotoxin (5 mg/kg) or saline. (d) Plasma concentration of IL-10, TNF, IL-1β, IL-6 and KC. n = 2 in saline groups, n = 6 in wild type endotoxin groups, n = 8 in A2B −/− normoxic endotoxin group, n = 7 in normoxic endotoxin A2B−/− group. Data are expressed as mean (±s.e.m.). *P < .05, **P < .01, ***P < .001, # P = .05–0.1 (unpaired Student's t-test).
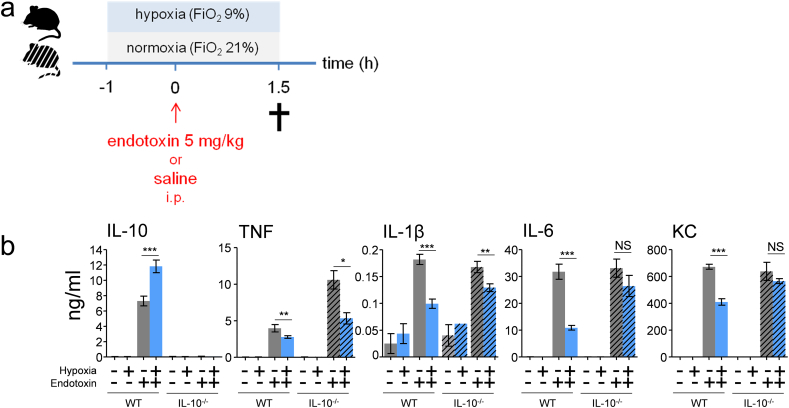
Fig. 5.
Hypoxia-induced attenuation of pro-inflammatory cytokines involves IL-10.
(a) Experimental setup. IL-10−/− and genetically matched wild type mice (C57BL/6 background) were randomized to short-term hypoxia or normoxia and intraperitoneally (i.p.) injected with endotoxin (5 mg/kg) or saline. (b) Plasma concentration of IL-10, TNF, IL-1β, IL-6 and KC. n = 3 in wild type saline groups, n = 4 in IL-10−/− saline groups, n = 8 in all endotoxin groups. Data expressed as mean (±s.e.m.). **P < .01, ***P < .001 (unpaired Student's t-test).
2.2. Experimental Protocol
After randomization using the sealed envelope method, mice were placed in an air-tight cage that was continuously flushed with either medical air (normoxia) or an hypoxic nitrogen/medical air mixture (fraction of inspired oxygen [FiO2] of 9%) at the same airflow rate. Endotoxin (E. coli, serotype 0111:B4, Sigma-Aldrich, St Louis, MO, USA) dissolved in normal saline was administered intraperitoneally at a dose of 5 mg/kg. Mice were sacrificed by exsanguination through orbita extraction under deep isoflurane anesthesia. Blood was collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes, centrifuged (14,000 g, 5 min, room temperature), was and plasma was stored at −80 °C until analysis. Splenic tissue was snap-frozen in liquid nitrogen and stored at −80 °C until analysis.
2.3. Cytokine Analysis
Plasma concentrations of TNF, IL-1β, IL-6, KC, and IL-10 were measured using a Luminex assay (Milliplex, Merck Millipore, Billerica, MA, USA) or ELISA (Duoset or Quantikine, R&D systems, Minneapolis, MN, USA).
2.4. mRNA Expression Analysis
Spleen tissue was homogenized using a Tissuelyzer LT instrument (Qiagen, Venlo, the Netherlands) and RNA was isolated with the RNeasy kit (Qiagen). Up to 1 μg of RNA was used for cDNA synthesis with iScript (Bio-Rad, Veenendaal, the Netherlands).
qPCR was performed on a CFX96 (Bio-Rad, Hercules, CA, USA). The following primer-probe sets were used (all from Life Technologies, Carlsbad, CA, USA): TNF: Mm00443258_m1, IL-1β: Mm00434228_m1, IL-6: Mm00446190_m1, KC: Mm04207460_m1, IL-10: Mm00439614_m1, VEGF (Mm00437306_m1), B2M (Mm00437762_m1) and HRPT (Mm01545399_m1). ΔCt values were calculated as the difference between the Ct value of the target gene and the geometric mean of the Ct values of two housekeeping genes (B2M and HRPT). Fold regulation (2|ΔΔCt|) was determined by normalizing ΔCt to the reference group (normoxia-saline).
2.5. Tissue Adenosine Measurements
Nucleosides were extracted from spleen tissue by sonification in 850 μL of ice cold 0.4 N perchloric acid. The mixture was vortexed and 10 μL of this solution was used for protein assay. The remaining volume was centrifuged at 14000 rpm at 4 °C for 10 min and 710 μL of the supernatant was vortexed with 40 μL phenol red (0.1 mg/mL in water) and 356ul 0.6 N KHCO3/KOH. After addition of 111 μL ammonium phosphate and 50 μL of 0.18 N H3PO4 the sample was vortexed, and centrifuged at 14000 rpm for 5 min. 1000 uL of the supernatant was used for adenosine measurements by HPLC [26].
2.6. Human Studies
2.6.1. Subjects and Study Design
The human experiments were carried out in two phases (ClinicalTrials.gov identifiers NCT01889823 and NCT01978158) for safety reasons. All experiments were in accordance with the declaration of Helsinki. After approval from the local ethics committee of the Radboud University Medical Center, thirty healthy, male volunteers gave written informed consent to participate in the experiments. Subjects were screened and had a normal physical examination, electrocardiography, and routine laboratory. Exclusion criteria were febrile illness during the 2 weeks before the experiment, high altitude exposure in the three months prior to the experiment, use of prescription drugs, history of spontaneous vagal collapse, and participation in a previous trial with endotoxin administration.
In the first phase, effects of short-term hypoxia in the absence of systemic inflammation were studied. Ten subjects were exposed to hypoxia for 3.5 h by titration of FiO2 to a peripheral saturation (SaO2) of 80–85%, using an nitrogen/medical air mixture and an air-tight respiratory helmet (CaStar, StarMed, Italy). The airflow was adjusted to prevent carbon dioxide rebreating and prevent hypercapnia. In the second phase, 20 subjects participated in endotoxemia experiments. These subjects were randomized using the sealed envelope method to hypoxia (n = 10) as described above, or normoxia (medical air, FiO2 of 21%, also using the respiratory helmet and the same airflow rate as in hypoxic subjects, n = 10). One hour after initiation of hypoxia or normoxia, 2 ng/kg U.S. Reference Escherichia coli endotoxin (serotype O:113, Clinical Center Reference Endotoxin, National Institute of Health, Bethesda, USA) was administered intravenously to elicit a systemic inflammatory response. Due to continuous monitoring of SaO2, blinding of the researchers performing the procedures on the healthy volunteers was not possible. All laboratory analyses were performed by blinded personnel. A depiction of the experimental setup is provided in Fig. S2.
2.6.2. Procedures and Recording of Vital Signs
Subjects refrained from caffeine and alcohol 24 h before the experiment, and refrained from food and drinks 10 h before the experiment. A venous cannula was placed for fluid infusion (prehydration with 1.5 L 2.5% glucose/0.45% saline in the hour preceding endotoxin administration followed by hydration with 150 mL/h for 6 h, and 75 mL/h for the rest of the experiment) and endotoxin administration. An arterial cannula was placed for monitoring of blood pressure and blood withdrawal. Heart rate and SaO2 were monitored using a three-lead electrocardiogram and a pulse oximeter connected to a Philips MP50 patient monitor. Body temperature was measured every 30 min using an infrared tympanic thermometer (FirstTemp Genius 2; Covidien, Ireland).
2.6.3. Plasma Cytokines and PaO2 Measurements
Blood was collected in EDTA tubes and centrifuged immediately at 2000 g at 4 °C for 10 min after which plasma was stored at −80 °C until analysis by Luminex assay (Milliplex). PaO2 was analyzed in lithium heparin anticoagulated arterial blood using CG4+ cartridges and a point-of-care i-STAT blood gas analyzer (Abbott, Abbott Park, IL, USA).
2.6.4. HIF-1α Protein Expression in Leukocytes
After lysis of erythrocytes in lithium heparin-anticoagulated blood using Pharm Lyse solution (Becton Dickinson, San Jose, CA), surface staining was performed using fluorochrome-conjugated antibodies (CD15-FITC (Clone H198), CD3-PECγ7 (Clone HIT3a), CD14-APC (Clone M5E2) (BioLegend Cat# 301807, RRID:AB_314189) (all obtained from BioLegend, San Diego, USA) for neutrophils, lymphocytes, and monocytes, respectively. After fixation and permeabilization with Transcription Factor Fixation/Permeabilization buffer (eBioscience Inc., San Diego, USA), PE-conjugated anti-HIF-1α and corresponding isotype control (Clone 241812, RD Systems, Minneapolis, USA)were used to stain intracellular HIF-1α and correct for aspecific binding. Leukocytes were fixed and stored overnight in PBS containing 1% bovine serum albumin and 1% paraformaldehyde. The data were acquired with Cytomics FC500 (Beckman Coulter, Brea, CA, USA) and analyzed with Kaluza software (Beckman Coulter). Cell subtypes were identified by surface staining and sideward scatter. Mean PE fluorescence, after subtraction of isotype-fluorescence, was used as a measure of HIF-1α expression. The gating strategy used is depicted in Fig. S3.
2.6.5. mRNA Expression of HIF-1α, and Hypoxia Signaling-Related Genes
Blood was collected in PAXgene tubes (PreAnalytiX, Venlo, The Netherlands), which contain a proprietary reagent that immediately stabilizes leukocyte RNA at the moment of blood withdrawal, and kept at room temperature for 24 h before storage at −80 °C. Total RNA was isolated using the PAXgene Blood RNA kit (PreAnalytiX) and up to 1 μg of RNA was reverse transcribed into cDNA using the i-script cDNA synthesis kit (Biorad), and stored at −20 °C until analysis. mRNA expression of HIF-1α was analyzed with qPCR on a Bio-Rad CFX96 (Bio-Rad). HIF-1α expression was normalized to the expression of the housekeeping gene B2M. The following primer-probe sets were used (from Life Technologies): HIF-1α: Hs00153153_m1 and B2M: Hs00984230_m1.
Expression of hypoxia signaling-related genes was analyzed in 3 subjects from each group using the RT2 Profiler hypoxia signaling Plus PCR Array (Qiagen) according to the manufacturer's protocol on a CFX96 (Bio-Rad). The array plate was customized by replacing 4 controls by 4 extra genes (Von Hippel Lindau [VHL], granulocyte macrophage colony-stimulating factor [GM-CSF], granulocyte colony-stimulating factor [G-CSF] and soluble VEGF receptor [FLT1]). Genes of interest were normalized to 4 housekeeping genes (GAPDH, B2M, RPLP0 and ACTB) present on the PCR array. A <35 cycles cut off was applied.
2.6.6. Plasma Adenosine Measurements
Plasma adenosine concentrations were measured using an in-house developed method described previously [36]. Briefly, blood was immediately mixed at the tip of the syringe with a 2.5 mL solution containing pharmacological blockers of adenosine formation, transport and degradation.Blood samples were centrifuged for 10 min at 1000 g at 4 °C and plasma was stored at −80 °C until analysis. Plasma adenosine concentrations were determined by high performance liquid chromatography (HPLC).
2.7. Whole Blood Stimulation Experiments
After approval from the local ethics committee of the Radboud university medical center (CMO 2010/10), lithium heparin-anticoagulated blood was obtained from 8 healthy volunteers who provided written informed consent. Blood was diluted 5 times in culture medium (RPMI [Invitrogen, Carlsbad, California, USA] supplemented with 10 μg/mL gentamicin, 10 mM Glutamax and 10 mM pyruvate) and incubated in 48-well plates in the presence of 1 or 10 μM PSB1115 (Tocris BioScience, Abingdon, UK) or vehicle (DMSO, final concentration of 0.1% in all experimental conditions) for 30 min at 37 °C and 5% CO2. Hereafter, 10 μM 5′-N-Ethylcarboxamidoadenosine (NECA) or vehicle (DMSO) was added and cultures were again incubated for 30 min. Subsequently, 10 ng/mL endotoxin (E. coli, serotype O55:B5, Sigma Aldrich) or vehicle (RPMI) was added and cultures were incubated for 24 h, after which plates were centrifuged for 8 min at 1400 RPM at room temperature and supernatant collected and stored at −80 °C until analysis. Cytokine concentrations were measured using ELISA according to the manufacturer's instructions (Human Duoset, R&D systems).
2.8. Calculations and Statistical Analysis
For both the human and murine experiments a power calculations were performed. In previous human endotoxemia experiments performed by our group, the standard deviation (s.d.) for peak plasma TNF concentration was 30% of the mean. Using an two-sided α of 0.05, a power of 80% (β of 0.2), and an expected detectable contrast of 40% in an unpaired t-test design, 10 subjects per group were required.
For the initial murine experiments, a provisional power calculations were based on previous murine endotoxemia experiments performed by our group, the s.d. for plasma IL-6 concentration was 25% of the mean. Using an two-sided α of 0.05, a power of 80% (β of 0.2), and an expected detectable contrast of 40% in an unpaired t-test design, 8 animals per group were required. To reduce the number of animals needed, we used the data from the primary murine experiment to calculate the lowest number of animals necessary. Plasma IL-6 concentrations were normally distributed, with a mean of 38,019 pg/mL in the normoxic group, and 11,126 pg/mL in the hypoxia group, and a pooled standard deviation of 6994. A 2-group 2-sided power calculation with these actual data shows that n = 2 would be sufficient to identify this difference. Nevertheless, we deemed this group size insufficient to draw robust conclusions and therefore have used a minimum of n = 5 for all endotoxin-treated groups. Because saline treatment was shown to not induce cytokine responses under normoxic or hypoxic conditions, we applied the ‘refinement’ dogma; by reducing the number of animals in this group to n = 2–6. Data are presented as median and interquartile range, or mean (±s.e.m.) based on their distribution (calculated by the Shapiro-Wilk test). Differences between groups in demographic characteristics were calculated using Kruskall-Wallis tests. Except for demographic characteristics, all non-parametric data were log-transformed before statistical analysis The human in vivo cytokine data were analyzed using unpaired Student's t-tests on Area Under Curve (AUC) of time-concentration curves to assess differences in total amount of cytokines released. For other human in vivo data, repeated measures one-way analysis of variance (ANOVA) followed by Dunnett's post-hoc test was used to assess within-group differences over time and make comparisons to baseline, whereas between-group differences over time were assessed using repeated measures two-way ANOVA (interaction term). Independent groups were compared using Student's t-test, multiple independent groups were compared using one way ANOVA with Dunnett's post-hoc test to compare with control group. All tests were two-sided, and a p-value of <0.05 was considered statistically significant. Statistical calculations were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA).
For the RT2 Profiler hypoxia signaling Plus PCR Array data, differences between groups were analyzed using repeated measures two-way ANOVA (interaction term) on ΔCT values. To analyze differences over time within one group or in the pooled dataset of hypoxic and normoxic endotoxemic subjects, paired Student t-tests were used on ΔCT values, comparing the different time-points against baseline (t = −1, just before initiation of hypoxia or normoxia using the respiratory helmet). A 2-fold change cut off compared to baseline was applied and p-values were corrected for the false discovery rate (FDR) using Benjamini Hochberg correction. qPCR array data were analyzed using TIGR Multiexperiment viewer 4.0 (TMeV4.0) and are presented as fold change compared to baseline.
3. Results
3.1. Short-term hypoxia exerts anti-inflammatory effects during murine endotoxemia
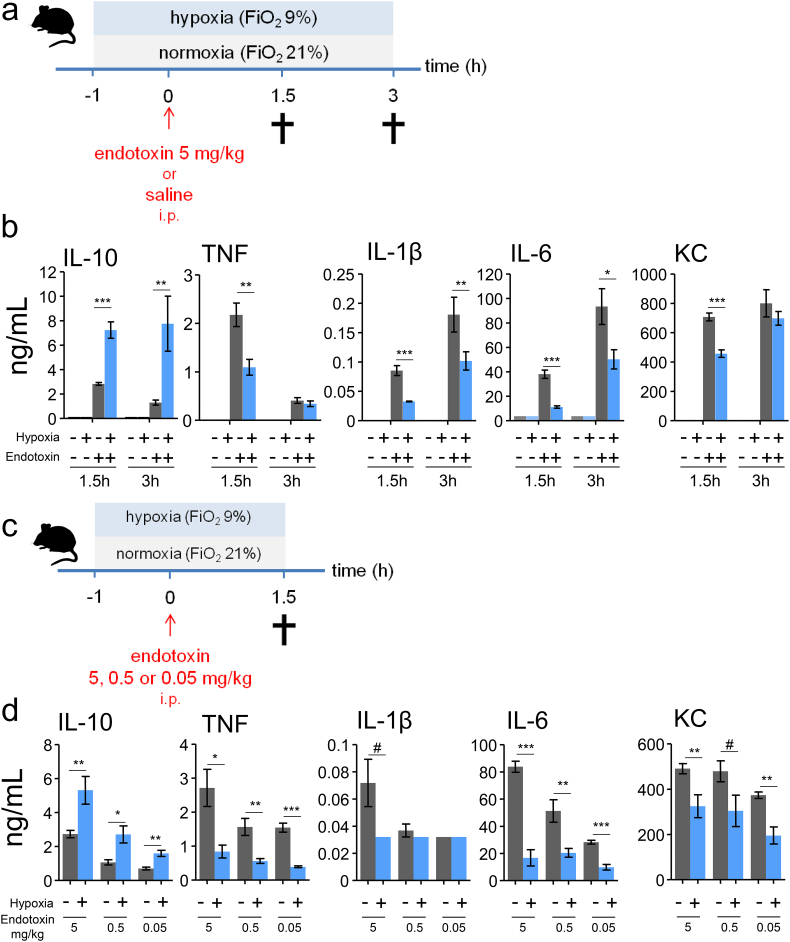
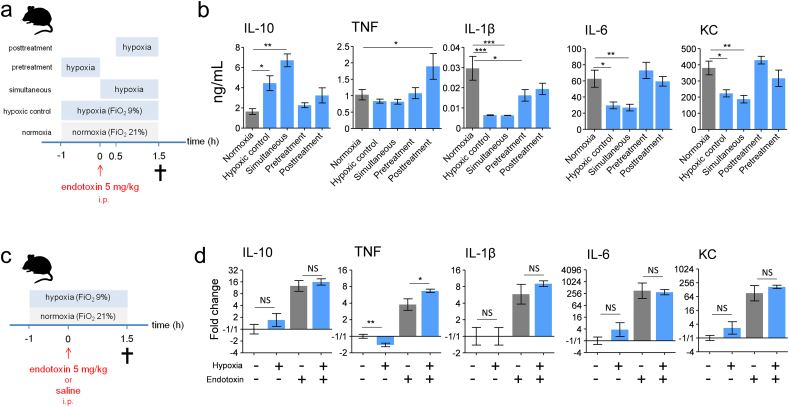
Binding of lipopolysaccharide (LPS, endotoxin) to Toll-like receptor 4 is a critical step in orchestrating of the inflammatory response in Gram-negative sepsis, and increased levels of plasma cytokines are one of the key hallmarks of this response. We used a murine model in which intraperitoneal injection with E. coli-derived endotoxin was employed to elicit a systemic inflammatory response, and studied the effects of short-term hypoxia (fraction of inspired oxygen [FiO2] of 9%) on the systemic cytokine response (experimental design depicted in Fig. 1a). Plasma levels of both pro- (tumor necrosis factor alpha [TNF], interleukin-1β [IL-1β], IL-6, and keratinocyte-derived chemokine [KC]) and anti- inflammatory (IL-10) cytokines were increased at both 1.5 and 3 h after endotoxin administration (Fig. 1b). Hypoxia initiated 1 h before endotoxin administration resulted in profound augmentation of plasma IL-10 levels and attenuation of the pro-inflammatory cytokine response (Fig. 1b). These effects were most pronounced at 1.5 h after endotoxin administration (Fig. 1b), and were present to a similar extent when mice were challenged with lower doses of endotoxin (Fig. 1c and d).
Fig. 1.
Short-term hypoxia exerts anti-inflammatory effects during murine endotoxemia.
(a) Experimental setup. C57BL/6 mice were randomized to short-term hypoxia or normoxia and intraperitoneally (i.p) injected with endotoxin (5 mg/kg) or saline. (b) Plasma concentrations of interleukin-10 (IL-10), tumor necrosis factor alpha (TNF), IL-1β, IL-6 and keratinocyte-derived chemokine (KC). n = 4 in saline groups, n = 8 in endotoxin groups. (c) Experimental setup. C57BL/6 mice were randomized to short-term hypoxia or normoxia and intraperitoneally injected with endotoxin (5, 0.5 or 0.05 mg/kg). (d). Plasma concentrations of IL-10, TNF, IL-1β, IL-6 and KC. n = 5 per group. Data expressed as mean (± s.e.m.). *P < .05,**P < .01, ***P < .001, #P = .05–0.10 (unpaired Student's t-test).
3.2. The Effects of Short-Term Hypoxia Are Rapidly Constituted and Post-Transcriptionally Regulated
Subsequently, we assessed the time window during which hypoxia exerts the observed anti-inflammatory effects by varying the timing of hypoxia relative to endotoxin administration (experimental design depicted in Fig. 2a). Except for TNF, which was not affected, the previously found anti-inflammatory phenotype was also observed when hypoxia was initiated at the time of endotoxin administration (“simultaneous” condition, Fig. 2b). In contrast, anti-inflammatory effects were neither identified when hypoxia was applied only in the hour before endotoxin administration (hypoxia “pretreatment” condition), nor when it was initiated 0.5 h after endotoxin administration (hypoxia “posttreatment” condition, Fig. 2b). From these results, we infer that the first 30 min following endotoxin administration are critical for the anti-inflammatory effects of short-term hypoxia. To investigate whether these effects are transcriptionally regulated, we measured mRNA expression of IL-10, TNF, IL-1β, IL-6 and KC in splenic tissue (experimental design depicted in Fig. 2c). In mice that were not challenged with endotoxin, hypoxia alone did not affect expression of any of the genes, except for a slight but significant decrease in TNF mRNA (Fig. 2d). Expression of all cytokine genes was strongly induced by endotoxin administration, but was not attenuated by hypoxia (Fig. 2d); TNF mRNA levels were even slightly enhanced. To ascertain that the spleen becomes hypoxic in our model, we also determined mRNA expression of the classic hypoxia-induced gene vascular endothelial growth factor (VEGF). As depicted in Fig. S4, hypoxia significantly increased VEGF expression, both in the presence and absence of endotoxin-induced inflammation.
Fig. 2.
The anti-inflammatory effects of short-term hypoxia are rapidly constituted and post-transcriptionally regulated.
(a) Experimental setup. C57BL/6 mice were randomized to normoxia or one of the following hypoxia modalities: hypoxic control: hypoxia starting 1 h before endotoxin administration until 1.5 h thereafter; simultaneous: hypoxia starting simultaneous with endotoxin administration until 1.5 h thereafter; pretreatment: hypoxia only in the hour before endotoxin administration; posttreatment: hypoxia starting 0.5 h after endotoxin administration until 1.5 h thereafter. Endotoxin (5 mg/kg) was injected intraperitoneally (i.p). (b) Plasma concentration of IL-10, TNF, IL-1β, IL-6 and KC. (c) Experimental setup. C57BL/6 mice were randomized to short-term hypoxia or normoxia and intraperitoneally injected with endotoxin (5 mg/kg) or saline. (d) mRNA expression of IL-10, TNF, IL-1β, IL-6 and KC measured in splenic tissue. n = 8 per group in all experiments. Data expressed as mean (± s.e.m.) or mean (± s.e.m.) fold change related to normoxic-saline condition on a 2log scale. *P < .05, **P < .01, ***P < .001 (compared to normoxia calculated using one-way ANOVA with post-hoc Dunnett's test for panel b; unpaired Student's t-test for panel d).
3.3. Hypoxia Inducible Factors Are Not Involved in the Anti-Inflammatory Effects of Short-Term Hypoxia
Previous studies have revealed that hypoxia-inducible transcription factors (HIFs) are involved in regulation of immune responses [33]. To evaluate the involvement of HIFs in the anti-inflammatory effect of short-term hypoxia, we performed hypoxia experiments in mice deficient for HIF1α or HIF2α in their hematopoietic system and wild type littermates (experimental design depicted in Fig. 3a). As shown in Fig. 3b, hematopoietic deletion of HIF1α or HIF2α did not relevantly affect the hypoxia-induced differences in cytokine responses compared to wild type mice. These results indicate no involvement of hematopoietic HIF1α or HIF2α in the anti-inflammatory effects of short-term hypoxia, which is in line with the previous finding that these effects are post-transcriptionally mediated.
3.4. Short-Term Hypoxia Augments Adenosine Levels and Adenosine 2B Receptor-Dependent IL-10 Production
Purinergic signaling plays a critical role in adaptation to hypoxia. In vitro and ex vivo studies have shown that hypoxia as well as inflammation leads to increased levels of the anti-inflammatory and tissue-protective purine adenosine [3, 6, 9, 38, 46]. In addition, inflammation also increases the expression of adenosine receptors [38]. This may indicate that hypoxia and inflammation have additive or synergistic effects on adenosine signaling. Previous in vitro work revealed that stimulation of adenosine 2B (A2B) receptors results in increased IL-10 production via a post-transcriptional mechanism [30]. Therefore, we hypothesized that short-term hypoxia results in a rapid increase in adenosine, leading to an early and profound increase in plasma IL-10 levels through enhanced A2B receptor signaling, ultimately resulting in dampening of the pro-inflammatory cytokine response. To test these hypotheses, we first measured tissue adenosine levels during hypoxia, systemic inflammation and the combination of both conditions (experimental design depicted in Fig. 4a). Hypoxia in the absence of inflammation enhanced splenic levels of adenosine within 30 min, further increasing after 1.5 h of hypoxia (Fig. 4b). Systemic inflammation also increased adenosine levels, and concurrent hypoxia resulted in a synergistic increase (Fig. 4b).
Hereafter, we assessed the involvement of the A2B receptor in the observed enhanced IL-10 levels and subsequent attenuation of the pro-inflammatory cytokine response using germline-deficient A2B receptor mice and genetically matched controls (experimental design depicted in Fig. 4c). The hypoxia-induced increase in plasma IL-10 observed in wild type mice was nullified in A2B-deficient mice (Fig. 4d). Furthermore, the hypoxia-induced attenuation of IL-1β and KC was less pronounced and did not reach statistical significance in these mice, whereas the trend towards lower TNF levels was absent (Fig. 4d). Thus, the adenosine 2B receptor is crucial for the enhanced IL-10 levels induced by short-term hypoxia. Because IL-10 potently attenuates pro-inflammatory cytokine production during endotoxemia in mice [22] and humans [32], we evaluated whether IL-10 is involved in the hypoxia-induced attenuation of pro-inflammatory cytokines. To this end, we performed experiments in germline IL-10-deficient mice (experimental design depicted in Fig. 5a). In these mice, the hypoxia-induced anti-inflammatory phenotype less pronounced for IL-1β and absent for IL-6 and KC (Fig. 5b).
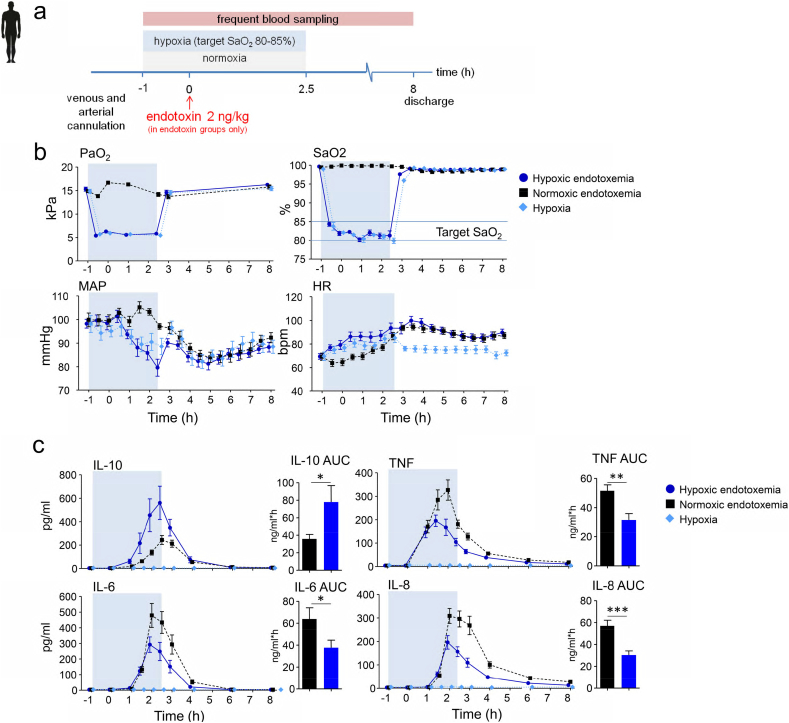
3.5. Short-Term Hypoxia Dampens the Endotoxin-Induced Pro-Inflammatory Cytokine Response in Humans in vivo
To translate our findings obtained in mice to humans, we performed hypoxia experiments in healthy volunteers who were intravenously injected with endotoxin. Thirty healthy subjects were exposed to either endotoxemia alone, hypoxia (target peripheral saturation [SaO2] of 80–85%) alone, or hypoxia combined with endotoxemia. (See Fig. 6a for the experimental design, Fig. S2 for a depiction of the experimental setup, and Table S1 for demographic characteristics of the study subjects). Hypoxia (FiO2 of 11–12%) resulted in decreased arterial oxygen pressures accomplishing the peripheral saturation (SaO2) target of 80–85%, with a concurrent decrease in mean arterial pressure and an increase in heart rate (Fig. 6b). Hypoxia was well-tolerated and no serious adverse events occurred during the study. In the hypoxia-only group, plasma cytokine concentrations were below the lower detection limit throughout the experiment, indicating that short-term hypoxia exerts no inflammatory effects by itself (Fig. 6c). Endotoxin administration elicited a typical systemic inflammatory response characterized by an increase in body temperature (Δ temperature 1.6 ± 0 0.2 °C in both groups, mean ± s.e.m.) and flu-like symptoms. Furthermore, plasma concentrations of TNF, IL-6, IL-8, and IL-10 increased profoundly after endotoxin administration (Fig. 6c). Hypoxia during endotoxemia resulted in an early and two-fold increase of IL-10 compared with the normoxia group, while pro-inflammatory cytokine levels were attenuated by 30 to 50% (Fig. 6c).
Fig. 6.
Short-term hypoxia dampens the endotoxin-induced pro-inflammatory cytokine response in humans.
(a) Experimental setup. Healthy volunteers were randomized to either short-term hypoxia or normoxia in combination with intravenous administration of 2 ng/kg endotoxin to elicit systemic inflammation, or to short-term hypoxia without endotoxin administration. (b) Arterial oxygen pressure (PaO2) peripheral oxygen saturation (SaO2), mean arterial pressure (MAP) and heart rate (HR). (c) IL-10, TNF, IL-6 and IL-8 plasma concentrations over time and area under the time-concentration curves (AUC). The blue shaded box indicates the period of hypoxia. Data are expressed as mean (± s.e.m.). P-values indicate the difference between the AUCs of the normoxic endotoxemia and hypoxic endotoxemia groups, calculated using unpaired Student's t-tests. *P < .05, **P < .01, ***P < .001. n = 10 per group.
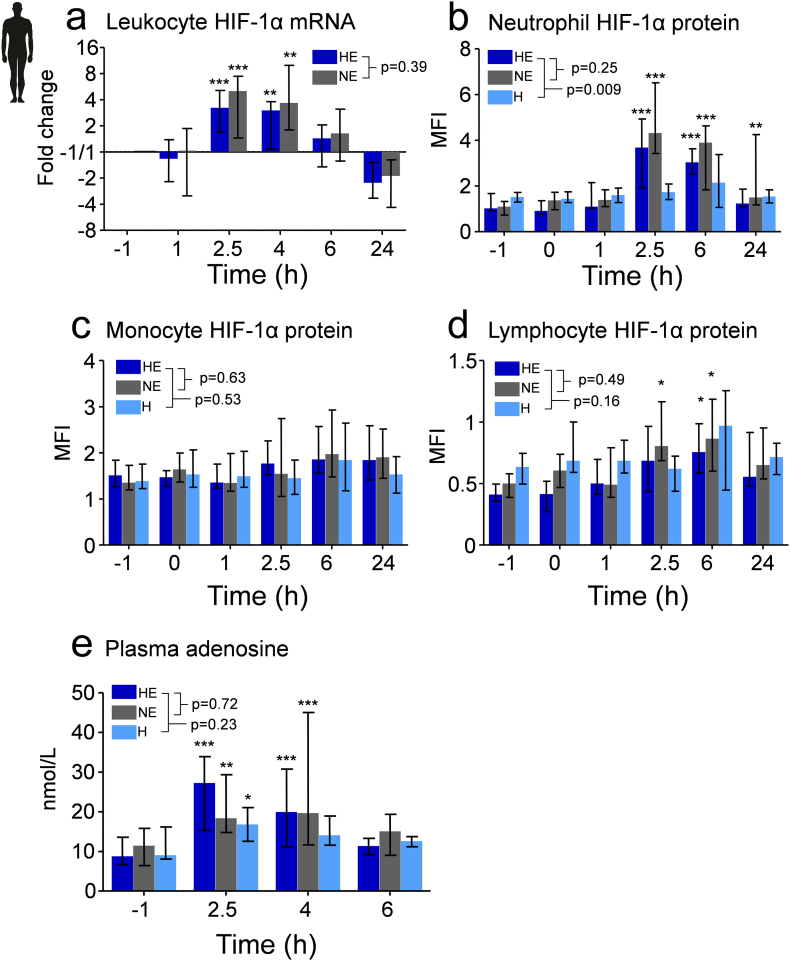
3.6. Hypoxia Does Not Affect Leukocyte HIF-1α mRNA and Protein Expression, and HIF Activity in Humans
Although HIFs were not involved in the anti-inflammatory effects of short-term hypoxia in mice, we nevertheless assessed the possible involvement of these transcription factors in the human setting. To this end, we measured HIF-1α mRNA and protein expression in circulating leukocytes. HIF-1α mRNA in leukocytes and HIF-1α protein levels in neutrophils and lymphocytes, but not monocytes, increased after endotoxin administration (Fig. 7a–d). This endotoxin-induced increase in HIF-1α expression is in line with previous in vitro studies showing that endotoxin induces HIF-1α activity under normoxic conditions [35]. Hypoxia in the presence and absence of endotoxemia did not affect HIF-1α mRNA and protein expression (Fig. 7a). In addition, we assessed hypoxia-induced transcriptional activity by determining the expression of 86 hypoxia-signaling-related genes in leukocytes, many of which are downstream targets of HIFs. This analysis revealed no significant changes in expression of any of the genes in the hypoxia only group. Furthermore, there were no significant differences in expression of any of the genes between hypoxic and normoxic endotoxemic subjects (Table S2). Taken together, short-term hypoxia neither results in enhanced HIF-1α levels, nor in HIF-dependent transcriptional activity in circulating leukocytes.
Fig. 7.
Short-term hypoxia neither affects leukocyte HIF-1α mRNA and protein expression, nor HIF transcriptional activity, and augments plasma adenosine levels in humans.
Measurements were performed on samples obtained from the experiments depicted in Fig. 6a. (a) Leukocyte HIF-1α mRNA change over time expressed as change from baseline (timepoint -1, just before initiation of hypoxia or normoxia using the respiratory helmet) on a 2log scale. (b, c and d) Change in neutrophil, monocyte and lymphocyte HIF-1α protein expression over time as measured by flow cytometry. Neutrophils, monocytes, and lymphocytes were identified with CD15, CD14, and CD3 fluorochrome-conjugated antibodies, respectively. Intracellular HIF-1α protein expression is expressed as the difference between mean fluorescence intensity (MFI) of HIF-1α antibody-stained and isotype control-stained cells. The gating strategy is provided in Fig. S3. (e) Plasma concentrations of adenosine. Data are expressed as median [IQR]. Within-group changes over time were analyzed using one way ANOVA followed by Dunnett's post-hoc test on log-transformed data. Between-group differences over time were analyzed using repeated measures two-way ANOVA on log transformed data (interaction term). *P < .05, **P < .01, ***P < .001 compared to baseline (timepoint -1 h). HE; hypoxic endotoxemia, NE; normoxic endotoxemia, H; hypoxia. MFI; mean fluorescence intensity. n = 10 per group.
3.7. Short-Term Hypoxia Augments Plasma Adenosine Levels in Humans in vivo
Plasma adenosine levels were measured to evaluate the role of this purine in the human experiments. Hypoxia in the absence of systemic inflammation resulted in a significant increase in plasma adenosine levels at the end of the hypoxic period (t = 2.5 h, Fig. 7e), which normalized after reoxygenation. Systemic inflammation in the presence as well as the absence of hypoxia resulted in a more prolonged increase in plasma adenosine levels.
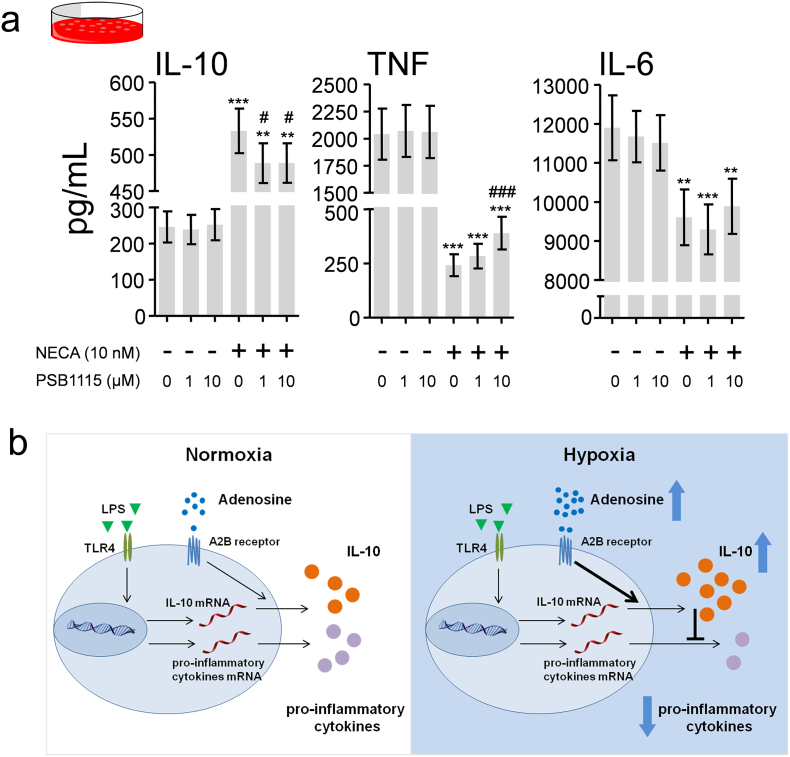
3.8. A2B Receptor Stimulation in Human Whole Blood Mimics the Anti-Inflammatory Effects of Hypoxia
Finally, to investigate the involvement of the A2B receptor in the human setting, we stimulated human whole blood with endotoxin in the presence or absence of the nonselective adenosine receptor agonist 5′-N-Ethylcarboxamidoadenosine (NECA) and the selective human A2B receptor antagonist PSB1115. As depicted in Fig. 8a, NECA potently enhanced IL-10 production, while attenuating TNF and IL-6 release. A2B receptor blockade partially counteracted NECA's effects on IL-10 and TNF.
Fig. 8.
A2B blockade partially reverses the anti-inflammatory effects of the adenosine receptor agonist NECA in endotoxin-stimulated human whole blood.
(a) Concentrations of IL-10, TNF and IL-6 in supernatants of whole blood cultures stimulated with endotoxin (10 ng/mL) in the presence and absence of the nonselective adenosine receptor agonist 5′-N-Ethylcarboxamidoadenosine (NECA) and the selective human A2B receptor antagonist PSB1115. N = 8, Data expressed as mean (±s.e.m.). **P < .01, ***P < .001 (compared with cultures incubated with the same dose of PSB1115 in the absence of NECA, calculated using paired Student's t-tests). # P < .05, ### P < .001 (compared with cultures incubated with NECA in the absence of PSB1115, calculated using repeated measures one-way ANOVA with Dunnett's post-hoc tests). (b) Proposed mechanism of hypoxia-induced anti-inflammation. Short-term hypoxia results in augmented adenosine levels and A2B receptor signaling. This results in enhanced IL-10 release via a post-transcriptional mechanism, in turn attenuating the pro-inflammatory cytokine response.
Taken together, our data reveal that short-term hypoxia dampens the endotoxin-induced systemic inflammatory response through a rapid increase in adenosine levels, resulting in an A2B-receptor dependent increase of IL-10 concentrations and subsequent attenuation of pro-inflammatory cytokines (Fig. 8b).
4. Discussion
Hypoxia and inflammation are key characteristics of many pathological processes, and they influence each other considerably. Herein, we demonstrate that short-term hypoxia exerts profound anti-inflammatory effects during systemic inflammation in vivo in both mice and humans. The underlying mechanism involves enhanced adenosine release and stimulation of the adenosine A2B receptor, resulting in augmented IL-10 production and subsequent dampening of the pro-inflammatory cytokine response. Our data further demonstrate that HIFs are not involved in the immunologic effects of short-term hypoxia.
Previous work demonstrated that hypoxia increases extracellular adenosine concentrations in vitro [3] and ex vivo [6, 9, 46]. We show that clinically relevant levels of hypoxia result in a rapid increase of adenosine levels in vivo, in both humans and mice. Hypoxia can enhance extracellular adenosine levels through several pathways. During cellular stress, such as hypoxia or inflammation, precursor nucleotides (e.g. ATP) are released, and hypoxia enhances their conversion to adenosine [13]. In addition, hypoxia inhibits adenosine uptake of the equilibrative-nucleotide-transporter [10]. Nevertheless, both these processes take several hours to constitute, as they involve alterations of the transcription and translation of the proteins involved. However, in ex vivo experiments, hypoxia was shown to attenuate adenosine kinase activity within minutes, resulting in a rapid increase of extracellular adenosine [9, 46]. It appears plausible that this mechanism accounts for the swift hypoxia-induced increase of adenosine in the present work.
We identify that the hypoxia-induced enhanced IL-10 production is mediated via an A2B receptor-dependent post-transcriptional mechanism; findings which are corroborated by our in vitro data obtained in human leukocytes. Furthermore, anti-inflammatory effects through A2B receptor signaling have been reported by others. For example, mice deficient for A2B display an augmented TNF response after renal ischemia and reperfusion [17], and increased influx and delayed clearance of neutrophils in a lung injury model [27]. Along these lines, an important role for A2B receptors in improving outcome was demonstrated in murine models of pleural inflammation [8], renal [17], intestinal [20], and hepatic [5] ischemia-reperfusion, and multimicrobial abdominal sepsis [7]. Previous human work is in support of the identified adenosine-induced IL-10-dependent anti-inflammatory effects. First, the infusion of recombinant IL-10 during human endotoxemia resulted in decreased plasma concentrations of pro-inflammatory cytokines [28]. Second, treatment with the adenosine reuptake inhibitor dipyridamole during human endotoxemia caused an early and pronounced increase of plasma IL-10 levels and attenuation of TNF and IL-6 plasma concentrations [37]. In contrast, intravenous infusion of adenosine during human endotoxemia did not result in a clear anti-inflammatory phenotype [42, 43]. This may be explained by the fact that adenosine infusion started 30 min after endotoxin administration in these studies, whereas we show that the first 30 min are critical for its anti-inflammatorily effects. In addition, plasma adenosine is known to have a very short half-life [39], and the infused adenosine may therefore not have reached the target tissue. It needs to be acknowledged that although A2B and IL-10 deficient mice clearly display attenuation of the hypoxia-induced anti-inflammatory phenotype, it was not completely abrogated, implying that other mechanisms may also contribute to hypoxia-induced anti-inflammatory effects, albeit to a much lesser extent.
We found no involvement of HIFs in the anti-inflammatory effects of short-term hypoxia. Our initial murine data revealed that the anti-inflammatory effects exerted by short-term hypoxia are very rapid and post-transcriptionally regulated, already rendering the critical involvement of a transcription factor unlikely, and the subsequent experiments in hematopoietic HIF-1α- and HIF-2α-deficient mice confirm this. Nevertheless, it needs to be acknowledged that HIFs are known to exert immunomodulatory effects, but that these mainly appear to be of relevance in the setting of chronic hypoxia. For example, a recent murine study showed that prolonged hypoxia (12 h up to several days) in the context of a concurrent skin or pulmonary infection leads to increased morbidity and mortality, in a HIF-dependent manner [47]. Another study, in which mice were exposed to hypoxia for 11–27 days, demonstrated an enhanced plasma TNFα response upon challenge with endotoxin [2]. These findings signify that distinctly different mechanisms underlie the effects of short-term and chronic hypoxia. Finally, administration of the hypoxia-mimetic DMOG, a prolyl hydroxylase inhibitor which stabilizes HIFs, resulted in a similar anti-inflammatory cytokine profile in endotoxemic mice as observed in the present study, although the in vivo involvement of HIFs was not determined and additional off-target effects cannot be excluded when using these pharmacological mimetics [19].
Our work demonstrates that short-term hypoxia attenuates the inflammatory response in vivo consistently across species. The relevance of hypoxia-induced anti-inflammation remains to be determined, but speculatively, it may represent a mechanism aimed at limiting inflammation-induced tissue injury, thereby improving outcome of severe infections with concurrent tissue hypoxia. Hosts can respond to invading pathogens by mounting an immunologic response, which may result in collateral damage known as immunopathology [16, 29]. Tolerance to the consequences of pathogen invasion and immunopathology are mechanisms that provide an effective adaptation to disease. As such, hypoxia-induced anti-inflammation may represent a mechanism to improve the host's tolerance to immunopathology, and theoretically, accepting or inducing systemic hypoxia could therefore curtail excessive inflammation. Previous implementation studies [21, 45] and clinical trials [15, 34] have demonstrated safety and feasibility of conservative oxygenation strategies in the intensive care unit (ICU), paving the way for further exploration of variable oxygenation targets. Intriguingly, recent work revealed that short-lived hypoxia at the emergency department appeared to be associated with a lower mortality compared to normoxia or hyperoxia [31]. In that study, patients in the hypoxia group displayed a median PaO2 of 54 mmHg (7.7 kPa) at the emergency department [31], which is in the same range as what the hypoxic subjects were exposed to in our study. Based on the data presented in the present work, it is tempting to speculate that this observed beneficial effect on outcome is due to reduced immunopathology. Nevertheless, we wish to emphasize that caution should be taken in vulnerable patients due to the risk of inadequate tissue oxygenation and organ failure. The adenosine signaling pathway may therefore represent another therapeutic target. Unfortunately, direct systemic infusion of adenosine as an immunomodulatory therapy is limited by its short half-life [39] and undesired cardiovascular side effects [24]. Direct, unselective pharmacologic stimulation of adenosine receptors may result in a similar harmful profile. Theoretically, allosteric modulation of the A2B receptor, thereby increasing its binding affinity and/or functional efficacy, may be more successful. Under physiologic conditions with low levels of adenosine, the adenosine receptor is not activated, preventing its disadvantageous systemic effects. Under local inflammatory or hypoxic conditions however, tissue levels of adenosine increase, and the allosterically modulated A2B receptor will be more sensitive to adenosine and/or cause stronger anti-inflammatory effects at similar adenosine concentrations, resulting in site-specific anti-inflammatory effects [1] without systemic side effects. Although the development of this class of drugs is in its infancy, efforts aimed at pharmacologic targeting of the adenosine signaling pathway to achieve anti-inflammatory effects are being made [4, 11].
In conclusion, we demonstrate that short-term hypoxia profoundly attenuates the pro-inflammatory cytokine response during systemic inflammation in mice, and translate these findings to humans by showing identical results in healthy volunteers. The underlying mechanism involves increased adenosine levels and subsequent adenosine 2B receptor-mediated enhanced release of IL-10. Hypoxia-induced anti-inflammation may constitute a mechanism to improve the host's tolerance to immunopathology, but this requires further study. As short-term hypoxia is common in critically ill patients, its immunomodulatory effects may affect outcome. Finally, hypoxia and the adenosine pathway might represent promising therapeutic modalities to modulate the immune response.
Acknowledgments
Acknowledgments
The authors thank the research nurses Marieke van der A, Chantal Luijten-Arts, Hellen van Wezel of the ICU department for help during the endotoxemia experiments and Cor Jacobs for help with the flow cytometry analysis. We are indebted to Bart Ramakers for his advice on adenosine measurements.
Funding Sources
This work was supported by a PhD grant from the Radboud Centre for Infectious Diseases grant 2013 and a Young Investigator Grant from the Dutch Society of Anesthesiology Young Investigator Grant 2013 to MK, by National Institute of Health Grants R01-DK097075, R01-HL098294, POI-HL114457, R01-DK082509, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900 to HKE, by grants from the German Research Society (DFG) WI 3291/1-1, 1-2, 3 and 5 to BW, and by an ERC Consolidator Grant (#310372) and a Spinoza Grant of the Netherlands Organization for Scientific Research Spinoza Grant 2016 to MGN. BW has been supported by grants from the DFG (WI 3291/1-1, 1-2, 3, 5 and SFB/TRR 205 Die Nebenniere: Zentrales Relais in Gesundheit und Krankheit) and Seed grants from the Dresden International Graduate School for Biomedicine and Bioengineering (DIGS-BB, TU-Dresden).
Conflicts of Interest
All the authors declare that they have no conflict of interest.
Author Contributions
DK, PP, and MK designed the study. DK, BW, EP, LTvE, JG, AJ, EJ, MP, RG, LD, AMM, AK, JDL, ALZ, MRB and MK performed experiments. DK and MK analyzed and interpreted the data and drafted the manuscript. HE provided materials. BW, LABJ, MGN, NPR, JGvdH, GJS, HKE and PP critically revised the manuscript. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.06.021.
Appendix A. Supplementary data
Supplementary material
References
- 1.Antonioli L., Csóka B., Fornai M., Colucci R., Kókai E., Blandizzi C. Adenosine and inflammation: What's new on the horizon? Drug Discov Today. 2014;19:1051–1068. doi: 10.1016/j.drudis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Baze M.M., Hunter K., Hayes J.P. Chronic hypoxia stimulates an enhanced response to immune challenge without evidence of an energetic tradeoff. J Exp Biol. 2011;214:3255–3268. doi: 10.1242/jeb.054544. [DOI] [PubMed] [Google Scholar]
- 3.Casanello P., Torres A., Sanhueza F., González M., Farías M., Gallardo V. Equilibrative nucleoside transporter 1 expression is downregulated by hypoxia in human umbilical vein endothelium. Circ Res. 2005;97:16–24. doi: 10.1161/01.RES.0000172568.49367.f8. [DOI] [PubMed] [Google Scholar]
- 4.Chen J.-F., Eltzschig H.K., Fredholm B.B. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choukèr A., Ohta A., Martignoni A., Lukashev D., Zacharia L.C., Jackson E.K. In vivo hypoxic preconditioning protects from warm liver ischemia-reperfusion injury through the adenosine A2B receptor. Transplantation. 2012;94:894–902. doi: 10.1097/TP.0b013e31826a9a46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conde S.V., Monteiro E.C. Hypoxia induces adenosine release from the rat carotid body. J Neurochem. 2004;89:1148–1156. doi: 10.1111/j.1471-4159.2004.02380.x. [DOI] [PubMed] [Google Scholar]
- 7.Csóka B., Németh Z.H., Rosenberger P., Eltzschig H.K., Spolarics Z., Pacher P. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Rocha Lapa F., da Silva M.D., de Almeida Cabrini D., Santos A.R.S. Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: evidence for the role of adenosine A2 receptors. Purinergic Signal. 2012;8:693–704. doi: 10.1007/s11302-012-9299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decking U.K.M., Schlieper G., Kroll K., Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81:154–164. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- 10.Eltzschig H.K., Abdulla P., Hoffman E., Hamilton K.E., Daniels D., Schönfeld C. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eltzschig H.K., Bratton D.L., Colgan S.P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13:852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eltzschig H.K., Ko D., Eckle T., Kong T., Robson S.C., Colgan S.P. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltzschig H.K., Sitkovsky M.V., Robson S.C. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girardis M., Busani S., Damiani E., Donati A., Rinaldi L., Marudi A. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;47:717–720. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 16.Graham A.L., Allen J.E., Read A.F. Evolutionary causes and consequences of immunopathology. Annu Rev Ecol Evol Syst. 2005;36:373–397. [Google Scholar]
- 17.Grenz a., Kim J.-H., Bauerle J.D., Tak E., Eltzschig H.K., Clambey E.T. Adora2b adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF- release. J Immunol. 2012;189:4566–4573. doi: 10.4049/jimmunol.1201651. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Gruber M., Hu C., Johnson R.S., Brown E.J., Keith B., Simon M.C. Acute postnatal ablation of Hif-2 - results in anemia. Proc Natl Acad Sci. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hams E., Sauners S.P., Cummins E.P., O'Connor A., Tambuwala M.T., Gallagher W.M. The hydroxylase inhibitor dimethyloxallyl glycine attenuates endotoxic shock via alternative activation of macrophages and IL-10 production by B1 cells. Shock. 2011;36:295–302. doi: 10.1097/SHK.0b013e318225ad7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart M.L., Jacobi B., Schittenhelm J., Henn M., Eltzschig H.K. Cutting edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182:3965–3968. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 21.Helmerhorst H.J.F., Schultz M.J., van der Voort P.H.J., Bosman R.J., Juffermans N.P., de Wilde R.B.P. Effectiveness and clinical outcomes of a two-step implementation of conservative oxygenation targets in critically ill patients: a before and after trial. Crit Care Med. 2015;44:1. doi: 10.1097/CCM.0000000000001461. [DOI] [PubMed] [Google Scholar]
- 22.Howard M., Muchamuel T., Andrade S., Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imtiyaz H.Z., Williams E.P., Hickey M.M., Patel S.A., Durham A.C., Yuan L. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanei Y., Hanon S., Van-Tosh A., Schweitzer P. Adenosine-induced atrial fibrillation during pharmacologic stress testing: report of eight cases and review of the literature. Int J Cardiol. 2008;129:2007–2009. doi: 10.1016/j.ijcard.2007.05.090. [DOI] [PubMed] [Google Scholar]
- 25.Kiers H.D., Scheffer G.-J., van der Hoeven J.G., Eltzschig H.K., Pickkers P., Kox M. Immunologic consequences of hypoxia during critical illness. Anesthesiology. 2016:1073–1090. doi: 10.1097/ALN.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudsen T., Winters R., Otey S., Blackburn M., Airhart M., Church J. Effects of (R)-deoxycoformycin (pentostatin) on intrauterin nucleoside catabolism and embryo viability in the pregnant mouse. Teratology. 1992;45:91–103. doi: 10.1002/tera.1420450109. [DOI] [PubMed] [Google Scholar]
- 27.Konrad F.M., Witte E., Vollmer I., Stark S., Reutershan J. Adenosine receptor A2b on hematopoietic cells mediates LPS-induced migration of PMNs into the lung interstitium. AJP Lung Cell Mol Physiol. 2012;303:L425–L438. doi: 10.1152/ajplung.00387.2011. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A., Zanotti S., Bunnell G., Habet K., Anel R., Neumann A. Interleukin-10 blunts the human inflammatory response to lipopolysaccharide without affecting the cardiovascular response. Crit Care Med. 2005;33:331–340. doi: 10.1097/01.ccm.0000152229.69180.2. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335(80):936–942. doi: 10.1126/science.1214935. https://doi.org/335/6071/936 [pii]\r10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemeth Z.H., Lutz C.S., Csoka B., Deitch E.A., Leibovich S.J., Gause W.C. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. https://doi.org/175/12/8260 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page D., Ablordeppey E., Wessman B.T., Mohr N.M., Trzeciak S., Kollef M.H. Emergency department hyperoxia is associated with increased mortality in mechanically ventilated patients: a cohort study. Crit Care. 2018;22 doi: 10.1186/s13054-017-1926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pajkrt D., van der Poll T., Levi M., Cutler D.L., Affrime M.B., van den Ende A. Interleukin-10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood. 1997;89:2701–2705. [PubMed] [Google Scholar]
- 33.Palazon A., Goldrath A.W., Nizet V., Johnson R.S. Review HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panwar R., Hardie M., Bellomo R., Barrot L., Eastwood G.M., Young P.J. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193:43–51. doi: 10.1164/rccm.201505-1019OC. [DOI] [PubMed] [Google Scholar]
- 35.Peyssonnaux C., Cejudo-Martin P., Doedens A., Zinkernagel A.S., Johnson R.S., Nizet V. Cutting edge : essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 36.Ramakers B.P., Pickkers P., Deussen A., Rongen G., van den Broek P., van der Hoeven J.G. Measurement of the endogenous adenosine concentration in humans in vivo: methodological considerations. Curr Drug Metab. 2008;9:679–685. doi: 10.2174/138920008786049249. [DOI] [PubMed] [Google Scholar]
- 37.Ramakers B.P., Riksen N.P., Stal T.H., Heemskerk S., van den Broek P., Peters W.H.M. Dipyridamole augments the antiinflammatory response during human endotoxemia. Crit Care. 2011;15:R289. doi: 10.1186/cc10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramakers B.P., Wever K.E., Kox M., van den Broek P.H., Mbuyi F., Rongen G. How systemic inflammation modulates adenosine metabolism and adenosine receptor expression in humans in vivo. Crit Care Med. 2012;40:2609–2616. doi: 10.1097/CCM.0b013e318259205b. [DOI] [PubMed] [Google Scholar]
- 39.Riksen N.P., Rongen G.A., Yellon D., Smits P. Human in vivo research on the vascular effects of adenosine. Eur J Pharmacol. 2008;585:220–227. doi: 10.1016/j.ejphar.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 40.Ryan H.E., Lo J., Johnson R.S. HIF-1 α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjöberg F., Singer M. The medical use of oxygen: a time for critical reappraisal. J Intern Med. 2013;274:505–528. doi: 10.1111/joim.12139. [DOI] [PubMed] [Google Scholar]
- 42.Soop A., Johansson C., Hjemdahl P., Kristiansson M., Gyllenhammar H., Li N. Adenosine treatment attenuates cytokine interleukin-6 responses to endotoxin challenge in healthy volunteers. Shock. 2003;19:503–507. doi: 10.1097/01.shk.0000051756.08171.11. [DOI] [PubMed] [Google Scholar]
- 43.Soop A., Sundén-Cullberg J., Albert J., Hållström L., Treutiger C.J., Sollevi A. Adenosine infusion attenuates soluble RAGE in endotoxin-induced inflammation in human volunteers. Acta Physiol. 2009;197:47–53. doi: 10.1111/j.1748-1716.2009.01985.x. [DOI] [PubMed] [Google Scholar]
- 44.Stadtfeld M., Graf T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development. 2005;132:203–213. doi: 10.1242/dev.01558. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki S., Eastwood G.M., Glassford N.J., Peck L., Young H., Garcia-Alvarez M. Conservative oxygen therapy in mechanically ventilated patients: a pilot before-and-after trial. Crit Care Med. 2014;42:1414–1422. doi: 10.1097/CCM.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T., Otsuguro K., Ohta T., Ito S. Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br J Pharmacol. 2010;161:1806–1816. doi: 10.1111/j.1476-5381.2010.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson A.A.R., Dickinson R.S., Murphy F., Thomson J.P., Marriott H.M. Hypoxia determines survival outcomes of bacterial infection through HIF-1alpha dependent re-programming of leukocyte metabolism. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aal2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material