Abstract

Many factors affect the variation in the exposome. We examined the influence of shared household and partner’s sex in relation to the variation in 128 endocrine disrupting chemical (EDC) exposures among couples. In a cohort comprising of 501 couples trying for pregnancy, we measured 128 (13 chemical classes) persistent and nonpersistent EDCs and estimated 1) sex-specific differences; 2) variance explained by shared household; and 3) Spearman’s rank correlation coefficients (rs) for females, males, and couples’ exposures. Sex was correlated with 8 EDCs including per- and polyfluoroalkyl substances (PFASs) (p < 0.05). Shared household explained 43% and 41% of the total variance for PFASs and blood metals, respectively, but less than 20% for the remaining 11 EDC classes. Coexposure patterns of the exposome were similar between females and males, with within-class rs higher for persistent than for nonpersistent chemicals. Median rss of polybrominated compounds and urine metalloids were 0.45 and 0.09, respectively, for females (0.41 and 0.08 for males; 0.21 and 0.04 for couples). Our findings suggest that individual, rather than shared environment, could be a major factor influencing the covariation of the exposome. Understanding the correlations of exposures has important analytical and sampling implications for exposomics research.
Introduction
Variation of human disease and phenotype is typically partitioned into genetic and environmental components, with the latter fraction being further divided into shared and unique environments. Therefore, understanding the effects of shared factors are important for environmental epidemiologic investigation. Variation of environmental exposure levels in the population is a complex phenomenon and is influenced by factors shared by individuals — such as those within a household — and nonshared factors specific to individuals, such as sex and individual lifestyle. One approach to investigate the role of environment is the paradigm of the exposome,1 a concept that attempts to capture the totality of exposures from conception onward to comprehensively characterize the individual-level and shared differences of exposure. However, the exposome is a dynamic entity with variations across time (temporal) and place (spatial)2, underscoring the importance of considering sources of variability when assessing human health.
Instead of trying to measure “all” — if possible — lifetime exposures, investigators may focus on critical and sensitive time windows in human development such as pregnancy, infancy, childhood, and adolescence to capture temporal complexity efficiently.3 Furthermore, household-level ascertainments of exposure (i.e., sampling individuals from households) have been posited to be sufficient surrogates for all individuals in the household.4 These assumptions may help characterize the exposome and study its time-dependent relationship with health outcomes. For example, the Human Early-Life Exposome (HELIX) project seeks to define the pregnancy and early life exposomes and health,5 while the EXPOsOMICS project has its conceptual framework of a life-course approach to a broader range of exposures.6
However, one outstanding challenge of implementing the exposome paradigm in research includes the difficulty of interpreting exposure-disease associations due to the dense correlations among all exposures.7 The dense correlation pattern between exposure levels makes it hard to identify the directionality of the potential causal relation between exposures and outcome.8 Second, correlations between exposures vary (e.g., absolute median correlation from almost 0 to above 0.5), and, thus, there is no universal scale to assess the biological significance.9 In addition, exposome-wide, or equivalently, environment-wide association studies (EWASs) assess all the associations between exposures and an outcome to identify potential association signals.10 The data-driven approach assumes little to no collinearity between environmental predictors, but it is almost impossible to select any single uncorrelated exposures out from the dense exposome. One strategy for addressing these analytical issues is to characterize the correlations in diverse cohorts to provide reference levels to gauge biological significance of associations.
We investigated whether cohabiting couples trying for pregnancy would have similar concentrations of endocrine disrupting chemicals (EDCs) given their shared households and whether concentrations varied across partners in light of their individual exposures arising from other environments such as lifestyle, recreation, or occupation. We posit that EDC levels are highly correlated between partners of the same household given their shared exposures. This avenue of study is important given that EDCs have been found to affect human fecundity and fertility,11,12 though much of the available evidence relies on research conducted in either men or women but not couples. We utilized the Longitudinal Investigation of Fertility and the Environment (LIFE) Study to empirically assess couples’ shared and individual variations in a mixture of EDCs. We selected the LIFE Study because it has 13 classes of EDCs quantified in both partners of the couple in keeping with the couple based nature of human reproduction.13−18 Although the scope of exposure assessment in LIFE is far from reaching the all-encompassing exposome, we can still sample the prepregnancy exposome in the context of shared environment. Specifically, we sought to 1) estimate the EDC variability explained by shared environment; 2) test the sex-specific differences of EDCs; and 3) characterize the correlation structure of couples’ EDC concentrations to understand the potential covariations influenced by sex. Lastly, we discuss the important implications of the findings relative to sampling and analytical considerations for future exposome-related research.
Methods
Study Design and Cohort
Briefly, the LIFE Study enrolled 501 couples planning to discontinue contraception to become pregnant from 16 counties in Michigan and Texas, 2005–2009. Couples were followed daily until pregnant or up to 12 months of trying to become pregnant (infertile). Study participants were screened for eligibility criteria: 1) married or in a committed relationship; 2) females aged 18–40 years and men aged 18+ years; 3) capable of communicating in English or Spanish; and 4) no physician-diagnosed infertility. A complete description of study methods including enrollment has been previously published.19
Data Collection and Toxicologic Analysis
Following enrollment and completion of the baseline interview, nurses obtained preconception nonfasting blood (20 mL) and urine (120 mL) samples from each partner of the couple using equipment free of the contaminants under study. Samples were frozen at −20 °C or colder prior to the quantification of EDCs or naturally occurring phytoestrogens. We also included serum cotinine, a metabolite of nicotine, along with 128 chemicals representing 13 different chemical classes (Table 1). Persistent EDCs included 4 classes of serum persistent organic compounds, 36 congeners of polychlorinated biphenyls (PCBs), 9 organochlorine pesticides (OCPs), 11 polybrominated chemicals [polybrominated diphenyl ethers (PBDEs) and 1 polybrominated biphenyl (PBB)], and 7 per- and polyfluoroalkyl substances (PFASs), and were quantified by a single laboratory using published standard operating procedures. In short, after solid-phase extraction, serum concentrations of PCBs, OCPs, PBDEs, and PBB were measured by high resolution gas chromatography-high resolution mass spectrometry using an isotope dilution method and of PFASs using liquid chromatography-tandem spectrometry using an isotope dilution method.20,21
Table 1. List of Chemical Classes and Measured Chemicals in the Current Study19.
| EDC class | no. | individual chemicals | medium | LOQe |
|---|---|---|---|---|
| polychlorinated biphenyls (PCBs) | 36 | congeners: 28, 44, 49, 52, 66, 74, 87, 99, 101, 105, 110, 114, 118, 128, 138, 146, 149, 151, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 196, 201, 206, 209 | serum | 1–3 pg/g, wet weight |
| organochlorine pesticides (OCPs) | 9 | hexachlorobenzene (HCB), β-hexachlorocyclohexane (β-HCH), γ-hexachlorocyclohexane (γ-HCH), oxychlordane, trans-nonachlor, p,p′-DDT, o,p′-DDT, p,p′-DDE, and mirex | serum | 1–3 pg/g, wet weight |
| polybrominated chemicalsa | 11 | brominated biphenyl (BB 153); brominated diphenyl ethers (BDEs) congeners: 17, 28, 47, 66, 85, 99, 100, 153, 154, 183 | serum | 5 pg/g, wet weight |
| per- and polyfluoroalkyl substances (PFASs) | 7 | 2-(N-ethyl-perfluorooctane sulfonamido) acetate (Et-PFOSA-AcOH), 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH), perfluorodecanoate (PFDeA), perfluorononanoate (PFNA), perfluorooctane sulfonamide (PFOSA), perfluorooctanesulfonate (PFOS), and perfluorooctanoate (PFOA) | serum | 0.04–0.1 ng/mL, wet weight |
| phytoestrogens | 6 | genistein, daidzein, O-desmethylangolensin, equol, enterodiol, and enterolactone | urine | 0.2–0.6 ng/mL |
| phthalate metabolites | 14 | mono (3-carboxypropyl) phthalate (mCPP), monomethyl phthalate (mMP), monoethyl phthalate (mEP), mono (2-isobutyl phthalate) (miBP), mono-n-butyl phthalate (mBP), mono (2-ethyl-5-carboxyphentyl) phthalate (mECPP), mono-[(2-carboxymethyl) hexyl] phthalate (mCMHP), mono (2-ethyl-5-oxohexyl) phthalate (mEOHP), mono (2-ethyl-5-hydroxyhexyl) phthalate (mEHHP), monocyclohexyl phthalate (mCHP), monobenzyl phthalate (mBzP), mono (2-ethylhexyl) phthalate (mEHP), monoisononyl phthalate (mNP), and monooctyl phthalate (mOP) | urine | 0.2–2 ng/mL |
| phenolsb | 6 | total bisphenol A (BPA); benzophenones (BPs): 4-hydroxybenzophenone (4-OH-BP), 2,4-dihydroxybenzophenone (2,4-OH-BP), 2,2′,4,4′-tetrahydroxybenzophenone (2,2′4,4′-OH-BP), 2-hydroxy-4-methoxybenzophenone (2-OH-4-MeO-BP), and 2,2′-dihydroxy-4-methoxybenzophenone (2,2′-OH-4-MeO-BP) | urine | 0.02–0.05 ng/mL |
| antimicrobial chemicalsc | 12 | triclosan (TCS) and triclocarban (TCC); parabens: methyl paraben (MP), ethyl paraben (EP), propyl paraben (PP), butyl paraben (BP), benzyl paraben (BzP), heptyl paraben (HP), 4-hydroxy benzoic acid (4-HB), 3,4-dihydroxy benzoic (3,4-DHB), methyl-protocatechuic acid (OH-Me-P), and ethyl-protocatechuic acid (OH-Et-P) | urine | 0.02–0.05 ng/mL |
| paracetamol and derivatives | 2 | paracetamol and 4-aminophenol | urine | 0.5 ng/mL and 0.25 ng/mL |
| blood metals | 3 | cadmium (Cd), lead (Pb), and mercury (Hg) | 10 ng/L to 10 μg/L | |
| cotinined | 1 | cotinine | serum | 0.01 ng/mL |
| urine metals | 17 | manganese (Mn), chromium (Cr), beryllium (Be), cobalt (Co), molybdenum (Mo), cadmium (Cd), tin (Sn), cesium (Cs), barium (Ba), nickel (Ni), copper (Cu), zinc (Zn), tungsten (W), platinum (Pt), thallium (Tl), lead (Pb), and uranium (U) | urine | 10 ng/L to 10 μg/L |
| urine metalloids | 4 | selenium (Se), arsenic (As), antimony (Sb), and tellurium (Te) | urine | 10 ng/L to 10 μg/L |
Polybrominated chemicals contain mostly PBDEs with one PBB.
Phenols contain mostly benzophenones with one BPA.
Antimicrobial chemicals contain mostly parabens with TCS and TCC.
Serum cotinine is not an EDC but is included for comprehensive investigation.
LOQ, limits of quantification.
Nonpersistent ECDs included 5 classes of urinary nonpersistent organic compounds, 6 phytoestrogens, 14 phthalate metabolites, 6 phenols [bisphenol A (BPA) and benzophenones (BPs)], 12 antimicrobial chemicals [parabens, triclosan (TCS), and triclocarban (TCC)], 2 paracetamol and derivatives, and were quantified by another laboratory with liquid chromatography tandem mass spectrometry using published standard operating procedures.22−25 Three other classes of EDCs including 3 blood metals, 17 urinary metals, and 4 urinary metalloids were measured using inductively coupled plasma mass spectrometry.26
Serum lipids were obtained by measuring total cholesterol, free cholesterol, triglycerides, and phospholipids using enzymatic methods;27 total serum lipids were estimated as described by Phillips et al.28 Creatinine was measured by a Roche/Hitachi Model 912 clinical analyzer (Dalla, TX, USA) using the Creatinine Plus Assay (Roche Diagnostics). Serum cotinine was quantified by isotope dilution tandem mass spectrometry.29
Statistical Analysis
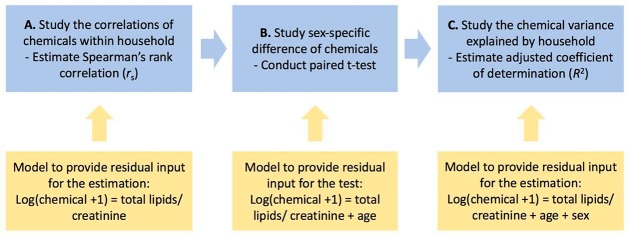
Our overall analytical scheme, as well as the statistical models and tests used, is shown in Figure 1. First, we adjusted each chemical for potential a priori defined potential confounders in the model in addition to total lipids (for lipophilic chemicals, n = 56) and creatinine (for urinary chemicals, n = 61) to reduce the estimated variability and susceptibility to bias.30,31 Specifically, we adjusted PCBs, OCPs, and polybrominated chemicals for total lipids and all other urinary chemicals for creatinine. All chemicals were log-transformed (x + 1), and continuous covariates were rescaled to have a zero mean and unit variance (Figure 1A). Since the biomarkers of chemical exposure need not follow a normal distribution, we calculated the Spearman’s rank correlation (rs) matrices for EDCs in females, males, and couples after extracting the residuals.
Figure 1.
Analytical scheme to investigate the exposures’ variability and correlations. A) We first extract the residuals from a linear model after adjusting for the base covariates (total lipids or creatinine) to calculate the Spearman’s rank correlation (rs). B) Then, we used another linear model with an additional age variable to obtain residuals and conducted a paired t test to test the difference of chemicals between female and male partners in the same household. C) Afterward, we further adjusted for sex prior to extracting residuals to calculate the percentage of chemical variance explained by the shared environment.
We estimated the sex-specific difference with a paired t test (by household) after extracting the residuals from a linear model adjusted for age (Figure 1B). We used a similar approach to estimate the percentage of variance explained by the shared environment. However, sex and age variables were excluded in the adjustment step to isolate their effects (Figure 1C). Afterward, we extracted and regressed the residuals against the household variable to obtain the adjusted coefficient of determination (R2).
We leverage the family based design and diverse chemical measurements in the LIFE Study to study household correlations of exposures. It is important to know how generalizable our findings are to the U.S. population and therefore we computed concordance as the Pearson correlation coefficient (r) between the chemical relatedness rs in this study and that in the 2003–2004 National Health and Nutrition Examination Survey (NHANES).9 We used r as a measure of concordance to model the linear relationships between chemicals. First, we estimated the rs of 4292 unique chemical pairs in NHANES without filtering by age, sex, and race (median sample size was 1923). Then, we estimated the concordance based on a total of 101 matched biomarkers between the two studies. We chose the 2003–2004 NHANES because of the close temporal nature with the implementation of the LIFE Study and given that the same laboratory measured persistent EDCs.
We used all the instrument derived concentrations for the analyses.32,33 For missing values, we substituted them by multiple imputation, assuming a missing-at-random scenario.17 We conducted imputation based on the information from available demographics, previous history of clinical symptoms, and all other chemical variables and created a total of 10 imputed data sets for males and females separately.
We visualized the correlations between exposures as exposome globe using the R package Circlize (v 0.3.1).34 EDCs were sorted from lipophilic to hydrophilic to aid visual interpretation of the patterns. We combined the final estimates from imputations using Rubin’s method35 and calculated the p values of correlations by permutation tests. To adjust for multiple testing, we used the false discovery rate (FDR) q values unless otherwise specified. We executed all analyses using the computing environment R (v 3.3.1).36 For reproducibility purposes, all analytic code is publicly available on GitHub via an MIT license (https://github.com/jakemkc/exposome_variability).
Results
Important differences were observed between partners (Table 2). Overall, female partners were younger, had a lower body mass index (BMI) (<25), consumed fewer alcoholic drinks, were less likely to report a hypertension or high cholesterol, and had lower serum cotinine and lipids and creatinine than male partners (p < 0.01).
Table 2. Sociodemographic and Lifestyle Characteristics of Females and Males in the LIFE Study17,19.
| characteristics | females (n = 501) | males (n = 501) |
|---|---|---|
| age (year)d | 29.99 ± 4.14 | 31.77 ± 4.92 |
| BMI (kg/m2)b,d | ||
| <25 | 230 (46) | 84 (17) |
| ≥25 and <30 | 136 (27) | 206 (41) |
| ≥30 | 135 (27) | 311 (62) |
| non-Hispanic white | 396 (79) | 397 (79) |
| college graduate or higherc | 474 (95) | 457 (91) |
| yearly income $80,000 or over | 297 (59) | 298 (59) |
| regular vigorous exercise in the past 12 months | 200 (40) | 211 (42) |
| smoke at the time of study | ||
| no | 445 (89) | 440 (88) |
| yes (no. of cigarettes on a typical day) | ||
| 1–3 | 19 (4) | 26 (5) |
| 4–6 | 8 (2) | 11 (2) |
| 7–10 | 15 (3) | 8 (2) |
| >10 | 14 (3) | 16 (3) |
| ≥12 alcoholic drinks in the past 12 monthsd | 374 (75) | 428 (85) |
| no. of alcoholic drinks on a typical occasiond | ||
| 0 | 128 (26) | 73 (15) |
| 1 | 108 (22) | 63 (13) |
| 2 | 169 (34) | 150 (30) |
| 3 | 68 (14) | 99 (20) |
| 4 | 19 (4) | 62 (12) |
| 5 | 9 (2) | 54 (11) |
| history of diabetes | 6 (1) | 14 (3) |
| history of high blood pressured | 20 (4) | 52 (10) |
| history of high cholesterold | 41 (8) | 78 (16) |
| serum cotinine (ng/mL)a,d | 0.62 ± 0.23 | 1.24 ± 2.17 |
| serum total lipids (mg/dL)a,d | 2.00 ± 0.03 | 6.56 ± 0.26 |
| urinary creatinine (mg/dL)a,d | 4.22 ± 0.86 | 4.76 ± 0.73 |
log+1 transformed values.
BMI, body mass index.
p < 0.05.
p < 0.01. Values in mean ± SD or n (%).
Nine (7%) chemicals were correlated with partner’s sex, i.e., cotinine (p = 7.38 × 10–11), blood lead (p = 7.34 × 10–64), mercury (p = 2.37 × 10–9), and cadmium (p = 6.84 × 10–3), and 5 PFASs: perfluorodecanoate (PFDeA, p = 1.94 × 10–6), perfluorononanoate (PFNA, p = 1.04 × 10–22), perfluorooctane sulfonamide (PFOSA, p = 9.31 × 10–4), perfluorooctanesulfonate (PFOS, p = 1.94 × 10–52), and perfluorooctanoate (PFOA, p = 3.85 × 10–41). Of note, findings were robust to the FDR correction with the exception of blood cadmium.
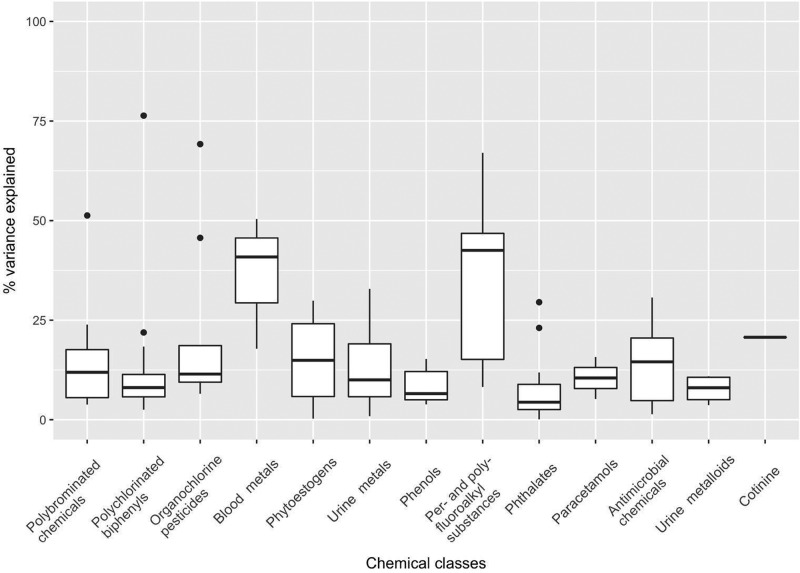
Figure 2 shows the boxplot summary of the chemical variance explained by the shared environment (i.e., household). We estimated that two classes of chemicals, PFASs and blood metals, had higher levels of explained variance (median 0.43 and 0.41 respectively) than the remaining chemical classes. For the remaining 11 classes, median explained variances ranged from 0.04 (phthalates) to 0.21 (cotinine). A few persistent organic compounds, namely PCB congener 28, PBDE congener 47, and β-hexachlorocyclohexane(β-HCH), had an explained variance over 50%.
Figure 2.
Summary of the percentage of chemical variance explained by the shared (household) environment. Boxplots of the adjusted coefficient of determination (R2) within different chemical classes are shown. Interquartile range is not shown for the cotinine class because it contains only 1 compound. For each box, median and interquartile ranges are drawn, and the whiskers are extended to the largest values within 1.5*interquartile range. Black dots denote correlations outside of the range covered by the whiskers.
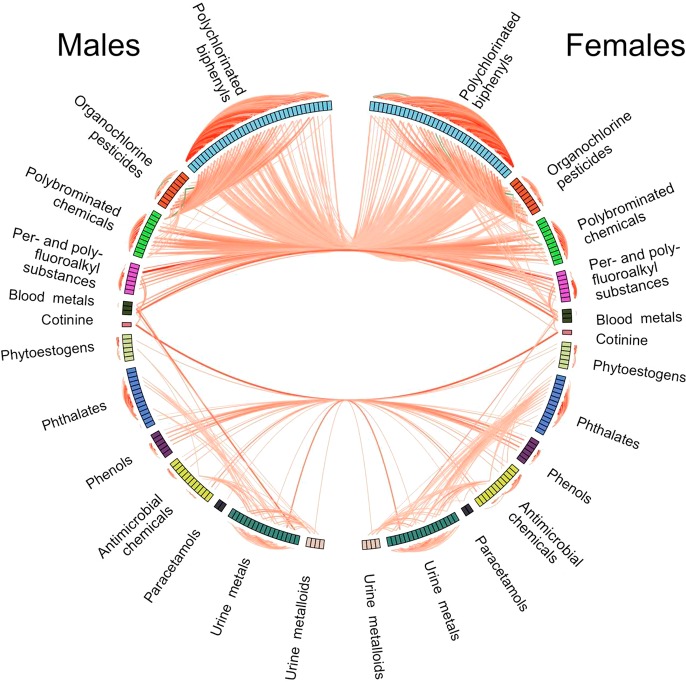
The exposome globe37 displays the rss for females (right-half), for males (left-half), and for couples (across the left–right of the globe) (Figure 3). To assist interpretation, we only presented the rss outside the range of −0.25 to 0.25 as lines connecting different parts on the track, and they represent less than 10% of all the rss. For females, we observed two larger positively correlated “clusters” across EDC classes: A) a dense cluster with serum persistent organic compounds such as PCBs and OCPs (upper right of Figure 3); B) another loosely packed cluster with urinary EDCs such as phytoestrogens, phthalates, phenols, and antimicrobial compounds (lower right of Figure 3). Correlations between serum and urinary EDCs were mostly small and distributed between −0.25 and 0.25. For males, there were similar coexposure patterns to females.
Figure 3.
Exposome correlation globe showing the relationships of biomarkers between females, males, and couples. Right-half represents biomarkers in females; left-half represents biomarkers in males. Only Spearman’s rank correlations greater than 0.25 and smaller than −0.25 were shown as connections in the globe. Red line denotes positive correlation, and dark green line denotes a negative one. Color intensity and line width are proportional to the size of the correlation. Within-class and between-class correlations are shown outside and inside of the track respectively. Correlations in couples are indicated by the lines linking females and males (i.e., crossing the vertical-half of the globe).
While we found similar correlations in the population of males and females separately, we found that correlations in couples living in the same household were, in fact, less densely packed and with values attenuated toward the null (Figure 3; see Figure S1).
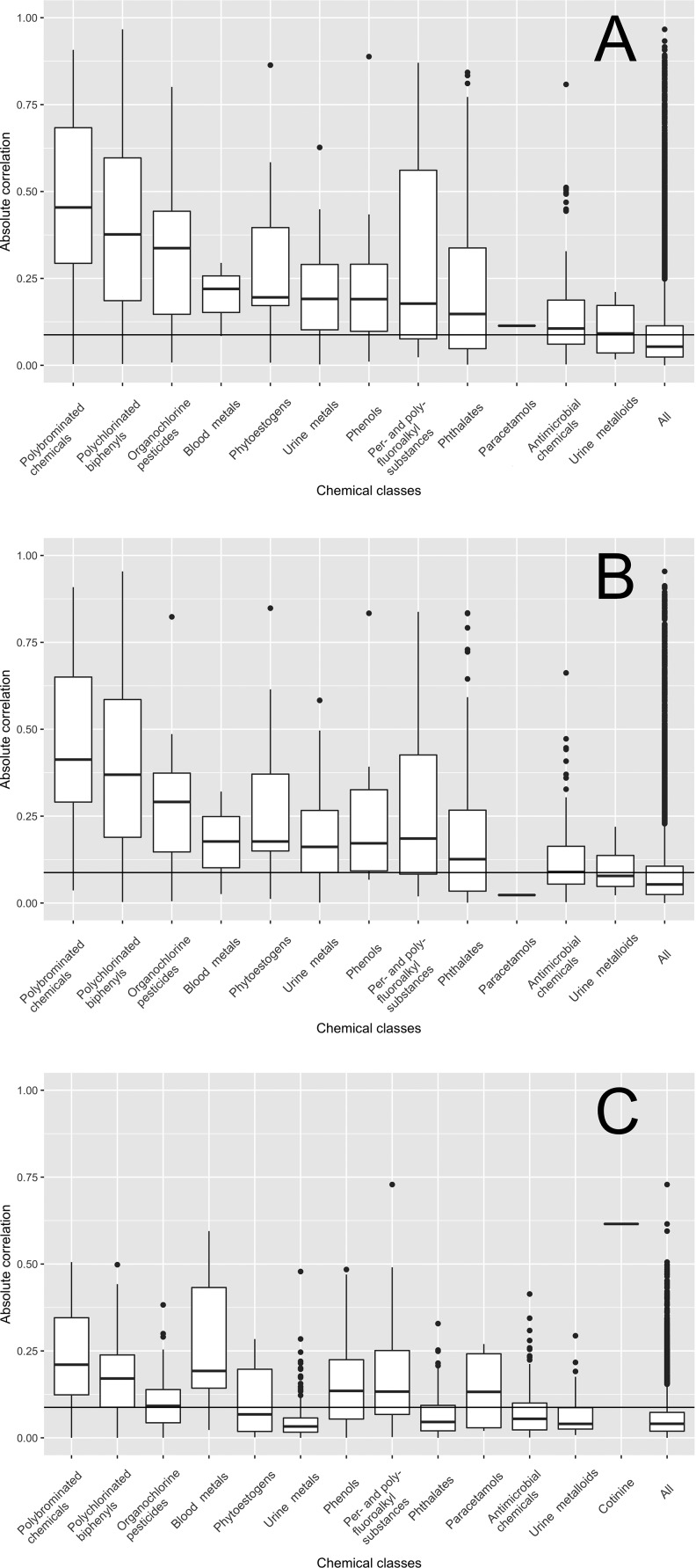
A summary of the within-class correlations as absolute magnitude is shown in Figure 4. For females (Figure 4A), we found that polybrominated compounds had the highest median correlation (rs = 0.45) while urine metalloids had the lowest (rs = 0.09). Across different chemical classes, persistent organic compounds such as polybrominated compounds, PCBs, and OCPs had higher median correlations (0.45, 0.38, and 0.34 respectively). For the rest of the classes, the median correlations were all below 0.25. Males (Figure 4B) had similar within-class correlation distributions as were found in females. The classes with highest and lowest median correlations were polybrominated compounds (rs = 0.41) and paracetamols (rs = 0.02) respectively.
Figure 4.
Boxplots of Spearman’s rank correlations (rs) within different chemical classes: A) females; B) males; and C) couples. For couples, summary statistics were estimated with the full 128 × 128 correlation matrix instead of with the half triangle. Certain classes contain only 1 pair of correlation (paracetamols in females, paracetamols in males, and cotinine in couples). “All” represents the grouping by the correlation of all pairs of chemicals available. The horizontal line drawn across the chemical classes is equal to the 95th percentile of the null distribution obtained from permuting the concentrations of all chemicals. For each box, median and interquartile ranges are drawn, and the whiskers are extended to the largest values within 1.5*interquartile range. Black dots denote correlations outside of the range covered by the whiskers.
We also assessed whether exposure in one person of the couple was associated with their partner’s exposure level. For couples, we found a strong diminishing effect for the within-class correlations, both in terms of the median and interquartile range (IQR) (Figure 4C). Comparing the couples with those between females and males (interhousehold), all chemical classes had the median correlations below 0.25, and urine metals were the class with the greatest percentage drop (83%). We also observed substantial reduction in the IQRs of some chemical classes within a couple. For example, the IQR of polybrominated compounds was 0.21, which corresponds to an over 35% drop in females versus males. We found that urine metals had the largest drop in IQR (77%). The correlations between the same chemicals in couples (i.e., the diagonal of the correlation matrix of couples) were generally higher than that among the same class members. Because of the low number of exposure indicators in blood metals (3 chemicals), paracetamols (2 chemicals), and cotinine (1 chemical), the diminishing effect to within-class correlations was countered, and thus the drop in medians and IQRs of these classes was not as prominent as others.
We estimated the concordances between correlations in the LIFE study (as females, males, and couples) and the 2003–2004 NHANES (Table 3). Correlations r were more consistent with correlations observed in the NHANES 2003–2004 females: 0.88; males: 0.84; couples: 0.67. We concluded the exposure correlation patterns captured in LIFE are comparable to that in the U.S. population. However, correlations r varied greatly between chemical groups, from −0.78 to 0.98, potentially due to the small number of chemicals being correlated within particular groups.
Table 3. Correlations of Chemical Relatedness between 2003–2004 NHANES and Current Study by Different Chemical Classes.
| females |
males |
couples |
||||
|---|---|---|---|---|---|---|
| chemical classb | Pearson r | n | Pearson r | n | Pearson r | n |
| blood metals | 0.07 | 3 | 0.13 | 3 | –0.04 | 6 |
| OCPs | 0.69 | 36 | 0.51 | 36 | 0.25 | 72 |
| PCBs | 0.88 | 561 | 0.88 | 561 | 0.77 | 1122 |
| PFASs | 0.31 | 21 | 0.29 | 21 | 0.32 | 42 |
| phthalates | 0.90 | 78 | 0.89 | 78 | 0.34 | 156 |
| phytoestogens | 0.98 | 15 | 0.97 | 15 | 0.86 | 30 |
| polybrominated chemicals | 0.76 | 55 | 0.74 | 55 | 0.77 | 110 |
| urine metalloids | 0.41 | 3 | –0.78 | 3 | 0.34 | 6 |
| urine metals | 0.82 | 55 | 0.78 | 55 | –0.01 | 110 |
| total | 0.84 | 827 | 0.84 | 827 | 0.67 | 1654 |
Pearson r, Pearson correlation coefficients of the chemical relatedness (spearman correlations of chemicals) between National Health and Nutrition Examination Survey (NHANES) and this study; n, sample size.
Restricted to 9 chemical classes that were measured in both studies.
Discussion
Findings
Understanding the coexposure patterns is an important step toward investigating the joint health effects of chemical mixtures and for statistical design of exposome-related investigations. We describe our high-level findings here. First, exposure concentrations were modestly correlated (low absolute value of correlation) between partners for many EDCs. Second, the percentages of significant rs (q < 0.05) in males and females were 25.3 and 23.1, respectively. These percentages contrast to the 9.5% observed between couples in the same household (Figure 4). Overall, chemical correlations for households were similar to those observed in 2003–2004 NHANES. Although we studied only a small fraction of the exposome (128 EDCs) with 501 participants, the comparison indicates reproducible coexposure correlations with respect to the patterns sought in a noninstitutionalized U.S. population.
Although couples in our study hypothetically potentially share a large degree of dietary and indoor environmental factors, their exposures were only modestly correlated (low rs). We believe that there are two additional factors affecting the familial coexposure patterns in our investigation. The first one is concerned with how long the couples have been living together. In the U.S., the median age of first marriage is over 24 since 2000,38 and newly married couples could show a greater discordance in chemical coexposure relationships due to different premarriage exposures. The second factor is potential physiological dampening of exposure variability related to the half-life of the target chemicals.39,40 Polybrominated compounds, PCBs, and OCPs are persistent chemicals with high lipophilicity and longer half-lives (on the order of years). Their serum concentrations are integrated over a period of time and not completely associated with recent exposure.41 Since most of the couples recruited in LIFE Study were living together, we claim this phenomenon can explain the drop in rs of persistent chemicals relative to the short-lived urinary chemicals (such as phthalate metabolites, whose half-lives are on the order of days) in couples (Figure 4). These factors should be considered when using surrogate measurement to assess the exposures of family members.
Wu et al. conducted a household-based study42,43 and measured serum perfluorinated and PBDE compounds in family members. They found that the rss between child and parent (n = 68) ranged between 0.26 and 0.79 (median = 0.31) for perfluorinated compounds and 0.66 and 0.74 (median = 0.68) for PBDEs. Within couples 55+ years of age (n = 10), higher rss were observed for both perfluorinated compounds (0.24 to 0.79, median = 0.53) and PBDEs (0.45 to 0.78, median = 0.72) in comparison to those observed between child and parent. These findings are consistent with our observations.
Persistent organic chemicals are known to cause adverse health effects and are prioritized by the U.S. Centers for Disease Control and Prevention for health monitoring.44 Many of them had wide applications in electrical/electronic equipment, agricultural chemicals, and furniture.45,46 Although other emerging EDCs such as BPA and phthalates have short half-lives, they have extensive modern applications in cosmetics and consumer products.15,47−49 The ubiquity of these chemicals in different microenvironments such as schools and offices suggests that household environment alone is not a major contributor to body burden, consistent with our results (Figure 2). PFASs and blood metals had higher variance explained by the shared environment than other chemical classes, and we believe that this could be related to the lifestyle of the subjects in the LIFE cohort (e.g., PFAS exposures via food items and personal care products). Elimination of persistent chemicals in women due to breastfeeding could be a factor confounding the effect of shared environment. We studied the influence of parity, a proxy for breastfeeding, and found that it has little impact on the levels of EDCs and variance explained by the shared environment (results are shown as sensitivity analyses in the Supporting Information).
Strength and Limitations
Our investigation includes 128 chemical biomarkers with diverse physicochemical properties that span from persistent lipophilic to nonpersistent hydrophilic chemicals and is one of the first attempts to systematically characterize their correlations in a household setting. However, we do not know how long the couples have been living together at the baseline of the study to quantitatively assess how this factor affects coexposure patterns. Second, we only collected biological samples at the baseline; thus, it is not possible to study how exposure levels and coexposure patterns change longitudinally with time, which could be an important piece of information for assessing fecundity outcome and chemical exposures. Third, technical variation may have biased our findings. However, since samples were acquired with predefined protocols and later measured by accredited analytical laboratories with standard operating procedures, we assumed that the influence from technical sources was negligible. Finally, we only had EDC measurements at baseline. Findings for less-persistent EDCs were more modest than those of persistent EDCs and may be less precise.
Analytical and Sampling Implications for Exposome-Wide Investigations
Our findings have implications for high-throughput association tests between correlated exposures and health outcomes and phenotypes. One of the typical approaches to adjust for multiple hypothesis tests in EWASs includes controlling the family wise error rate. For example, the Bonferroni correction provides a new significance level by dividing the original threshold by the number of tests, assuming that they are independent. Nyholt50 devised a method to address correlation among variables to estimate the effective number of variables in a data set, which is then used to correct the family wise error rate. When many things are correlated with one another, the total number of variables is decreased. Benjamini and Yekutieli51 and Fan et al.52 documented a procedure that considers nonindependence among statistical tests in updated estimates of the FDR.
In the future, exposome-related investigations will include many more variables than ever before with the emergence of highly sensitive high-resolution mass spectrometry techniques53 and availability of large data sets from reproducibility and precision medicine or biobank initiatives.10 Dense correlations among exposures indicate that multicollinearity may also influence the reliability of the association size in multiple regression and/or potential confounding. We claim that this could be ameliorated through understanding coexposure patterns. For example, statistical approaches for evaluating the effects of mixture exposures such as principal component analysis generally involve dimensionality reduction that relies on estimating the correlation structure to reduce the number of exposures being considered.54 Other regression approaches, such as the Elastic Net,55 can consider highly colinear exposure variables while estimating interpretable coefficients that are similar to that delivered from a typical regression model.
Capturing population-level exposure variability — and the demographic variables that are associated with the variation, such as sex, location, and time — is a grand ambition in the exposome concept.10 Given people spend over 90% of their time indoors and more than 12 h a day at home (https://www.bls.gov/tus), household samples might be a reasonable surrogate to represent home exposures to family members who share the same living environment. Linking cluster exposure information with individual health outcome is a common practice.56−58 While unit-based exposure assessment is a tempting approach because of the simplicity and cost-savings over personal measurement, it may fail to catch a significant fraction of exposure variability in the population as we found that shared environment could explain a small percentage of biomarker variance (Figure 2). For epidemiological investigations that sample study participants who are nested in a unit such as homes or schools, searching the literature for appropriate surrogates or conducting preliminary measurements to assess the influence of shared environment are ways to justify unit-based measurement. For example, our study suggests that 1) total time of participants spent in a shared unit, 2) sex of participants, and 3) half-lives of chemicals under investigation are important factors to consider for taking unit-based measurements.
Finally, correlations of chemical mixtures can also be an important tool used in exposure science and cumulative risk assessment as groups of correlated chemicals are often released from a single source (e.g., power plant and vehicular exhaust).59 Thus, studying the coexposure patterns is the first step to identify the sources prior to more in-depth source apportionment methods such as positive matrix factorization and chemical mass balance receptor models.60 In cumulative risk assessment, dose addition is one of the common approaches to estimate the risks from mixture exposures by assuming a shared toxicity mechanism between chemicals (e.g., binding to the same receptor).61 Knowing the coexposure correlations could be a first step toward identification of exposures with similar physicochemical properties to guide follow-up investigations.
Acknowledgments
This study is supported by the NIH grants (ES025052 and ES023504) and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts # N01-HD-3-3355; N01-HD-3-3356; N01-HD-3-3358; HHSN27500002; HHSN27500003; HHSN27500006).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.8b01467.
Correlation heat maps showing the Spearman’s rank correlations (rs) across 128 chemicals grouped in 13 different classes and sensitivity analysis of the parity variable (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wild C. P. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol., Biomarkers Prev. 2005, 14 (8), 1847–1850. 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Wild C. P.; Scalbert A.; Herceg Z. Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Environ. Mol. Mutagen 2013, 54 (7), 480–499. 10.1002/em.21777. [DOI] [PubMed] [Google Scholar]
- Stingone J. A.; Buck Louis G. M.; Nakayama S. F.; Vermeulen R. C. H.; Kwok R. K.; Cui Y.; Balshaw D. M.; Teitelbaum S. L. Toward greater implementation of the exposome research paradigm within environmental epidemiology. Annu. Rev. Public Health 2017, 38, 315–327. 10.1146/annurev-publhealth-082516-012750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potera C. The helix project: tracking the exposome in real time. Environ. Health Perspect 2014, 122 (6), A169. 10.1289/ehp.122-A169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M.; Slama R.; Robinson O.; Chatzi L.; Coen M.; van den Hazel P.; Thomsen C.; Wright J.; Athersuch T. J.; Avellana N.; Basagaña X.; Brochot C.; Bucchini L.; Bustamante M.; Carracedo A.; Casas M.; Estivill X.; Fairley L.; van Gent D.; Gonzalez J. R.; Granum B.; Gražulevičienė R.; Gutzkow K. B.; Julvez J.; Keun H. C.; Kogevinas M.; McEachan R. R. C.; Meltzer H. M.; Sabidó E.; Schwarze P. E.; Siroux V.; Sunyer J.; Want E. J.; Zeman F.; Nieuwenhuijsen M. J. The human early-life exposome (helix): project rationale and design. Environ. Health Perspect 2014, 122 (6), 535–544. 10.1289/ehp.1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P.; Chadeau-Hyam M.; Gmuender H.; Gulliver J.; Herceg Z.; Kleinjans J.; Kogevinas M.; Kyrtopoulos S.; Nieuwenhuijsen M.; Phillips D. H.; Probst-Hensch N.; Scalbert A.; Vermeulen R.; Wild C. P. EXPOsOMICS Consortium. The exposome in practice: design of the exposomics project. Int. J. Hyg. Environ. Health 2017, 220 (2), 142–151. 10.1016/j.ijheh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J. P. A. Exposure-wide epidemiology: revisiting bradford hill. Stat. Med. 2016, 35 (11), 1749–1762. 10.1002/sim.6825. [DOI] [PubMed] [Google Scholar]
- Ioannidis J. P. A.; Loy E. Y.; Poulton R.; Chia K. S. Researching genetic versus nongenetic determinants of disease: a comparison and proposed unification. Sci. Transl. Med. 2009, 1 (7), 7ps8. 10.1126/scitranslmed.3000247. [DOI] [PubMed] [Google Scholar]
- Patel C. J.; Ioannidis J. P. A. Placing epidemiological results in the context of multiplicity and typical correlations of exposures. J. Epidemiol. Community Health 2014, 68 (11), 1096–1100. 10.1136/jech-2014-204195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrai A. K.; Cui Y.; Bushel P. R.; Hall M.; Karakitsios S.; Mattingly C. J.; Ritchie M.; Schmitt C.; Sarigiannis D. A.; Thomas D. C.; Wishart D.; Balshaw D. M.; Patel C. J. Informatics and data analytics to support exposome-based discovery for public health. Annu. Rev. Public Health 2017, 38, 279–294. 10.1146/annurev-publhealth-082516-012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde E.; Burdorf A.; Nieschlag E.; Eijkemans R.; Kremer J. A. M.; Roeleveld N.; Habbema D. Is human fecundity declining in western countries?. Hum. Reprod. 2010, 25 (6), 1348–1353. 10.1093/humrep/deq085. [DOI] [PubMed] [Google Scholar]
- Hauser R. The environment and male fertility: recent research on emerging chemicals and semen quality. Semin. Reprod. Med. 2006, 24 (3), 156–167. 10.1055/s-2006-944422. [DOI] [PubMed] [Google Scholar]
- Patel C. J.; Sundaram R.; Buck Louis G. M. A data-driven search for semen-related phenotypes in conception delay. Andrology 2017, 5 (1), 95–102. 10.1111/andr.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis G. M.; Barr D. B.; Kannan K.; Chen Z.; Kim S.; Sundaram R. Paternal exposures to environmental chemicals and time-to-pregnancy: overview of results from the life study. Andrology 2016, 4 (4), 639–647. 10.1111/andr.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis G. M.; Sundaram R.; Sweeney A. M.; Schisterman E. F.; Maisog J.; Kannan K. Urinary bisphenol a, phthalates, and couple fecundity: the longitudinal investigation of fertility and the environment (life) study. Fertil. Steril. 2014, 101 (5), 1359–1366. 10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford S. L.; Kim S.; Chen Z.; Gore-Langton R. E.; Boyd Barr D.; Buck Louis G. M. Persistent organic pollutants and semen quality: the life study. Chemosphere 2015, 135, 427–435. 10.1016/j.chemosphere.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis G. M. B.; Sundaram R.; Schisterman E. F.; Sweeney A. M.; Lynch C. D.; Gore-Langton R. E.; Maisog J.; Kim S.; Chen Z.; Barr D. B. Persistent environmental pollutants and couple fecundity: the life study. Environ. Health Perspect 2013, 121 (2), 231–236. 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaacks L. M.; Barr D. B.; Sundaram R.; Maisog J. M.; Zhang C.; Buck Louis G. M. Pre-pregnancy maternal exposure to polybrominated and polychlorinated biphenyls and gestational diabetes: a prospective cohort study. Environ. Health 2016, 15, 11. 10.1186/s12940-016-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis G. M.; Schisterman E. F.; Sweeney A. M.; Wilcosky T. C.; Gore-Langton R. E.; Lynch C. D.; Boyd Barr D.; Schrader S. M.; Kim S.; Chen Z.; Sundaram R. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development – the life study. Paediatr. Perinat. Epidemiol 2011, 25 (5), 413–424. 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A.; Jones R. S.; Lapeza C. R.; Focant J.-F.; McGahee E. E. 3rd; Patterson D. G. Jr Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal. Chem. 2004, 76 (7), 1921–1927. 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z.; Needham L. L.; Calafat A. M. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal. Chem. 2005, 77 (18), 6085–6091. 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- Mumford S. L.; Kim S.; Chen Z.; Boyd Barr D.; Buck Louis G. M. Urinary phytoestrogens are associated with subtle indicators of semen quality among male partners of couples desiring pregnancy. J. Nutr. 2015, 145 (11), 2535–2541. 10.3945/jn.115.214973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.; Alomirah H.; Cho H.-S.; Minh T. B.; Mohd M. A.; Nakata H.; Kannan K. Occurrence of phthalate metabolites in human urine from several asian countries. Environ. Sci. Technol. 2011, 45 (7), 3138–3144. 10.1021/es103879m. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos A. G.; Wang L.; Thomaidis N. S.; Kannan K. A multi-class bioanalytical methodology for the determination of bisphenol a diglycidyl ethers, p-hydroxybenzoic acid esters, benzophenone-type ultraviolet filters, triclosan, and triclocarban in human urine by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1324, 141–148. 10.1016/j.chroma.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Smarr M. M.; Grantz K. L.; Sundaram R.; Maisog J. M.; Honda M.; Kannan K.; Buck Louis G. M. Urinary paracetamol and time-to-pregnancy. Hum. Reprod. 2016, 31 (9), 2119–2127. 10.1093/humrep/dew172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. S.; Buck Louis G. M.; Sundaram R.; Maisog J. M.; Steuerwald A. J.; Parsons P. J. Birth outcomes and background exposures to select elements, the longitudinal investigation of fertility and the environment (life). Environ. Res. 2015, 138, 118–129. 10.1016/j.envres.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins J. R.; Waldrep K.; Bernert J. T. The estimation of total serum lipids by a completely enzymatic “summation” method. Clin. Chim. Acta 1989, 184 (3), 219–226. 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Phillips D. L.; Pirkle J. L.; Burse V. W.; Bernert J. T. Jr; Henderson L. O.; Needham L. L. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Arch. Environ. Contam. Toxicol. 1989, 18 (4), 495–500. 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Bernert J. T. Jr.; Turner W. E.; Pirkle J. L.; Sosnoff C. S.; Akins J. R.; Waldrep M. K.; Ann Q.; Covey T. R.; Whitfield W. E.; Gunter E. W.; Miller B. B.; Patterson D. G. Jr; Needham L. L.; Hannon W. H.; Sampson E. J. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin. Chem. 1997, 43 (12), 2281–2291. [PubMed] [Google Scholar]
- Schisterman E. F.; Whitcomb B. W.; Louis G. M. B.; Louis T. A. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ. Health Perspect 2005, 113 (7), 853–857. 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavner D. L.; Morgan W. T.; Sears S. B.; Richardson J. D.; Byrd G. D.; Ogden M. W. Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers’ spot and 24-h urines. J. Pharm. Biomed. Anal. 2006, 40 (4), 928–942. 10.1016/j.jpba.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Schisterman E. F.; Vexler A.; Whitcomb B. W.; Liu A. The limitations due to exposure detection limits for regression models. Am. J. Epidemiol. 2006, 163 (4), 374–383. 10.1093/aje/kwj039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D. B.; Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am. J. Epidemiol. 2003, 157 (4), 355–363. 10.1093/aje/kwf217. [DOI] [PubMed] [Google Scholar]
- Gu Z.; Gu L.; Eils R.; Schlesner M.; Brors B. Circlize implements and enhances circular visualization in r. Bioinformatics 2014, 30 (19), 2811–2812. 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- Schafer J. L. Multiple imputation: a primer. Stat. Methods Med. Res. 1999, 8 (1), 3–15. 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2016.
- Patel C. J.; Manrai A. K. Development of exposome correlation globes to map out environment-wide associations. Pac. Symp. Biocomput 2015, 231–242. 10.1142/9789814644730_0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau . Current population survey: annual social and economic supplement. U.S. Census Bureau: 2016.
- Makey C. M.; McClean M. D.; Sjödin A.; Weinberg J.; Carignan C. C.; Webster T. F. Temporal variability of polybrominated diphenyl ether (pbde) serum concentrations over one year. Environ. Sci. Technol. 2014, 48 (24), 14642–14649. 10.1021/es5026118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport S. M.; Kupper L. L.. Quantitative Exposure Assessment; Rappaport: El Cerrito, CA, 2008. [Google Scholar]
- Aylward L. L.; Hays S. M.; Smolders R.; Koch H. M.; Cocker J.; Jones K.; Warren N.; Levy L.; Bevan R. Sources of variability in biomarker concentrations. J. Toxicol. Environ. Health, Part B 2014, 17 (1), 45–61. 10.1080/10937404.2013.864250. [DOI] [PubMed] [Google Scholar]
- Wu X. M.; Bennett D. H.; Moran R. E.; Sjödin A.; Jones R. S.; Tancredi D. J.; Tulve N. S.; Clifton M. S.; Colón M.; Weathers W.; Hertz-Picciotto I. Polybrominated diphenyl ether serum concentrations in a californian population of children, their parents, and older adults: an exposure assessment study. Environ. Health 2015, 14, 23. 10.1186/s12940-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. M.; Bennett D. H.; Calafat A. M.; Kato K.; Strynar M.; Andersen E.; Moran R. E.; Tancredi D. J.; Tulve N. S.; Hertz-Picciotto I. Serum concentrations of perfluorinated compounds (pfc) among selected populations of children and adults in california. Environ. Res. 2015, 136, 264–273. 10.1016/j.envres.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Q.; Loganath A.; Chong Y. S.; Tan J.; Obbard J. P. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health A 2006, 69 (21), 1987–2005. [DOI] [PubMed] [Google Scholar]
- Whitehead T.; Metayer C.; Buffler P.; Rappaport S. M. Estimating exposures to indoor contaminants using residential dust. J. Exposure Sci. Environ. Epidemiol. 2011, 21 (6), 549–564. 10.1038/jes.2011.11. [DOI] [PubMed] [Google Scholar]
- Dodson R. E.; Perovich L. J.; Covaci A.; Van den Eede N.; Ionas A. C.; Dirtu A. C.; Brody J. G.; Rudel R. A. After the pbde phase-out: a broad suite of flame retardants in repeat house dust samples from california. Environ. Sci. Technol. 2012, 46 (24), 13056–13066. 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. S.; Whitcomb B. W.; Chen Z.; Ye A.; Kannan K.; Buck Louis G. M. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum. Reprod. 2015, 30 (11), 2645–2657. 10.1093/humrep/dev219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr M. M.; Sundaram R.; Honda M.; Kannan K.; Louis G. M. B. Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples’ fecundity. Environ. Health Perspect 2017, 125 (4), 730–736. 10.1289/EHP189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis G. M. B.; Kannan K.; Sapra K. J.; Maisog J.; Sundaram R. Urinary concentrations of benzophenone-type ultraviolet radiation filters and couples’ fecundity. Am. J. Epidemiol. 2014, 180 (12), 1168–1175. 10.1093/aje/kwu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt D. R. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am. J. Hum. Genet. 2004, 74 (4), 765–769. 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y.; Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat 2001, 29 (4), 1165–1188. 10.1214/aos/1013699998. [DOI] [Google Scholar]
- Fan J.; Han X.; Gu W. Estimating false discovery proportion under arbitrary covariance dependence. J. Am. Stat. Assoc. 2012, 107 (499), 1019–1035. 10.1080/01621459.2012.720478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. P. Sequencing the exposome: a call to action. Toxicol Rep 2016, 3, 29–45. 10.1016/j.toxrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. W.; Joubert B. R.; Braun J. M.; Dilworth C.; Gennings C.; Hauser R.; Heindel J. J.; Rider C. V.; Webster T. F.; Carlin D. J. Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environ. Health Perspect 2016, 124 (12), A227–A229. 10.1289/EHP547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H.; Hastie T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Series B Stat. Methodol 2005, 67 (2), 301–320. 10.1111/j.1467-9868.2005.00503.x. [DOI] [Google Scholar]
- Cui X.; Li F.; Xiang J.; Fang L.; Chung M. K.; Day D. B.; Mo J.; Weschler C. J.; Gong J.; He L.; Zhu D.; Lu C.; Han H.; Zhang Y.; Zhang J. J. Cardiopulmonary effects of overnight indoor air filtration in healthy non-smoking adults: a double-blind randomized crossover study. Environ. Int. 2018, 114, 27–36. 10.1016/j.envint.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Day D. B.; Xiang J.; Mo J.; Li F.; Chung M.; Gong J.; Weschler C. J.; Ohman-Strickland P. A.; Sundell J.; Weng W.; Zhang Y.; Zhang J. J. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Int. Med. 2017, 177 (9), 1344–1353. 10.1001/jamainternmed.2017.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q.; Wang Y.; Zanobetti A.; Wang Y.; Koutrakis P.; Choirat C.; Dominici F.; Schwartz J. D. Air pollution and mortality in the medicare population. N. Engl. J. Med. 2017, 376 (26), 2513–2522. 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra K.; Sokhi R.; Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos. Environ. 2008, 42 (13), 2895–2921. 10.1016/j.atmosenv.2007.12.010. [DOI] [Google Scholar]
- Rizzo M. J.; Scheff P. A. Utilizing the chemical mass balance and positive matrix factorization models to determine influential species and examine possible rotations in receptor modeling results. Atmos. Environ. 2007, 41 (33), 6986–6998. 10.1016/j.atmosenv.2007.05.008. [DOI] [Google Scholar]
- Chen J. J.; Chen Y. J.; Rice G.; Teuschler L. K.; Hamernik K.; Protzel A.; Kodell R. L. Using dose addition to estimate cumulative risks from exposures to multiple chemicals. Regul. Toxicol. Pharmacol. 2001, 34 (1), 35–41. 10.1006/rtph.2001.1485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.