Abstract
IL-1β-converting enzyme (ICE; caspase-1) is the intracellular protease that cleaves the precursors of IL-1β and IL-18 into active cytokines. In the present study, the effect of ICE deficiency was evaluated during experimental colitis in mice. In acute dextran sulfate sodium-induced colitis, ICE-deficient (ICE KO) mice exhibited a greater than 50% decrease of the clinical scores weight loss, diarrhea, rectal bleeding, and colon length, whereas daily treatment with IL-1 receptor antagonist revealed a modest reduction in colitis severity. To further characterize the function of ICE and its role in intestinal inflammation, chronic colitis was induced over a 30-day time period. During this chronic time course, ICE KO mice exhibited a near complete protection, as reflected by significantly reduced clinical scores and almost absent histological signs of colitis. Consistently, colon shortening occurred only in dextran sulfate sodium-exposed wild-type mice but not in ICE KO mice. Protection was accompanied by reduced spontaneous release of the proinflammatory cytokines IL-18, IL-1β, and IFN-γ from total colon cultures. In addition, flow cytometric analysis of isolated mesenteric lymph node cells revealed evidence of reduced cell activation in ICE KO mice as evaluated by surface expression of CD3 CD69 and CD4 CD25. We conclude that inhibition of ICE represents a novel anti-inflammatory strategy for intestinal inflammation.
Interleukin-1β-converting enzyme (ICE), also known as caspase-1, is a specific intracellular cysteine protease required for the processing of some cytokines lacking a signal peptide to allow for release of the mature proteins from the intracellular compartment (1). The precursors of IL-1β and IL-18 have been identified as substrates for ICE (1–3). Both pro-IL-1β and pro-IL-18 are inactive until cleavage by ICE occurs (4–6). Therefore, mice deficient in ICE (ICE KO) have a defective production and release of mature, bioactive IL-1β and IL-18, whereas the precursor forms are normally synthesized (4–6). ICE itself exists as inactive precursor and requires two internal cleavages before becoming enzymatically active; this activation can be induced by a variety of proinflammatory stimuli (1, 7).
IL-18 is constitutively expressed in the inactive precursor form mainly in monocytes/macrophages and epithelial cells (8). IL-18 acts as an important costimulus for production of IFN-γ and other T helper type (Th) 1 cytokines (9). In addition, IL-18, together with IL-12, facilitates T lymphocyte activation and the production of IFN-γ (10). Neutralization of IL-18 exerts beneficial effects in Con A-induced hepatitis, streptococcal cell wall arthritis, hepatic metastases of murine B16 melanoma, and experimental autoimmune encephalomyelitis (11–14). Increased IL-18 levels have been observed in lesions of patients with Crohn's disease, but not ulcerative colitis (15, 16). Furthermore, increased concentrations of IL-18 were demonstrated in a dose-dependent manner in colon sections of mice with acute dextran sulfate sodium (DSS)-induced colitis (17). Blockade of IL-18 in two different models, acute DSS- and trinitrobenzene sulfonic acid-induced colitis, resulted in a significant amelioration of intestinal inflammation (17).§
The role of IL-1β in intestinal inflammation depends both on the up-regulation of IL-1β production as well as the level of its naturally occurring inhibitor, the IL-1 receptor antagonist (IL-1Ra). Indeed, there is evidence that the balance of IL-1 and IL-1Ra may affect disease outcome. For example, mice deficient in IL-1Ra develop spontaneous rheumatoid arthritis and lethal arteritis (18, 19). Administration of IL-1Ra reduces disease severity in several models (20), including immune complex-induced colitis in rabbits (21).
Because of the role of ICE in production of bioactive IL-1β and IL-18, inhibition of this protease might result in a synergistic anti-inflammatory effect. In the present study, the role of ICE in acute and chronic DSS-induced colitis was investigated by using ICE KO mice.
Materials and Methods
Reagents and Mice.
Human IL-1Ra prepared in hyaluronic acid was a kind gift of Amgen Biologicals. RPMI 1640 medium and fetal calf serum were from Life Technologies (Grand Island, NY). The generation and genetic background of ICE KO mice have been described (22). Six- to 8-week-old female mice were used. The wild-type (WT) mice used as controls and for IL-1Ra treatment were of the same genetic background, sex, and age as the ICE KO mice, although they were not littermates.
Induction of Colitis and Experimental Design.
Studies were approved by the Animal Use and Care Committee at the University of Colorado Health Sciences Center. For acute colitis induction, mice were fed 3.5% of DSS (molecular mass 40 kDa; ICN) dissolved in sterile, distilled water ad libitum for the experimental days 1–5 followed by 5 days of regular drinking water. The DSS solutions were made fresh on day 3. Control mice had access to water (without DSS). For chronic colitis induction, mice were fed 2% DSS dissolved in sterile, distilled water ad libitum for 5 days followed by 5 days of normal drinking water; this cycle was repeated three times, resulting in a 30-day experimental period.
Determination of Clinical Scores.
Body weight, the presence of occult or gross blood per rectum, and stool consistency were determined daily during the acute course and every other day during the chronic colitis induction. The clinical score was assessed by trained individuals blinded to the treatment groups (23). The baseline clinical score was determined on day 1. Briefly, no weight loss was registered as 0, weight loss of 1–5% from baseline was assigned 1 point, 6–10% 2 points, 11–20% 3 points, and more than 20% 4 points. For stool consistency, 0 points were assigned for well-formed pellets, 2 points for pasty and semiformed stools that did not adhere to the anus, and 4 points for liquid stools that did adhere to the anus. For bleeding, 0 was assigned for no blood by using hemoccult (Beckman Coulter), 2 points for positive hemoccult, and 4 points for gross bleeding.
Histological Scoring.
Postmortem, the entire colon was excised, and a 1-cm segment of the transverse colon was fixed in 10% buffered formalin for histological analysis. Paraffin sections were stained with hematoxylin/eosin. Four to six colon rings were obtained from each 1-cm colon segment and were thus available for histological examination. Histological scoring was performed in a blinded fashion by a pathologist (H.A.L.) as a combined score of inflammatory cell infiltration (score 0–3) and tissue damage (score 0–3) (23, 24). The presence of occasional inflammatory cells in the lamina propria was scored as 0, increased numbers of inflammatory cells in the lamina propria was scored as 1, confluence of inflammatory cells extending into the submucosa was scored as 2, and transmural extension of the infiltrate was scored as 3. For tissue damage, no mucosal damage was scored as 0, lymphoepithelial lesions were scored as 1, surface mucosal erosion or focal ulceration was scored as 2, and extensive mucosal damage and extension into deeper structures of the bowel wall was scored as 3. The combined histological score ranged from 0 (no changes) to 6 (extensive cell infiltration and tissue damage).
Colon Organ Culture.
A segment of the colon was removed, cut open longitudinally, and washed in PBS containing penicillin and streptomycin. The colon was then further cut into segments of ≈1 cm2 and placed in 24 flat-bottom well culture plates containing fresh RPMI 1640 medium supplemented with penicillin and streptomycin. The colon segments were incubated at 37°C in 1 ml of fresh supplemented RPMI 1640 medium for 24 h. Culture supernatants were harvested and assayed for cytokines. Protein concentration was determined by using the Bio-Rad protein assay.
Mesenteric Lymph Node (MLN) Cell Preparation.
At the end of each experiment, the MLNs were removed. MLNs were pressed through a 100-μm nylon cell strainer to isolate single cell suspensions. Cells were washed once and counted before analysis for flow cytometry.
Cytokine Measurements.
Murine IL-18 and IL-1β levels were measured by using an electrochemiluminescence method as described (25, 26). Samples were analyzed by using an Origen 1.5 analyzer (Igen, Gaithersburg, MD). The range of detection is 20 pg/ml to 10 ng/ml for both IL-1β and IL-18. IFN-γ was determined by using a specific ELISA (PharMingen).
Flow Cytometry.
The isolated MLNs were washed twice in staining buffer consisting of PBS supplemented with 1% fetal calf serum and 0.1% azide. Flow cytometry followed routine procedures by using 1 × 105 cells per sample. To measure the expression of CD3ɛ (clone 145–2C11), CD69 (clone H1.2F3), CD4 (clone L3T4), and CD25 (clone PC61), cells were labeled with either a FITC- or a phycoerythrin-labeled antibody (PharMingen). Flow cytometric analysis was conducted on a FACSCalibur (PharMingen) and analyzed by using the CELLQUEST analysis program (PharMingen).
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical significance of differences between treatment and control groups were determined by factorial ANOVA and a Bonferroni–Dunn procedure as a posthoc test. Statistical analyses were performed by using STAT-VIEW 4.51 software (Abacus Concepts, Berkeley, CA).
Results
Dominance of ICE Deficiency Over IL-1Ra Treatment in the Protection of Acute Colitis.
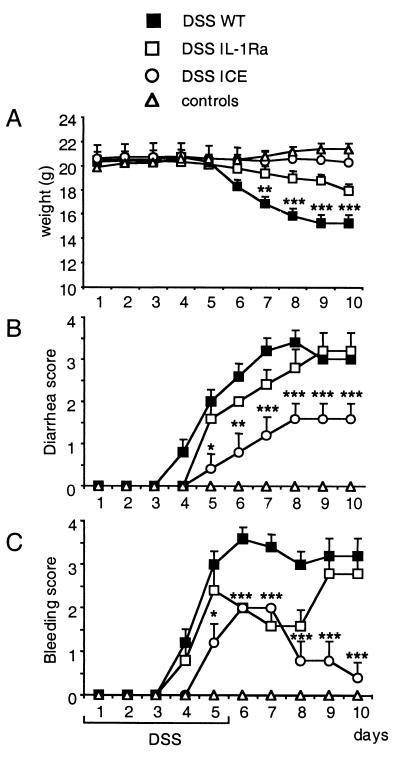
The role of ICE in acute DSS-induced colitis was explored. We first conducted an experiment in the acute model comparing ICE KO mice with WT mice. ICE KO mice showed a strong protection from colitis as evaluated by the clinical scores of weight loss, rectal bleeding, and diarrhea. No weight loss was observed in ICE KO mice, whereas WT mice lost an average of 30% body weight (Fig. 1). A significant protection was also observed in the bleeding and diarrhea score for ICE KO mice.
Figure 1.
Effect of ICE deficiency and IL-1Ra treatment during acute colitis. Untreated or hyaluronic acid-treated WT, ICE KO, and WT mice injected i.p. with IL-1Ra (100 mg/kg) once daily were fed with 3.5% DSS for 5 days followed by 5 days of regular drinking water. (A) Change in body weight. (B) Diarrhea score. (C) Bleeding score. Each score was assessed daily as described in Materials and Methods. Non-DSS control mice, either ICE KO or WT, are summarized as controls, as no differences among these groups could be detected. Data are mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P <0.001 vs. DSS WT.
Because ICE cleaves the precursor of IL-1β as well as the IL-18 precursor to the active proinflammatory cytokines, the effect observed in ICE KO mice was compared with blockade of IL-1 by the antagonist IL-1Ra. We have recently reported the protective effect of IL-18 blockade in this same model (17). For IL-1 blockade, mice were injected once daily for 10 days with 100 mg/kg body weight of IL-1Ra or hyaluronic acid as the control. IL-1Ra-treated mice presented with less weight loss (14%) when compared with the WT mice (Fig. 1). Although IL-1Ra-treated mice initially showed the same onset of diarrhea as WT mice, after day 6 these mice developed less severe diarrhea. Treatment with IL-1Ra reduced the bleeding score. In summary, ICE KO mice exhibited a significant reduction of colitis severity, whereas IL-1Ra-treated DSS-fed mice only revealed a modest effect when compared with DSS-fed WT mice.
ICE Deficiency Prevents Colon Shortening in the Acute DSS Model.
The evaluation of colon length is the parameter with the lowest variability in the model of DSS-induced colitis (23, 27). DSS exposure in WT mice resulted in a 39% reduction of colon length after the 10-day experimental period. In contrast, DSS administration to ICE KO mice did not result in a significant reduction of colon length, whereas treatment with IL-Ra partially protected from colon shortening (27% shortening; data not shown).
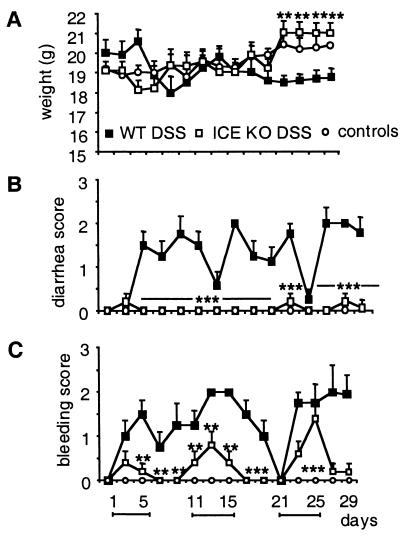
ICE Deficiency Protects Mice from Chronic Colitis.
The results obtained in the acute model strongly indicate a role for ICE in intestinal inflammation. To evaluate the role of ICE in more detail, we used a model of chronic colitis by exposing mice to 2% DSS for 5 days, followed by 5 days of regular drinking water (27). This cycle was repeated three times, resulting in a 30-day experimental period as described in Materials and Methods. As shown in Fig. 2A, DSS-fed ICE KO and control non-DSS WT and ICE KO mice exhibited a weight increase over the experimental time course (4% and 7%, respectively), whereas DSS-fed WT mice presented with a 7% weight loss. In addition, DSS-fed ICE KO mice showed a significantly reduced bleeding score and no diarrhea throughout the entire experimental period. In contrast, DSS-fed WT mice had an elevated bleeding and diarrhea score during the same time period (Fig. 2 B and C).
Figure 2.
Effect of ICE deficiency on chronic colitis. WT and ICE KO mice were fed with 2% DSS for 5 days followed by 5 days of regular water. This cycle was repeated three time resulting in a 30-day experimental period. (A) Change in body weight. (B) Diarrhea score. (C) Bleeding score. Each score was assessed every other day as described in Materials and Methods. Data are mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P <0.001 vs. DSS WT.
Absence of Colon Shortening in ICE KO Mice After Chronic DSS Exposure.
DSS-fed WT mice presented with a 30% reduction of colon length on day 30 after the chronic time course, whereas there was no difference in colon length in DSS-fed ICE KO mice compared with the non-DSS control groups (data not shown).
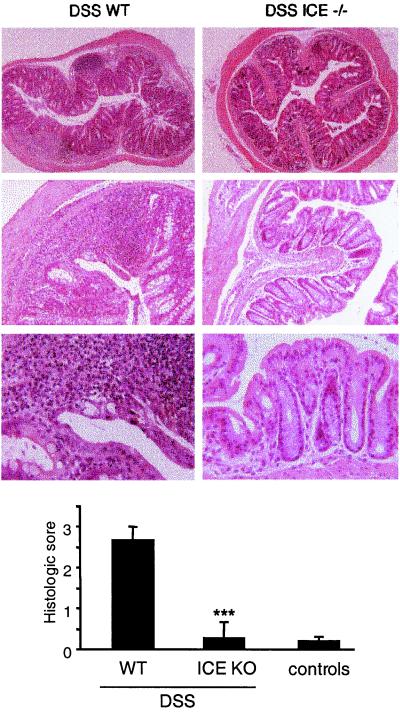
Lack of Inflammation in ICE KO Mice After Chronic DSS Exposure.
On day 30, histology of rings of the transverse part of the colon in DSS-fed WT mice revealed multiple erosive lesions and inflammatory cell infiltrations composed of macrophages, lymphocytes, eosinophils, and occasional neutrophils. However, following the same time course of DSS administration, ICE KO mice showed nearly no signs of histological inflammation (Fig. 3 Upper). A histological score of 0.3 ± 0.4 was observed in ICE KO mice compared with a score of 2.7 ± 0.3 in the WT DSS group (n = 14, P = 0.001, Fig. 3 Lower). Histological signs of inflammation were not detected in the non-DSS control groups (0.2 ± 0.1 in the ICE KO group and 0.1 ± 0.1 in the WT group).
Figure 3.
Histological score in the chronic DSS model. WT and ICE KO mice were fed with 2% DSS for 5 days followed by 5 days of regular water. This cycle was repeated three times, resulting in a 30-day experimental period. A 1-cm segment of the transverse colon was fixed in 10% buffered formalin. Paraffin sections were stained with hematoxylin/eosin. (Magnifications are 52-, 130-, and 325-fold.) Histologic scoring was performed in a blinded fashion as described in Materials and Methods. (Upper) Histological sections from DSS-fed WT and ICE KO mice with increasing magnifications are shown. As there was no difference between the DSS-exposed ICE KO and non-DSS control mice, control sections are not shown. Bars are mean ± SEM. ***, P < 0.001 vs. DSS WT.
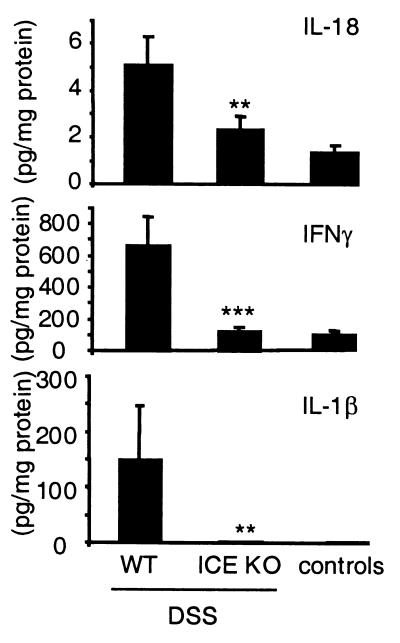
Protection from Colitis in ICE KO Mice Is Associated with Reduced Production of Proinflammatory Cytokines.
To evaluate cytokine production in DSS-fed ICE KO mice, spontaneous release of IL-18, IFN-γ, and IL-1β were evaluated in the supernatant of colon culture at the end of the experiment (Fig. 4). The highest release of the three proinflammatory cytokines was detected in the DSS-exposed WT group. For IL-18, a 55% reduction occurred in DSS-fed ICE KO mice. IFN-γ showed a 73% decrease, whereas production of IL-1β was almost completely suppressed.
Figure 4.
Spontaneous colon cytokine production after chronic DSS-induced colitis. WT and ICE KO mice were fed with 2% DSS for 5 days followed by 5 days of regular water. This cycle was repeated three times, resulting in a 30-day experimental period. The colon was removed and washed extensively, and 1-cm2 pieces were cultured for 24 h. Cytokines were measured in the supernatant, and protein concentration was determined as described in Materials and Methods. Bars are mean ± SEM. **, P < 0.01; ***, P < 0.001 vs. DSS WT.
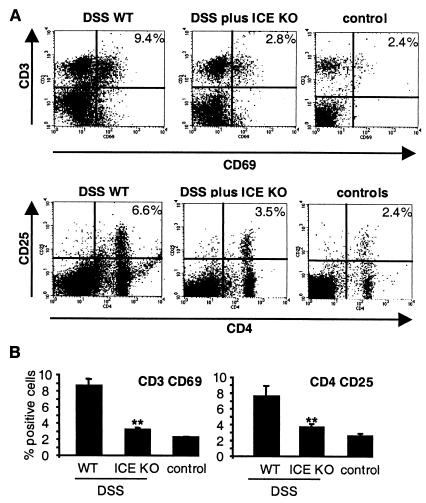
ICE Deficiency Results in Reduced Expression of CD69 and CD25 on MLNs.
It has been demonstrated that the expression of the early activation antigen CD69 on T cells and the expression of the IL-2Rα chain CD25 on CD4 cells of the intestinal draining MLNs is associated with increased disease activity in experimental models of colitis (28, 29). To evaluate the presence of these surface antigens, MLN were isolated after the 30-day experimental time course and stained for either CD3 and CD69 or CD4 and CD25. In Fig. 5A, a representative analysis for both stainings is shown. DSS-fed ICE KO mice exhibited a significantly lower population of CD3 CD69 (3.1 ± 0.2%; n = 5, P = 0.004) and CD4 CD25 (3.7 ± 0.4%, n = 5, P = 0.003) double positive cells compared with DSS-fed WT mice (8.6 ± 0.8% and 7.6 ± 1.3%, for CD3 CD69 and CD4 CD25, respectively). As differences between the various non-DSS controls were not observed, these groups were summarized as controls.
Figure 5.
Flow cytometric analysis of CD4 CD25 and CD3 CD69 expression of MLNs. WT and ICE KO mice were fed with 2% DSS for 5 days followed by 5 days of regular water. This cycle was repeated three times, resulting in a 30-day experimental period. MLNs were prepared, and cells were isolated and double-stained for either CD4 CD25 or CD3 CD69 for flow cytometry as described in Materials and Methods. (A) Representative staining for each experimental group. (B) Mean ± SEM of five mice per group. **, P < 0.01 vs. WT.
Discussion
In the present study, ICE was identified as a key contributor in intestinal inflammation. During acute colitis, ICE deficiency was shown to exert greater anti-inflammatory effects compared with treatment with IL-1Ra or as previously reported, with neutralization of IL-18 (17), as assessed by the macroscopic scores weight loss, stool consistency, rectal bleeding, and colon length. Because IL-1Ra has a short half-life, daily administration might not have provided complete or prolonged IL-1 receptor blockade (30). The previously described effect of anti-IL-18 treatment resulted in a 40% reduction of the clinical scores (17), whereas blockade of IL-1 by administration of IL-1Ra resulted in a 30% effect. Therefore, ICE deficiency is associated with a more pronounced effect compared with single blockade of either IL-1 or IL-18. Although the marked protection from acute DSS-induced colitis observed in ICE KO mice can be attributed to the combined blockade of IL-1β and IL-18, the possibility remains that ICE could cleave additional—as yet unknown—substrates that participate in intestinal inflammation.
Compared with the model of acute DSS-induced colitis, during chronic colitis ICE KO mice showed an even more pronounced protection, as shown by the reduction in clinical and histological scores as well as reduced production of the proinflammatory cytokines IL-1β, IL-18, and IFN-γ from total colon cultures. Furthermore, after chronic DSS exposure, ICE KO mice had significantly less CD69-activated T cells and no increase in CD4+CD25+ cells when compared with WT mice.
The role of ICE in intestinal inflammation has only been described during infection with Entamoeba histolytica, in which cysteine proteinases with ICE activity cause intestinal inflammation and tissue damage (31). In other models of in vivo inflammation, the role of ICE has been characterized in more detail. For instance, beneficial effects of ICE blockade or ICE deficiency have been demonstrated in toxin-induced pancreatitis (32, 33) and collagen-induced arthritis (34).
Interestingly, in the context of intestinal inflammation, ICE inhibits apoptosis of inflammatory neutrophils (35). Although ICE (caspase-1) belongs to the family of caspases, which play important roles in apoptosis, the inhibition of cell death in inflammatory neutrophils is not a direct effect of ICE but rather is mediated indirectly through release of IL-1β (35). Moreover, the constitutive apoptotic program in inflammatory neutrophils can be inhibited by a variety of signals that are associated with the expression of an inflammatory response, including an array of proinflammatory cytokines (36). Blockade of cytokines results in increased apoptosis of intestinal lymphocytes. In fact, administration of neutralizing anti-IL-12 antibodies during trinitrobenzene sulfonic acid-induced colitis is followed by the appearance of apoptotic CD4+ T cells at the site of inflammation in the colon (37). Furthermore, the anti-tumor necrosis factor α antibodies, currently used with considerable therapeutic efficacy in the treatment of Crohn's disease, have been suggested to act by inducing apoptosis of T cells (38). Thus, induction of apoptosis is regarded as a new strategy to intervene in intestinal inflammation (38, 39). Therefore, an increased rate of neutrophil apoptosis in ICE KO mice is a potential mechanism contributing to protection against DSS-induced colitis.
ICE cleaves the precursors of both IL-1β and IL-18, leading to the production of the respective bioactive cytokines (1). IL-1β could also be cleaved by other enzymes, such as proteinase-3, cathepsin G, chymotrypsin, elastase, a mast cell chymase, various matrix metalloproteinases, and granzyme A to yield active IL-1β (40, 41). The complete suppression of IL-1β release into the colon culture supernatant in ICE KO mice demonstrates that ICE cleavage is the mechanism responsible for the generation of mature IL-1β in chronic DSS-induced colitis.
IFN-γ synthesis was evaluated in the colon culture supernatant because IL-18, in synergism with IL-12, results in a strong induction of this cytokine. The significant reduction observed in ICE KO mice can be explained by the lack of mature IL-18 (1) and, to a lesser degree, of IL-1β.
IL-18 plays a significant role during intestinal inflammation. Increased concentrations of IL-18 were detected in the inflammatory lesions of patients with Crohn's disease but not ulcerative colitis (15, 16). Moreover, IL-18 expression could be localized to the intestinal epithelium and macrophages in inflammatory granulomas (15, 16). We have observed a significant increase of IL-18 in the colonic epithelium during acute DSS-induced colitis (17). Moreover, neutralization of IL-18 in DSS- and trinitrobenzene sulfonic acid-induced colitis ameliorated the severity of disease (17).§ IL-18 is synthesized mainly by epithelial cells and macrophages, both cell populations contributing to intestinal inflammation. Under inflammatory conditions in the intestine, the maintenance of the epithelial barrier can be severely reduced; this represents a crucial step in unprotected exposure of lamina propria lymphocytes to antigens and microbial toxins (42, 43). Macrophages involved in intestinal inflammation are recruited mostly from the blood; these macrophages are phenotypically different from the resident population. These blood-derived macrophages produce several cytokines, such as IL-18, IL-1β, IL-12, and tumor necrosis factor α, that are important in the inflammatory response (44). In fact, recent data suggest that macrophages might represent a crucial cell population in intestinal inflammation. Hugot et al. (45) and Ogura et al. (46) have reported that mutations of the gene encoding the NOD2 locus are strongly associated with the development of Crohn's disease. It was previously demonstrated that NOD2 expression is highly restricted to monocytes, thus emphasizing the role of macrophages in intestinal inflammation (47).
Although the goal of the present study was to investigate the role of ICE in intestinal inflammation rather than in a specifically T cell-dependent model, T lymphocytes may contribute to DSS-induced colitis. Although disease can be induced by acute administration of DSS in severe combined immunodeficient mice (48), other studies provide evidence that cyclosporin and FK506 are protective in the short-term acute DSS model, suggesting that T cells participate in disease development (49, 50). Furthermore, specific T cell subsets exert protective effects after acute administration of DSS (51). To evaluate whether T cell activation differs in ICE KO and WT mice after the chronic time course, MLNs were isolated and characterized by flow cytometry. Expression of CD69 on CD3+ T cells was significantly higher in DSS-fed WT compared with DSS-fed ICE KO mice. CD69 is an activation marker that is up-regulated by a variety of stimuli (52). Evaluation of CD69 expression is a commonly used method for analysis of cell activation, in particular in MLNs during intestinal inflammation (29). CD4+ CD25+ cells represent a population of suppressor T cells that exhibit anti-inflammatory properties and can prevent colitis induction (53). In fact, the DSS WT mice present with an increase of CD4+ CD25+ population. However, no increase could be observed in ICE-deficient mice, suggesting that induction of inflammation was prevented before stimulating this T cell population to infiltrate the draining lymph nodes.
In conclusion, the present study demonstrates that ICE deficiency results in protection from acute and chronic DSS-induced colitis. ICE deficiency is clearly superior to the single blockade of either IL-18 or IL-1β in the acute DSS-induced colitis model. In addition, a more pronounced protection, accompanied by reduced T cell activation in the MLNs, is observed in the chronic model of DSS-induced colitis. These results indicate that inhibition of ICE might represent a novel approach in the therapy of intestinal inflammation.
Acknowledgments
These studies were supported by National Institutes of Health Grant AI-15614 (to C.A.D.) and Deutsche Forschungsgemeinschaft Grant DFG SI 749/2-1 (to B.S.).
Abbreviations
- ICE
IL-1β-converting enzyme
- DSS
dextran sulfate sodium
- WT
wild type
- MLN
mesenteric lymph node
- IL-1Ra
IL-1 receptor antagonist
Footnotes
ten Hove, T., Corbaz, A., Chvastok, Y., Drillenburg, P., van Deventer, S. J. H. & te Velde, A. A. (2000) Eur. Cytokine Netw. 11, A17005 (abstr.).
References
- 1.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 2.Bazan J F, Timans J C, Kastelein R A. Nature (London) 1996;379:591. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- 3.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Nature (London) 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 4.Cerretti D P, Kozlosky C J, Mosley B, Nelson N, Van Ness K, Greenstreet T A, March C J, Kronheim S R, Druck T, Cannizzaro L A, et al. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 5.Puren A J, Razeghi P, Fantuzzi G, Dinarello C A. J Infect Dis. 1998;178:1830–1834. doi: 10.1086/314481. [DOI] [PubMed] [Google Scholar]
- 6.Thornberry N A, Bull H G, Calaycay J R, Chapman K T, Howard A D, Kostura M J, Miller D K, Molineaux S M, Weidner J R, Aunins J, et al. Nature (London) 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 7.Wilson K P, Black J A, Thomson J A, Kim E E, Griffith J P, Navia M A, Murcko M A, Chambers S P, Aldape R A, Raybuck S A, et al. Nature (London) 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello C A. Methods. 1999;19:121–132. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- 9.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Nature (London) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 10.Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashino K. J Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- 11.Faggioni R, Jones-Carson J, Reed D A, Dinarello C A, Feingold K R, Grunfeld C, Fantuzzi G. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. . (First Published February 11, 2000; 10.1073/pnas.040561297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joosten L A, van De Loo F A, Lubberts E, Helsen M M, Netea M G, van Der Meer J W, Dinarello C A, van Den Berg W B. J Immunol. 2000;165:6553–6558. doi: 10.4049/jimmunol.165.11.6553. [DOI] [PubMed] [Google Scholar]
- 13.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes A M, Anasagasti M J, Martin J, Carrascal T, Walsh P, Reznikov L L, Kim S H, et al. Proc Natl Acad Sci USA. 2000;97:734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wildbaum G, Youssef S, Grabie N, Karin N. J Immunol. 1998;161:6368–6374. [PubMed] [Google Scholar]
- 15.Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, Luzza F, Fusco A, Pallone F. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- 16.Pizarro T T, Michie M H, Bentz M, Woraratanadharm J, Smith M F J, Foley E, Moskaluk C A, Bickston S J, Cominelli F. J Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- 17.Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr H A, Hartmann G, Dinarello C A, Endres S, Eigler A. Am J Physiol. 2001;281:R1264–R1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 18.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicklin M J, Hughes D E, Barton J L, Ure J M, Duff G W. J Exp Med. 2000;191:303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 21.Cominelli F, Nast C C, Duchini A, Lee M. Gastroenterology. 1992;103:65–71. doi: 10.1016/0016-5085(92)91096-m. [DOI] [PubMed] [Google Scholar]
- 22.Kuida K, Lippke J A, Ku G, Harding M W, Livingston D J, Su M S, Flavell R A. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 23.Siegmund B, Rieder F, Albrich S, Wolf K, Bidlingmaier C, Firestein G S, Boyle D, Lehr H A, Loher F, Hartmann G, et al. J Pharmacol Exp Ther. 2001;296:99–105. [PubMed] [Google Scholar]
- 24.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, et al. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Sacco S, Ghezzi P, Dinarello C A. Am J Physiol. 1997;273:R400–R406. doi: 10.1152/ajpregu.1997.273.1.R400. [DOI] [PubMed] [Google Scholar]
- 26.Fantuzzi G, Reed D A, Dinarello C A. J Clin Invest. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inahgaki Y, Nakaya R. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 28.Stallmach A, Wittig B, Giese T, Pfister K, Hoffmann J C, Bulfone-Paus S, Kunzendorf U, Meuer S C, Zeitz M. Gastroenterology. 1999;117:866–876. doi: 10.1016/s0016-5085(99)70345-8. [DOI] [PubMed] [Google Scholar]
- 29.Veltkamp C, Tonkonogy S L, De Jong Y P, Albright C, Grenther W B, Balish E, Terhorst C, Sartor R B. Gastroenterology. 2001;120:900–913. doi: 10.1053/gast.2001.22547. [DOI] [PubMed] [Google Scholar]
- 30.Granowitz E V, Porat R, Mier J W, Pribble J P, Stiles D M, Bloedow D C, Catalano M A, Wolff S M, Dinarello C A. Cytokine. 1992;4:353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Wang L, Seydel K B, Li E, Ankri S, Mirelman D, Stanley S L., Jr Mol Microbiol. 2000;37:542–548. doi: 10.1046/j.1365-2958.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 32.Norman J, Yang J, Fink G, Carter G, Ku G, Denham W, Livingston D. J Interferon Cytokine Res. 1997;17:113–118. doi: 10.1089/jir.1997.17.113. [DOI] [PubMed] [Google Scholar]
- 33.Rau B, Paszkowski A, Lillich S, Baumgart K, Moller P, Beger H G. Lab Invest. 2001;81:1001–1013. doi: 10.1038/labinvest.3780312. [DOI] [PubMed] [Google Scholar]
- 34.Ku G, Faust T, Lauffer L L, Livingston D J, Harding M W. Cytokine. 1996;8:377–386. doi: 10.1006/cyto.1996.0052. [DOI] [PubMed] [Google Scholar]
- 35.Watson R W, Rotstein O D, Parodo J, Bitar R, Marshall J C, William R, Watson G. J Immunol. 1998;161:957–962. [PubMed] [Google Scholar]
- 36.Cox G, Gauldie J, Jordana M. Am J Respir Cell Mol Biol. 1992;7:507–513. doi: 10.1165/ajrcmb/7.5.507. [DOI] [PubMed] [Google Scholar]
- 37.Fuss I J, Marth T, Neurath M F, Pearlstein G R, Jain A, Strober W. Gastroenterology. 1999;117:1078–1088. doi: 10.1016/s0016-5085(99)70392-6. [DOI] [PubMed] [Google Scholar]
- 38.Neurath M F, Finotto S, Fuss I, Boirivant M, Galle P R, Strober W. Trends Immunol. 2001;22:21–26. doi: 10.1016/s1471-4906(00)01798-1. [DOI] [PubMed] [Google Scholar]
- 39.Beutler B. Immunity. 2001;15:5–14. doi: 10.1016/s1074-7613(01)00176-5. [DOI] [PubMed] [Google Scholar]
- 40.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer A H, Cheronis J. Proc Natl Acad Sci USA. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fantuzzi G, Dinarello C A. J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 42.Groot J, Bijlsma P, Van Kalkeren A, Kiliaan A, Saunders P, Perdue M. Ann NY Acad Sci. 2000;915:237–246. doi: 10.1111/j.1749-6632.2000.tb05247.x. [DOI] [PubMed] [Google Scholar]
- 43.Kucharzik T, Lugering N, Rautenberg K, Lugering A, Schmidt M A, Stoll R, Domschke W. Ann NY Acad Sci. 2000;915:171–183. doi: 10.1111/j.1749-6632.2000.tb05240.x. [DOI] [PubMed] [Google Scholar]
- 44.Mahida Y R. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Hugot J P, Chamaillard M, Zouali H, Lesage S, Cezard J P, Belaiche J, Almer S, Tysk C, O'Morain C A, Gassull M, et al. Nature (London) 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 46.Ogura Y, Bonen D K, Inohara N, Nicolae D L, Chen F F, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr R H, et al. Nature (London) 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 47.Ogura Y, Inohara N, Benito A, Chen F F, Yamaoka S, Nunez G. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 48.Dieleman L A, Ridwan B U, Tennyson G S, Beagley K W, Bucy R P, Elson C O. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 49.Murthy S N, Cooper H S, Shim H, Shah R S, Ibrahim S A, Sedergran D J. Dig Dis Sci. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 50.Takizawa H, Shintani N, Natsui M, Sasakawa T, Nakakubo H, Nakajima T, Asakura H. Digestion. 1995;56:259–264. doi: 10.1159/000201253. [DOI] [PubMed] [Google Scholar]
- 51.Saubermann L J, Beck P, De Jong Y P, Pitman R S, Ryan M S, Kim H S, Exley M, Snapper S, Balk S P, Hagen S J, et al. Gastroenterology. 2000;119:119–128. doi: 10.1053/gast.2000.9114. [DOI] [PubMed] [Google Scholar]
- 52.Marzio R, Mauel J, Betz-Corradin S. Immunopharmacol Immunotoxicol. 1999;21:565–582. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 53.Read S, Malmstrom V, Powrie F. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]