Abstract
Background
Plant lipoxygenase (LOX) genes are members of the non-haeme iron-containing dioxygenase family that catalyze the oxidation of polyunsaturated fatty acids into functionally diverse oxylipins. The LOX family genes have been extensively studied under biotic and abiotic stresses, both in model and non-model plant species; however, information on their roles in cotton is still limited.
Results
A total of 64 putative LOX genes were identified in four cotton species (Gossypium (G. hirsutum, G. barbadense, G. arboreum, and G. raimondii)). In the phylogenetic tree, these genes were clustered into three categories (9-LOX, 13-LOX type I, and 13-LOX type II). Segmental duplication of putative LOX genes was observed between homologues from A2 to At and D5 to Dt hinting at allopolyploidy in cultivated tetraploid species (G. hirsutum and G. barbadense). The structure and motif composition of GhLOX genes appears to be relatively conserved among the subfamilies. Moreover, many cis-acting elements related to growth, stresses, and phytohormone signaling were found in the promoter regions of GhLOX genes. Gene expression analysis revealed that all GhLOX genes were induced in at least two tissues and the majority of GhLOX genes were up-regulated in response to heat and salinity stress. Specific expressions of some genes in response to exogenous phytohormones suggest their potential roles in regulating growth and stress responses. In addition, functional characterization of two candidate genes (GhLOX12 and GhLOX13) using virus induced gene silencing (VIGS) approach revealed their increased sensitivity to salinity stress in target gene-silenced cotton. Compared with controls, target gene-silenced plants showed significantly higher chlorophyll degradation, higher H2O2, malondialdehyde (MDA) and proline accumulation but significantly reduced superoxide dismutase (SOD) activity, suggesting their reduced ability to effectively degrade accumulated reactive oxygen species (ROS).
Conclusion
This genome-wide study provides a systematic analysis of the cotton LOX gene family using bioinformatics tools. Differential expression patterns of cotton LOX genes in different tissues and under various abiotic stress conditions provide insights towards understanding the potential functions of candidate genes. Beyond the findings reported here, our study provides a basis for further uncovering the biological roles of LOX genes in cotton development and adaptation to stress conditions.
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4985-2) contains supplementary material, which is available to authorized users.
Keywords: Cotton, Lipoxygenase gene family, Promoter, Abiotic stress, Gene expression, Virus-induced gene silencing
Background
As an important cash crop, cotton is regarded as the backbone in the economy of many developing countries. It not only provides the quality fiber but also cottonseed oil to the world economy [1]. However, during its growth period it encounters various biotic and abiotic stresses. Among the abiotic stresses, salinity causes severe problems in arid and semi-arid regions; it limits crop production by interfering with germination, growth and fertility. Depending on the intensity it causes ion toxicity, water stress, membrane damage, oxidative stress, nutritional imbalances and several cellular and metabolic dysfunctions that can result in the death of plants [2].
The outcome of salinity is increased concentrations of Na+ and Cl− ions in plants, which interfere with the normal defense mechanisms against abiotic stresses. In addition, salinity causes hyper-accumulation of reactive oxygen species (ROS), such as overproduction of H2O2, O2−, and OH. H2O2 regulates several physiological processes in plants; however, its overproduction results in several deleterious effects on plants cells. Thus, in order to maintain a balanced level of cellular ROS, plants have evolved mechanisms to detoxify ROS, either enzymatically or non-enzymatically [3]. Generally, cotton is regarded as moderately salt-tolerant crop, but its growth, yield and fiber quality are substantially affected by salt stress [4]. Increasing tolerance to abiotic stresses in order to improve both the yield and fiber quality is one the major challenges for scientists working in this field.
Oxylipins and their derivatives such as jasmonic acid, divinyl ethers, and volatile aldehydes play significant roles during plant responses to biotic and abiotic stresses [5–7]. The synthesis of these oxylipins is catalyzed by LOXs, which are non haeme, iron-containing dioxygenases, ubiquitous in plants and animals [8]. The LOXs catalyze the oxidation of polyunsaturated fatty acids either at carbon atom 9 or at carbon atom 13 and justify their roles as 9-LOX and 13-LOX, respectively. The function of LOX can differ between 9-LOX to 13-LOX due to variations in particular motifs at the specific binding site [9]. The 9-LOX subfamily includes genes which share a high similarity with each other, whereas, 13-LOX subfamily is further categorized into two types according to their similarity and structure. 13-LOX type I genes are more similar to each other (more than 75%) and lack a chloroplast transit peptide; and 13-LOX type II genes show less similarity with each other (up to 35%) and possess a chloroplast transit peptide [10, 11].
The isomers resulting from the functions of 9-LOX or 13-LOX, (9S)-hydroperoxyoctadecadienoic acid (9-HPOD) or (13S)-hydroperoxyoctadecadienoic acid (13-HPOD), further leads to numerous branches of enzymes for the synthesis of different oxylipins [12]. The LOX genes and their derived oxylipins play substantial roles during all stages of plant life such as seed germination, growth, development, and response to stresses [13, 14]. More importantly, the 13-LOX derived oxylipins JA and its precursor (+)-12-oxo-phytodienoic acid (OPDA) have been well characterized for their significant roles during development and response to abiotic stresses [15]. Several reports have highlighted the involvement of JA signaling in salt stress in plants [16–19].
Similarly, the LOX genes have been well documented during plant responses to biotic and abiotic stresses. For example, in pepper, CaLOX1 plays a crucial role during modulation of abiotic stress responses via rapid scavenging of ROS and activation of defense-related marker genes [20]. In persimmon, DkLOX3 substantially enhances tolerance to senescence and salt stress by accumulating less O2− and H2O2 along with the activation of stress-responsive genes [21]. Expression analysis studies also suggest the possible involvement of LOX genes during development and response to biotic and abiotic stress conditions [22–25]. Increased LOX activity is considered to correlate directly with plant salt tolerance mechanisms; such increased LOX activity was observed in many plants, as in rice [26] citrus [27] and tomato [28, 29].
LOX family genes have been comprehensively studied in various plants species, such as in Arabidopsis [30], rice [31] soybean, [32] maize [33] tomato [34], cucumber [35] and others, due to their potential function in various physiological and molecular events. Previous studies have also reported the functions of two LOX genes in cotton during bacterial infection [36, 37]. However, to date, there is no information about genome-wide identification and characterization of LOX gene family members in cotton. With the release of the genomic sequences of four cotton species and the availability of transcriptomic data, it has now become possible to comprehensively characterize and analyze the LOX gene family, which is an essential step to explore the functions of LOX genes in cotton.
In the present study, 64 putative LOX genes were identified during genome-wide screening in four species of cotton. Their phylogenetic relationship, chromosomal distribution, synteny, structure, and expression patterns in various organs, under different abiotic stresses and in response to exogenous phytohormones, were determined. Moreover, we functionally characterized selective LOX genes in response to salt stress using virus induced gene silencing (VIGS). Our study provides insights that will be helpful for further characterization of cotton LOX genes in future.
Results
Genome-wide identification of LOX gene family in Gossypium spp
To identify LOX family genes in cotton, we initially used the Arabidopsis LOX domain as query to BLAST four cotton genome databases. As a result 21, 18, 11 and 14 putative LOX genes were identified from G. hirsutum, G. barbadense, G. arboreum and G. raimondii, respectively (Additional file 1: Table S1). All of these putative genes fulfilled the criteria of LOX genes as described by Feussner and Wasternack [38]. Multiple sequence alignment (MSA) depicted the presence of representative 38 amino acids motif (His-(X)4-His-(X)4-His-(X)17-His-(X)8-His), highly conserved among all the predicted LOX genes of cotton spp. (Additional file 2: Figure S1). This motif was considered to be essential for enzyme stability and activity; and also to provide binding sites for non-haeme iron-containing dioxygenases [38]. Further properties of these predicted genes, including protein lengths, molecular weights, isoelectric points, functional domains and proposed functionality are provided in Additional file 3: Table S2.
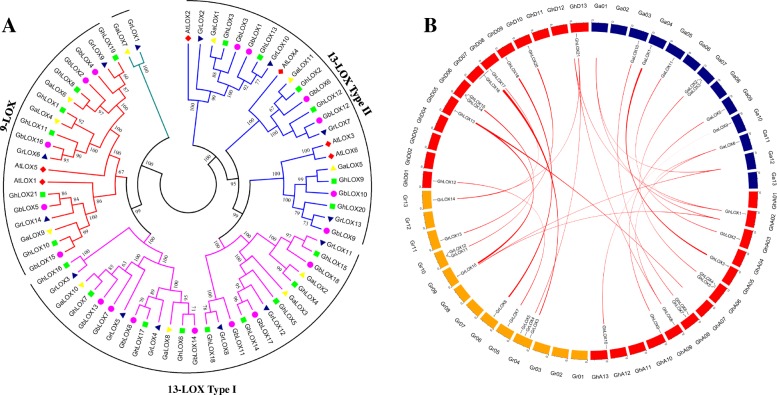
The evolutionary dynamics and syntenic relationships of putative LOX genes among two diploid genomes (G. arboreum, G. raimondii) and sub-genomes in cultivated allotetraploid (G. hirsutum) were visualized by generating a circos plot [39] (Fig. 1). The results show that GaLOX, GrLOX and GhLOX genes are distributed among seven chromosomes of A, D and At subgenomes, respectively, whereas GhLOX genes of Dt and At subgenomes were distributed among 7 and 8 chromosomes, respectively. The distribution was uneven, with chromosomes A06, A08, D02, D06, D08 and D09 possessing two GhLOX genes, while others (A02, A03, A05, A10, A13, D05 and D13) harbored one GhLOX gene (Fig.1b, Additional file 4: Table S3). Moreover, 19 and 29 duplicated gene pairs were identified from At to A2 and Dt to D5, respectively. The genes of each duplicated pair showed greater similarity to each other (Additional file 5: Table S4).
Fig. 1.
Phylogenetic and synteny analysis of LOX family genes. a Neighbor-joining phylogenetic tree was generated with protein sequences of four species of cotton and one model plant Arabidopsis. b Syntenic relationships among LOX genes of two diploid (G. arboreum, G. raimondii) and one allotetraploid (G. hirsutum) cotton was visualized in a circos plot. The chromosomes of G. arboreum, G. raimondii, and G. hirsutum were shaded with blue, yellow, and red colors, respectively
Phylogenetic analysis of LOX gene family in cotton
To understand the evolutionary relationships of the LOX gene family in the Gossypium lineage we constructed a phylogenetic tree for 4 species of cotton and the model plant Arabidopsis using conserved amino acid sequences (Additional file 6: Table S5). As mentioned above, the plant LOX gene family can be grouped into two subfamilies (9-LOX and 13-LOX), according to their positional specificity and catalysis of hydrocarbon backbone [10]. The 13-LOX genes are further categorized into type I and type II, based on their protein structure and similarity [40]. As shown in Fig. 1, all the LOX family genes cluster into three different groups and LOX genes of each species are randomly distributed. Upon inspection of sequence features, we determined that 9-LOX category genes harbor a valine (V) residue (9-LOX motif) at one specific site, responsible for their functional regiospecificity, whereas all the other LOX genes contained phenylalanine (F) (13-LOX motif) and are categorized into 13-LOX subfamily, with the exception of GaLOX7 and GrLOX1 (Additional file 7: Figure S2). Both of these genes possess a leucine (L) residue at the specific site instead of phenylalanine or valine, and form a distinct branch in the phylogenetic tree. There are only few reports about the categorization of such kinds of LOX genes in plants [22].
Subcellular localization of LOX proteins was predicted using three different publicly available programs. The results revealed that 13-LOX type I proteins are preferentially found in the cytoplasm, while type II 13-LOXs are in chloroplast (Additional file 8: Table S6). More investigation showed that cotton 9-LOX genes formed association with Arabidopsis 9-LOX genes AtLOX1 and AtLOX5, reported to play roles in protection of plants against various biotic and abiotic stresses through the synthesis of diverse oxylipins [41, 42]. The 13-LOX subfamily clustered with AtLOX2, AtLOX3, AtLOX4, and AtLOX6, whose roles are well established in growth, development, jasmonic acid biosynthesis, and defense signaling against stress conditions [43–45]. More often, it is considered that genes with similar functions cluster together; the distribution of cotton LOX genes in different clusters might explain their probable functions.
Structural and motif analysis of GhLOX genes
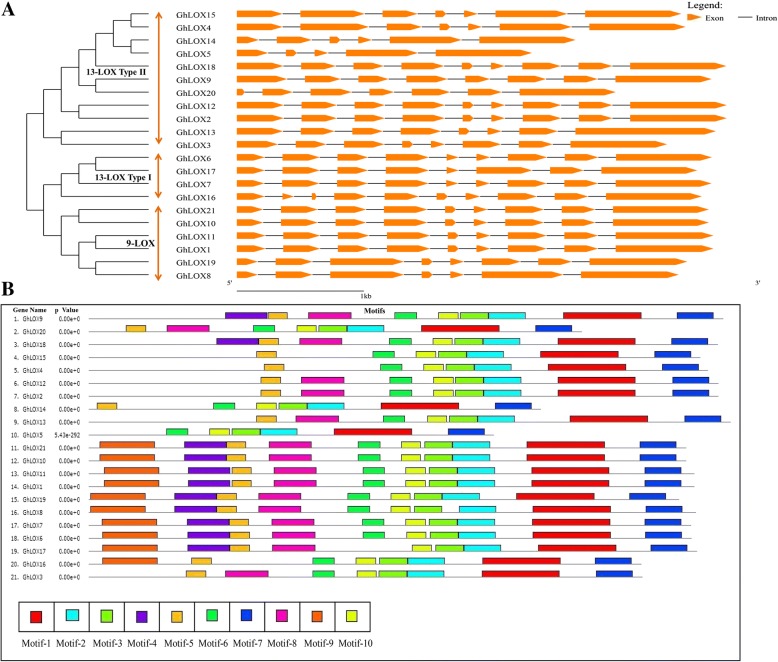
To gain further knowledge about the structure and motif composition of GhLOX genes, we constructed a separate phylogenetic tree and analyzed their structural characteristics through the Gene Structure Display web portal. Based on the results of phylogenetic tree, GhLOX genes were divided into three subfamilies (9-LOX, 13-LOX type I and type II). The number of introns and length of exons among most of the GhLOX genes either 9-LOX or 13-LOX were found to be similar (Fig. 2a). However, we observed variation in gene structure of some 13-LOX type II genes. The minimum number of introns (4 introns) was found in GhLOX5 and maximum number in GhLOX16 (9 introns).
Fig. 2.
Structural and motif analysis of GhLOX genes. a Structural analysis. Neighbor-joining phylogenetic tree was constructed among GhLOX genes with MEGA7. The subfamilies were marked correspondingly. The sizes of exons are relative to their sequence length. b Motif analysis. Ten distinct motifs were identified with MEME suite and each motif was represented with different color
To explore conserved motifs within GhLOX subfamilies, we used MEME Suite (Ver. 4.12.0) to predict distinct motifs shared by the GhLOX family. We identified 10 distinct motifs. Most of these motifs encoded a LOX domain (Fig. 2b and Additional file 9: Table S7). As expected, most of the structurally similar members shared common motifs within the same subfamily, suggesting their similar functions. Upon closer inspection, it was found that 5 motifs (motifs 1–3, 7, 10) were shared by all the GhLOX genes. However, it is noteworthy that some of the specific motifs were absent in specific subfamilies. For example, motif 9 was absent in all the members of 13-LOX subfamily type II, and motif 4 was absent in most of the members of this subfamily. However, whether the presence or absence of these specific motifs confers unique functional roles to LOX genes need further research. In any case, the structure and motif conservation within subfamilies may additionally support the results of phylogenetic analysis. Alternatively, variations in motif composition between subfamilies might be explained by their functional diversification.
Cis- elements analysis of GhLOX genes
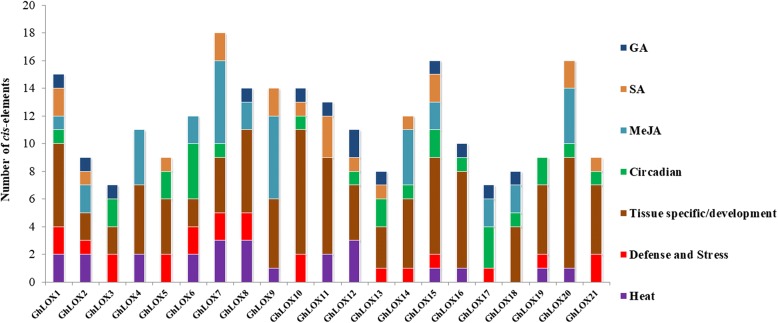
Cis-elements in promoters play vital roles in regulating the expression of genes. These are in regions of non-coding DNA, usually found upstream of transcriptional start sites. In most of cases, gene expression depends on the presence or absence of these elements [46]. To analyze cis-elements potentially involved in the regulation of GhLOX genes, we selected a 1.5 kb 5′ flanking region upstream from the start codon of each GhLOX gene. The PLANTCARE database identified several stress, phytohormone, developmental and light responsive cis-regulatory elements in their promoter regions (Fig. 3 and Additional file 10: Table S8). Out of 21 GhLOX genes, 20 possessed tissue-specific or development-responsive elements, with 16 enriched in circadian-responsive elements, 15 contained ARE (anaerobic induction-responsive element), 14 had TC-rich repeats (defense or stress-related), 13 harbored the HSE (heat stress element), and 8 genes possessed MBS (MYB-binding sites), responsible for abiotic stress responses. All the GhLOX genes contained large numbers of light-responsive elements. Moreover, many of the GhLOX genes also harbored the TCA, CGTCA/TGACG, GARE/P-box encoding cis-acting elements in their promoter regions, responsible for SA (salicylic acid), JA (jasmonic acid), and GA (gibberellic acid) signaling, respectively.
Fig. 3.
Promoter analysis of GhLOX genes. The numbers of different cis-elements were presented in the form of bar graphs; similar cis-elements were shown with same colors
Tissue specific expression patterns of GhLOX genes
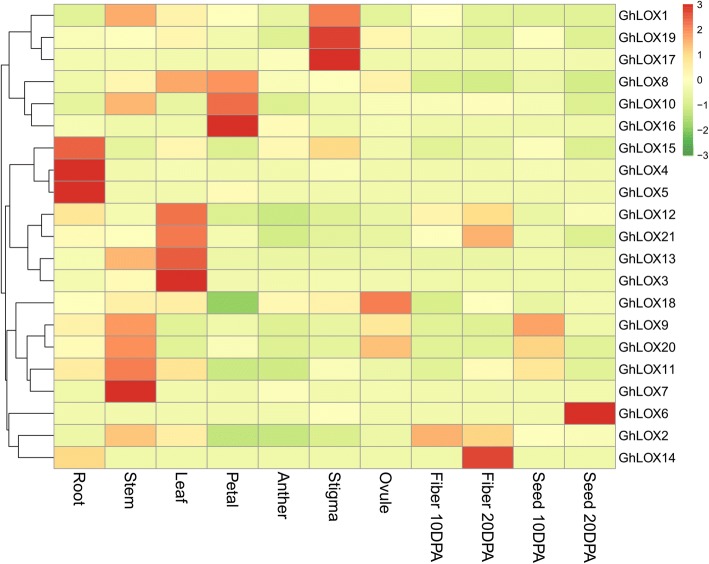
To determine the expression of GhLOX genes in various tissues of G. hirsutum (root, stem, leaves, flowers, fiber and seeds), we investigated the transcriptomic data provided by Zhang et al. [47]. The expression analysis showed that transcripts of all the GhLOX genes were detected in at least two tissues (Fig. 4 and Additional file 11: Table S9). However, 3 genes (GhLOX10, GhLOX19, and GhLOX21) were expressed in all the tested tissues [Fragments per kilobase of transcript per million mapped reads (FPKM ≥ 1)]. In addition, 6 GhLOX genes (GhLOX2, GhLOX9–10, GhLOX13, and GhLOX20–21) were highly expressed in stem, with highest expression noted for GhLOX20 (FPKM ≥ 46) and 7 genes (GhLOX2–3, GhLOX8, GhLOX12–13, GhLOX19 and GhLOX21) strongly induced in leaves, with highest expression observed for GhLOX21 (FPKM ≥ 61). Based on the expression pattern of GhLOX genes in cotton and the reported roles of their orthologues in Arabidopsis, we can assume the probable functions of these genes. GhLOX21 is highly expressed in leaves and also had strong expression in roots (FPKM ≥ 23), and its ortholog is AtLOX1, reported to be involved in lateral root development [41]. Similarly, along with higher expression of GhLOX9 and GhLOX20 in stem, both of these genes are also strongly induced in seeds at 10 DPA (FPKM ≥ 30), and more strongly than other GhLOX genes. Their ortholog is AtLOX3, reported to regulate seed development [30]. This suggests that GhLOX21, GhLOX9 and GhLOX20 perform similar functions in cotton to AtLOX1 and AtLOX3 in Arabidopsis. However, this expression trend was not observed in all the GhLOX genes, suggesting that some LOX genes may adopt different physiological functions across species.
Fig. 4.
Expression pattern of GhLOX genes in different tissues of G. hirsutum. The transcriptomic data related to tissue expression were accessed from NCBI and the pheatmap package was used for the generation of heatmaps
Expression analysis of GhLOX genes under different abiotic stresses
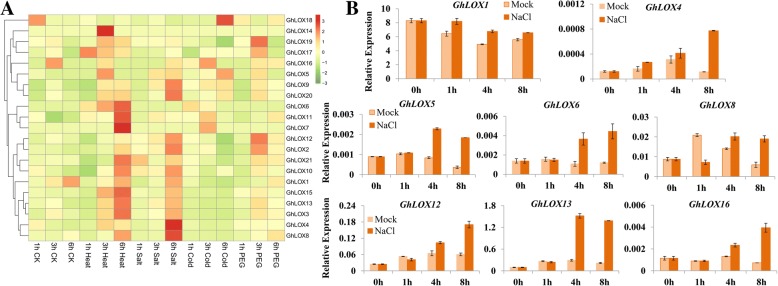
Considering potential roles of LOX genes and their metabolites in various plant species against biotic and abiotic stresses, we investigated the transcript abundance of GhLOX genes in response to heat, salt, polyethylene glycol (PEG) and cold, using transcriptomic data of G. hirsutum provided by Zhang et al. [47]. The results show that all the GhLOX genes were differentially expressed under one or more stresses (Fig. 5a). Comparing four stress factors, more GhLOX genes showed altered expression in response to heat and salinity than to osmotic (PEG) and cold stresses. Interestingly, most of the GhLOX genes which showed altered expression in response to heat, also expressed differentially to salinity stress, suggesting the existence of similar biological processes affected by GhLOX genes in modulating these stress responses in cotton. Eight GhLOX genes were also induced significantly (treatment RPKM/control RPKM ≥ 2) under heat stress, with highest fold change observed for GhLOX7. In response to salinity stress, nine GhLOX genes showed increased expression (treatment RPKM/control RPKM ≥ 1.5) over the 3 h to 6 h time period. Some genes demonstrated interesting expression profiles. For example GhLOX18 was only induced under 6 h cold stress, and GhLOX19 under 3 h PEG stress (treatment RPKM/control RPKM ≥ 2), suggesting potential functions for these genes in cold and osmotic stress in cotton. The expression profiles of selected genes, assessed through quantitative real time polymerase chain reaction (qRT-PCR), further verified the results of the transcriptomic datasets. The results show that most of the selected GhLOX genes were induced after 4 h of salt treatment and maintained high levels of expression up to 8 h (Fig. 5b).
Fig. 5.
Expression profile of GhLOX genes in response to different abiotic stresses. a Abiotic stress related transcriptomic data were obtained from NCBI and its normalization and visualization was performed using the pheatmap package. b The relative expression of selected GhLOX genes under 200 mM salt stress was determined by qRT-PCR. The cotton UBQ7 gene was used as internal control. Error bars denotes the standard deviation calculated from three independent experiments
Expression patterns of GhLOX genes under different exogenous phytohormone treatments
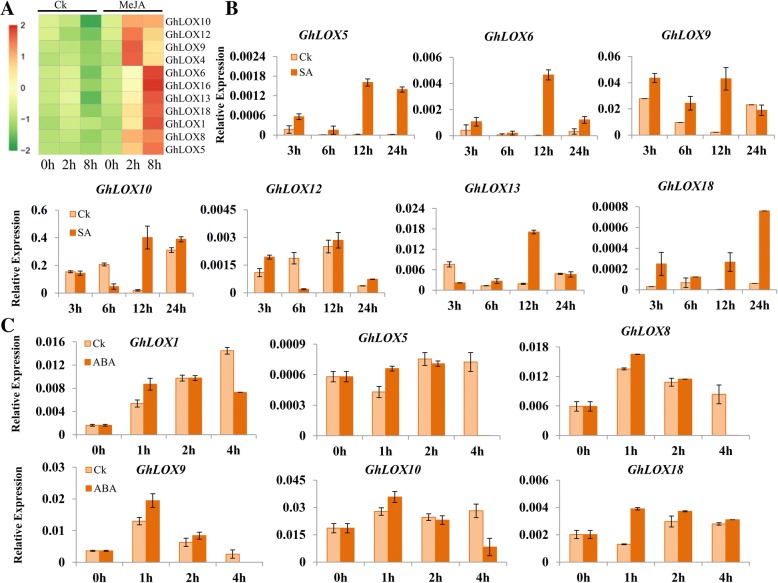
Phytohormones play important roles in alleviating adverse abiotic stresses in plants, along with many other biological functions [17]. To elucidate the potential functions of GhLOX genes in response to exogenous phytohormones, we determined the expression pattern of GhLOX genes by qRT-PCR. From the homologous genes pairs, we selected one pair and designed gene-specific primers. The expression of all the GhLOX genes was significantly induced in response to MeJA (methyl jasmonate) treatment compared to controls (Fig. 6a). Differential expression of some selected genes was observed following SA treatment. Most genes exhibited higher expression by 12 h after treatment (Fig. 6b). Interestingly, following ABA (abscisic acid) treatment most of the selected GhLOX genes were highly induced during the early time periods and reduced during the later time periods (Fig. 6c).
Fig. 6.
Expression analysis of GhLOX genes following treatment with exogenous phytohormones. a Expression levels of GhLOX genes in response to 100 μM MeJA treatment were determined by qRT-PCR analysis and visualized as heat map using the pheatmap package. b Responses of selective GhLOX genes under 1 mM SA were measured by qRT-PCR. c Expression analysis of selective GhLOX genes under 0.5 μM ABA treatment was performed by qRT-PCR. The cotton UBQ7 gene was used as internal control. Error bars denotes the standard deviation calculated from three independent experiments
Silencing of GhLOX12 and GhLOX13 compromises cotton tolerance to salt stress
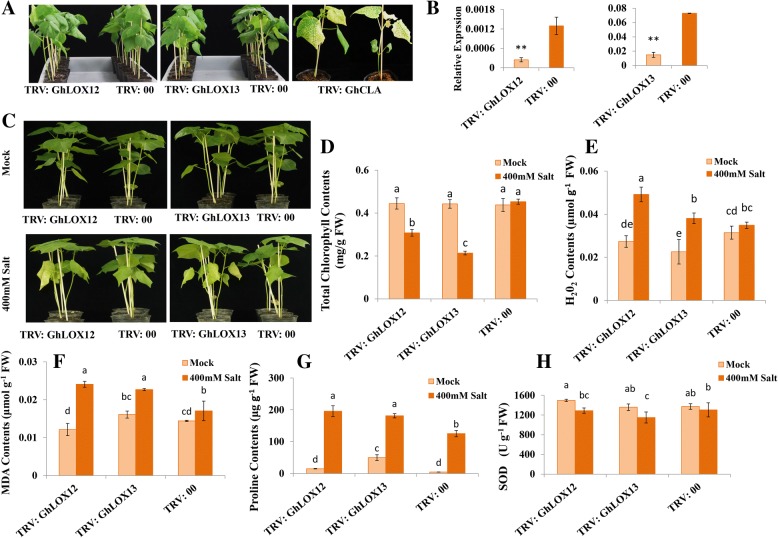
Based on the integration of promoter analysis and differential expression patterns under different abiotic stresses we hypothesized that GhLOX genes are potentially important in the regulation of stress responses. Further salt-induced expression patterns of selective candidate genes inspired us to investigate their functional importance in cotton. To verify our hypothesis, we adopted a VIGS approach to knock down the expression of two LOX genes, GhLOX12 and GhLOX13 using TRV vectors, (TRV: GhLOX12) and (TRV: GhLOX13) respectively. The results revealed that GhLOX12 and GhLOX13 were successfully knocked down in cotton 2 weeks after VIGS operation (Fig. 7b). TRV: GhCLA was used as positive control. Two weeks following VIGS, when positive control plants changed phenotype (Fig. 7a), qRT-PCR was employed to determine the expression levels in the leaves of TRV: GhLOX12, TRV: GhLOX13 and TRV: 00 control plants. The results show that the transcript levels of both GhLOX12 and GhLOX13 were significantly reduced following 2 weeks of VIGS.
Fig. 7.
Silencing of GhLOX12, GhLOX13 compromises tolerance to salt stress in target gene-silenced cotton. a The target gene-silenced, control and positive control plants before salt stress. b Relative expression levels of target gene-silenced and control plants. c Phenotype (d) total chlorophyll contents, e H2O2 contents (f) MDA contents (g) proline contents and (h) SOD activity between target gene-silenced and control plants under mock and salt stress treatment. Experiments were repeated three times, the values represent the means and the error bars show standard deviation and asterisks represents the significant difference at *P ≥ 0.05, **P ≥ 0.01, Student’s t-test, different letters denote the significant difference (P ≥ 0.05) between each other according to LSD test
Half of the target gene-silenced (TRV: GhLOX12, TRV: GhLOX13) and control plants (TRV: 00) were then irrigated with water as mock and half with 400 mM NaCl as salt stress treatment. After 10 days, there was no phenotypic difference between control and target gene-silenced plants in response to water treatment. However, target gene-silenced plants displayed severe wilting and yellowing of leaves compared to control plants (TRV: 00) under salt stress, consistent with an observed decrease in chlorophyll contents (Fig. 7c and d). Abiotic stresses in plants elevate the level of ROS (mainly of O2− and H2O2); which ultimately affect plant metabolism, signal transduction and damages the plant cells. To determine the possible roles of ROS in salt stressed plants; we measured the H2O2 contents in target gene-silenced and control plants under salinity and mock conditions. The H2O2 contents were induced significantly in the leaves of both target gene-silenced plants as compared with control plants under salt stress (Fig. 7e). The malondialdehyde (MDA) level in plants is considered as a marker of abiotic stress response, reflecting membrane damage or injury. Similar to H2O2, MDA levels was significantly higher in target gene-silenced plants as compared to control plants under salt stress (Fig. 7f). Interestingly, proline, which is a non-toxic osmolyte and plays important roles in osmotic adjustment and turgidity of cells under stress conditions, also increased dramatically following salinity treatment both in target gene-silenced and control plants, however there was significant difference between them (Fig. 7g). Furthermore, to explore the function of target gene-silencing in the modulation of antioxidant enzymes, we measured the superoxide dismutase (SOD) activity of salt stressed and mock treated plants. As expected, SOD activity decreased under salt treatment in both target gene-silenced and control plants as compared to mock. The target gene-silenced plants displayed a significantly reduced SOD activity as compared to control plants under salt stress (Fig. 7h).
Discussion
The LOX gene family has been extensively studied in model and non-model plant species for their potential functions in growth, development, and responses to biotic and abiotic stresses. However, previously such efforts were not directed towards understanding the cotton LOX gene family. By using Arabidopsis LOX genes as query, we searched the four cotton genomes, and identified 64 homologous LOX genes. In G. hirsutum, we identified 21 genes, which is a comparatively large gene family relative to the other three species of cotton. Additionally, the numbers of GhLOX genes were not twice that of two diploid cotton species, presumably due to genomic variations occurring during paleopolyploidization in the Gossypium lineage, which ultimately led to the evolution of cultivated tetraploid cotton. Of 13 chromosomes, all the GaLOX and GrLOX genes were distributed among 7 chromosomes while GhLOX genes were found on 7 chromosomes of the Dt subgenome and on 8 chromosomes of the At subgenome. Further systematic analysis is needed to unfold insights into LOX gene family evolution in cotton.
During phylogenetic analysis, the putative LOX genes were grouped into three subfamilies based on their protein structure and similarity. Gene prediction analysis showed that all the 13-LOX type II genes encode chloroplastic proteins, while most of 9-LOX and 13-LOX type I proteins are preferentially localized in the cytoplasm. Multiple sequence alignment of conserved amino acids residues revealed the presence of conserved motifs corresponding to these categories, suggesting the functional conservation among these subfamilies. Gene structure analysis showed that most of the 9-LOX and 13-LOX type I GhLOX genes shared similar structures. However, we observed some variation in the structure of 13-LOX type II genes, which might be explained by their functional diversification. To gain further insights into the regulation of GhLOX genes under changing environmental conditions, we investigated the cis-regulatory elements inside their promoter regions. Mainly five types of cis-acting elements were identified, including defense/stress-related (biotic and abiotic), tissue specific/development-related, hormones-responsive, light-responsive and circadian-related. Such a wide spectrum of cis-acting elements is consistent with the published reports about the multifunctional roles of LOX genes in plants [13].
The expression profile of GhLOX genes in different tissues revealed that most of the genes are expressed in vegetative tissues, suggesting that GhLOX genes play important roles in the development of vegetative tissues in cotton. In addition, significantly higher expression of some genes in only specific tissues, such as GhLOX14 in fiber at 20 DPA, GhLOX6 in seed at 20 DPA, and GhLOX17 and GhLOX19 in stigma suggests likely functional importance in these organs.
The expression pattern of GhLOX genes obtained from transcriptomic data under abiotic stresses revealed that most of the genes are associated with heat and salt stress simultaneously and there might be a similar mechanism of tolerance prevailing in cotton under these stresses. However, we observed different expression pattern of some genes during heat and salt stress, such as GhLOX6–7 and GhLOX14, which were only responsive to heat stress, and no changes in expression were observed for salt stress. Moreover, the results obtained in expression analysis of selective LOX genes under salt stress through qRT-PCR were in agreement with the transcriptomic dataset. The observed significant induction of GhLOX genes in response to phytohormones further supports their probable involvement in modulating stress conditions. More intriguingly, the comparative analysis of significantly induced genes under heat and salt stress and their promoter sequences might help explain the reasons for their responsiveness to these stresses. For example, GhLOX12 harbored 3 HSE, 1 MBS, 1 LTR (low temperature-responsive), 2 ARE and 2 TC-rich repeat elements.
Salinity is an increasing problem for world agriculture; it affects more than 20% of irrigated areas [48]. Cotton, a relatively salt tolerant crop is also nevertheless adversely affected by salinity during germination and seedling stages of development. Losses can reach 50% of seed yield, if the salinity is above a threshold level [49]. Our genome-wide screening of LOX genes in cotton, their phylogeny, promoter and expression analysis suggests their potential in modulating salinity tolerance. VIGS silencing of two LOX genes, GhLOX12 and GhLOX13 led to a compromised plant tolerance to salt stress, confirming their functional importance.
To explore the possible mechanism of their sensitivity, we analyzed some physiological parameters including total chlorophyll contents, accumulation of H2O2, MDA, proline, and activity of SOD both under salt stress and mock treatment. The results showed that silencing of the target LOX genes in cotton significantly effects the growth and physiology under salinity. Some physiological traits were particularly adversely affected by silencing of GhLOX12 and GhLOX13. Chlorophyll contents and activity of antioxidant enzyme (SOD) were significantly decreased in LOX gene-silenced plants, whereas H2O2, MDA, and proline contents were found to be increased significantly under salt stress. Abiotic stresses often result in overproduction of ROS, which cause the lipid peroxidation, interfering with normal physiological processes and ultimately leading to programmed cell death. The scavenging of ROS is an important mechanism of tolerance against salt and osmotic stresses [50]. The higher accumulation of H2O2, MDA and reduced SOD activity after silencing of GhLOX12 and GhLOX13 suggests the reduced ability of silenced plants to properly scavenge ROS that leads to membrane damages and chlorophyll reduction. These results are consistent with previous studies where LOX genes have been implicated in salt stress tolerance by modulating ROS [20, 21]. The increased contents of proline in silenced plants following salinity treatment might be explained by the need of a substitution process to effectively detoxify elevated ROS. Substitution processes may be necessary when the activities of antioxidant enzymes such as SOD are decreased in silenced plants [51].
Conclusion
The decoding of cotton genomes allowed us to perform a genome-wide screening for LOX family genes. Through a systematic approach, comprising analysis of gene structure, phylogeny, motif composition, promoter structure, and expression patterns in diverse tissues, in response to signaling molecules, and under various abiotic stress factors, we were able to comprehensively characterize the LOX family genes in cotton. Results suggest the conservation and diversity of sequence features and functions among LOX family genes in different species of cotton. Moreover, the functional characterization of two LOX genes (GhLOX12 and GhLOX13) through a VIGS approach supported their role in salinity tolerance, possibly via regulating ROS. This comprehensive analysis provides a foundation for future functional studies to decipher the functional roles of all LOX genes in cotton and potentially other species. The data presented here may help in the selection of appropriate candidate genes for further functional characterization related to a specific aspect of cotton development or under stress conditions.
Methods
Isolation and sequence retrieval of LOX gene family from four species of cotton
The genome assemblies of four cotton species (G. arboreum, G. raimondii, G. barbadense and G. hirsutum) were retrieved from cottongen (https://www.cottongen.org). The sequences of Arabidopsis were accessed from TAIR website (https://www.Arabidopsis.org/). By employing the BLAST+ program [52] Arabidopsis LOX genes were used as query to identify putative LOX genes in four cotton genomes. All the putative LOX genes were checked for the presence of LOX domain through pfam database (http://pfam.sanger.ac.uk/) [53], NCBI Conserved Domain Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) [54], SMART (http://smart.embl-heidelberg.de/) [55] and InterProScan programs (http://www.ebi.ac.uk/Tools/InterProScan/) [56]. Moreover, KEGG pathway numbers were determined by extracting and mapping gene ontology (GO) terms through Blast2GO program [57, 58]. Several of the redundant sequences were removed for not having the complete domain or shortness.
Phylogenetic and subcellular localization analysis
Multiple sequence alignments of selected LOX genes were performed through ClustalX software (ver. 1.83) and conserved motifs were manually screened and highlighted using online shading tool (http://www.bioinformatics.org/sms2/color_align_cons.html). Further, conserved amino acid sequences were employed to Molecular Evolutionary Genetics Analysis (MEGA 7) software for construction of phylogenetic tree, with Neighbor Joining method having 1000 bootstrap values [59]. Subcellular localization prediction of LOX genes were carried out using three publically available web tools ProtComp 9.0 (http://linux1.softberry.com/berry.phtml), Target P1.1 (http://www.cbs.dtu.dk/services/TargetP) and PSORT (http://wolfpsort.org/) [60].
Genes structure, conserved motifs and promoter analysis
The organization of exon/intron boundaries was carried out online via accessing Gene Structure Display Server (GSDS, V.2) (http://gsds.cbi.pku.edu.cn/) [61]. MEME Suite (http://meme-suite.org/index.html) [62] was employed for identification of conserved motifs, with default setting except changes in number of motifs (10) and width (upto 200 residues). The structural annotation of distinct motifs was carried out using Pfam database [53]. The potential cis-elements in the promoter sequences of GhLOX genes were identified using PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Plant material and treatment for expression analysis
The transcriptomic data of G. hirsutum ‘TM1’ related to tissue expression and in response to various abiotic stress factors were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA248163). The resulting expression data were then used for generation of heatmaps by pheatmap package (pheatmap: R package version 1.0.8, https://CRAN.R-project.org/package=pheatmap).
To check the expression profile of GhLOX genes by qRT-PCR, G. hirsutum cv. YZ1 seedlings were grown in Hoagland solution under controlled conditions (25 °C,16 h light/8 h dark cycle). After 4 weeks, plants were subjected to Hoagland solution containing water as mock treatment or 200 mM NaCl as salt stress treatment, or 100 μM MeJA, 1 mM SA and 0.5 μM ABA as hormones treatments. Leaf samples were taken at desired time points and stored for subsequent RNA extraction.
Vector construction and procedure for VIGS in cotton
Three hundred seventy-seven bp and 400 bp fragments from the coding DNA sequence of GhLOX12 and GhLOX13 were amplified from G. hirsutum cv. Y668 cDNA, respectively and subsequently introduced into the TRV: 00 plasmid. DNA was digested with restriction enzymes BamHI and KpnI to generate the TRV: GhLOX12 and TRV: GhLOX13. The TRV: GhLOX12, TRV: GhLOX13 and TRV1 construct were inserted into A. tumefaciens strain GV3101 by electroporation. We followed the same procedure for VIGS in cotton as mentioned previously by Gao et al. [63]. The primers used for vectors construction are mentioned in Additional file 12: Table S10.
Plant material and salt tolerance assays for functional analysis of GhLOX genes
G. hirsutum cv. Y668 seedlings were grown in small pots of soil under controlled condition (25 °C, 16 h/8 h light/dark period) in incubator. After verifying the VIGS efficiency through qRT-PCR, the roots of both control and target gene-silenced plants were irrigated with water as mock treatments and 400 mM NaCl as salt stress up to 10 days. The VIGS experiments were repeated with three replicates, and 40 plants were used during each replication. Half of the plants were treated with NaCl for salt stress tolerance assay and half of the plants were treated with water as mock.
Determination of salt stress-related physiological parameters
Total chlorophyll contents were determined from 0.1 g cotton leaf tissues and 80% acetone was used as extraction buffer. The absorbance was measured at 663 nm and 645 nm using a Multimode plate reader. For calculation of total chlorophyll contents, we followed the method described by Sun et al. [64]. For quantification of H2O2 contents, 0.1 g samples of cotton leaves were prepared and followed the procedure as described in H2O2 Quantification Assay Kit (Sangon Biotech, China). The measurement of proline contents and SOD enzyme activity from stressed and control plants were as described by Yu et al. [65]. To assess the lipid peroxidation, we measured the MDA contents following the procedure of Hodges et al. [66].
qRT-PCR analysis
To investigate gene expression patterns, total RNA was extracted from leaves of G. hirsutum following the protocol of Tu et al. [67]. RNA was reverse transcribed into cDNA using M-mlv reverse transcript system (Promega, USA). qRT-PCR was performed on an ABI 7500 real time PCR system (Applied Biosystem, Foster City, CA, USA) as described by Xu et al. [68]. The fold changes were calculated using the comparative CT method (2-∆∆Ct) and GhUBQ7 was amplified as an internal control. Gene-specific primers were designed based on cDNA sequences. All the primers used in this study were designed using the software primer premier 5 and listed in Additional file 12: Table S10.
Additional files
Table S1. Nomenclature of LOX gene family in four species of cotton. (XLSX 11 kb)
Figure S1. Multiple sequence alignment of LOX gene family in four species of cotton. Circled boxes show the representative 38 amino acids motif in G. arboreum (A), G. raimondii (B), G. barbadense (C), and G. hirsutum (D). (PDF 653 kb)
Table S2. Properties of LOX gene family in four species of cotton. (XLSX 13 kb)
Table S3. Chromosomal location of LOX gene family in four species of cotton. (XLSX 11 kb)
Table S4. Similarity index of duplicated gene pairs between At to A2 and Dt to D5 subgenomes. (XLSX 9 kb)
Table S5. Protein sequences used for construction of phylogenetic tree. (XLSX 31 kb)
Figure S2. The 9-LOX and 13-LOX specific motif among LOX gene family in four species of cotton. The encircled column represents the specific motif in G. arboreum (A), G. raimondii (B), G. barbadense (C), and G. hirsutum (D). (PDF 478 kb)
Table S6. Subcellular localization of LOX gene family in four species of cotton. (XLSX 47 kb)
Table S7. The 10 distinct motifs found in GhLOX genes. (XLSX 10 kb)
Table S8. cis-acting elements in the promoters of GhLOX genes. The values represent the numbers of specific cis-acting elements in each GhLOX genes. (XLSX 11 kb)
Table S9. GhLOX genes tissue expression profile. The data represents the FPKM values. (XLSX 12 kb)
Table S10. Primers used in this Study. (DOCX 16 kb)
Acknowledgements
The author is thankful to Huazhong Agricultural University for providing scholarship during the study and Professor Keith Lindsay for revising the manuscript.
Funding
Funding support was provided by the Natural Science Foundation of Hubei (2016CFA054) and the basic research project of the Xinjiang production and Construction Corps (2016AG001). The funding agencies have no role in designing of study, data collection, analysis, decision to publish and in the preparation of the manuscript. There is no conflict of interest.
Availability of data and materials
The data supporting the results of this study are included in manuscript and its additional files.
Abbreviations
- 13-HPOD
(13S)-hydroperoxyoctadecadienoic acid
- 9-HPOD
(9S)-hydroperoxyoctadecadienoic acid
- ABA
Abscisic acid
- FPKM
Fragments per kilobase of transcript per million mapped reads
- GAs
Gibberellins
- JA
Jasmonic acid
- LOX
Lipoxygenase
- MeJA
Methyl jasmonate
- OPDA
(+)-12-oxo-phytodienoic acid
- qRT-PCR
Quantitative Real time Polymerase Chain Reaction
- ROS
Reactive Oxygen Species
- SA
Salicylic acid
- SOD
Superoxide dismutase
- VIGS
Virus induced gene silencing
Authors’ contributions
MS designed and carried out the experiments, and also wrote the manuscript, MMA and HS contributed in bioinformatics analysis, AU participated in data analysis and LZ directed and revised the manuscript. All the authors read, discussed and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang N, Ma J, Pei W, Wu M, Li H, Li X, Yu S, Zhang J, Yu J. A genome-wide analysis of the lysophosphatidate acyltransferase (LPAAT) gene family in cotton: organization, expression, sequence variation, and association with seed oil content and fiber quality. BMC Genomics. 2017;18(1):218. doi: 10.1186/s12864-017-3594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djanaguiraman M, Prasad PV. Effects of salinity on ion transport, water relations and oxidative damage. In: Ecophysiology and Responses of Plants under Salt Stress. New York: Springer; 2013. p. 89–114.
- 3.Hasanuzzaman M, Alam MM, Nahar K, Ahamed KU, Fujita M. Exogenous salicylic acid alleviates salt stress-induced oxidative damage in Brassica napus by enhancing the antioxidant defense and glyoxalase systems. Aust J Crop Sci. 2014;8(4):631. [Google Scholar]
- 4.Ashraf M. Salt tolerance of cotton: some new advances. Crit Rev Plant Sci. 2002;21(1):1–30. doi: 10.1080/0735-260291044160. [DOI] [Google Scholar]
- 5.Nafie E, Hathout T, Mokadem A, Shyma A. Jasmonic acid elicits oxidative defense and detoxification systems in Cucumis melo L. cells. Braz J Plant Physiol. 2011;23(2):161–174. doi: 10.1590/S1677-04202011000200008. [DOI] [Google Scholar]
- 6.Itoh A, Howe GA. Molecular cloning of a Divinyl ether synthase identification as a CYP74 cytochrome P-450. J Biol Chem. 2001;276(5):3620–3627. doi: 10.1074/jbc.M008964200. [DOI] [PubMed] [Google Scholar]
- 7.Gouinguene SP, Turlings TC. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002;129(3):1296–1307. doi: 10.1104/pp.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreou A, Brodhun F, Feussner I. Biosynthesis of oxylipins in non-mammals. Prog Lipid Res. 2009;48(3–4):148–170. doi: 10.1016/j.plipres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Hornung E, Walther M, Kühn H, Feussner I. Conversion of cucumber linoleate 13-lipoxygenase to a 9-lipoxygenating species by site-directed mutagenesis. Proc Natl Acad Sci. 1999;96(7):4192–4197. doi: 10.1073/pnas.96.7.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274(34):23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 11.Shibata D, Axelrod B. Plant lipoxygenases. J Lipid Mediat Cell Signal. 1995;12(2–3):213–228. doi: 10.1016/0929-7855(95)00020-Q. [DOI] [PubMed] [Google Scholar]
- 12.Blee E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002;7(7):315–322. doi: 10.1016/S1360-1385(02)02290-2. [DOI] [PubMed] [Google Scholar]
- 13.Porta H, Rocha-Sosa M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002;130(1):15–21. doi: 10.1104/pp.010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosblech A, Feussner I, Heilmann I. Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem. 2009;47(6):511–517. doi: 10.1016/j.plaphy.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- 16.Elkahoui S, Hernández JA, Abdelly C, Ghrir R, Limam F. Effects of salt on lipid peroxidation and antioxidant enzyme activities of Catharanthus roseus suspension cells. Plant Sci. 2005;168(3):607–613. doi: 10.1016/j.plantsci.2004.09.006. [DOI] [Google Scholar]
- 17.Sun H, Chen L, Li J, Hu M, Ullah A, He X, Yang X, Zhang X. The JASMONATE ZIM-domain gene family mediates JA signaling and stress response in cotton. Plant Cell Physiol. 2017;58(12):2139–2154. doi: 10.1093/pcp/pcx148. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Dong W, Zhang N, Ai X, Wang M, Huang Z, Xiao L, Xia G. A wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiol. 2014;164(2):1068–1076. doi: 10.1104/pp.113.227595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu Z, Guo J, Zhu A, Zhang L, Zhang M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol Environ Saf. 2014;104:202–208. doi: 10.1016/j.ecoenv.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Lim CW, Han SW, Hwang IS, Kim DS, Hwang BK, Lee SC. The pepper lipoxygenase CaLOX1 plays a role in osmotic, drought and high salinity stress response. Plant Cell Physiol. 2015;56(5):930–942. doi: 10.1093/pcp/pcv020. [DOI] [PubMed] [Google Scholar]
- 21.Hou Y, Meng K, Han Y, Ban Q, Wang B, Suo J, Lv J, Rao J. The persimmon 9-lipoxygenase gene DkLOX3 plays positive roles in both promoting senescence and enhancing tolerance to abiotic stress. Front Plant Sci. 2015;6:1073. doi: 10.3389/fpls.2015.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Jin YZ, Liu JY, Tang YF, Cao SX, Qi HY. The phylogeny and expression profiles of the lipoxygenase (LOX) family genes in the melon (Cucumis melo L.) genome. Sci Hortic. 2014;170:94–102. doi: 10.1016/j.scienta.2014.03.005. [DOI] [Google Scholar]
- 23.Bae KS, Rahimi S, Kim YJ, Devi BSR, Khorolragchaa A, Sukweenadhi J, Silva J, Myagmarjav D, Yang DC. Molecular characterization of lipoxygenase genes and their expression analysis against biotic and abiotic stresses in Panax ginseng. Eur J Plant Pathol. 2016;145(2):331–343. doi: 10.1007/s10658-015-0847-9. [DOI] [Google Scholar]
- 24.Yang XY, Jiang WJ, Yu HJ. The expression profiling of the lipoxygenase (LOX) family genes during fruit development, abiotic stress and hormonal treatments in cucumber (Cucumis sativus L.) Int J Mol Sci. 2012;13(2):2481–2500. doi: 10.3390/ijms13022481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Chen X, Yan H, Li W, Li Y, Cai R, Xiang Y. The lipoxygenase gene family in poplar: identification, classification, and expression in response to MeJA treatment. PLoS One. 2015;10(4):e0125526. doi: 10.1371/journal.pone.0125526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mostofa MG, Hossain MA, Fujita M. Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma. 2015;252(2):461–475. doi: 10.1007/s00709-014-0691-3. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Hayyim G, Gueta-Dahan Y, Avsian-Kretchmer O, Weichert H, Feussner I. Preferential induction of a 9-lipoxygenase by salt in salt-tolerant cells of Citrus sinensis L. Osbeck. Planta. 2001;212(3):367–375. doi: 10.1007/s004250000397. [DOI] [PubMed] [Google Scholar]
- 28.Delaplace P, Frettinger P, Ghanem ME, Blondiaux A, Bauwens J, Cotton S, De Clerck C, Dewalque A, Guy J, Heuze F. Lipoxygenase pathway and antioxidant system in salt stressed tomato seedlings (Lycopersicon esculentum Mill.) Biotechnol Agron Soc Environ. 2009;13(4):529. [Google Scholar]
- 29.Molina A, Bueno P, Marín MC, Rodríguez-Rosales MP, Belver A, Venema K, Donaire JP. Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl. New Phytol. 2002;156(3):409–415. doi: 10.1046/j.1469-8137.2002.00527.x. [DOI] [PubMed] [Google Scholar]
- 30.Bannenberg G, Martinez M, Hamberg M, Castresana C. Diversity of the enzymatic activity in the lipoxygenase gene family of Arabidopsis thaliana. Lipids. 2009;44(2):85–95. doi: 10.1007/s11745-008-3245-7. [DOI] [PubMed] [Google Scholar]
- 31.Umate P. Genome-wide analysis of lipoxygenase gene family in Arabidopsis and rice. Plant Signal Behav. 2011;6(3):335–338. doi: 10.4161/psb.6.3.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin JH, Van K, Kim DH, Do Kim K, Jang YE, Choi B-S, Kim MY, Lee S-H. The lipoxygenase gene family: a genomic fossil of shared polyploidy between Glycine max and Medicago truncatula. BMC Plant Biol. 2008;8(1):133. doi: 10.1186/1471-2229-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolomiets M, Navarro P, Zhang J, Yalpani N, Simmons C, Meeley R, Duvick J. Identification and characterization of the lipoxygenase gene family of maize. Mycopathologia. 2004;157(4):501. [Google Scholar]
- 34.Mariutto M, Duby F, Adam A, Bureau C, Fauconnier ML, Ongena M, Thonart P, Dommes J. The elicitation of a systemic resistance by Pseudomonas putida BTP1 in tomato involves the stimulation of two lipoxygenase isoforms. BMC Plant Biol. 2011;11(1):29. doi: 10.1186/1471-2229-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu SQ, Liu XH, Jiang LW. Genome-wide identification, phylogeny and expression analysis of the lipoxygenase gene family in cucumber. Genet Mol Res. 2011;10(4):2613–2636. doi: 10.4238/2011.October.25.9. [DOI] [PubMed] [Google Scholar]
- 36.Sanier C, Sayegh-Alhamdia M, Jalloul A, Clerivet A, Nicole M, Marmey P. A 13-lipoxygenase is expressed early in the hypersensitive reaction of cotton plants to Xanthomonas campestris pv. Malvacearum. J Phytopathol. 2012;160(6):286–293. doi: 10.1111/j.1439-0434.2012.01900.x. [DOI] [Google Scholar]
- 37.Marmey P, Jalloul A, Alhamdia M, Assigbetse K, Cacas JL, Voloudakis AE, Champion A, Clerivet A, Montillet JL, Nicole M. The 9-lipoxygenase GhLOX1 gene is associated with the hypersensitive reaction of cotton Gossypium hirsutum to Xanthomonas campestris pv malvacearum. Plant Physiol Biochem. 2007;45(8):596–606. doi: 10.1016/j.plaphy.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Feussner I, Wasternack C. The lipoxygenase pathway. Annu Rev Plant Biol. 2002;53(1):275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Ouyang Y, Yao W. shinyCircos: an R/Shiny application for interactive creation of Circos plot. Bioinformatics. 2017;1:3. doi: 10.1093/bioinformatics/btx763. [DOI] [PubMed] [Google Scholar]
- 40.Andreou A, Feussner I. Lipoxygenases–structure and reaction mechanism. Phytochemistry. 2009;70(13–14):1504–1510. doi: 10.1016/j.phytochem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Vellosillo T, Martinez M, Lopez MA, Vicente J, Cascon T, Dolan L, Hamberg M, Castresana C. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell. 2007;19(3):831–846. doi: 10.1105/tpc.106.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez MA, Vicente J, Kulasekaran S, Vellosillo T, Martinez M, Irigoyen ML, Cascon T, Bannenberg G, Hamberg M, Castresana C. Antagonistic role of 9-lipoxygenase-derived oxylipins and ethylene in the control of oxidative stress, lipid peroxidation and plant defence. Plant J. 2011;67(3):447–458. doi: 10.1111/j.1365-313X.2011.04608.x. [DOI] [PubMed] [Google Scholar]
- 43.Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S. Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol. 2013;161(4):2159–2170. doi: 10.1104/pp.113.214544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caldelari D, Wang G, Farmer EE, Dong X. Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol Biol. 2011;75(1–2):25–33. doi: 10.1007/s11103-010-9701-9. [DOI] [PubMed] [Google Scholar]
- 45.Mochizuki S, Sugimoto K, Koeduka T, Matsui K. Arabidopsis lipoxygenase 2 is essential for formation of green leaf volatiles and five-carbon volatiles. FEBS Lett. 2016;590(7):1017–1027. doi: 10.1002/1873-3468.12133. [DOI] [PubMed] [Google Scholar]
- 46.Biłas R, Szafran K, Hnatuszko-Konka K, Kononowicz AK. Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult. 2016;127(2):269–287. doi: 10.1007/s11240-016-1057-7. [DOI] [Google Scholar]
- 47.Zhang T, Hu Y, Jiang W, Fang L, Guan X, Chen J, Zhang J, Saski CA, Scheffler BE, Stelly DM, et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat Biotechnol. 2015;33(5):531–537. doi: 10.1038/nbt.3207. [DOI] [PubMed] [Google Scholar]
- 48.Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD. Economics of salt-induced land degradation and restoration. Natural Resources Forum. 2014;38(4):282-95.
- 49.Ahmad S, Khan N, Iqbal MZ, Hussain A, Hassan M. Salt tolerance of cotton (Gossypium hirsutum L.) Asian J Plant Sci. 2002;1(6):715–719. doi: 10.3923/ajps.2002.715.719. [DOI] [Google Scholar]
- 50.Ullah A, Sun H, Hakim YX, Zhang X. A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol Plant. 2018;162(4):439–454. doi: 10.1111/ppl.12651. [DOI] [PubMed] [Google Scholar]
- 51.Anaraki ZE, Shariati M, Tafreshi SAH. Transient silencing of phytoene desaturase reveals critical roles on plant response to salinity stress. Acta Physiol Plant. 2017;39(8):161.
- 52.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinf. 2009;10(1):421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finn RD, Mistry J, Schuster-Bockler B, Griffiths-Jones S, Hollich V, Lassmann T, Moxon S, Marshall M, Khanna A, Durbin R, et al. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34(Database issue):D247–D251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchler-Bauer A, Anderson JB, Cherukuri PF, DeWeese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, et al. CDD: a conserved domain database for protein classification. Nucleic Acids Res. 2005;33(Database issue):D192–D196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40(Database issue):D302–D305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R. InterProScan: protein domains identifier. Nucleic Acids Res. 2005;33(Web Server issue):W116–W120. doi: 10.1093/nar/gki442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conesa A, Gotz S. Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics. 2008;2008:619832. doi: 10.1155/2008/619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35(Web Server issue):W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo AY, Zhu QH, Chen X, Luo JC. GSDS: a gene structure display server. Yi chuan = Hereditas. 2007;29(8):1023–1026. doi: 10.1360/yc-007-1023. [DOI] [PubMed] [Google Scholar]
- 62.Bailey TL, Williams N, Misleh C, Li WW. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34(Web Server issue):W369–W373. doi: 10.1093/nar/gkl198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao W, Long L, Xu L, Lindsey K, Zhang X, Zhu L. Suppression of the homeobox gene HDTF1 enhances resistance to Verticillium dahliae and Botrytis cinerea in cotton. J Integr Plant Biol. 2016;58(5):503–513. doi: 10.1111/jipb.12432. [DOI] [PubMed] [Google Scholar]
- 64.Sun XL, Ji W, Ding XD, Bai X, Cai H, Yang SS, Qian X, Sun MZ, Zhu YM. GsVAMP72, a novel Glycine soja R-SNARE protein, is involved in regulating plant salt tolerance and ABA sensitivity. Plant Cell Tissue Organ Cult. 2013;113(2):199–215. doi: 10.1007/s11240-012-0260-4. [DOI] [Google Scholar]
- 65.Yu LH, Wu SJ, Peng YS, Liu RN, Chen X, Zhao P, Xu P, Zhu JB, Jiao GL, Pei Y, et al. Arabidopsis EDT1/HDG11 improves drought and salt tolerance in cotton and poplar and increases cotton yield in the field. Plant Biotechnol J. 2016;14(1):72–84. doi: 10.1111/pbi.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207(4):604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- 67.Tu LL, Zhang XL, Liang SG, Liu DQ, Zhu LF, Zeng FC, Nie YC, Guo XP, Deng FL, Tan JF, et al. Genes expression analyses of sea-island cotton (Gossypium barbadense L.) during fiber development. Plant Cell Rep. 2007;26(8):1309–1320. doi: 10.1007/s00299-007-0337-4. [DOI] [PubMed] [Google Scholar]
- 68.Xu L, Zhang W, He X, Liu M, Zhang K, Shaban M, Sun L, Zhu J, Luo Y, Yuan D, et al. Functional characterization of cotton genes responsive to Verticillium dahliae through bioinformatics and reverse genetics strategies. J Exp Bot. 2014;65(22):6679–6692. doi: 10.1093/jxb/eru393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Nomenclature of LOX gene family in four species of cotton. (XLSX 11 kb)
Figure S1. Multiple sequence alignment of LOX gene family in four species of cotton. Circled boxes show the representative 38 amino acids motif in G. arboreum (A), G. raimondii (B), G. barbadense (C), and G. hirsutum (D). (PDF 653 kb)
Table S2. Properties of LOX gene family in four species of cotton. (XLSX 13 kb)
Table S3. Chromosomal location of LOX gene family in four species of cotton. (XLSX 11 kb)
Table S4. Similarity index of duplicated gene pairs between At to A2 and Dt to D5 subgenomes. (XLSX 9 kb)
Table S5. Protein sequences used for construction of phylogenetic tree. (XLSX 31 kb)
Figure S2. The 9-LOX and 13-LOX specific motif among LOX gene family in four species of cotton. The encircled column represents the specific motif in G. arboreum (A), G. raimondii (B), G. barbadense (C), and G. hirsutum (D). (PDF 478 kb)
Table S6. Subcellular localization of LOX gene family in four species of cotton. (XLSX 47 kb)
Table S7. The 10 distinct motifs found in GhLOX genes. (XLSX 10 kb)
Table S8. cis-acting elements in the promoters of GhLOX genes. The values represent the numbers of specific cis-acting elements in each GhLOX genes. (XLSX 11 kb)
Table S9. GhLOX genes tissue expression profile. The data represents the FPKM values. (XLSX 12 kb)
Table S10. Primers used in this Study. (DOCX 16 kb)
Data Availability Statement
The data supporting the results of this study are included in manuscript and its additional files.