Abstract
As a widely used anticancer and immunosuppressive agent, methotrexate (MTX) can induce multiple adverse drug reactions (ADRs), such as gastrointestinal toxicity, the mechanisms are poorly understood. Gut microbiota has been widely reported to be associated with the onset of multiple diseases as well as treatment outcomes of different drugs. In this study, mucosal injury was observed in MTX-treated mice, leading to significant changes in macrophages (i.e., M1/M2 ratio, P < 0.05) but not in dendritic cells. Moreover, the population, diversity and principal components of the gut microbiota in mice were dramatically altered after MTX treatment in a time-dependent manner, and Bacteroidales exhibited the most distinct variation among all the taxa (P < 0.05). Bacteroides fragilis was significantly decreased with MTX treatment (P < 0.01) and tended to decrease proportionately with increasing macrophage density. Gavage of mice with B. fragilis ameliorated MTX-induced inflammatory reactions and modulate macrophage polarization. In conclusion, our results delineate a strong impact of the gut microbiota on MTX-induced intestinal mucositis and provide a potential method for the prevention of such ADRs.
Keywords: Methotrexate, Gastrointestinal toxicity, Gut microbiota, Mononuclear phagocyte
Research in context.
Methotrexate has been successfully used to treat various cancers and autoimmune diseases either alone or in combination with other agents. Unfortunately, intestinal toxicity is the major dose-limiting factor for MTX administration, and MTX-induced intestinal mucositis represents a significant burden to patients. Our results delineate a strong impact of the gut microbiota on MTX-induced intestinal mucositis and provide a potential method for the prevention of such adverse drug reactions.
1. Introduction
Methotrexate (MTX), a structural analog of folic acid, blocks folate metabolism via competitive inhibition of dihydrofolate reductase (DHFR), thus leading to the suppression of de novo synthesis of purines and pyrimidines [[1], [2], [3]]. Over the past decades, MTX has been successfully used to treat various cancers and autoimmune diseases either alone or in combination with other agents [4]. Unfortunately, the curative potential of MTX is sometimes reduced due to its morbid multi-organ toxicity, including gastrointestinal toxicity, bone marrow toxicity, cardiotoxicity, nephrotoxicity and hepatotoxicity [[5], [6], [7]]. Currently, intestinal toxicity is the major dose-limiting factor for MTX administration, and MTX-induced intestinal mucositis represents a significant burden to patients. The condition may affect the entire gastrointestinal tract and is typically accompanied with nausea, bloating, abdominal pain and diarrhea, which often result in malabsorption, weight loss and disrupted chemotherapy [[8], [9], [10]]. Despite all the efforts being made to ameliorate MTX-induced intestinal damage [[11], [12], [13], [14]], there is no satisfactory therapeutic intervention so far that prevents or treats all the symptoms [15]. This is due, at least in part, to the lack of understanding of the mechanisms by which MTX induces intestinal impairment. A limited number of studies suggest that the administration of MTX induces DNA strand breaks in rapidly proliferating intestinal epithelial cells [16] and causes significant oxidative stress [17, 18]. More importantly, MTX may exert deleterious effects through a dynamic sequence of complex inflammatory events initiated by direct cellular injury in the intestinal epithelium and submucosal tissues [16, 19]. According to various reports, the mononuclear phagocyte system (MPS) plays a key role in the maintenance of gut homeostasis and exhibits multiple functions during immune responses in the intestine. The MPS mainly comprises dendritic cells (DCs) and macrophages [20], which initiate adaptive immune responses [21] and act as innate effector cells [22], respectively. When tissues are damaged following infection or injury, inflammatory monocytes are recruited from the circulation or the intestinal reservoir for homeostatic adaptation. Therefore, it is possible that the MTX-induced intestinal damage may cause dysregulation of the intestinal MPS, thus leading to a vicious inflammatory cycle.
On the other hand, the activated immune system in the intestine can also impact the gut microbiota, a diverse microbial community mainly composed of bacteria that colonize the gastrointestinal tract. Despite the complexity of the gut microbiota, there is a mutualistic relationship between the host and microorganisms in which the microbiota contributes to many physiological processes of the host, and in turn the host provides niches and nutrients for microbial survival [23, 24]. As the homeostasis of the gut microbiota is important to the intestinal epithelium and immune system and may modulate the intestinal metabolism of drugs, aberrant changes of the gut microbiota can result in altered drug response, including treatment inefficiency as well as adverse drug reactions (ADRs) [25]. For instance, the antitumor efficacy of CTLA-4 blockade-based immunotherapy is dependent on distinct Bacteroides species in the intestine, and response to CTLA-4 inhibitors may be lost in antibiotic-treated or germ-free mice [26]. Therefore, it is worthwhile to investigate the relationship among MTX-induced intestinal toxicity, immune responses and disruption of the gut microbiota.
In this study, we determined the impact of MTX on the intestinal mucosal damage, alteration in the MPS properties, and consequent imbalance in the host gut microbiota. We also observed that via a dynamic sequence of detrimental intestinal inflammatory reactions, MTX profoundly aggravates intestinal toxicity, which can be ameliorated by gavage with specific Bacteroides species. The current study may fuel subsequent studies investigating the prevention of treatment of MTX-induced intestinal damage.
2. Methods
2.1. Mice and Treatment
Eight-week-old male Balb/c mice were purchased from Beijing Vital River Laboratory Animal Co. Ltd. (Beijing, China). All mice were maintained in a pathogen-free animal facility. All experimental procedures and animal care were carried out in compliance with the regulations of the Animal Care Committee of Sichuan University. The mice were intraperitoneally (i.p.) injected with 1 mg/kg of MTX (Sigma-Aldrich, USA) or PBS every 3 days. The mice were treated with or without metronidazole (Sigma-Aldrich, USA) for 2 weeks before MTX injection and given antibiotics until the beginning of the experiment. Metronidazole (1 mg/ml) was added to sterile drinking water. The solutions and bottles were changed 2 times a week.
2.2. Cell Culture and Reagents
Colon adenocarcinoma Caco2 cells and intestinal epithelial IEC6 cells were cultured at 37 °C in 5% CO2 in DMEM (Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, USA), 100 units/ml penicillin (Beyotime Biotechnology, China) and 100 μg/ml streptomycin (Beyotime Biotechnology, China). Macrophage RAW264.7 cells were cultured at 37 °C in 5% CO2 in RPMI-1640 (Gibco, USA) medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco, USA), 100 units/ml penicillin (Beyotime Biotechnology, China) and 100 μg/ml streptomycin (Beyotime Biotechnology, China).
2.3. Microbial DNA Extraction, 16S rDNA Amplicon Sequencing
The fecal samples used in this study were collected before or after injection of MTX (or PBS) from mice under metronidazole regimen or water and kept at −80 °C until further analysis. Frozen fecal samples were processed for DNA isolation using the Stool DNA Isolation Kit according to manufacturer's instructions. One nanogram of purified fecal DNA was used for PCR amplification. Amplicons spanning the variable region 4 (V4) of the 16S rRNA gene were generated by using the following primers: forward, 5′-GTGCCAGCMGCCGCGGTAA-3′; reverse, 5′-GGACTACHVGGGTWTCTAAT-3′. The PCR products were then sequenced on an Illumina Hi-seq sequencer at Novogene (Novogene, Beijing, China).
2.4. Taxonomic Microbiota Analysis
FLASH (V 1.2.7, http://ccb.jhu.edu/software/FLASH/) [27] was used to obtain assembly reads, i.e., the raw tags, for each sample, from which the sequences of barcodes and primers were truncated. The raw tags were then processed using the QIIME (Quantitative Insights Into Microbial Ecology, http://www.qiime.org) analysis pipeline to obtain clean tags [[28], [29], [30]]. Using Uparse (V 7.0.1001, http://drive5.com/uparse/) [31], sequences sharing 97% nucleotide sequence identity in the 16S region were binned into operational taxonomic units (OTUs). Taxonomical classification was performed using the RDP-classifier (V 2.2, http://sourceforge.net/projects/rdp-classifier/) [32], and OTU mapping was employed using the SILVA database (http://www.arb-silva.de/) [33]. For alpha diversity, QIIME and R (V 2.15.3) were used to construct the Chao1 curve. For beta diversity, the Unifrac distance was calculated using QIIME, and PCA was performed using R.
2.5. Co-Culture of B. fragilis with RAW264.7 Macrophages
B. fragilis (ATCC 25285) were plated at 1 × 106 per well with 100 μl BHI and cultured with or without 1 × 106/ml RAW264.7 cells. After 16 h, the B. fragilis was collected, diluted and cultured on sheep blood agar plates for 24 h.
2.6. Gut Colonization with Dedicated Bacterial Species
For inoculation of mice untreated or treated with metronidazole, colonization was performed on the day following the first MTX injection by oral gavage with 100 μl PBS containing 1 × 109 bacterial cells. B. fragilis was grown on sheep blood agar plates for 48 h at 37 °C under anaerobic conditions. B. fragilis was harvested from the sheep blood agar plates, suspended in sterile PBS, centrifuged and washed with PBS and then resuspended in sterile PBS at an optical density (600 nm) of 1, which corresponds to approximately 1 × 109 colony-forming units (CFUs) per ml.
2.7. Flow Cytometry
Mesenteric lymph nodes (MLNs) and spleens were harvested from mice on day 7 or day 14 after the first injection of MTX. The tissues were cut into small pieces and digested with type IV collagenase (Sigma, USA) at 37 °C for 30 min with shaking. The mixture was subsequently filtered through a 70 μm cell strainer. The cells were stained with antibodies against the following surface markers for flow cytometry: CD11b, F4/80, CD206 and CD11c. The antibodies were purchased from BD Biosciences and BioLegend. Cell populations were gated as follows: M1 macrophage (CD11b+F4/80+), M2 macrophage (CD11b+CD206+) and DC (CD11c+). Flow cytometry was performed on a FACSCalibur (BD, USA) flow cytometer, and the data were analyzed with the software FlowJo 6.0.
2.8. Histology of Gut Tissue and Immunofluorescence Staining of the Gut Leukocytes
The whole tissue of the small intestine (duodenum, jejunum and ileum) and colon was harvested, cleaned from feces, fixed in 4% paraformaldehyde for 24 h and then embedded longitudinally in paraffin. Small intestine or colon tissues were cut into 4 μm longitudinal sections and stained with hematoxylin and eosin for histological analyses. To evaluate lymphocytic infiltration, the longitudinal sections were blocked with 5% bovine serum albumin (BSA) for 1 h. Then, FITC-conjugated anti-mouse CD4 (1:100 for 2 h, BD Biosciences), PE-conjugated anti-mouse CD11b (1:100 for 2 h, BD Biosciences) and DAPI (Beyotime Biotechnology) were used. All steps were performed at 4 °C in the dark. Thickness of muscularis mucosae was assessed using Image J.
2.9. Gene Expression Analysis
Total RNA was extracted from intestinal tissues or cells using RNAiso Plus (TaKaRa, Japan). Complementary DNA was generated using the PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa, Japan) according to the manufacturer's instructions. Quantification of gene expression was performed on Bio-rad CFX Connect platform using the SYBR® Fast qPCR Mix (TaKaRa, Japan). The gene-specific primer sequences are shown in Supplementary Table 1.
2.10. Quantification of Bacteria by qPCR
Genomic DNA was isolated from fecal samples using the Stool DNA Isolation Kit (Foregene, China) following the manufacturer's instructions. qPCR was performed using SYBR Green for different Bacteroides species. The gene-specific primer sequences are shown in Supplementary Table 1.
2.11. Cytotoxicity Tests and Cell Treatment With B. fragilis
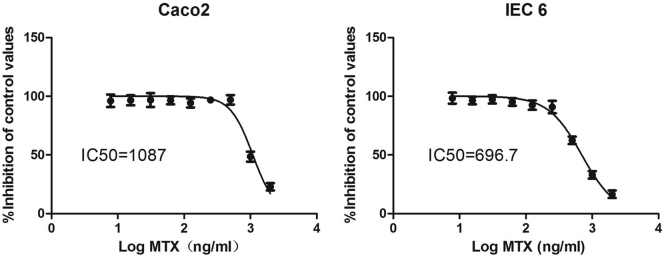
The ability of MTX to inhibit the replication of cells was assayed in 96-well plates using a cell counting kit-8 (CCK-8, Dojindo, Japan). Cells were plated at a density of 1 × 104 per well with 100 μl cell culture medium and treated with or without 7.8–2000 ng/ml MTX. After 24 h, the cells were further incubated with 10 μl CCK-8 for 4 h at 37 °C. The percentage of cells that stained positively with CCK-8 compared with the control cells were used to quantify the half-maximal inhibitory concentration (IC50). Cells were plated at a density of 2 × 105 per well in 6-well plates with MTX (according to IC50) and incubated with or without 1 × 107 CFUs/ml B. fragilis for 4 h and then were collected and evaluated for gene expression analysis.
2.12. Statistical Analyses
The data were analyzed with Prism 5 (GraphPad). The data are presented as the means ± SEM, and P values were computed using unpaired two-way analysis of variance (ANOVA). Corrected p values were shown to account for multiple testing. All reported analyses were considered significant at P values <0.05.
3. Results
3.1. MTX Induced Inflammatory Response in Intestinal Tissues
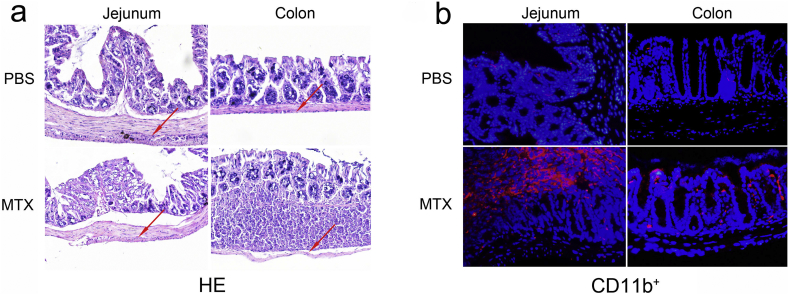
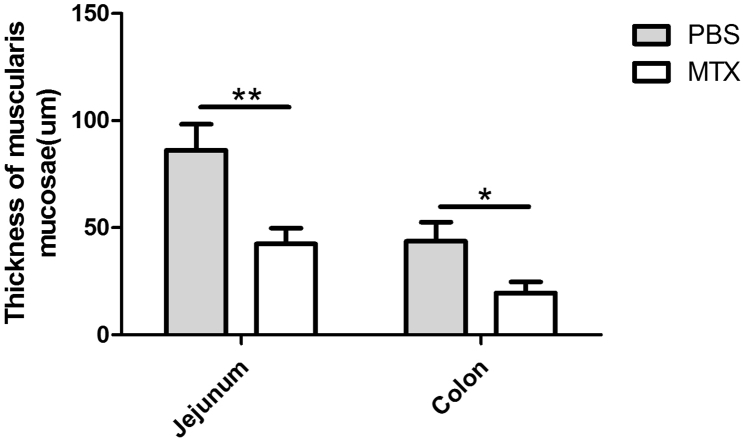
To investigate the effects of MTX on the intestinal tissue, intestinal samples were collected from Balb/c mice after systemic administration of MTX for 14 days and prepared for histological analyses. In comparison with the PBS-treated controls, the MTX-treated mice tended to exhibit distinct villous atrophy, increased goblet cell accumulation and collapsed muscularis mucosae in the jejunum. In addition to these alterations, the MTX-induced mucosal damage in the colon was accompanied with inflammatory cell infiltration (Fig. 1a and Supplementary Fig. 1), indicating that MTX induces mucosal inflammation. In line with these results, immunofluorescence staining of jejunum and colon showed aberrant accumulation of CD11b+ myeloid cells in the MTX-treated mice but not in the PBS-treated mice (Fig. 1b), while no difference in CD4+ T cells was detected. These data suggest that the MTX-induced intestinal damage may be attributed to a series of intestinal inflammatory changes including CD11b+ myeloid cell infiltration.
Fig. 1.
MTX induces inflammatory responses in intestinal tissues. (a) Representative hematoxylin-eosin (HE) staining of the jejunum and colon in control and MTX-treated mice on day 14. (b) Representative immunofluorescence staining of the monocyte surface marker (CD11b) in the jejunum and colon of Balb/c mice treated with or without MTX on day 14. Nuclei were counterstained with DAPI (blue); magnification 200×.
Supplementary Fig. 1.
Thickness of muscularis mucosae (n = 3). *P < 0.05 and **P < 0.01 versus PBS.
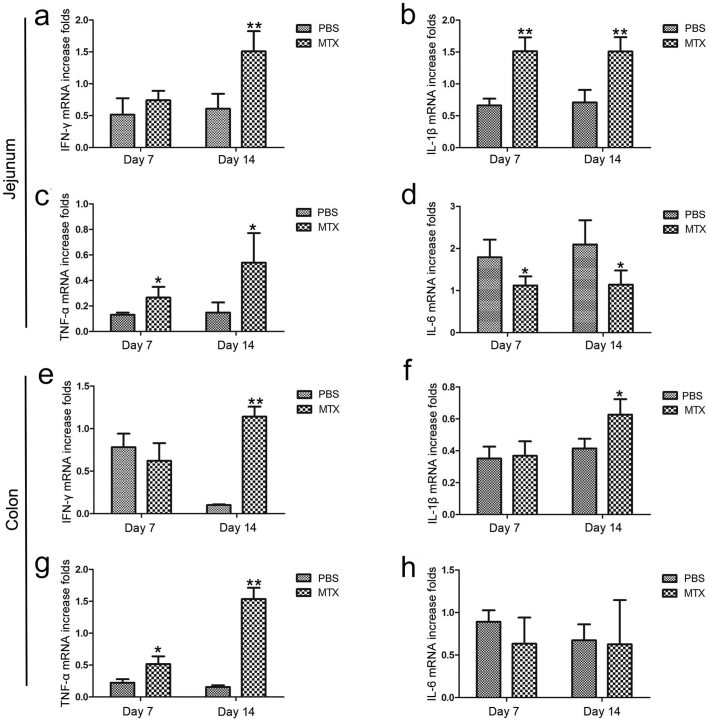
To further confirm the hypothesis that MTX promotes intestinal inflammatory response, the mRNA expression levels of inflammatory markers in jejunal and colonic tissues were detected in the MTX-treated mice and PBS-treated mice using real-time PCR. We observed an up-regulation of IL-1β, TNF-α and IFN-γ mRNA expression in both jejunum and colon of the MTX-treated mice, while lower levels of IL-6 were observed in the jejunum of the same groups (Fig. 2). However, no IL-10 was detectable in the intestinal tissues.
Fig. 2.
MTX induces alterations in intestinal cytokines. (a-d) Real-time PCR analyses of expression of cytokines (a) IFN-γ, (B) IL-1β, (c) TNF-α, and (d) IL-6 in the mouse jejunum after treatment with or without MTX (n = 3/group). (e-h) Expression levels of cytokines (e) IFN-γ, (f) IL-1β, (g) TNF-α, and (h) IL-6 in the mouse colon (n = 3/group). Shown are the means ± SEM; *P < 0.05 and **P < 0.01 versus PBS treatment (normal).
3.2. MTX Induced Changes in Macrophage Subsets
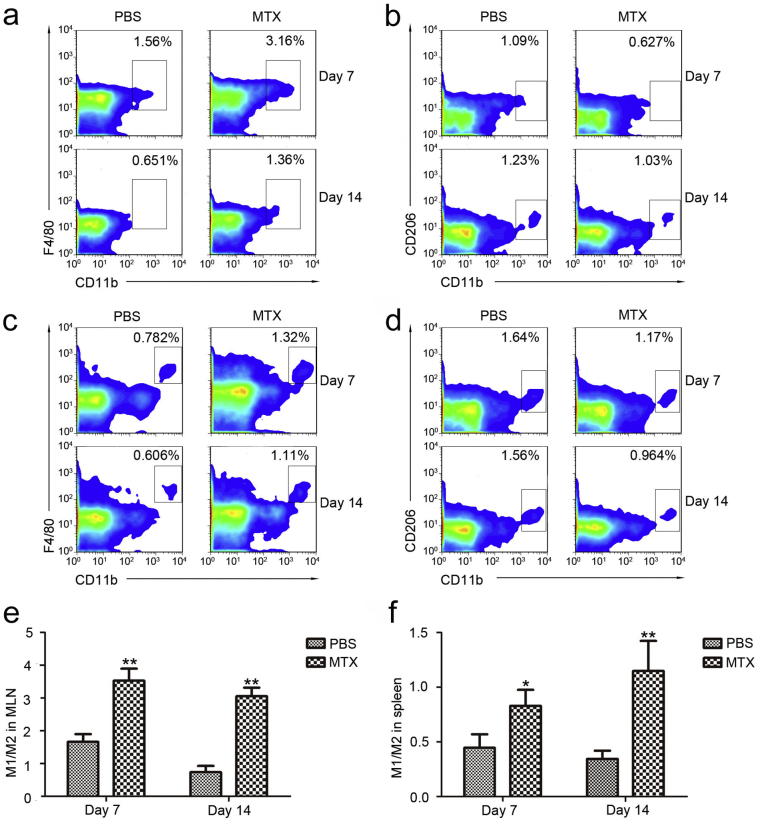
To explore the changes in immune cell activities, we focused on the increased proportions of CD11b+ mononuclear phagocytes, which can be subdivided into macrophages and DCs. There are two main groups of macrophages, designated as activated macrophages (also known as M1 macrophages, promoting inflammatory responses by producing substantial amounts of nitric oxide and pro-inflammatory cytokines such as IL-12 and TNF-α) and alternatively activated macrophages (also known as M2 macrophages, serving anti-inflammatory functions by producing anti-inflammatory cytokines such as IL-10) [[34], [35], [36], [37]]. Due to the plasticity of environment-dependent M1 or M2 polarization and their diverse roles in inflammation induction/homeostasis maintenance, we quantified the M1 and M2 macrophages in the mesenteric lymph nodes (MLNs) and spleens to investigate the effect of MTX on the intestinal and extra-intestinal lymphoid tissues, respectively. The MTX-treated mice exhibited fewer CD11b+F4/80+ M1 macrophages and more CD11b+CD206+ M2 macrophages in both MLNs and spleens according to flow cytometric examination (Fig. 3a–d). The M1/M2 ratio-based differences were markedly pronounced in the MTX-treated group than in the PBS-treated group (Fig. 3e and d), indicating that MTX facilitates the polarization of pro-inflammatory M1 macrophages and decreases the anti-inflammatory M2 phenotype, thereby promoting intestinal inflammation. Concomitantly, MTX can also be implicated in the regulation of monocyte polarization in the spleen.
Fig. 3.
MTX induces changes in macrophage subsets in the mesenteric lymph nodes (MLNs) and spleens. (a, b) MTX-associated changes in (a) CD11b+F4/80+ M1 macrophages and (b) CD11b+CD206+ M2 macrophages in MLNs on day 7 and day 14. (c, d) MTX-associated changes in (c) CD11b+F4/80+ M1 macrophages and (d) CD11b+CD206+ M2 macrophages in the spleens on day 7 and day 14. (e) M1/M2 ratio in the MLNs on day 7 and day 14. (f) M1/M2 ratio in the spleens on day 7 and day 14. (A-F, n = 3/group) Shown are the means ±SEM; *P < 0.05 and **P < 0.01 versus PBS treatment.
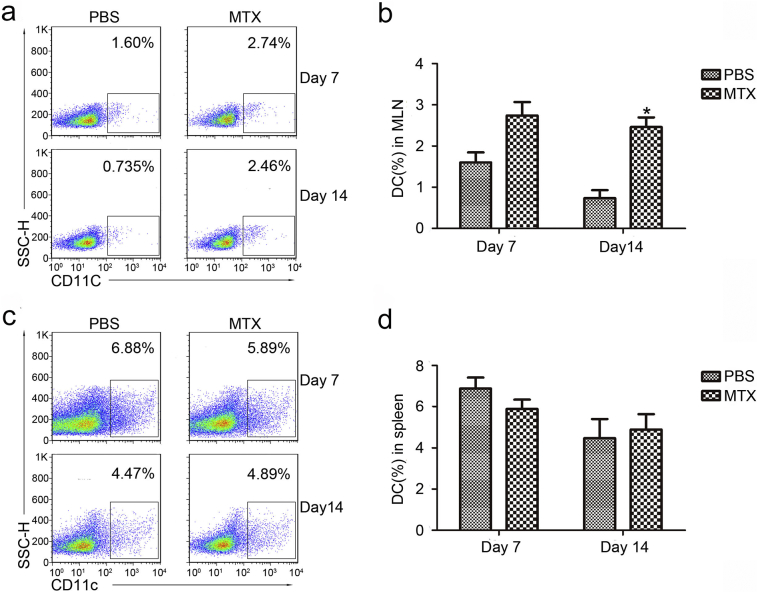
Given that dendritic cells are also essential for active immunity, changes in the CD11c+ dendritic cells were also monitored in the MLNs and spleens using flow cytometry. Increasing numbers of DCs were observed in the MLNs after 14 days of MTX treatment, whereas no significant difference could be detected in the spleens (Supplementary Fig. 2), suggesting that long-term MTX treatment can increase the number of DCs in the MLNs but not in spleens.
Supplementary Fig. 2.
MTX induces changes in dendritic cells (DCs) in the mesenteric lymph nodes (MLNs) but not in the spleen.
3.3. MTX Induced Changes in Intestinal Microbial Components
Considering that M1 macrophages function as phagocytes and are specialized in the recognition and elimination of microbes [38] and the gut microbiota is closely associated with the intestinal epithelium and immune system, we hypothesized that MTX would stimulate M1 macrophage growth and causally disturb the balance of the gut microbiota. Therefore, feces of mice with/without MTX treatment were collected on day 0, day 7 and day 14 for analyses of the gut microbiota by performing high throughput pyrosequencing of 16S ribosomal RNA (rRNA) gene amplicons.
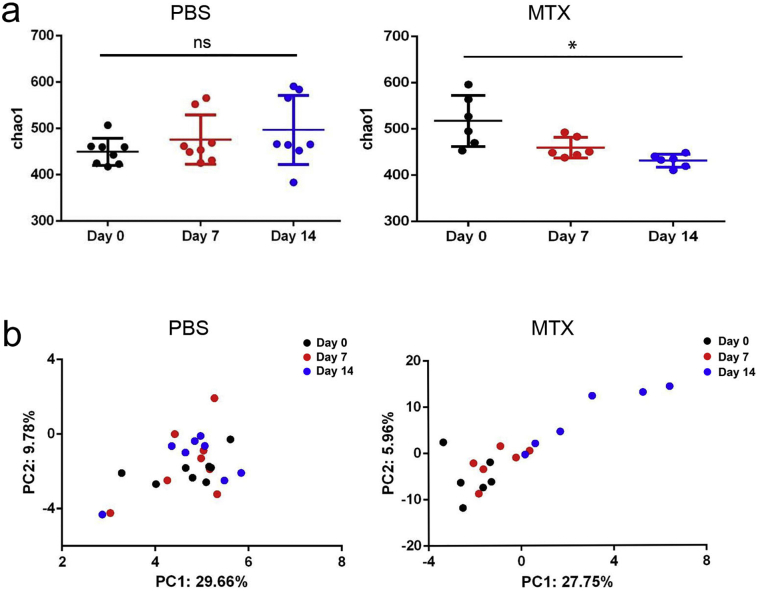
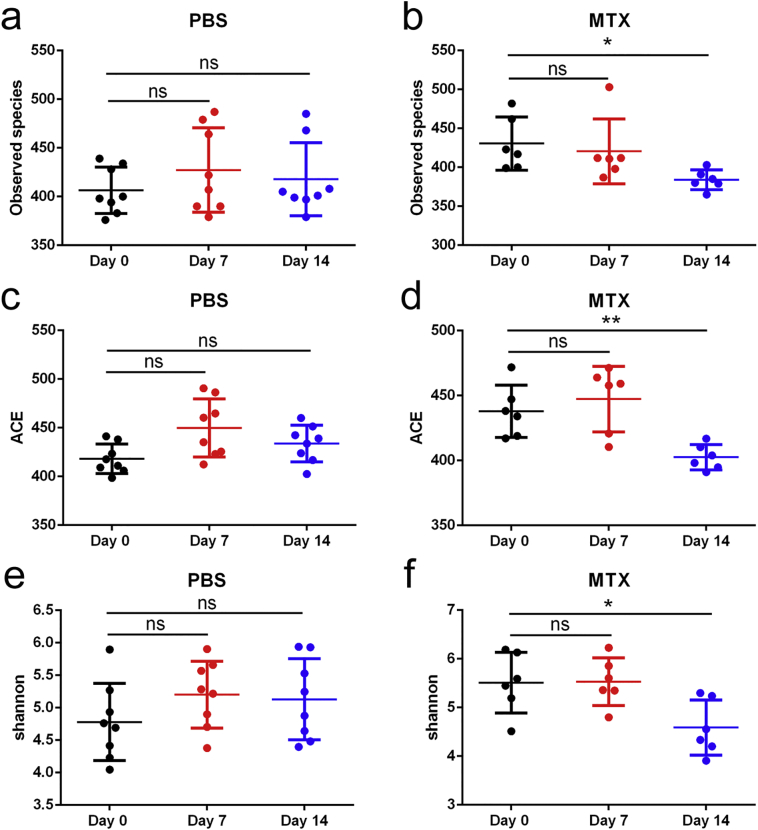
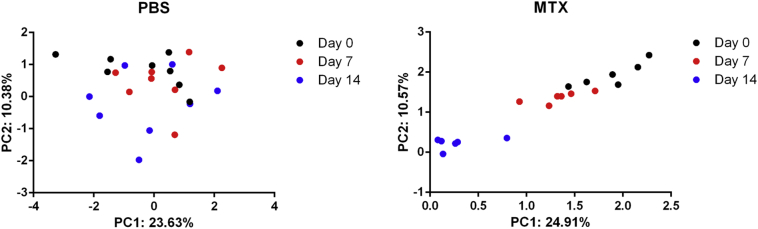
Analyses of alpha diversity of bacteria with the Chao1 index revealed gradually reduced diversity of the total microbiota in the MTX-treated groups, whereas the species richness of microbial communities in the PBS-treated controls showed little difference over time (Fig. 4a), the analyses of Observed species, abundance-based coverage estimator (ACE) and Shannon diversity were in accord with Chao 1(Supplementary Fig. 3). Additionally, principal component analysis (PCA) demonstrated that the composition of the gut microbiome of mice treated with MTX for 14 days was significantly altered compared with that of the PBS-treated mice on day 0 (Fig. 4b and Supplementary Fig. 4). These findings are consistent with our hypothesis that MTX contributes to perturbations of microbiota composition.
Fig. 4.
MTX induces changes in intestinal microbial components. (a) Alpha diversity and (b) principal component analysis (PCA) of the gut microbiota in MTX-treated and PBS-treated mice on day 0, day 7, and day 14. Shown are the means ± SEM; ns, not significant; *adjusted P < 0.05 versus day 0.
Supplementary Fig. 3.
Observed species, ACE and Shannon diversity analysis.
Supplementary Fig. 4.
Weighted UniFrac analysis.
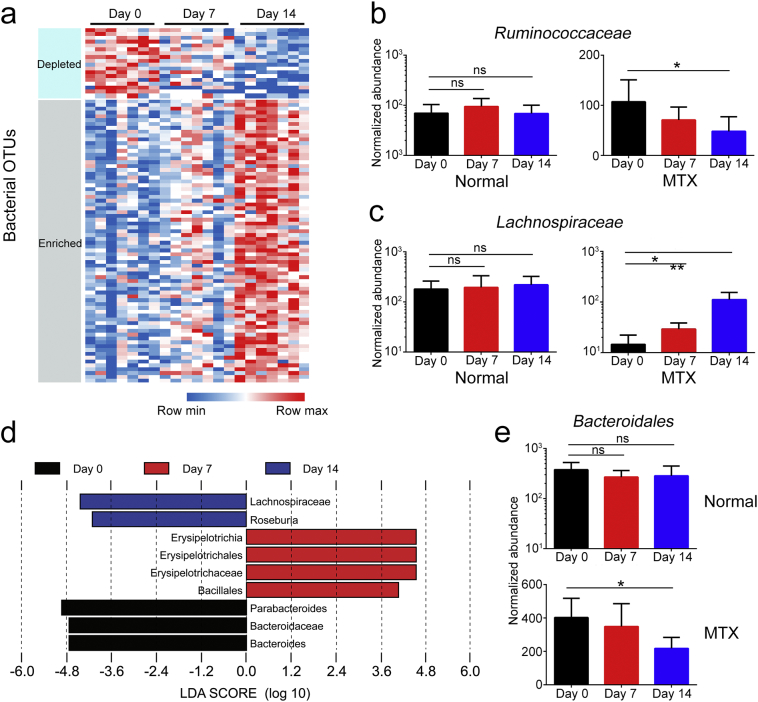
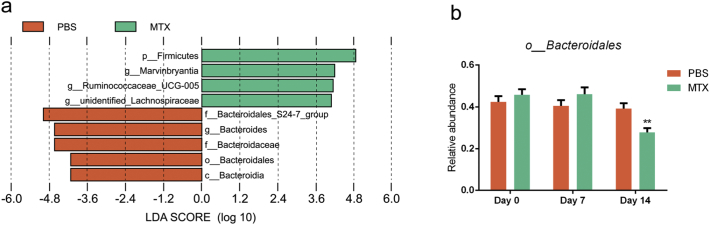
Further, the significantly altered components of the intestinal microbiota were estimated by comparing the MTX-induced discrimination of normalized operational taxonomic units (OTUs) in mice. The OTUs of different microbiota were gradually increased or decreased during MTX treatment (Supplementary Table 2), which is illustrated in the form of a heatmap (Fig. 5a). For instance, the Ruminococcaceae family underwent a sharp decrease (Fig. 5b), while the number of Lachnospiraceae family members was dramatically increased (Fig. 5c). To identify the most differentially abundant taxa in the MTX-treated mice over time, the linear discriminant analysis (LDA) effect size (LEfSe) method was applied to assess the effect size of each taxon. The output revealed that mice treated with MTX for 7 days and 14 days had fewer Bacteroidetes in the feces (Fig. 5d). Consistent with this finding, statistical analysis indicated a reduction in Bacteroidales in the MTX group (Fig. 5e). In addition, LefSe and statistical analysis for MTX-treated mice versus PBS-treated mice had been proceeded with timepoint as covariate, the results indicated that Bacteroidales was decreased in MTX-treated mice compared to PBS-treated mice (Supplementary Fig. 5a–b). Linear mixed effect model was conducted at the same time, MTX treatment and timepoint were considered as fixed effect, Bacteroidales was also changed significantly in this analysis (Supplementary Table 3). Collectively, we demonstrate a profound role of MTX in the quantitative alteration of a diverse range of microbial taxa, among which Bacteroidales exhibited the most distinct variation.
Fig. 5.
MTX induces alterations in various microbial taxa over time. (a) Heatmap of OTUs of intestinal microbiota in the MTX-treated mice (n = 7). (b, c) Examples of OTUs for which the abundance (b) decreased or (c) increased after MTX treatment. (d) Linear discriminant analysis (LDA) effect size (LEfSe) of the differentially abundant microbial taxa on day 0, day 7, and day 14 in the MTX-treated mice. Only taxa meeting an LDA significant threshold of >3.6 are shown. (e) Changes in Bacteroidales in mice treated with or without MTX. Shown are the means ±SEM; ns, not significant; * adjusted P < 0.05 and ** adjusted P < 0.01 versus day 0.
Supplementary Fig. 5.
LefSe analysis for MTX-treated mice versus PBS-treated mice.
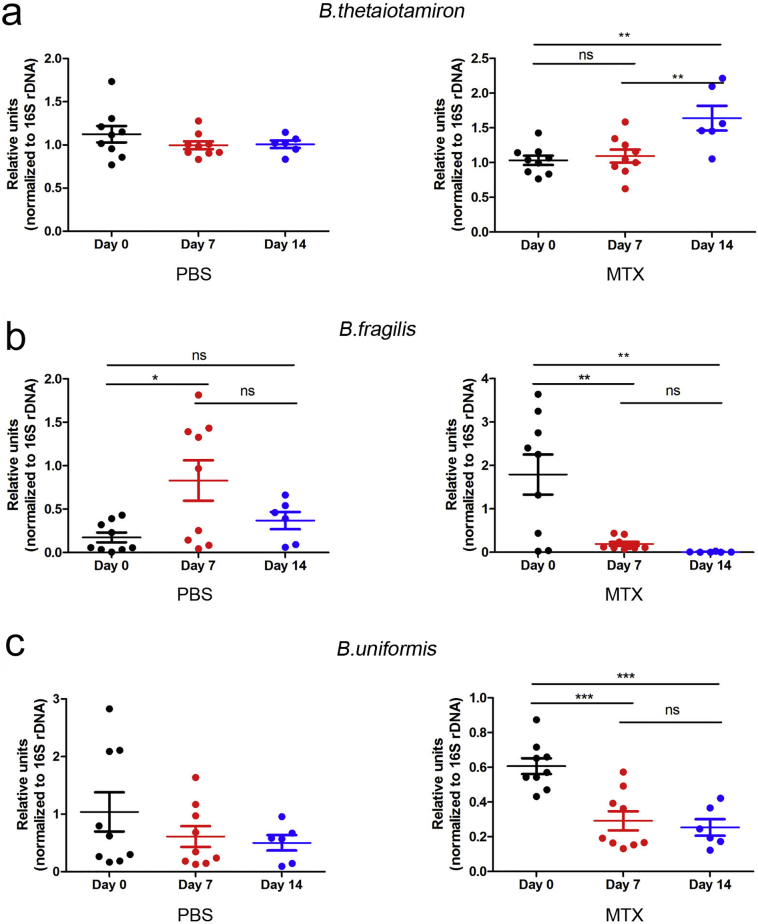
Since Bacteroides serves as a representative genus of the Bacteroidaceae family, we performed real-time PCR targeting specific Bacteroides species in fecal contents. The content of B. thetaiotamicron distinctly increased (Fig. 6a), while that of B. uniforms and B. fragilis significantly decreased after MTX but not PBS treatment (Fig. 6b–c). Notably, one of the well-reported regulatory Bacteroides isolates, B. fragilis [39], was the most significantly impacted in terms of fold change (Fig. 6b).
Fig. 6.
Trend towards a decrease in Bacteroidales in feces post MTX treatment. Real-time PCR analyses of feces DNA targeting (a) B. thetaiotamicron, (b) B. fragilis, and (c) B. uniforms in the MTX-treated PBS-treated mice. Ns, not significant; *P < 0.05, **P < 0.01 and ***P < 0.001 versus day 0.
3.4. B. fragilis Ameliorated MTX-Associated Inflammatory Damage
B. fragilis, a member of the normal colon microbiata, has been demonstrated to play a vital role in immune regulation and associated with auto-immune diseases [40] as well as to the drug response against CTLA-4 [26]. Therefore, we focused on B. fragilis to explore the relationship between the intestinal microbiota and MTX-induced inflammatory response.
First, we performed co-culture of RAW264.7 macrophages and B. fragilis to validate the causal effect of promotion of M1 macrophages by Bacteroides. Our data showed that the proliferation rate of B. fragilis tended to decrease with the increase in macrophage density, suggesting that the proliferation of B. fragilis was inhibited by RAW264.7 macrophages (Supplementary Fig. 6). Accordingly, the MTX-associated decrease in intestinal B. fragilis was likely mediated by M1 macrophage polarization.
Supplementary Fig. 6.
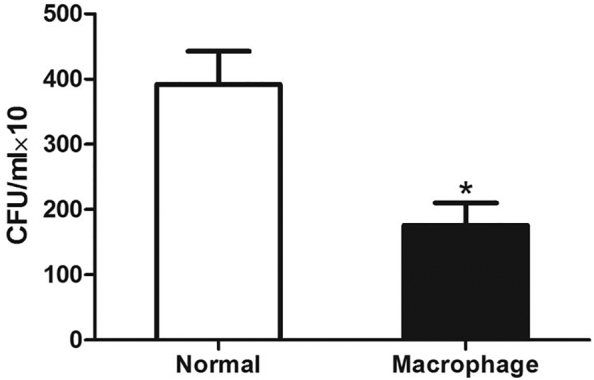
RAW264.7 macrophages inhibit B. fragilis proliferation.
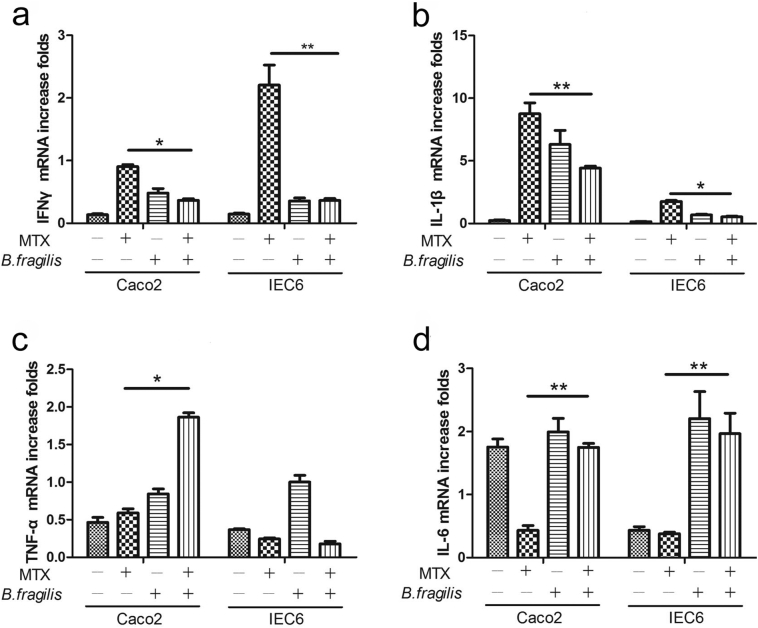
These findings prompted us to ask whether the decrease in B. fragilis promoted MTX-induced inflammation. Therefore, real-time PCR was performed to monitor the expression of inflammatory cytokines in Caco2 and IEC6 cells treated with or without B. fragilis and MTX at the half-maximal inhibitory concentration (IC50) of MTX for these two cell lines (Supplementary Fig. 7). It was illustrated that B. fragilis could efficiently decrease the MTX-induced secretion of IFN-γ and IL-1β (Fig. 7a and b) and increase the MTX-induced reduction in the expression of IL-6 (Fig. 7d) in both cell lines. However, the expression of TNF-α was significantly increased by B. fragilis in Caco2 but not in IEC6 cells (Fig. 7c). Accordingly, we found that B. fragilis could profoundly ameliorate the inflammatory process promoted by MTX.
Supplementary Fig. 7.
MTX inhibits the proliferation of Caco2 cells and IEC6 cells.
Fig. 7.
B. fragilis protects from MTX-induced inflammatory responses. (a-d) Effects of B. fragilis and MTX on the expression of cytokines (a) IFN-γ, (b) IL-1β, (c) TNF-α, and (d) IL-6 in Caco2 cells and IEC6 cells. *P < 0.05 and **P < 0.01 versus the MTX group.
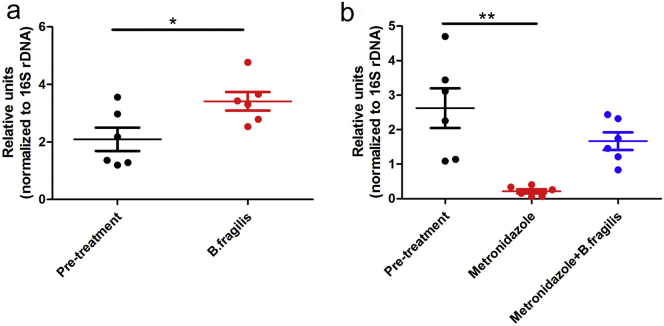
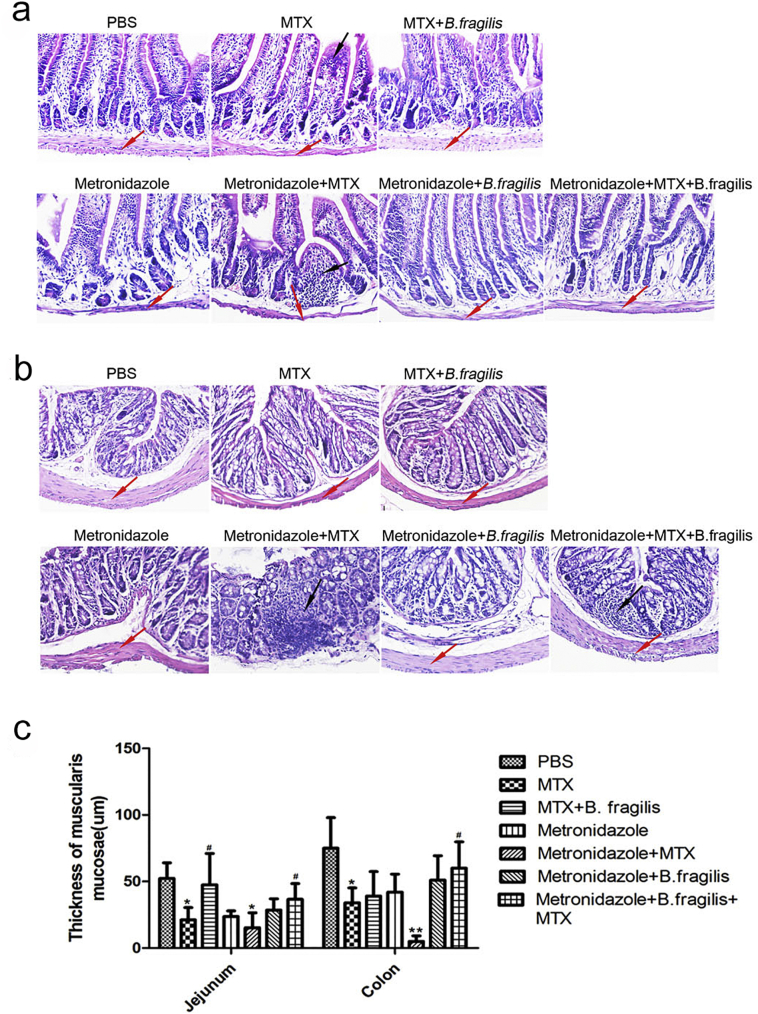
To further validate the hypothesis that B. fragilis can rescue the intestine from MTX-induced inflammatory damage, we performed histological analyses on intestinal tissues. Since oral gavage with B. fragilis led to an increase in the intestinal B. fragilis quantity in mice pre-treated with or without the antibiotic metronidazole (Supplementary Fig. 8), the metronidazole-treated mice were gavaged with bacteria of this taxon to verify the effect of B. fragilis. In line with previous studies, MTX-treated mice exhibited damage to the muscularis mucosae, which was more severe in mice pre-treated with metronidazole and significantly alleviated by gavage with B. fragilis (Supplementary Fig. 9). The results confirmed a protective role of B. fragilis against the MTX-induced inflammatory damage, and thus we can confidently conclude that MTX-associated reduction in B. fragilis aggravates the detrimental inflammatory response.
Supplementary Fig. 8.
Oral gavage with B. fragilis increases intestinal B. fragilis content.
Supplementary Fig. 9.
B. fragilis protects from MTX-associated tissue injury. (a) Hematoxylin-eosin (HE) staining of the jejunum and colon in control and MTX-treated mice. (b) Thickness of muscularis mucosae (n = 3). *P < 0.05 and **P < 0.01 versus PBS. #P < 0.05, ##P < 0.01 versus MTX.
3.5. B. fragilis Ameliorated MTX-Associated Macrophage Alteration
As stated earlier, MTX-induced macrophage polarization tended to lead to the decrease in Bacteroides, and B. fragilis protected cells from MTX-associated inflammatory response in vitro, so we next investigated whether B. fragilis had any impact on macrophages.
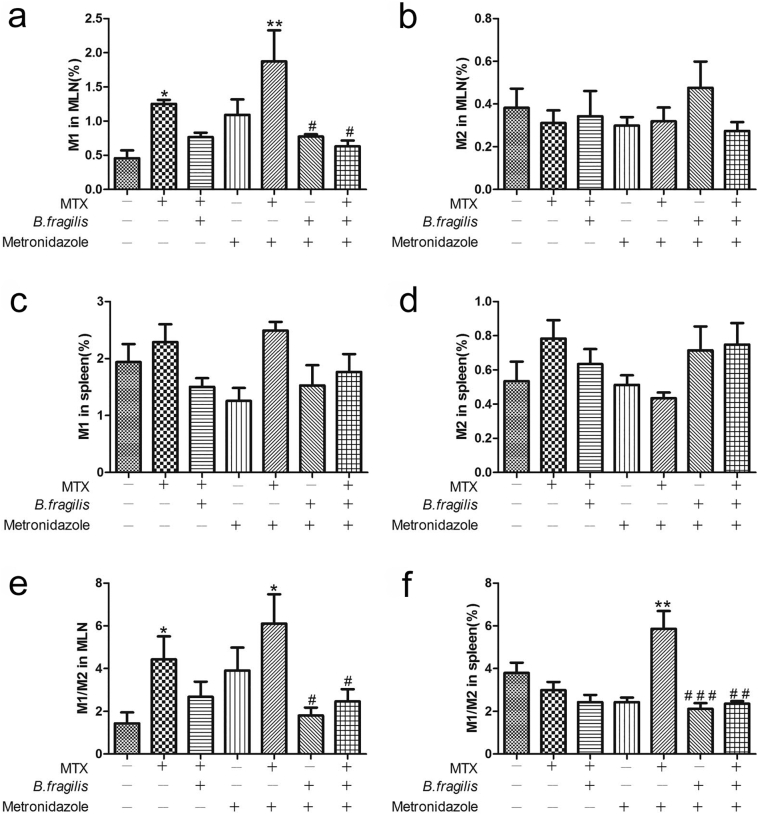
MTX treatment alone induced M1 macrophage proliferation in MLNs, especially in mice pre-treated with metronidazole, and addition of B. fragilis significantly attenuated this increase in M1 macrophages induced by MTX (Fig. 8a). However, no significant difference was detected in M2 macrophages and M1 macrophages in the spleen (Fig. 8b–d). Meanwhile, gavage with B. fragilis also mitigated the increase in the M1/M2 ratio as a result of MTX administration in both MLNs and spleen, especially in the metronidazole-treated mice that had reduced number of B. fragilis (Fig. 8e and f), suggesting that B. fragilis can modulate the MTX-induced macrophage imbalance and that reduction of B. fragilis can in turn aggravate this imbalance resulting from MTX administration, thus leading to a positive feedback, which contributes to intestinal tissue damage.
Fig. 8.
B. fragilis ameliorates MTX-induced macrophage alterations. (a) M1 macrophages and (b) M2 macrophages in the MLNs. (c) M1 macrophages and (d) M2 macrophages in the spleen. (A-D, n = 3/group). *P < 0.05 and **P < 0.01 versus blank control. #P < 0.05, ##P < 0.01 and ###P < 0.001 versus metronidazole + MTX.
4. Discussion
MTX is considered a cornerstone of many multidrug-based chemotherapeutic regimens and is used for the treatment of immune diseases, mainly targeting the folate metabolite pathway. Nevertheless, the administration of MTX is often associated with multiple adverse events, among which gastrointestinal toxicity is the major dose-limiting factor. As with many other chemotherapeutic agents, MTX can induce intestinal mucositis, which can affect the entire gastrointestinal tract and cause malabsorption, diarrhea, and severe pain [16, 41]. Although the precise pathogenic mechanism underlying the chemotherapy-induced intestinal mucositis remains unknown, several studies have suggested the disruption of immune regulation initiated by direct chemotherapy-induced injury to intestinal mucosa [42]. In this study, we demonstrated that MTX-induced intestinal mucosal injury can facilitate the recruitment of mononuclear phagocytes, which release additional pro-inflammatory cytokines, thus potentiating tissue damage.
Notably, the human intestinal tract hosts >1012 microorganisms per gram of luminal content [43]. Differences are reported between mucosal (in both colon and jejunum) and fecal bacteria in terms of relative abundance. However, they are correlated, and fecal bacteria is mostly used to reflect the status of mucosal bacteria, due to the easy collections. This large arsenal of microbes promotes a broad range of biochemical and metabolic activities to complement the host physiology. For instance, the microbiota facilitates the digestion of polysaccharides, production of essential vitamins, development of the host's intestinal epithelium and immune system, and prevention of pathogenic colonization [[44], [45], [46]]. In turn, the dynamically and rapidly changing intestinal habitat plays a major part in regulating both the composition of the microbial community and the individual genomes. Accordingly, when the delicate balance between the host and flora is disrupted, the gut microbiota may cause or contribute to diseases [47, 48]. It has been demonstrated that the gut microbiota is associated with a diverse array of intestinal and extra-intestinal diseases, such as inflammatory bowel disease, multiple sclerosis, arthritis, type 1 diabetes, and allergic inflammation [49, 50]. Moreover, disruption of the microbiota has been shown to hinder disease rehabilitation. For example, persistent alterations in the intestinal microbiome have been shown to contribute to obesity-associated weight regain after dieting [51]. Our data from in vivo and in vitro studies suggested that MTX administration lead to alterations in the population, diversity, and major components of the intestinal microbiota, especially Bacteroides. To reduce the technical artifacts of UPARSE, we also conducted DADA2 to re-analyzed our data and got the similar conclusion (Supplementary Table 4). Along with previously confirmed mechanisms such as high levels of oxidative and nitrosative stress, distortion in the gut microbial community exacerbates the existing intestinal damage. Intriguingly, disruption of the gut microbiota has been linked to drug responses, for example, the antitumor effects of CTLA-4 blockade has been recently found to be influenced by the composition of the microbiota (B. fragilis and/or B. thetaiotaomicron and Burkholderiales) [26]. Although we did not investigate the impact of intestinal microbes on the efficiency of MTX treatment, we have indicated the protective role of B. fragilis against MTX-induced inflammatory responses, suggesting the important role of such Bacteroides species in response to multiple drugs. Collectively, we infer that the gut microbiota may be involved in the improvement of antitumor therapeutic effects either by directly strengthening drug efficiency or by alleviating toxicities, prompting new resolutions for MTX-induced gastrointestinal toxicity and future studies on the mechanisms of chemotherapy-induced complications. Moreover, our results suggested that co-treatment of MTX and metronidazole results in severe intestinal damage partly due to the lack of protective microbes in the intestine, and the combinatorial use of specific antibiotics (e.g., metronidazole) and MTX is inevitable, exogenous supplementation of B. fragilis may ameliorate the intestinal damage. Hence, retrospective investigations may be needed to evaluate the side effect of antibiotics on MTX treatment patients to avoid drug-induced severe intestinal mucositis.
It should be noted that our analyses at the species level only focused on Bacteroides species due to their significant variance in quantity. Nevertheless, the intestinal microbiota represents a complicated ecologic niche in which a wide range of microorganisms are correlated to the host's immune system. Supportive evidence has revealed complicated interactions among these microbial taxa, which play an important part in the metabolism of food and drugs [49]. It is possible that a combined effect of a variety of microbes played a role in the amelioration of MTX-induced inflammation. Therefore, studies on single species without deciphering the interplay with other taxa may be inadequate in explaining the underlying mechanisms. Although we have indicated that the MTX-induced gastrointestinal toxicity was exacerbated through the alteration of the gut microbiota and that B. fragilis can ameliorate the damage, we did not investigate the underlying mechanisms. Limited evidence indicates that polysaccharide A (PSA) produced by B. fragilis may act as the causal component in the protection against Helicobacter hepaticus-induced experimental colitis [52], so it is worth testing whether PSA can ameliorate MTX-induced ADRs instead of B. fragilis. In that case, taking PSA together with MTX could be a strategy to reduce the toxicity of MTX-based therapy. Notably, the risk of ADRs to MTX is also associated with germline single nucleotide polymorphisms (SNPs). For example, SNPs in the SLCO1B1 gene (e.g., rs11045879) are related to the toxicity of MTX, with the C allele being associated with higher risk for delayed clearance and higher plasma concentration of MTX [53]. Moreover, relationship between SNPs in the MTHFR gene (a key enzyme for MTX pharmacokinetics) and MTX-induced toxicities in patients with acute lymphoblastic leukemia has also been evaluated in various populations. Although the results remain conflicting, the C677T variant of the MTHFR gene has been associated with increased risk of severe gastrointestinal mucositis [54]. As the reported SNPs have potential influence on gene or protein expression, it is worthwhile to further investigate the association between the gut microbiota and related genes.
Our study was conducted with the hypothesis that MTX initiates mucositis by directly impairing basal epithelial cells and cells in the submucosal tissues via DNA and non-DNA injury [16]. Subsequently, mucosal integrity is further damaged via up-regulation of pro-inflammatory cytokines, resulting in higher susceptibility to bacterial colonization and enhanced recruitment of immunocytes such as mononuclear phagocytes. After being activated by microbial products, mononuclear phagocytes produce additional pro-inflammatory cytokines and disrupt the gut microbial community, which in turn enhance immunocyte recruitment and contribute to imbalanced macrophage composition. The whole process results in a vicious inflammatory cycle to amplify the primary damage. Therefore, our results essentially indicate an indirect way by which MTX serves a pro-inflammatory function that is not contradictory to the conventional knowledge of MTX as an immunosuppressive agent.
The following are the supplementary data related to this article.
Gene specific primers.
Significantly altered OTUs of microbiota with MTX treatment.
Linear mixed effect models.
DADA2 analysis.
Funding source
This study was supported by grants from the “Leading Academic of Ten-thousand Talents Program”, National Science and Technology Major Project (No. 2012ZX09103301-025), National Key Research Development Program (No. 2016YFC0905000) and National Natural Science Foundation of China (No. 81522028, No. 81673452). Heng Xu is also supported by a grant from “The Recruitment Program of Global Young Experts” (known as “The Thousand Young Talents Plan”). All funding sources were used for data collection and analysis.
Conflict of Interests
None.
Author's contribution
Li Yang, Heng Xu contributed to study design. Bailing Zhou,Shuang Chen, Chaoheng Yu, Rong Huang,Rui Zhang,Yantai Wang contributed to data and figures collection. Xuyang Xia, Yang Shu, Shouyue Zhang contributed to data analysis. Peiqi Wang, Ding Bai contributed to writing. Lian Lu, Fengjiao Yuan, Yaomei Tian, Yingzi Fan, Xueyan Zhang, Lei Wu contributed to literature search.
Contributor Information
Heng Xu, Email: xuheng81916@scu.edu.cn.
Li Yang, Email: yl.tracy73@gmail.com.
References
- 1.Leitao R.F., Brito G.A., Oria R.B., Braga-Neto M.B., Bellaguarda E.A., Silva J.V. Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol. 2011;11:90. doi: 10.1186/1471-230X-11-90. [Epub 2011/08/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neradil J., Pavlasova G., Veselska R. New mechanisms for an old drug; DHFR- and non-DHFR-mediated effects of methotrexate in cancer cells. Klin Onkol. 2012;25(Suppl. 2) [2S87-92. Epub 2012/01/01] [PubMed] [Google Scholar]
- 3.Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009;146(5):489–503. doi: 10.1111/j.1365-2141.2009.07765.x. [Epub 2009/06/23] [DOI] [PubMed] [Google Scholar]
- 4.Paci A., Veal G., Bardin C., Leveque D., Widmer N., Beijnen J. Review of therapeutic drug monitoring of anticancer drugs part 1—cytotoxics. Eur J Cancer. 2014;50(12):2010–2019. doi: 10.1016/j.ejca.2014.04.014. [Epub 2014/06/04] [DOI] [PubMed] [Google Scholar]
- 5.Morsy M.A., Ibrahim S.A., Amin E.F., Kamel M.Y., Rifaai R.A., Hassan M.K. Curcumin ameliorates methotrexate-induced nephrotoxicity in rats. Adv Pharmacol Sci. 2013;2013:387071. doi: 10.1155/2013/387071. [Epub 2014/01/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widemann B.C., Balis F.M., Kempf-Bielack B., Bielack S., Pratt C.B., Ferrari S. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100(10):2222–2232. doi: 10.1002/cncr.20255. [Epub 2004/05/13] [DOI] [PubMed] [Google Scholar]
- 7.Perez-Verdia A., Angulo F., Hardwicke F.L., Nugent K.M. Acute cardiac toxicity associated with high-dose intravenous methotrexate therapy: case report and review of the literature. Pharmacotherapy. 2005;25(9):1271–1276. doi: 10.1592/phco.2005.25.9.1271. [Epub 2005/09/17] [DOI] [PubMed] [Google Scholar]
- 8.Kolli V.K., Abraham P., Isaac B., Kasthuri N. Preclinical efficacy of melatonin to reduce methotrexate-induced oxidative stress and small intestinal damage in rats. Dig Dis Sci. 2013;58(4):959–969. doi: 10.1007/s10620-012-2437-4. [Epub 2012/10/12] [DOI] [PubMed] [Google Scholar]
- 9.Avritscher E.B., Cooksley C.D., Elting L.S. Scope and epidemiology of cancer therapy-induced oral and gastrointestinal mucositis. Semin Oncol Nurs. 2004;20(1):3–10. doi: 10.1053/j.soncn.2003.10.002. [Epub 2004/03/25] [DOI] [PubMed] [Google Scholar]
- 10.Ben-Lulu S., Pollak Y., Mogilner J., Bejar J., GC Sukhotnik., I Dietary transforming growth factor-beta 2 (TGF-beta2) supplementation reduces methotrexate-induced intestinal mucosal injury in a rat. PloS one. 2012;7(9):e45221. doi: 10.1371/journal.pone.0045221. [Epub 2012/09/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C., Tian L., Zhang M., Sun Q., Zhang X., Li X. Protective effect of amifostine on high-dose methotrexate-induced small intestinal mucositis in mice. Dig Dis Sci. 2013;58(11):3134–3143. doi: 10.1007/s10620-013-2826-3. [Epub 2013/08/28] [DOI] [PubMed] [Google Scholar]
- 12.Kolli V.K., Kanakasabapathy I., Faith M., Ramamoorthy H., Isaac B., Natarajan K. A preclinical study on the protective effect of melatonin against methotrexate-induced small intestinal damage: effect mediated by attenuation of nitrosative stress, protein tyrosine nitration, and PARP activation. Cancer Chemother Pharmacol. 2013;71(5):1209–1218. doi: 10.1007/s00280-013-2115-z. [Epub 2013/02/20] [DOI] [PubMed] [Google Scholar]
- 13.Koppelmann T., Pollak Y., Mogilner J., Bejar J., Coran A.G., Sukhotnik I. Dietary L-arginine supplementation reduces methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol. 2012;12:41. doi: 10.1186/1471-230X-12-41. [Epub 2012/05/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugiyama A., Kimura H., Ogawa S., Yokota K., Takeuchi T. Effects of polyphenols from seed shells of Japanese horse chestnut (Aesculus turbinata BLUME) on methotrexate-induced intestinal injury in rats. J Vet Med Sci. 2011;73(5):673–678. doi: 10.1292/jvms.10-0423. [Epub 2010/12/22] [DOI] [PubMed] [Google Scholar]
- 15.Keefe D.M., Schubert M.M., Elting L.S., Sonis S.T., Epstein J.B., Raber-Durlacher J.E. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109(5):820–831. doi: 10.1002/cncr.22484. [Epub 2007/01/20] [DOI] [PubMed] [Google Scholar]
- 16.Sonis S.T. The pathobiology of mucositis. Nat Rev Cancer. 2004;4(4):277–284. doi: 10.1038/nrc1318. [Epub 2004/04/02] [DOI] [PubMed] [Google Scholar]
- 17.El-Sheikh A.A., Morsy M.A., Hamouda A.H. Protective mechanisms of thymoquinone on methotrexate-induced intestinal toxicity in rats. Pharmacognosy Magazine. 2016;12(Suppl. 1):S76–S81. doi: 10.4103/0973-1296.176106. [Epub 2016/04/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautam R., Singh M., Gautam S., Rawat J.K., Saraf S.A., Kaithwas G. Rutin attenuates intestinal toxicity induced by methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement Altern Med. 2016;16:99. doi: 10.1186/s12906-016-1069-1. [Epub 2016/03/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonis S.T., Elting L.S., Keefe D., Peterson D.E., Schubert M., Hauer-Jensen M. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 Suppl):1995–2025. doi: 10.1002/cncr.20162. [Epub 2004/04/27] [DOI] [PubMed] [Google Scholar]
- 20.Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [Epub 2010/02/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [Epub 1998/04/01] [DOI] [PubMed] [Google Scholar]
- 22.Lee S.H., Starkey P.M., Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161(3):475–489. doi: 10.1084/jem.161.3.475. [Epub 1985/03/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper L.V., Macpherson A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–169. doi: 10.1038/nri2710. [Epub 2010/02/26] [DOI] [PubMed] [Google Scholar]
- 24.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [Epub 2010/03/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haiser H.J., Turnbaugh P.J. Is it time for a metagenomic basis of therapeutics? Science. 2012;336(6086):1253–1255. doi: 10.1126/science.1224396. [Epub 2012/06/08] [DOI] [PubMed] [Google Scholar]
- 26.Vetizou M., Pitt J.M., Daillere R., Lepage P., Waldschmitt N., Flament C. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [Epub 2015/11/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magoc T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [Epub 2011/09/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [Epub 2010/04/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thaiss C.A., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A.C. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514–529. doi: 10.1016/j.cell.2014.09.048. [Epub 2014/11/25] [DOI] [PubMed] [Google Scholar]
- 30.Levy M., Thaiss C.A., Zeevi D., Dohnalova L., Zilberman-Schapira G., Mahdi J.A. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163(6):1428–1443. doi: 10.1016/j.cell.2015.10.048. [Epub 2015/12/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998. doi: 10.1038/nmeth.2604. [Epub 2013/08/21] [DOI] [PubMed] [Google Scholar]
- 32.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [Epub 2007/06/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue) doi: 10.1093/nar/gks1219. [D590-D6. Epub 2012/11/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [Epub 2003/01/04] [DOI] [PubMed] [Google Scholar]
- 35.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [Epub 2012/03/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [Epub 2008/11/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dale D.C., Boxer L., Liles W.C. The phagocytes: neutrophils and monocytes. Blood. 2008;112(4):935–945. doi: 10.1182/blood-2007-12-077917. [Epub 2008/08/08] [DOI] [PubMed] [Google Scholar]
- 38.Smythies L.E., Sellers M., Clements R.H., Mosteller-Barnum M., Meng G., Benjamin W.H. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115(1):66–75. doi: 10.1172/JCI19229. [Epub 2005/01/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dasgupta S., Erturk-Hasdemir D., Ochoa-Reparaz J., Reinecker H.C., Kasper D.L. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe. 2014;15(4):413–423. doi: 10.1016/j.chom.2014.03.006. [Epub 2014/04/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazmanian S.K., Liu C.H., Tzianabos A.O., Kasper D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [Epub 2005/07/13] [DOI] [PubMed] [Google Scholar]
- 41.Stringer A.M., Al-Dasooqi N., Bowen J.M., Tan T.H., Radzuan M., Logan R.M. Biomarkers of chemotherapy-induced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support. Care Cancer. 2013;21(7):1843–1852. doi: 10.1007/s00520-013-1741-7. [Epub 2013/02/12] [DOI] [PubMed] [Google Scholar]
- 42.Chan E.S., Cronstein B.N. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002;4(4):266–273. doi: 10.1186/ar419. [Epub 2002/07/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savage D.C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [Epub 1977/01/01] [DOI] [PubMed] [Google Scholar]
- 44.Renz H., Brandtzaeg P., Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011;12(1):9–23. doi: 10.1038/nri3112. [Epub 2011/12/14] [DOI] [PubMed] [Google Scholar]
- 45.Stecher B., Hardt W.D. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14(1):82–91. doi: 10.1016/j.mib.2010.10.003. [Epub 2010/11/03] [DOI] [PubMed] [Google Scholar]
- 46.Smith K., Mccoy K.D., Macpherson A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19(2):59–69. doi: 10.1016/j.smim.2006.10.002. [Epub 2006/11/23] [DOI] [PubMed] [Google Scholar]
- 47.Littman D.R., Pamer E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10(4):311–323. doi: 10.1016/j.chom.2011.10.004. [Epub 2011/10/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honda K., Littman D.R. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [Epub 2012/01/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [Epub 2009/04/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamada N., Seo S.U., Chen G.Y., Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13(5):321–335. doi: 10.1038/nri3430. [Epub 2013/04/27] [DOI] [PubMed] [Google Scholar]
- 51.Thaiss C.A., Itav S., Rothschild D., Meijer M., Levy M., Moresi C. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540(7634):544–551. doi: 10.1038/nature20796. [Epub 2016/12/03] [DOI] [PubMed] [Google Scholar]
- 52.Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [Epub 2008/05/30] [DOI] [PubMed] [Google Scholar]
- 53.Li J., Wang X.R., Zhai X.W., Wang H.S., Qian X.W., Miao H. Association of SLCO1B1 gene polymorphisms with toxicity response of high dose methotrexate chemotherapy in childhood acute lymphoblastic leukemia. Int J Clin Exp Med. 2015;8(4):6109–6113. [Epub 2015/07/02] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L., Hu X., Xu L. Impact of methylenetetrahydrofolate reductase (MTHFR) polymorphisms on methotrexate-induced toxicities in acute lymphoblastic leukemia: a meta-analysis. Tumour Biol. 2012;33(5):1445–1454. doi: 10.1007/s13277-012-0395-2. [Epub 2012/04/25] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene specific primers.
Significantly altered OTUs of microbiota with MTX treatment.
Linear mixed effect models.
DADA2 analysis.