Abstract
Background: Chinese patent medicine Tongxinluo capsule (TXL) is commonly used for cardio-cerebrovascular diseases. Previous research had demonstrated that TXL exhibited great clinical effects on the treatment of acute myocardial infarction (AMI), however there is a lack of systematic review. The purpose of this study was to evaluate the potential effectiveness and safety of TXL for secondary prevention in patients with AMI.
Method: We searched 6 databases to identify relevant randomized controlled trials (RCTs) from inceptions to December 30, 2017. Two review authors independently assessed the methodological quality and analyzed data by the RevMan 5.3 software. The publication bias was assessed through funnel plot and Begg's test. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used for evaluating the quality of evidence.
Results: We included 19 RCTs in this review and performed a meta-analysis based on 16 studies. There were statistical differences of TXL treatment group in reducing primary cardiovascular events (cardiac death [RR = 0.27, 95%CI: 0.08~0.95, I2 = 0%], recurrent myocardial reinfarction [RR = 0.38, 95%CI: 0.20~0.74, I2 = 0%], arrhythmia [RR = 0.44, 95%CI: 0.30~0.66, I2 = 0%], recurrent angina pectoris [RR = 0.34, 95%CI: 0.17~0.69, I2 = 0%]). TXL could improve cardiac function (LVEF [MD = 4.10, 95%CI: 3.95~4.25, I2 = 0%]), regulate blood lipid TC [MD = −0.66, 95%CI: −0.94 ~ −0.37, I2 = 74%], TG [MD = −0.38, 95%CI: −0.62 ~ −0.14, I2 = 70%], LDL-C[−0.40, 95%CI: −0.65 ~ −0.16, I2 = 88%), decrease the level of hs-CRP (4-week: MD = −0.78, 95%CI: −0.97 ~ −0.60, I2 = 20%; Over 4-week: MD = −1.36, 95%CI: −1.55 ~ −1.17, I2 = 20%). However, TXL has little effects on revascularization [RR = 0.45, 95%CI: 0.13~1.56, I2 = 0%], recurrent heart failure (RR = 0.83, 95%CI: 0.27~2.57, I2 = 0%), and HDL-C (MD = 0.14, 95%CI: 0.00 ~0.29, I2 = 73%). Furthermore, TXL treatment group was more prone to suffer gastrointestinal discomfort.
Conclusion: Chinese patent medicine TXL seemed beneficial for secondary prevention after AMI. This potential benefit needs to be further assessed through more rigorous RCTs.
Systematic review registration number in the PROSPERO register: CRD42017068417.
Keywords: tongxinluo, TXL, acute myocardial infarction, systematic review, meta-analysis
Introduction
Cardiovascular diseases (CVDs) are the major public health problem and a chief cause of morbidity and mortality around the world. Approximately 17.7 million people died from CVDs in 2015, accounting for 31% of all global deaths (WHO, 2017). Acute myocardial infarction (AMI) is the most severe type of CVD, causing more than 4 million deaths in Europe and northern Asia (Thygesen et al., 2012; Nichols et al., 2014). In United States, there would be an American diagnosed as myocardial infarction (MI) approximately every 40 s and the overall prevalence for MI was 3.0% in adults (Benjamin et al., 2017). In China, the number of CVD events manifestly increased from 0.75 million in 1990 to 1.4 million in 2013, and one million deaths were caused by MI annually (Li J. et al., 2015; Zhou et al., 2016). According to the National Institute of Health and Care Excellence clinical guideline, the treatment of AMI mainly contains primary percutaneous coronary intervention (PPCI), fibrinolysis, coronary angiography and so forth (Carville et al., 2015). And with the development of these revascularization treatments, the short-term prognosis of AMI has been greatly improved, but mortality and recurrence rate of patients with MI remain high after the acute phase of MI (García-García et al., 2010; Andrés et al., 2012). Secondary prevention of MI has been defined as a kind of evidence-based guideline recommendation and comprehensive intervention strategy to control various cardiovascular risk factors, reduce the recurrence of MI, heart failure (HF), arrhythmia, angina and the incidence of sudden death, extend patients' life and improve their life quality via using non-medical and medical therapy (Perk et al., 2013; Piepoli et al., 2018). Thus, it is vital to conduct secondary prevention for patients who suffered the MI as early as possible.
Nowadays, to decrease risk factors and mortality, available guidelines for secondary prevention of MI worldwide uniformly recommend interventions: one is the optimal use of cardio-protective medical therapies [aspirin, statins, beta-blockers and angiotensin converting enzyme inhibitors/ angiotensin receptor blockers (ACEI/ARBs)], the other one is non-medicine strategy, like smoking cessation and the increase of physical activities (Zeymer et al., 2011; Petersen et al., 2012; Bauters et al., 2014). Traditional Chinese medicine (TCM) has been used in patients with CVDs for thousands of years and is still being commonly used in modern times in both China and elsewhere worldwide (Tachjian et al., 2010). However, to date, it is still uncertain whether TCM can be used as a kind of drug therapy for secondary prevention of CVDs.
Tongxinluo Capsule (TXL), passed appraisal by National Fund for new drug research and registered in the State Food and Drug Administration of China, is a formally classical Chinese patent medicine in treating unstable angina pectoris and acute coronary syndrome (Wang et al., 2003; Ma et al., 2009). The main ingredients of TXL consist of Ginseng Radix Et Rhizoma (Ren Shen), Paeoniae Radix Rubra (Chi Shao), Ziziphi Spinosae Semen (Suan Zao Ren), Dalberglae Odoriferae Lignum (Jiang Xiang), Santali Albi Lignum (Tan Xiang), Olibanum (Ru Xiang), Hirudo (Shui Zhi), Scorpio (Quan Xie), Scolopendra (Wu Gong), Cicadae Periostracum (Chan Tui), Eupolyphaga Steleophaga (Tu Bie Chong), and Borneolum Syntheticum (Bing Pian), which can supply qi, activate blood circulation, dredge collaterals and relieve pain according to the TCM theory (Pharmacopoeia, 2015). Besides, in clinical practice, TXL is taken orally 2-4 capsules per time, 3 times a day. The detailed production process and quality control of TXL was showed in Supplementary Material 1. Plenty of studies showed that TXL could enhance the stability of vulnerable plaques, inhibit inflammation, improve the left ventricular function and exhibit other pharmacological functions (Zhang L. et al., 2009; Zhang et al., 2010; Bai et al., 2013). A Cochrane systematic review indicated TXL could reduce the frequency of acute angina, improve electrocardiogram and angina symptoms (Wu et al., 2006). Currently, many studies reported TXL had good therapeutic effects on patients with MI, but there was little solid evidence about secondary prevention after AMI, thus, the purpose of this study was to perform a comprehensive review and meta-analysis of TXL for secondary prevention after AMI, especially for the primary cardiovascular events and heart functions.
Methods
The review protocol was registered on PROSPERO (CRD42017068417), and the review was constructed following the PRISMA guidelines (Supplementary Material 2).
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) with blind method or not and no limit of publishing language were included. The course of treatment was no less than 4–week, and the number of patients in treatment/control group was more than 30. We excluded the conference papers.
Types of participants
Patients diagnosed with AMI were included, not included those with severe liver and kidney dysfunction. Age and race were not limited.
Types of interventions
Based on the conventional western medicine treatment, the treatment group was given TXL alone or combined with western medicine, and the control group was given nothing, placebo or western medicine. According to clinical symptoms, the conventional western medicines are mainly for secondary prevention of MI, including aspirin (100 mg/d), clopidogrel (75 mg/d), metoprolol tartrate tablet (50 ~ 100 mg/bid), atorvastatin (20 mg/d) and so forth.
Types of outcome measures
Primary outcomes
①Primary cardiovascular events: cardiac death, recurrent myocardial infarction, revascularization, arrhythmia, heart failure and recurrent angina pectoris; ② Heart functions: left ventricular ejection fraction (LVEF). The included studies must contain the primary outcomes.
Secondary outcomes
①Lipid levels: total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C); ② Inflammatory response: hypersensitive C-reactive protein (hs-CRP); ③ Adverse events were calculated as follows: peripheral hemogram, liver function, renal function, gastrointestinal reactions and so forth.
Search methods for identification of studies
The search was applied to PubMed, The Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Database, and Chinese Scientific Journal Database (VIP) from inception to December 30, 2017. The search strategy used the following general terms individually or combined: “tong-xin-luo,” “Tong-xin-luo,” “Tong xin luo,” “tong xin luo,” “Tongxinluo,” “tongxinluo,” “myocardial infarct,*” “heart attack,*” “heart disease,*” “ischemia reperfusion,” “patient.*” The detail search strategy was shown in Supplementary Material 3.
Data extraction and management
Data extraction and management
Two investigators (QZ and JYH) independently conducted the literature searching, study selection, and data extraction. The extracted data of included studies was filled in a standardized form prepared for this review. The extracted data included the first author, year of publication, disease type, sample size, randomized method, average age of participants, treatment duration, interventions in the treatment and control group, dose of medicines, outcomes and so on. Disagreements were discussed and resolved in a consensus meeting with the corresponding author (HCS). If the literature has multiple time end indicators, we chose the longest one.
Unit of analysis issues
Subgroups were divided according to intervention or course of treatment and analyzed individually.
Assessment of quality of included studies
According to Cochrane Reviewer's Handbook, two of the authors (ML and RJQ) individually assessed the risk of bias. Six evaluation criteria of the quality of RCTs were used, which involved the generation of random sequence, randomization concealment, blind method, integrity of outcome data, selective reporting and other bias.
Measures of treatment effect
Revman 5.3 software provided by the Cochrane Collaboration was used for data analyses. Continuous outcomes were presented as weighted mean difference (WMD), dichotomous data as risk ratio (RR) and 95% confidence interval (CI). Means and standard deviations were extracted for continuous outcomes, and as for the dichotomous data, we recorded the number of patients who experienced the event in each group.
Assessment of heterogeneity
Clinical heterogeneity of included studies was analyzed with χ2 test. If I2 < 50%, then there is no statistical heterogeneity between studies, and fixed effect model was used for data analysis; If I2 > 50%, then statistical heterogeneity between studies exists, random effect model was used, and the cause of heterogeneity was analyzed. Subgroup analysis was used when clinical heterogeneity exists. Subgroup was divided according to the sources of heterogeneity, such as intervention measure, course, and dosage of medicines. Descriptive analysis was used if clinical heterogeneity still exists.
Assessment of publication biases
The potential publication bias was assessed using funnel plots and Begg's test by Stata 14 software (Begg and Mazumdar, 1994). If P > 0.05, there is no publication bias, whereas publication bias exists.
Sensitivity analysis
Compare the pooled statistics before and after excluding studies of low quality and great weight and those which have different results from other studies. If they have the same results (both results have differences or have no differences), then the meta-analysis result is stable and vice versa.
Statistical analysis
The primary and second outcomes from the included studies were entered into Revman 5.3 (Higgins and Green, 2011). P < 0.05 was considered statistically significant.
Grade
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to evaluate the quality of evidence by ML and XYZ, which was classified as high, moderate, low, or very low based on the judgment of the risk of bias, inconsistency, indirectness, imprecision and other considerations (Schunemann et al., 2013). Summary of Findings (SOF) tables were prepared using the software program “GRADE pro GDT.”
Results
Description of included trials
Search process
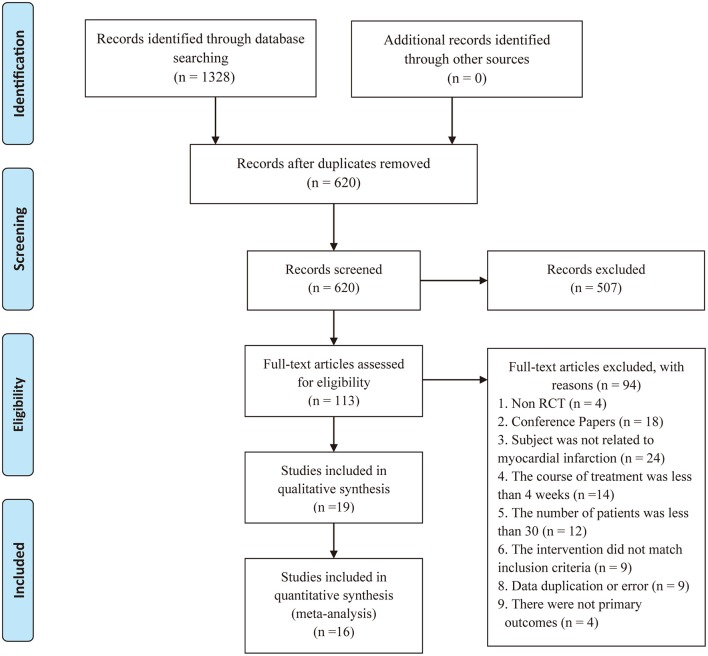
The initial search using the electronic search strategies yielded 1328 studies. After removing 708 duplicates from different databases, we evaluated 620 potentially relevant articles for eligibility. After screening the titles and abstracts, we excluded 507 studies. Of the 113 remaining studies, we further excluded 94 studies after screening the full-text articles. Eventually, we included 19 studies. There may exist the clinical heterogeneity between long-term intervention (≥1–year) and short-term duration (< 1–year) in included studies. Thus, we conducted a descriptive analysis of 3 articles (Zhang and Zhang, 2009; Shen, 2011; Wang Y. L. et al., 2016) with long-term intervention and do a meta-analysis of the remaining 16 studies (You et al., 2005, 2012; Chen et al., 2007, 2008; Huang, 2010; Liang et al., 2010; Liao et al., 2010; Tian and Li, 2011; Yang and Yu, 2011; Tian et al., 2014; Qin, 2015; Xia et al., 2016; Fan et al., 2017; Li X. F. et al., 2017; Peng et al., 2017; Wu, 2017). A flow chart (Figure 1) illustrates our search process and study selection.
Figure 1.
Process of searching and screening studies.
Included studies
We included 19 studies in this review. All studies were conducted in China and published in full.
Participants
Total of 1877 participants with AMI were included. The average sample size of the trials was 99 participants (ranging from 60 to 190 participants per trial). The course of treatment fluctuated between 4–week and 2–year.
Interventions
Based on the conventional western medicine treatment, there were 12 studies (You et al., 2005, 2012; Chen et al., 2007; Zhang and Zhang, 2009; Huang, 2010; Liang et al., 2010; Liao et al., 2010; Tian et al., 2014; Qin, 2015; Wang Y. L. et al., 2016; Xia et al., 2016; Peng et al., 2017) for TXL vs. blank control. 6 studies (Shen, 2011; Tian and Li, 2011; Yang and Yu, 2011; Fan et al., 2017; Li X. F. et al., 2017; Wu, 2017) made a comparison between TXL plus western medicine and western medicine. One study (Chen et al., 2008) showed TXL versus placebo. Further details were given in Table 1.
Table 1.
The detailed information of included studies.
| Studies | Disease | Sample (T/C) | Age (T/C) | Interventions (T/C) | Duration | Outcomes |
|---|---|---|---|---|---|---|
| Fan et al., 2017 | STEMI+PCI | 95/95 | 32~73/34~74 | TXL (3 capsules/tid) + Trimetazidine (20 mg/tid) +CWMT/ Trimetazidine (20 mg/tid) + CWMT | 12-week | ⑦ ⑫ |
| Li X. F. et al., 2017 | STEMI+PCI | 50/50 | 70 ± 9/72 ± 8 | TXL (3 capsules/tid) + Trimetazidine (20 mg/tid) +CWMT/Trimetazidine (20 mg/tid) + CWMT | 12-week | ⑦ |
| Peng et al., 2017 | AMI+PCI | 85/85 | 61.3 ± 3.8/62.8 ± 3.5 | TXL (4 capsules/tid) + CWMT/ CWMT | 6-month | ①②③⑥ |
| Wu, 2017 | STEMI+PCI | 30/30 | 65.03 ± 8.81/63.23 ± 9.50 | TXL (4 capsules/tid) + Atorvastatin Calcium (10 mg/d) + CWMT/ Atorvastatin Calcium (10 mg/d) + CWMT | 4-week | ①②⑦⑧⑨⑩ ⑪ ⑫ ⑬ |
| Wang Y. L. et al., 2016 | AMI+PCI | 30/30 | 58 ± 7/58 ± 6 | TXL (4 capsules/tid) + CWMT/CWMT | 1-year | ⑦ |
| Xia et al., 2016 | AMI+PCI | 37/37 | 51.5 ± 13.5/51.5 ± 15.2 | TXL (4 capsules/tid) + CWMT/CWMT | 4-week | ② ⑫ |
| Qin, 2015 | STEMI+PCI | 50/50 | 56.5 ± 11.2/58.1 ± 11.4 | TXL (4 capsules/tid) + CWMT/CWMT | 60-day | ①②④⑤⑥⑦⑧⑨⑩ ⑪ |
| Tian et al., 2014 | AMI+PCI | 30/30 | 54.9 ± 10.4/54.5 ± 9.8 | TXL (4 capsules/tid) + CWMT/CWMT | 3-month | ①②③⑥⑦ ⑫ |
| You et al., 2012 | AMI | 45/46 | 46 ± 8.6/47 ± 5.2 | TXL (4 capsules/tid) + CWMT/CWMT | 8-week | ⑦ |
| Yang and Yu, 2011 | AMI | 42/34 | 62.48 ± 10.05/63.37 ± 9.27 | TXL (4 capsules/tid) + Metoprolol sustained release tablets (23.75–47.5 mg/d) + CWMT/Metoprolol tablets (6.26-12.5 mg/d) +CWMT | 4-week | ④ ⑬ |
| Tian and Li, 2011 | AMI+ thrombolysis | 51/51 | 57 ± 12/61 ± 11 | TXL (4 capsules/tid) + Urokinase + CWMT/Urokinase + CWMT | 4-week | ④⑦ ⑬ |
| Shen, 2011 | AMI+ thrombolysis | 51/47 | 62.87 ± 3.65/63.21 ± 3.92 | TXL (3 capsules/tid) + Simvastatin (80 mg/d) + CWMT/ Simvastatin (20 mg/d) + CWMT | 1-year | ①③ ⑪ ⑬ |
| Liao et al., 2010 | AMI | 39/37 | 60.3 ± 9.9/64.3 ±11.7 | TXL (3–4 capsules/tid) + CWMT/CWMT | 4-week | ⑦ ⑫ |
| Huang, 2010 | STEMI+PCI | 62/58 | 58.3 ± 12.6 | TXL (4 capsules/tid) + CWMT/CWMT | 6-month | ①②③⑧⑨ ⑫ ⑬ |
| Liang et al., 2010 | AMI+PCI | 42/38 | 40~70 | TXL (2 capsules/tid) + CWMT/CWMT | 6-month | ②⑥⑧⑨⑩ ⑪ |
| Zhang and Zhang, 2009 | AMI+PCI | 96/82 | 43~70 | TXL (3 capsules/tid) + CWMT/CWMT | 24-month | ①②③⑤⑥ ⑬ |
| Chen et al., 2008 | AMI | 35/35 | 68 ± 7/69 ± 7 | TXL (3 capsules/tid) + CWMT/Placebo (3 capsules/tid) + CWMT | 6-week | ⑦ |
| Chen et al., 2007 | AMI | 30/30 | 58 ± 11 | TXL (2-4 capsules/tid) + CWMT/CWMT | 8-week | ①②④⑤⑥⑦⑧⑨⑩ ⑪ ⑬ |
| You et al., 2005 | STEMI+PCI | 60/52 | Unclear | TXL (4 capsules/tid) + CWMT/CWMT | 6-month | ⑦ |
PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; CWMT, Conventional western medicine treatment; Tid, Three times a day; Qd, One time a day; ① Cardiac death; ② Recurrent myocardial infarction; ③ Revascularization; ④ Arrhythmia; ⑤ Heart failure; ⑥ Recurrent angina pectoris; ⑦ LVEF; ⑧ TC; ⑨ TG; ⑩ HDL-C; ⑪ LDL-C; ⑫ hs-CRP; ⑬ Adverse reactions.
Risk of bias assessment
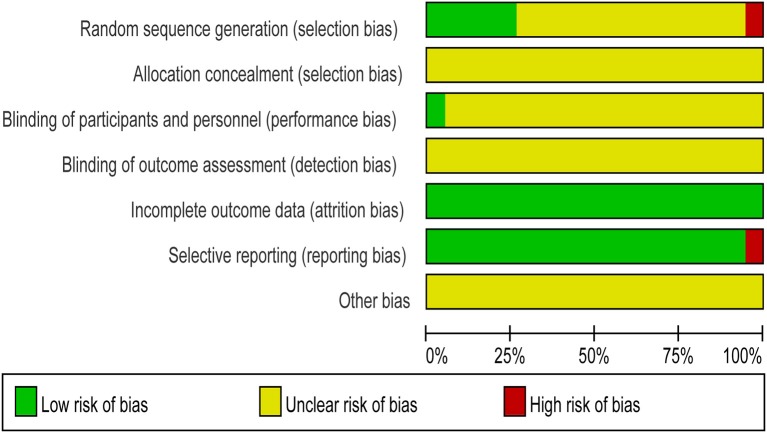
All the trials provided very limited information about design and methodology. Six studies (Chen et al., 2008; Zhang and Zhang, 2009; Tian et al., 2014; Qin, 2015; Xia et al., 2016; Wu, 2017) described the random sequence generation methods, among which 5 articles (Chen et al., 2008; Tian et al., 2014; Qin, 2015; Xia et al., 2016; Wu, 2017) showed the random number table, the other one used lottery (Zhang and Zhang, 2009). The rest included studies only mentioned random, did not reported it in detail. In addition, allocation concealment and blind were not very clear. Only one study used a double-blind method (Chen et al., 2008). None of the trials had a pretrial estimation of sample size, which indicated the lack of statistical power to ensure appropriate estimation of the therapeutic effect, as shown in Figure 2.
Figure 2.
Risk of bias graph.
Effects of interventions
We conducted a descriptive analysis of 3 studies. A study (Wang Y. L. et al., 2016) showed TXL could increase LVEF (P < 0.05), and the other 2 articles mainly reported the role of TXL on primary cardiovascular events. One study (Shen, 2011) exhibited TXL was beneficial to reduce the total cardiovascular events, the other (Zhang and Zhang, 2009) demonstrated TXL had no effects on cardiac death, non-fatal MI, HF, recurrent angina pectoris except revascularization. Then we did a meta-analysis of the remaining 16 studies with a short-term duration and assessed the clinical functions of TXL by primary/s outcomes.
Primary outcomes
Primary cardiovascular events
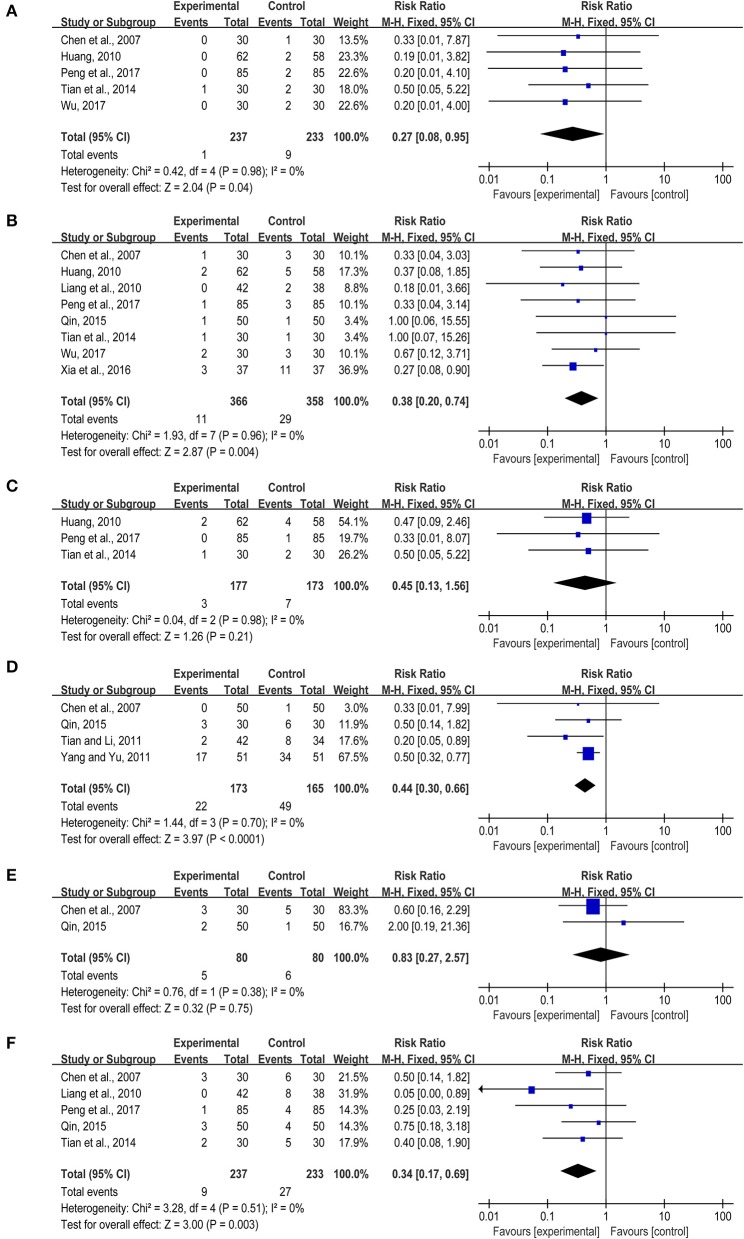
Based on the western conventional treatments, TXL alone or combined with other western medicine could lower the risk of cardiovascular events effectively. Six studies (Chen et al., 2007; Huang, 2010; Tian et al., 2014; Qin, 2015; Peng et al., 2017; Wu, 2017), including 470 patients, reported cardiac death. One of the researches (Qin, 2015) showed no death in either treatment group or control group, so we conducted the meta-analysis on the other 5 studies (Chen et al., 2007; Huang, 2010; Tian et al., 2014; Peng et al., 2017; Wu, 2017), indicating that TXL had better efficacy on lowering the cardiac death rate compared with the control group (RR = 0.27, 95% CI: 0.08~0.95, I2 = 0%) (Figure 3A). Eight articles (Chen et al., 2007; Huang, 2010; Liang et al., 2010; Tian et al., 2014; Qin, 2015; Xia et al., 2016; Peng et al., 2017; Wu, 2017) reported the recurrent myocardial infarction, 11 cases happened in treatment group while 29 cases occurred in control group, showing that TXL had the lower rate of reinfarction (RR = 0.38, 95%CI: 0.20~0.74, I2 = 0%) (Figure 3B). Three studies revealed the revascularization of patients with myocardial infarction (Huang, 2010; Tian et al., 2014; Peng et al., 2017), there was no evidence that the treatment group was better than the control group (RR = 0.45, 95%CI: 0.13~1.56, I2 = 0%) (Figure 3C). Besides, TXL presented the less cardiac arrhythmia (RR = 0.44, 95%CI: 0.30~0.66, I2 = 0%) (Chen et al., 2007; Tian and Li, 2011; Yang and Yu, 2011; Qin, 2015) (Figure 3D). Eleven patients suffered from heart failure in 2 studies (Chen et al., 2007; Qin, 2015), the number of treatment group and control group was 5 and 6, respectively. There was no statistical significance in heart failure (RR = 0.83, 95%CI: 0.27~2.57, I2 = 0%) (Figure 3E). In addition, 5 articles (Chen et al., 2007; Liang et al., 2010; Tian et al., 2014; Qin, 2015; Peng et al., 2017) reported recurrent angina pectoris, involving in 470 participants, indicating TXL could markedly lower relapse rate than the control group (RR = 0.34, 95%CI: 0.17~0.69, I2 = 0%) (Figure 3F).
Figure 3.
(A–F) Primary cardiovascular events.
Heart function
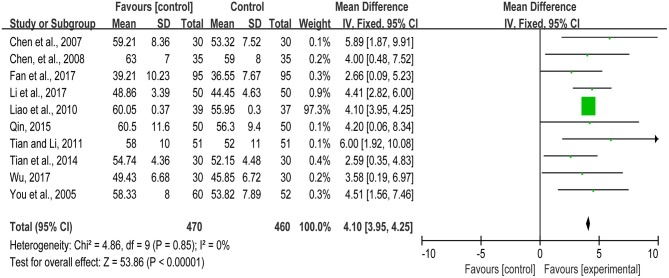
AMI is the most severe manifestation of coronary artery disease, which has a negative effect on the myocardial function and blood supply. LVEF is closely related to the myocardial contractility, reflecting myocardial systolic function and bloodstream of the coronary arteries. Eleven studies (You et al., 2005, 2012; Chen et al., 2007, 2008; Liao et al., 2010; Tian and Li, 2011; Tian et al., 2014; Qin, 2015; Fan et al., 2017; Li X. F. et al., 2017; Wu, 2017) reported the LVEF of the patients with myocardial infarction. While there was one study (You et al., 2012) detecting LVEF by radionuclide ventriculography, others used the doppler echocardiography. Thus, the meta-analysis we conducted from the remaining ten studies. The meta-analysis showed that TXL treatment group could increase the LVEF significantly and improve heart function (MD = 4.10, 95%CI: 3.95~4.25, I2 = 0%) (Figure 4).
Figure 4.
The LVEF of included studies.
Secondary outcomes
The blood lipid levels
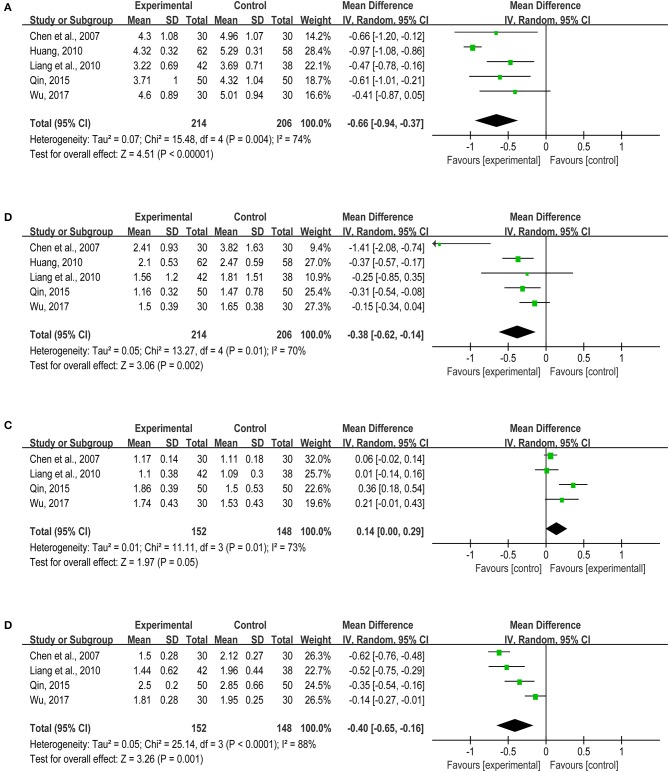
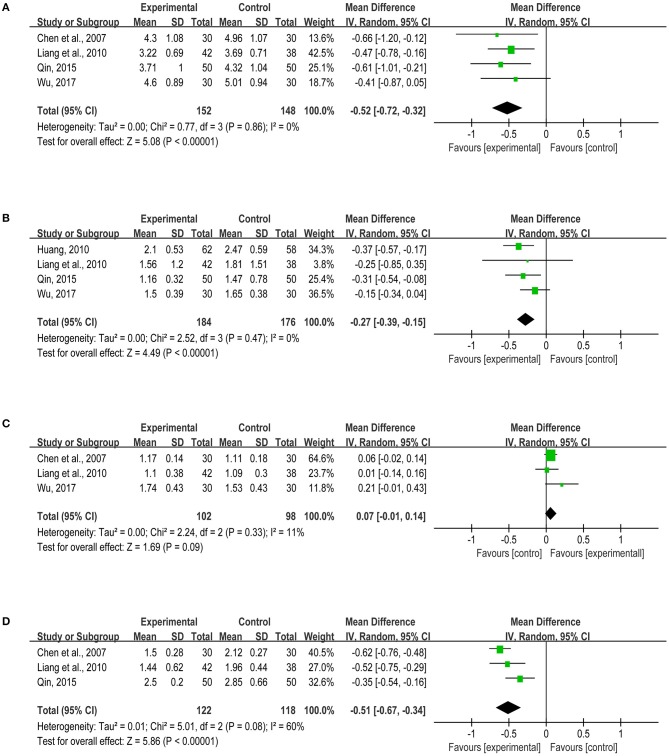
Atherosclerosis is the main pathogenesis of myocardial infarction in coronary heart disease, which is affected by many factors, such as lipid metabolism disorder. Current researches indicated that dyslipidemia is one of the risk factors causing atherosclerosis, myocardial infarction and other vascular diseases (Cheng et al., 2015; Górski et al., 2016). On the basis of statin therapy in both treatment and control groups, the included studies indicated that TXL could effectively regulate lipid metabolism. Five articles (Chen et al., 2007; Huang, 2010; Liang et al., 2010; Qin, 2015; Wu, 2017) reported TC and TG, including 214 patients in the treatment group and 206 sufferers in the control group. TXL could remarkably reduce the level of TC (MD = −0.66, 95%CI: −0.94 ~ −0.37, I2 = 74%) and TG (MD = −0.38, 95%CI: −0.62 ~ −0.14, I2 = 70%) (Figures 5A,B). Due to the large heterogeneity, the sensitivity analysis of TC was performed. When we removed one literature (Huang, 2010), the result did not change significantly (MD = −0.52, 95%CI: −0.72 ~ −0.32, I2 = 0%) (Figure 6A). The sensitivity analysis of TG presented the similar outcome after eliminating one study (Chen et al., 2007) (MD = −0.27, 95%CI: −0.39~-0.15, I2 = 0%) (Figure 6B). A low level of plasma HDL-C is a strong and independent risk factor for atherosclerotic cardiovascular disease. There was no statistical significance in HDL-C from four studies (Chen et al., 2007; Liang et al., 2010; Qin, 2015; Wu, 2017) (MD = 0.14, 95%CI: 0.00 ~0.29, I2 = 73%) (Figure 5C), and such difference between treatment group and control group was unchanged through sensitivity analysis when we removed one study (Qin, 2015) (MD = 0.07, 95%CI: −0.01~0.14, I2 = 11%) (Figure 6C). Moreover, according to the extracted data from four articles (Chen et al., 2007; Liang et al., 2010; Qin, 2015; Wu, 2017), TXL enabled to lower LDL-C significantly (MD = −0.40, 95%CI: −0.65 ~ −0.16, I2 = 88%) (Figure 5D). The sensitivity analysis of LDL-C presented the similar result after eliminating one study (Wu, 2017) (MD = −0.51, 95%CI: −0.67 ~ −0.34, I2 = 60%) (Figure 6D).
Figure 5.
(A–D) The blood lipid level.
Figure 6.
(A–D) The blood lipid level (sensitivity analysis).
The inflammation reaction
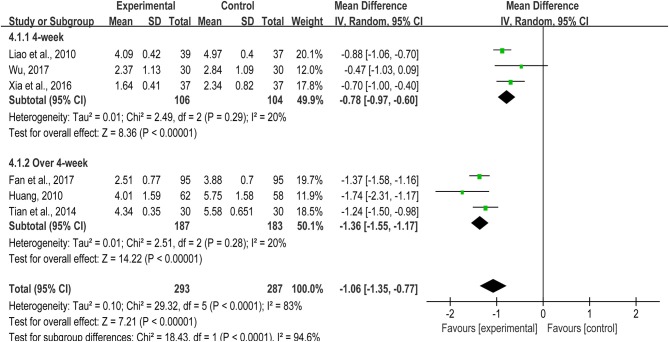
Inflammation reaction is one of the key links of AMI progression. The necrosis of numerous cardiomyocytes in the infarct tissue will lead to a sharp inflammation. The rapid accumulating of massive leukocytes and monocytes accompanied by the release of a great number of inflammatory factors, leading to further aggravate myocardial injury (Stirrat et al., 2017). Hs-CRP, one kind of C-reactive protein in plasma, is a non-specific marker of systemic inflammatory response in acute phase, could predict the risk of cardiovascular events. Six studies (Huang, 2010; Liao et al., 2010; Tian et al., 2014; Xia et al., 2016; Fan et al., 2017; Wu, 2017) revealed hs-CRP involving in 293/287 patients in both groups. Due to high heterogeneity, we carried out a subgroup analysis based on different treatment courses. When the intervention time was 4 weeks, TXL treatment group could effectively reduce the hs-CRP level (MD = −0.78, 95%CI: −0.97 ~ −0.06, I2 = 20%), and this therapeutic effect would get better along with the prolonging of treatment period (MD = −1.36, 95%CI: −1.55 ~ −1.17, I2 = 20%) (Figure 7).
Figure 7.
The hs-CRP of included studies.
Adverse reaction
Seven studies (Chen et al., 2007; Zhang and Zhang, 2009; Huang, 2010; Shen, 2011; Tian and Li, 2011; Yang and Yu, 2011; Wu, 2017), involving in 362/332 patients in two groups, have reported the adverse events of the TXL. Adverse drug reactions mainly included the gastrointestinal discomfort, transaminase elevation, stroke, pulmonary infection and so forth. Compared with the control group, the total risk rate of adverse events in the treatment group was relatively high (6.91% versus 4.22%), and the gastrointestinal discomfort accounted for the main proportion, as shown in Table 2.
Table 2.
The adverse reaction of seven included studies.
| Adverse events | Treatment group (362) | Control group (332) |
|---|---|---|
| Gastrointestinal discomfort | 16 | 3 |
| Transaminase elevation | 6 | 5 |
| Stroke | 0 | 1 |
| Hypotension | 1 | 1 |
| Pulmonary infection | 0 | 1 |
| Itch of skin | 2 | 2 |
| Allergic reaction | 0 | 1 |
| Total risk rate (%) | 6.91 | 4.22 |
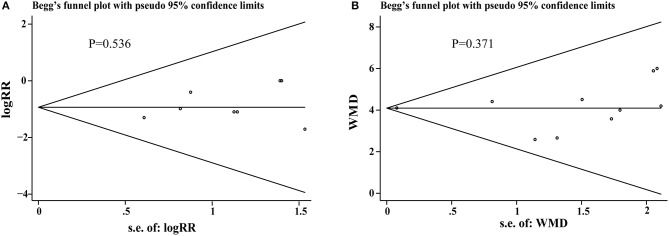
Publication biases
We proceed the publication bias (Recurrent myocardial reinfarction and LVEF) to evaluate the quality of our meta-analysis by funnel plot and Begg' test using State 14 software. Funnel chart analysis showed there were no obvious publication biases and P-values were 0.536, 0.371, respectively, as shown in Figures 8A,B.
Figure 8.
(A,B) The publication bias.
Grade
The quality of evidence was evaluated by ML and XYZ with GRADE approach, and most of cardiac events were considered as the moderate score. Some outcomes exhibited the low or very low evidence due to the large heterogeneity and/or the wide confidence interval (Table 3).
Table 3.
GRADE quality of evidence summary table.
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Control group | Treatment group | ||||
| Cardiac death | Study population | RR 0.27 (0.08 to 0.95) | 470 (5 studies) | ⊕⊕⊕⊖ moderatea | |
| 39 per 1000 | 10 per 1000 (3–37) | ||||
| Moderate | |||||
| 35 per 1000 | 9 per 1000 (3–33) | ||||
| Myocardial reinfarction | Study population | RR 0.38 (0.2–0.74) | 724 (8 studies) | ⊕⊕⊕⊖ moderatea | |
| 81 per 1000 | 31 per 1000 (16–60) | ||||
| Moderate | |||||
| 69 per 1000 | 26 per 1000 (14–51) | ||||
| Revascularization | Study population | RR 0.45 (0.13–1.56) | 350 (3 studies) | ⊕⊕⊕⊖ moderatea | |
| 40 per 1000 | 18 per 1000 (5–63) | ||||
| Moderate | |||||
| 67 per 1000 | 30 per 1000 (9–105) | ||||
| Arrhythmia | Study population | RR 0.44 (0.3–0.66) | 338 (4 studies) | ⊕⊕⊕⊖ moderatea | |
| 297 per 1000 | 131 per 1000 (89–196) | ||||
| Moderate | |||||
| 218 per 1000 | 96 per 1000 (65–144) | ||||
| Heart failure | Study population | RR 0.83 (0.27–2.57) | 160 (2 studies) | ⊕⊕⊖⊖ lowa,b | |
| 75 per 1000 | 62 per 1000 (20–193) | ||||
| Moderate | |||||
| 93 per 1000 | 77 per 1000 (25–239) | ||||
| Recurrent angina pectoris | Study population | RR 0.34 (0.17–0.69) | 470 (5 studies) | ⊕⊕⊕⊖ moderatea | |
| 116 per 1000 | 39 per 1000 (20–80) | ||||
| Moderate | |||||
| 167 per 1000 | 57 per 1000 (28–115) | ||||
| LVEF | The mean lvef in the intervention groups was 4.1 higher (3.95–4.25 higher) | 930 (10 studies) | ⊕⊕⊕⊖ moderatea | ||
| TC | The mean tc in the intervention groups was 0.66 lower (0.94–0.37 lower) | 420 (5 studies) | ⊕⊕⊖⊖ lowa,c | ||
| TG | The mean tg in the intervention groups was 0.38 lower (0.62–0.14 lower) | 420 (5 studies) | ⊕⊕⊖⊖ lowa,d | ||
| HDL | The mean hdl in the intervention groups was 0.14 higher (0–0.29 higher) | 300 (4 studies) | ⊕⊖⊖⊖ very lowa,b,e | ||
| LDL | The mean ldl in the intervention groups was 0.4 lower (0.65–0.16 lower) | 300 (4 studies) | ⊕⊕⊕⊖ moderatea,f | ||
| hs-CRP | The mean hs-crp in the intervention groups was 1.06 lower (1.35–0.77 lower) | 580 (6 studies) | ⊕⊖⊖⊖ very lowa,g | ||
The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI, Confidence interval; RR, Risk ratio.
GRADE Working Group grades of evidence.
High quality, Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality, Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality, Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality, We are very uncertain about the estimate.
The random and blind of some studies were not clear.
The confidence interval was wide.
I2 of 74% showed the inconsistence among studies.
I2 of 70% showed the inconsistence among studies.
I2 of 73% showed the inconsistence among studies.
I2 of 88% showed the inconsistence among studies.
I2 of 83% showed the inconsistence among studies.
Discussion
In this meta-analysis from 16 studies of TXL, we observed the results as following: There was significant difference of TXL in preventing restenosis and recurrence of cardiovascular events (cardiac death, myocardial reinfarction, arrhythmia, recurrent angina pectoris). TXL enabled to improve cardiac function (LVEF), regulate lipid metabolism (TC, TG, LDL), and inhibit inflammation reaction (hs-CRP). However, TXL has little effects on revascularization, recurrent heart failure, and HDL. Moreover, TXL treatment group was more prone to suffer gastrointestinal discomfort.
Effectiveness of TXL
AMI is a common cardiovascular disease, remaining a leading cause of morbidity and mortality worldwide. As we all know, AMI is caused by atherosclerotic coronary artery stenosis, plaque rupture and thrombosis appear, engendering insufficient blood supply of coronary, further resulting in myocardial ischemia or even necrosis. If AMI does not promptly recanalize the infarct-related coronary arteries after the onset of the disease, many patients eventually die of ventricular arrhythmias and heart failure. Despite substantial improvements in prognosis over the past decade, the progress is a result of several major trends, including improvements in risk stratification, more widespread use of an invasive strategy and medication (Kvakkestad et al., 2016). However, mortality and recurrence rate of the adverse cardiac events remain high after the acute phase of MI which will seriously affect the patients' quality of life and prognosis. Therefore, the second prevention of adverse cardiac events after MI is very important. Coronary artery in patients with MI has extensive lesions and multiple “vulnerable” plaques. While interventional therapy is often focusing on some single serious plaques, it is vital for many drugs to widely protect cardiovascular system through inhibiting vascular inflammation, improving myocardial energy metabolism, reducing myocardial remodeling, regulating blood lipids and so forth. Compared with the control group, TXL alone or combined with conventional western medicine exhibited a specific advantage in the secondary prevention of AMI, likely due to the comprehensive protection of blood vessels and myocardium. Many clinical trials and experimental evidence have just proved it.
Endothelial protective effect
Dysfunction of endothelial cells is directly associated with impaired vasorelaxation, increased inflammation, and increased migration and proliferation of smooth muscle cells, is the initiating factor in CVDs (Gutiérrez et al., 2013). And oxidized low-density lipoprotein- (ox-LDL-) induced dysfunction of the vascular endothelium is one of the key factors in the pathogenesis of many CVDs (Wang et al., 2009). It has been demonstrated that TXL alleviated ox-LDL-induced hyperpermeability via the activation of ERK1/2 in the vascular endothelial cell (Chang et al., 2017). Vascular injury after chronic hypoxia usually leads to endothelial injury and structural damage to tight junctions, TXL could promote endothelial cell proliferation and reverse the expression of tight junctions' proteins in vivo and in vitro (Zheng et al., 2015). Additionally, we found that TXL could regulate gene expression in hypoxia-treated human cardiac microvascular endothelial cells (CMECs) (Li Y. N. et al., 2015) and attenuate human CMEC injury via inhibiting peroxynitrite, which contributed to macrophage-mediated human CMEC injury (Wang et al., 2015).
Protection against ischemia-reperfusion (I/R) injury
When AMI occurs, reperfusion of the ischemic myocardium is a key approach for limiting infarct size. However, reperfusion itself paradoxically leads to further cardiovascular complications, which is called myocardial I/R injury. Nowadays, no effective therapy is available to protect the heart from this I/R injury (Eltzschig and Eckle, 2011). Previous studies have demonstrated that TXL enabled to effectively protect hearts against no-reflow and reperfusion injury in a protein kinase A-dependent manner (Li et al., 2010, 2013) and ameliorate myocardial I/R injury by promoting cardiac microvascular endothelial barrier function (Qi et al., 2015). Besides, as for cerebral I/R injury, TXL could reduce cell death via Cx43/Calpain II/Bax/Caspase-3 pathway (Cheng et al., 2017).
Anti-inflammation
The immune system and inflammation, through several cellular and soluble inflammatory mediators, play a great role in the local tissue structural changes of AMI (Swirski and Nahrendorf, 2013). TXL was able to decrease serum contents of P-selectin, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and pro-inflammatory cytokines (interleukin 6, interleukin 10), regulate anti-inflammatory factor levels in the early reperfusion of AMI (Zhang H. T. et al., 2009). As the above result showed, TXL treatment group could effectively reduce the hs-CRP level and this therapeutic effect would get better along with the prolonging of treatment period. Moreover, TXL exerted its vascular protective effects by inhibiting the vascular inflammatory response and suppressing the gene expression of miR-155 (Zhang et al., 2014).
Regulation of lipid metabolism
The pathogenesis of AMI has close links with diabolism of lipids. The rise of blood fat will easily lead to the occurrence and development of vulnerable plaques, further resulting in AMI. The clinical trials indicated that TXL could significantly reduce the level of TC, TG, and LDL. Relevant experimental study reported that TXL enabled to enhance the stability of vulnerable plaques and prevent plaques from rupture in a dose-dependent manner. In addition, high-dose TXL group showed a low serum level of low-density lipoprotein cholesterol and ox-LDL. Besides, TXL was effective for decreasing serum lipid levels and inhibiting plaque inflammation in the balloon-induced abdominal aortic endothelial injury rabbits model (Chen et al., 2009).
Others
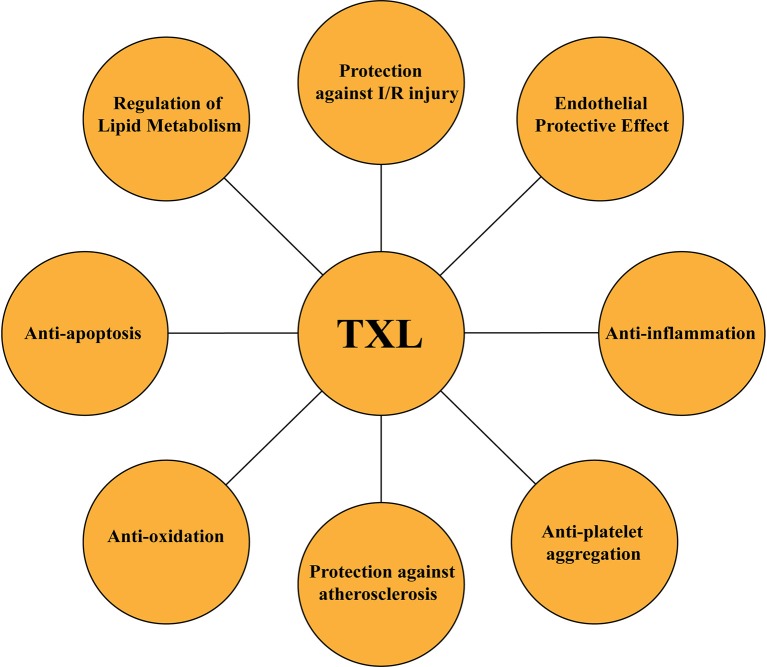
A large number of studies have reported that TXL presented various regulatory influences on CVDs. The cardioprotective benefits of TXL against MI were closely related to the inhibition of apoptosis and promotion of autophagy in rat hearts after AMI by activating AMPK signaling pathway (Li Q. et al., 2017). TXL also exhibited anti-platelet aggregation effects (Chen et al., 2016). TXL may probably exhibit a specific advantage in the prevention and treatment of myocardial fibrosis via mediating the expressions of TGF-beta1, Smad3, and Smad7 in diabetic rats (Wang X. et al., 2016). Furthermore, TXL could inhibit OX-LDL-induced maturation of dendritic cells by regulating PPAR gamma pathway (Su et al., 2010). So, in summary, TXL exerted its comprehensive protective effects on myocardium and blood vessels not only in clinical trials but also in basic experiments (Figure 9).
Figure 9.
Summary of pharmacological functions of TXL.
Nevertheless, there was no statistical difference between TXL treatment group and control group in some fields, such as revascularization, recurrent heart failure and HDL. The reasons may include the following two aspects. Firstly, TXL, a compound preparation consisting of many kinds of herbs and insects, presented limited therapeutic effects on revascularization, recurrent heart failure and HDL from the current literature.
Secondly, and most importantly, the intervention time of included studies to conduct meta-analysis was less than one year, it may be difficult to observe the occurrence of revascularization and heart failure as well as the increase of HDL during the short period. Thus, long-term studies need to be further implemented.
The safety of TXL
Seven studies reported adverse events in this review. Compared with the control group, the total risk rate of adverse events in the treatment group was relatively high, and the gastrointestinal discomfort accounted for the major proportion. We observed the reasons as following: ①TXL contains many animal medicines, such as Leech (Hirudo, Shui Zhi), Scorpion (Scorpio, Quan Xie), Centipede (Scolopendra, Wu Gong), and other insect drugs, which could dredge the channel and promote blood circulation, while on the other hand, this kind of function maybe partly cause stomach irritation. ② There were a few articles reporting adverse drug reactions which was not able to comprehensively reflect the safety of TXL. ③ Many studies did not show adverse events of control group. Additionally, there was not statistical analysis of untoward effects in original literature, all of such reasons contributed to the large adverse events in the experimental group.
Study limitations
With the limitation of small sample size, short-term duration and poor quality of methodology (unclear of random and blind methods), more large-scale perspective randomized double-blind long-term RCTs are needed to confirm the efficacy and safety of TXL in treating AMI. Furthermore, the included patients in our systematic review were all from China, so it is difficult to define the influence of race and region. Besides, there were not enough studies involving in dose-effect relationship of TXL.
Clinical practice and further research of this review
Given the available evidence by our work, the results of this review suggested that TXL alone or combined with conventional western medicine exhibited a specific advantage in the secondary prevention of AMI, especially in the decrease of primary cardiac events. Clinical practice could consider adding oral TXL to routine pharmacotherapy management, it may improve the living quality of the patients and clinical efficacy. Taking TXL for example, it also proved that traditional Chinese medicine, a kind of complementary and alternative medicine, has its unique advantages in the treatment of some diseases. Besides, some key points need to pay more attention. Firstly, lack of detailed description of randomization and blinding was the major methodological flaw of most included studies in this review, thus, clinical experts and statistical professors should have a better cooperation to design the high-quality clinical trials and make the blinding of participants and outcome assessors. Secondly, due to the limited evidence of safety of TXL, more research is worth further exploring. In addition, the researchers should conduct more trials in various countries to explore the influence of different regions and races. More importantly, the biological mechanism of TXL and its active compounds need to further explore, which will benefit to providing more evidence for clinical treatment in CVDs.
Conclusion
Chinese patent medicine TXL seemed beneficial for secondary prevention after AMI. This potential benefit needs to be further assessed through more rigorous RCTs. Furthermore, TXL may be safe for treating AMI due to the milder adverse events in clinic.
Author contributions
HS and GT provided guidelines for this systematic review and meta-analysis. ML, CL, SC, and YS wrote the main manuscript and prepared the Figures 3–7, 9 and Table 2. QZ and JH conducted the literature searching, study selection and data extraction. JH provided Table 1 and Figure 1. ML and RQ assessed the risk of bias and drew Figure 2. ML and XZ assessed the GRADE and made Table 3. CZ conducted the publication bias and prepared Figure 8.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JC declared a shared affiliation, though no other collaboration, with the authors to the handling Editor.
Footnotes
Funding. The present work was supported by grants from the National Natural Science Foundation of China (No. 81430098).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00830/full#supplementary-material
References
- Andrés E., Cordero A., Magán P., Alegría E., León M., Luengo E., et al. (2012). Long-term mortality and hospital readmission after acute myocardial infarction: an eight-year follow-up study. Rev. Esp. Cardiol. 65, 414–420. 10.1016/j.recesp.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Bai W. W., Xing Y. F., Wang B., Lu X. T., Wang Y. B., Sun Y. Y., et al. (2013). Tongxinluo improves cardiac function and ameliorates ventricular remodeling in mice model of myocardial infarction through enhancing angiogenesis. Evid. Based Complement Alternat. Med. 2013:813247 10.1155/2013/813247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauters C., Lemesle G., Meurice T., Tricot O., de Groote P., Lamblin N. (2014). Prognostic impact of β-blocker use in patients with stable coronary artery disease. Heart 100, 1757–1761. 10.1136/heartjnl-2014-305719 [DOI] [PubMed] [Google Scholar]
- Begg C. B., Mazumdar M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- Benjamin E. J., Blaha M. J., Chiuve S. E., Cushman M., Das S. R., Deo R., et al. (2017). Heart disease and stroke statistics-2017 update: a report from the american heart association. Circulation 135, e146–e603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carville S. F., Henderson R., Gray H. (2015). The acute management of ST-segment-elevation myocardial infarction. Clin. Med. 15, 362–367. 10.7861/clinmedicine.15-4-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Liu H., Wei C., Chang L., Liang J., Bei H., et al. (2017). Tongxinluo regulates expression of tight junction proteins and alleviates endothelial cell monolayer hyperpermeability via ERK-1/2 signaling pathway in oxidized low-density lipoprotein-induced human umbilical vein endothelial cells. Evid. Based Complement Alternat. Med. 2017:4198486 10.1155/2017/4198486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Cai S. H., Hong C. Z., Liu X. N., Wang Z. H. (2007). Clinical effects of Tongxinluo capsule on patients of acute myocardial infarction. J. Emerg. Trad. Chin. Med. 16, 823–824. 10.3969/j.issn.1004-745X.2007.07.032 [DOI] [Google Scholar]
- Chen W. Q., Zhong L., Zhang L., Ji X. P., Zhao Y. X., Zhang C., et al. (2009). Chinese medicine tongxinluo significantly lowers serum lipid levels and stabilizes vulnerable plaques in a rabbit model. J. Ethnopharmacol. 124, 103–110. 10.1016/j.jep.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Chen W., Sun X., Wang W. J., Fu X. D., Fu D. Y., Zhou J. M., et al. (2008). Effects of Tongxinluo capsules on cardiac ventricle remodeling after myocardial infarction: a multicenter clinical research. Natl. Med. J. China 88, 2271–2273. 10.3321/j.issn:0376-2491.2008.32.010 [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Chu C. S., Lin T. H., Lee K. T., Sheu S. H., Lai W. T. (2015). Lipid paradox in acute myocardial infarction-the association with 30-day in-hospital mortality. Crit. Care Med. 43, 1255–1264. 10.1097/CCM.0000000000000946 [DOI] [PubMed] [Google Scholar]
- Cheng X., Hou Z., Sun J., Huang Y., Wang L., Zhou Z., et al. (2017). Protective effects of Tongxinluo on cerebral ischemia/reperfusion injury related to Connexin 43/Calpain II/Bax/Caspase-3 pathway in rat. J. Ethnopharmacol. 2017, 148–157. 10.1016/j.jep.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Chen Z. Q., Hong L., Wang H., Yin Q. L. (2016). Effects of tongxinluo capsule on platelet activating factor, vascular endothelial function, flow of thrombosis in acute myocardial infarction patients after delayed percutaneous coronary intervention. Chin. J. Integr. Trad. West Med. 36, 415–420. 10.7661/CJIM.2016.04.0415 [DOI] [PubMed] [Google Scholar]
- Eltzschig H. K., Eckle T. (2011). Ischemia and reperfusion–from mechanism to translation. Nat. Med. 17, 1391–1401. 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q. X., Zhao J. X., Zhang H. X., Li B., Li Z. R. (2017). Effect of Tongxinluo combined with Trimetazidine on cardiac function after percutaneous coronary intervention in acute ST-elevation myocardial infarction. J. Hainan Med. Univ. 23, 1801–1804. 10.13210/j.cnki.jhmu.20170628.004 [DOI] [Google Scholar]
- García-García C., Sanz G., Valle V., Molina L., Sala J., Subirana I., et al. (2010). Trends in in-hospital mortality and six-month outcomes in patients with a first acute myocardial infarction. change over the last decade. Rev. Esp. Cardiol. 63, 1136–1144. 10.1016/S0300-8932(10)70245-1 [DOI] [PubMed] [Google Scholar]
- Górski B., Nargiełło E., Opolski G., Ganowicz E., Górska R. (2016). The association between dental status and systemic lipid profile and inflammatory mediators in patients after myocardial infarction. Adv. Clin. Exp. Med. 25, 625–630. 10.17219/acem/62937 [DOI] [PubMed] [Google Scholar]
- Gutiérrez E., Flammer A. J., Lerman L. O., Elízaga J., Lerman A., Fernández-Avilés F. (2013). Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 34, 3175–3181. 10.1093/eurheartj/eht351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S. (eds.). (2011). Cochrane Handbook for Systematic Reviews of Interventions. Available online at: http://handbook-5-1.cochrane.org/
- Huang B. (2010). Effects of Tongxinluo capsule on inflammatory response and vascular endothelial function in patients with acute myocardial infarction after intervention. J. New Chin. Med. 42, 22–24. 10.13457/j.cnki.jncm.2010.09.081 [DOI] [Google Scholar]
- Kvakkestad K. M., Abdelnoor M., Claussen P. A., Eritsland J., Fossum E., Halvorsen S. (2016). Long-term survival in octogenarians and older patients with ST-elevation myocardial infarction in the era of primary angioplasty: a prospective cohort study. Eur. Heart J. Acute Cardiovasc. Care 5, 243–252. 10.1177/2048872615574706 [DOI] [PubMed] [Google Scholar]
- Li J., Li X., Wang Q., Hu S., Wang Y., Masoudi F. A., et al. (2015). ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 385, 441–451. 10.1016/S0140-6736(14)60921-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li N., Cui H. H., Tian X. Q., Jin C., Chen G. H., et al. (2017). Tongxinluo exerts protective effects via anti-apoptotic and pro-autophagic mechanisms by activating AMPK pathway in infarcted rat hearts. Exp. Physiol. 102, 422–435. 10.1113/EP086192 [DOI] [PubMed] [Google Scholar]
- Li X. D., Yang Y. J., Cheng Y. T., Dou K. F., Tian Y., Meng X. M. (2013). Protein kinase A-mediated cardioprotection of Tongxinluo relates to the inhibition of myocardial inflammation, apoptosis, and edema in reperfused swine hearts. Chin. Med. J. 126, 1469–1479. 10.3760/cma.j.issn.0366-6999.20130224 [DOI] [PubMed] [Google Scholar]
- Li X. D., Yang Y. J., Geng Y. J., Jin C., Hu F. H., Zhao J. L., et al. (2010). Tongxinluo reduces myocardial no-reflow and ischemia-reperfusion injury by stimulating the phosphorylation of eNOS via the PKA pathway. Am. J. Physiol. Heart Circ. Physiol. 299, H1255–H1261. 10.1152/ajpheart.00459.2010 [DOI] [PubMed] [Google Scholar]
- Li X. F., Lu L., Paerhati T. (2017). Application effect of tongxinluo capsule combines with trimetazidine in postoperative ASETMI patients treated by PCI. Prac. J. Cardiac. Cereb. Pneumal Vasc. Dis. 25, 150–152. 10.3969/j.issn.1008-5971.2017.08.042 [DOI] [Google Scholar]
- Li Y. N., Wang X. J., Li B., Liu K., Qi J. S., Liu B. H., et al. (2015). Tongxinluo inhibits cyclooxygenase-2, inducible nitric oxide synthase, hypoxia-inducible factor-2a/vascular endothelial growth factor to antagonize injury in hypoxia-stimulated cardiac microvascular endothelial cells. Chin. Med. J. 128, 1114–1120. 10.4103/0366-6999.155119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. M., Wang Z. J., Su X. Y. (2010). The effect of Tongxinluo capsules on coronary restenosis of patients with acute myocardial infarction after percutaneoas coronary intervention. Chin. J. Integr. Trad. Western Med. Intens. Critic. Care 17, 175–176. 10.3969/j.issn.1008-9691.2010.03.017 [DOI] [Google Scholar]
- Liao C. L., Liang D., Pan G. Z., Huang M. Z., Huang H. H. (2010). Effects of Tongxinluo Capsule on CRP and ventricular remodeling in patients with acute myocardial infarction. Clin. J. Trad. Chin. Med. 22, 304–305. 10.16448/j.cjtcm.2010.04.050 [DOI] [Google Scholar]
- Ma Q., Zhang S., Ning Y., Pu X., Yu G., Zheng Z., et al. (2009). Effect of Tongxinluo on endothelial function and hypersensitive C-reactive protein in acute coronary syndrome patients undergoing percutaneous coronary intervention. J. Central South Univ. 34, 550–554. 10.3321/j.issn:1672-7347.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Nichols M., Townsend N., Scarborough P., Rayner M. (2014). Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J. 35, 2959–2959. 10.1093/eurheartj/ehu299 [DOI] [PubMed] [Google Scholar]
- Pharmacopoeia (2015). The Pharmacopoeia of the People's Republic of China. Beijing: China Medical Science Press. [Google Scholar]
- Peng Z. P., Yu Y., Zhu W. W. (2017). Effect of Tongxinluo capsule on serum EMPs and MMP-9 after PCI in patients with acute myocardial infarction. Mod. J. Integr. Trad. Chin. Western Med. 26, 3117–3119. 10.3969/j.issn.1008-8849.2017.28.012 [DOI] [Google Scholar]
- Perk J., De Backer G., Gohlke H., Graham I., Reiner Z., Verschuren W. M., et al. (2013). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). the fifth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). G Ital. Cardiol. 14, 328–392. 10.1714/1264.13964 [DOI] [PubMed] [Google Scholar]
- Petersen C. B., Grønbæk M., Helge J. W., Thygesen L. C., Schnohr P., Tolstrup J. S. (2012). Changes in physical activity in leisure time and the risk of myocardial infarction, ischemic heart disease, and all-cause mortality. Eur. J. Epidemiol. 27, 91–99. 10.1007/s10654-012-9656-z [DOI] [PubMed] [Google Scholar]
- Piepoli M. F., Hoes A. W., Brotons C., Hobbs R. F. D., Corra U. (2018). Main messages for primary care from the 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Gen. Pract. 24, 51–56. 10.1080/13814788.2017.1398320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi K., Li L., Li X., Zhao J., Wang Y., You S., et al. (2015). Cardiac microvascular barrier function mediates the protection of Tongxinluo against myocardial ischemia/reperfusion injury. PLoS ONE 10:e0119846. 10.1371/journal.pone.0119846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G. W. (2015). Master's thesis: Valuate the Effect of Tongxinluo Capsule on Patients With Acute Myocardial Infarction and Percutaneous Coronary Intervention Using QRS Score and Ultrasonic Cardiogram. Guangxi University of Chinese Medicine. [Google Scholar]
- Schunemann H., Brozek J., Guyatt G., Oxman A. (eds.). (2013). GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. The GRADE Working Group. Available online at: https://gdt.gradepro.org/app/handbook/handbook.html
- Shen J. J. (2011). Clinical effects of simvastatin combined with Tongxinluo Capsule on patients with acute myocardial infarction. Chin. J. Clin. Rat. Drug Use 4, 36–37. 10.3969/j.issn.1674-3296.2011.13.023 [DOI] [Google Scholar]
- Stirrat C. G., Alam S. R., MacGillivray T. J., Gray C. D., Dweck M. R., Raftis J., et al. (2017). Ferumoxytol-enhanced magnetic resonance imaging assessing inflammation after myocardial infarction. Heart 103, 1528–1535. 10.1136/heartjnl-2016-311018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W., Sun A., Xu D., Zhang H., Yang L., Yuan L., et al. (2010). Tongxinluo inhibits oxidized low-density lipoprotein-induced maturation of human dendritic cells via activating peroxisome proliferator-activated receptor gamma pathway. J. Cardiovasc. Pharmacol. 56, 177–183. 10.1097/FJC.0b013e3181e5f0f8 [DOI] [PubMed] [Google Scholar]
- Swirski F. K., Nahrendorf M. (2013). Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166. 10.1126/science.1230719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachjian A., Maria V., Jahangir A. (2010). Use of herbal products and potential interactions in patients with cardiovascular diseases. J. Am. Coll. Cardiol. 55, 515–525. 10.1016/j.jacc.2009.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen K., Alpert J. S., Jaffe A. S., Simoons M. L., Chaitman B. R., White H. D., et al. (2012). Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 60, 1581–1598. 10.1016/j.jacc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Tian C. M., Li S. X. (2011). Protective effect of Tongxinluo on myocardium in patients with acute myocardial infarction undergoing thrombolytic therapy. Chin. J. Prac. Med. 38, 82–84. 10.3760/cma.j.issn.1674-4756.2011.01.037 [DOI] [Google Scholar]
- Tian Z. T., Li H. L., Li K. (2014). Tongxinluo Capsule on Acute Myocardial Infarction after Percutaneous Coronary Artery Interventional Therapy after Operation in 30 Cases. Chin. J. Exp. Trad. Med. Formul. 20, 196–200. [Google Scholar]
- Wang H. J., Huang Y. W., Sun J. (2003). Effect of tongxinluo capsule on function of vascular endothelium in patients with unstable angina pectoris. Chin. J. Integr. Trad. Western Med. 23, 587–589. 10.3321/j.issn:1003-5370.2003.08.009 [DOI] [PubMed] [Google Scholar]
- Wang J. J., Zhang C. N., Meng Y., Han A. Z., Gong J. B., Li K. (2009). Elevated concentrations of oxidized lipoprotein(a) are associated with the presence and severity of acute coronary syndromes. Clin. Chim. Acta 408, 79–82. 10.1016/j.cca.2009.07.013 [DOI] [PubMed] [Google Scholar]
- Wang X., Liu K., Li B., Li Y., Ye K., Qi J., et al. (2015). Macrophages Aggravate Hypoxia-Induced Cardiac Microvascular Endothelial Cell Injury via Peroxynitrite: Protection by Tongxinluo. Cell Commun. Adhes. 22, 39–47. 10.3109/15419061.2016.1155565 [DOI] [PubMed] [Google Scholar]
- Wang X., Mu C., Mu T., Gao L., Zhao Y., Zhang Y., et al. (2016). Effects of Tongxinluo on myocardial fibrosis in diabetic rats. J. Chin. Med. Assoc. 79, 130–136. 10.1016/j.jcma.2015.06.022 [DOI] [PubMed] [Google Scholar]
- Wang Y. L., Cheng J. L., Li J. R., Zhou S., Qi J. W. (2016). Protective effect of myocardial and microvascular of tongxinluo capsule on patients of acute myocardial infarction after percutaneous coronary intervention. Pract. J. Cardiac Cereb. Pneumal Vasc. Dis. 24, 150–152. 10.3969/j.issn.1008-5971.2016.02.052 [DOI] [Google Scholar]
- WHO (2017). World Health Organization. Cardiovascular diseases (CVDs) [online]. Available online at: http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Google Scholar]
- Wu T., Harrison R. A., Chen X., Ni J., Zhou L., Qiao J., et al. (2006). Tongxinluo (Tong xin luo or Tong-xin-luo) capsule for unstable angina pectoris. Cochrane Database Syst Rev. 4:Cd004474 10.1002/14651858.CD004474.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Y. (2017). Master's thesis: Effect of Tongxinluo Combined With Atorvastatin on Myocardial Injury and Short-Term Prognosis in Patients With STEMI After PCI. Zhengzhou: Henan University of Chinese Medicine. [Google Scholar]
- Xia M. H., Bao X. J., Li P., Liu Q. M. (2016). Effects of Tongxinluo capsule combined with tirofiban on thrombus formation and inflammatory factors in patients with acute myocardial infarction after PCI. Guid. J. Trad. Chin. Med. Pharmacol. 22, 92–94. 10.13862/j.cnki.cn43-1446/r.2016.05.029 [DOI] [Google Scholar]
- Yang Y. Q., Yu M. M. (2011). Clinical effects of metoprolol sustained release tablets combined with Tongxinluo on patients of acute myocardial infarction. J. Shanxi Med. College Contin. Educ. 21, 25–26. 10.3969/j.issn.1671-0126.2011.01.014 [DOI] [Google Scholar]
- You M. S., Li X., Wang Y. Z., Zhao H. F., et al. (2012). Effects of tongxinluo on TNF-α expression and heart function after infarction in patients. Chin. J. Gerontol. 32, 231–232. 10.3969/j.issn.1005-9202.2012.02.004 [DOI] [Google Scholar]
- You S. J., Yang Y. J., Chen K. J., Gao R. L., Wu Y. J., Zhang J., et al. (2005). The protective effects of Tong-xin-luo on myocardium and microvasculature after reperfusion in acute myocardial infarction. Chin. J. Cardiol. 30, 433–437. 10.3760/j:issn:0253-3758.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Zeymer U., Jünger C., Zahn R., Bauer T., Bestehorn K., Senges J., et al. (2011). Effects of a secondary prevention combination therapy with an aspirin, an ACE inhibitor and a statin on 1-year mortality of patients with acute myocardial infarction treated with a beta-blocker. Support for a polypill approach. Curr. Med. Res. Opin. 27, 1563–1570. 10.1185/03007995.2011.590969 [DOI] [PubMed] [Google Scholar]
- Zhang H. T., Jia Z. H., Zhang J., Ye Z. K., Yang W. X., Tian Y. Q., et al. (2010). No-reflow protection and long-term efficacy for acute myocardial infarction with Tongxinluo: a randomized double-blind placebo-controlled multicenter clinical trial (ENLEAT Trial). Chin. Med. J. (Engl). 123, 2858–2864. 10.3760/cma.j.issn.0366-6999.2010.20.021 [DOI] [PubMed] [Google Scholar]
- Zhang H. T., Yang Y. J., Cheng Y. T. (2009). Effect of tongxinluo on mini-swine cytokines and myocardial no-reflow in early reperfusion of acute myocardial infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi. 29, 821–824. 10.3321/j.issn:1003-5370.2009.09.013 [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu Y., Lu X. T., Wu Y. L., Zhang C., Ji X. P., et al. (2009). Traditional Chinese medication Tongxinluo dose-dependently enhances stability of vulnerable plaques: a comparison with a high-dose simvastatin therapy. Am. J. Physio Heart Circ. Physiol. 297, H2004–H2014. 10.1152/ajpheart.00208.2009 [DOI] [PubMed] [Google Scholar]
- Zhang R. N., Zheng B., Li L. M., Zhang J., Zhang X. H., Wen J. K. (2014). Tongxinluo inhibits vascular inflammation and neointimal hyperplasia through blockade of the positive feedback loop between miR-155 and TNF-α. Am. J. Physiol. Heart Circ. Physiol. 307, H552–H562. 10.1152/ajpheart.00936.2013 [DOI] [PubMed] [Google Scholar]
- Zhang X. P., Zhang Y. (2009). Long term effects of Tongxinluo capsules on patients with acute myocardial infarction after stent implantation. Chin. Remed. Clin. 9, 243–244. 10.3969/j.issn.1671-2560.2009.03.038 [DOI] [Google Scholar]
- Zheng C. Y., Song L. L., Wen J. K., Li L. M., Guo Z. W., Zhou P. P., et al. (2015). Tongxinluo (TXL), a Traditional Chinese Medicinal Compound, Improves Endothelial Function After Chronic Hypoxia Both in vivo and in vitro. J. Cardiovasc. Pharmacol. 65, 579–586. 10.1097/FJC.0000000000000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Wang H., Zhu J., Chen W., Wang L., Liu S., et al. (2016). Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 387, 251–272. 10.1016/S0140-6736(15)00551-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.