Abstract
Background:
Africa is profoundly stricken by the HIV pandemic. People living with HIV/AIDS are more likely to be diagnosed with psychiatric disorders than the general population. We describe the prevalence of different mood and psychiatric disorders in HIV/AIDS infected patients with serious mental illness.
Materials and Methods:
We retrospectively sourced data from 105 patient files, at Weskoppies Hospital, between January 2012 and December 2016.
Results:
56 patients had a psychotic disorder; 27 patients had a mood disorder and three patients had a cognitive disorder. Multiple diagnoses were observed in seven patients with a mood and psychotic disorder; ten patients with a psychotic and cognitive disorder and one patient with a mood and cognitive disorder. One patient had all three diagnoses. The most common medical co-morbidities were hypertension (15.24%) and tuberculosis (13.33%).
Conclusion:
Mentally ill patients who are HIV positive mostly present with mood and psychotic disorders. Clinicians need to be vigilant to detect neuropsychiatric manifestations of HIV infection to effectively manage and optimise treatment. This study highlights the need for further intervention in these vulnerable patients.
Keywords: cognitive disorder, depression, HIV, psychosis, severe mental illness
Introduction
Psychiatric disorders are substantially more common in people living with HIV/AIDS compared to the general population (Sorsdahl et al., 2010; Freeman et al., 2008). In South Africa, almost half of people who are HIV positive have diagnosable mental disorders (Freeman et al., 2008). Mental illness increases the likelihood of high risk behaviors which increases the risk of infection with HIV, and, conversely, the presence of HIV/AIDS increases the lifetime prevalence of psychiatric illness (Hammond, 2007).
Patients who are HIV positive and who have psychiatric symptoms are often referred to psychiatrists for assessment and management. Clinicians need to know and understand the complications associated with HIV infection and mental illness. Timeous initiation of antiretroviral therapy and relevant psychotropic medication should improve the management of these patients. Severe mental illness (SMI) is the presence of a major psychiatric disorder (for e.g. schizophrenia or bipolar disorder) with significant disability (e.g. impairment of judgement and insight) and a tendency to chronicity (Joska et al., 2008). Adults with severe mental illness (SMI) (Joska et al., 2008) have a 2 – 36 times higher HIV seroprevalence than the general population (Singh et al., 2009). Patients with SMI may have considerable deficits in their knowledge of HIV and those who are knowledgeable may be unable to recognize their own level of risk (Popiel et al., 2016), which may affect whether or not they have been tested for HIV.
Given the high HIV burden in South Africa (UNAIDS, 2016) and the increasing pressure on mental health facilities, this study describes the clinical presentation of mental illness in a group of HIV positive patients admitted to a psychiatric hospital. In South Africa, depression, anxiety and substance abuse are prevalent among HIV patients already in antiretroviral treatment programs (Kagee et al., 2010; Myer et al., 2008), but little is known about the clinical presentation of psychiatric disorders among SMI patients who are HIV positive.
Materials and Methods
To describe the prevalence of psychotic and mood disorders in psychiatric patients who are HIV positive, we retrospectively accessed files of patients registered at the HIV Counselling and Testing Department of Weskoppies Hospital, Gauteng, South Africa. The register at the HIV Counselling and Testing Department lists patients who are HIV positive or tested positive for HIV during admission. Data was collected from January 2012 to December 2016.
Clinical files for HIV positive male and female psychiatric patients older than 18 years were included. Files with inadequate clinical information were excluded. 105 files were included.
Consent was obtained from the Chief Executive Officer of Weskoppies hospital. Confidentiality was maintained by omitting names and file numbers. Ethics approval was obtained from the Research Ethics Committee of the Faculty of Health Sciences at the University of Pretoria.
Data Collection
From the clinical files, we collated demographic information; adherence; substance use; past psychiatric and medical history; antiretroviral agents and investigations (baseline and special). Based on this information, patients were stratified into three groups, namely, psychotic, mood and cognitive disorders. Psychotic disorders included schizophrenia; schizoaffective disorder; psychotic disorder not otherwise specified; psychosis secondary to HIV; psychosis secondary to head injury and substance induced psychotic disorder.
Mood disorders included bipolar I manic, severe with psychotic features; bipolar I depressed, severe with psychotic features; bipolar disorder not otherwise specified; major depressive disorder, severe with psychotic features; depressive disorder not otherwise specified; mood disorder secondary to HIV and substance induced mood disorder.
Cognitive disorders included neurocognitive disorder secondary to HIV and cognitive disorder not otherwise specified.
Data Analysis
Summary statistics including mean, median, and standard deviation for continuous variables was calculated. For categorical variables, proportions were calculated, together with their associated 95% confidence intervals and frequency count as well as means and standard deviations. Associations between medical conditions and patient characteristics were described with the Pearson Chi-Square test. Data analysis were performed in STATA, where the p-value < 0.05 was statistically significant.
Results
Clinical diagnosis
105 clinical files were collected. 56 patients (53.33%) had a psychotic disorder (M=38.92, SD=10.46). 27 patients (25.71%) had a mood disorder (M=37.92, SD=10.42). Three patients (2.86%) had a cognitive disorder (M=42.4, SD=13.05).
Some patients had multiple diagnoses. Of the 19 patients shown in Table 1 below with a combination of disorders, seven patients (6.67%) had a mood and psychotic disorder. Ten patients (9.52%) had both a psychotic and cognitive disorder and there was one patient (0.95%) who had a mood and cognitive disorder. One patient (0.95%) had all three (psychotic, mood and cognitive) diagnoses.
Table 1.
Demographic profile psychiatric patients who are HIV positive, diagnosed with psychotic, mood and cognitive disorders admitted to Weskoppies hospital between January 2012 and December 2016
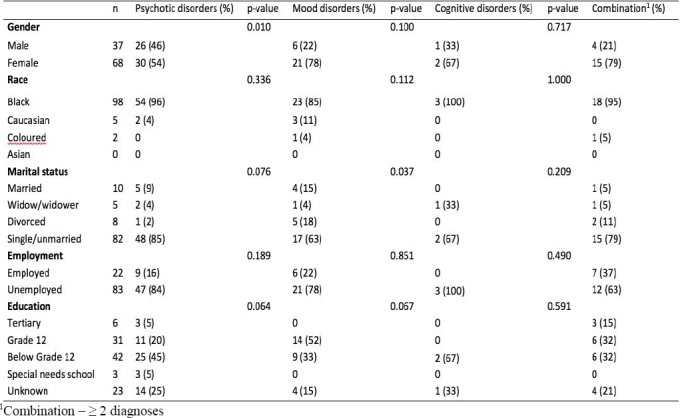
Demographic profile
Patients with psychotic disorders were between 21 and 59 years old (M=38.55, SD=9.88), while patients with mood disorders were between 21 and 57 years old (M=36.11, SD=8.96). The youngest person in the cognitive disorder group was 38 years old and the oldest was 72 years old (M=53.67, SD=17.16). Patients with two or more disorders (combination group) were between 21 and 67 years old (M=40.53, SD=12.16). See Table 1 below for the demographic data.
Clinical Profile
69 patients (65.71%) reportedly had a past psychiatric history (diagnosis and/or previous admission). 40 patients (38.10%) were adherent to medication, and 65 patients (61.90%) were reportedly non-adherent. 41 patients (39.05%) were on one or more substances, whilst 64 patients (60.95%) denied using any substances (Table 2). Tuberculosis and hypertension were reportedly the most common medical co-morbidities in our study (Table 3).
Table 2.
Substance use of psychiatric patients who were HIV positive and admitted to Weskoppies hospital between January 2012 and December 2016
| Substance | n | % | Psychotic | Mood | Cognitive | Combination |
|---|---|---|---|---|---|---|
| Alcohol | 12 | 11.4 | 5 | 6 | 0 | 1 |
| Cannabis | 7 | 6.7 | 4 | 3 | 0 | 0 |
| Methcathinone (CAT) | 1 | 1 | 0 | 1 | 0 | 0 |
| Multiple substances* | 17 | 16.2 | 10 | 4 | 0 | 3 |
| Unknown | 4 | 3.8 | 1 | 0 | 1 | 2 |
| No substances | 64 | 60.9 | 36 | 13 | 2 | 13 |
Multiple substances include: Alcohol, cannabis, CAT, nyaope, cocaine and heroin
Table 3.
Medical co-morbidities in psychiatric patients who were HIV positive and admitted to Weskoppies hospital between January 2012 and December 2016
| Medical condition | n | % |
|---|---|---|
| Hypertension | 16 | 15.2 |
| Tuberculosis (Pulmonary/Extrapulmonary) | 14 | 13.3 |
| Epilepsy | 9 | 8.6 |
| Other* | 4 | 3.8 |
| Asthma | 3 | 2.9 |
| Hyper/Hypothyroidism | 3 | 2.9 |
| Diabetes Mellitus | 2 | 1.9 |
| Dyslipidaemia | 2 | 1.9 |
| Anaemia/Thrombocytopenia | 1 | 1 |
| Malignancy | 1 | 1 |
| Total | 55 | 52.5 |
Diagnosis of other included: Peptic ulcer disease, sexually transmitted infection and head injury
Associations were tested for gender, race, marital status, employment and level of education, in each of the three groups. Due to the different pairings within the combination group, a p-value could not be obtained in this group. Only two statistically significant associations were found. The association between marital status and mood disorders was found to be significant (p-value = 0.037), with divorced patients being more prone to develop mood disorders than all other groups. An association was also found between gender and psychotic disorders (p-value = 0.010). Tests were performed for proportions within gender, which revealed a greater predominance in the male gender (prop = 0.70). No other associations were found in the various groups for the variables. See Table 1 for p-values.
Antiretroviral Therapy
All the patients were on antiretroviral therapy, either first or second line. There were no patients on a third line regimen (Table 4).
Table 4.
Description of antiretroviral therapy used to treat HIV infection in psychiatric patients admitted to Weskoppies Hospital between January 2012 and December 2016
| Regimen | n | % | |
|---|---|---|---|
| 1st line regimen | TDF + FTCa + EFV ABC + 3TC + EFVbV TDF + FTC + NVPV TDF + FTC + LPV/r |
75 | 71.4 |
| 2nd line regimen | AZT + 3TC + LPV/rV TDF + AZT + 3TC + LPV/rV TDF + 3TC + LPV/rV ABC + 3TC + LPV/r |
30 | 28.6 |
Regimens listed above are as per the South African Antiretroviral Treatment (Adult) Guidelines. 1st Line regimens are used for all new patients eligible for antiretroviral therapy including pregnant and breastfeeding women, HIV and TB co-infection and adolescents more than 15 years and more than 40 kg. 2nd Line regimen is used if the patients are failing on a TDF-based 1st line regimen or failing on a d4T- or AZT-based 1st line regimen. 3rd Line regimen if used if the patient is failing any 2nd line regimen. (Medicines Information Center, 2015, www.mic.uct.ac.za).
TDF – Tenofovir; FTC – Emtricitabine; 3TC – Lamivudine; EFV – Efavirenz; ABC – Abacavir; NVP – Nevirapine; LPV/r – Lopinavir/Ritonavir
a3TC used if FTC contraindicated and vice versa, bNVP used if EFV contraindicated
Other clinical investigations
104 patients had a full blood count performed. Only 40 (38.5%) patients were within normal limits, whilst 64 patients (61.5%) had abnormal results. Urea and electrolytes were tested in 104 patients, of which 36 (34.6%) were abnormal. 55 (56.1%) of 98 patients who had liver functions tests had abnormal results.
Thyroid function tests performed revealed 7.8% with abnormal results. Lumbar punctures were performed in 25 patients of which 5 (20%) were abnormal. 16 (39%) brain scans were found to be abnormal. Results are shown in Table 5.
Table 5.
Abnormal clinical investigations of psychiatric patients who were HIV positive and admitted to Weskoppies hospital between January 2012 and December 2016
| Components | Number of patients | Results | % | |
|---|---|---|---|---|
| Full blood count | Haemoglobin | 33 | ↓ | 31.7 |
| White cell count | 16 | ↓ | 15.4 | |
| Platelets | 8 | ↓ | 7.7 | |
| Platelets | 2 | ↑ | 1.9 | |
| MCV | 5 | ↓ | 4.8 | |
| MCV | 13 | ↑ | 12.5 | |
| Urea and electrolytes | Potassium | 10 | ↓ | 9.6 |
| Sodium | 8 | ↓ | 7.7 | |
| Urea and creatinine | 13 | ↑/↓ | 12.5 | |
| Liver function tests | ALT | 12 | ↑ | 11.5 |
| AST | 15 | ↑ | 14.5 | |
| ALP | 12 | ↑ | 11.5 | |
| Total protein | 23 | ↑ | 23.5 | |
| Albumin | 15 | ↓ | 15.3 | |
| GGT | 14 | ↑ | 14.3 | |
| Total and conjugated bilirubin | 2 | ↑ | 2 | |
| Thyroid function test | T4 | 5 | ↓ | 71.4 |
| TSH | 2 | ↑ | 28.6 | |
| Other | CRP | 23 | ↑ | 37 |
| Vitamin B12 | 3 | ↓ | 10.7 | |
| Syphilis | 5 | Reactive | 6 | |
| Iron studies | 9 | Abnormal | 50 | |
| CLAT | 8 | Negative | 100 | |
| CD4 | <500 | 71 | 72 | |
| >500 | 27 | 28 | ||
| Viral load | LDL | 16 | 50 | |
| High | 16 | 50 | ||
| Lumbar puncture | Protein | 2 | ↑ | 8 |
| Lymphocyte | 1 | ↑ | 4 | |
| Polymorph | 1 | ↑ | 4 | |
| ADA | 1 | ↑ | 4 | |
| Syphilis | 1 | Positive | 4 | |
↑ - increased; ↓ - decreased; MCV – mean cell volume; ALT - alanine aminotransferase; AST - aspartate aminotransferase; ALP - alkaline phosphatase; GGT - gamma-glutamyl transferase; TSH – thyroid stimulating hormone; CRP – C-reactive peptide; CLAT - Cryptococcal Latex Antigen; LDL – Lower than detectable limit; ADA - adenosine deaminase; EEG – Electroencephalogram
Discussion
In South Africa, at Weskoppies hospital, patients who are HIV positive almost always present with either psychotic or mood disorders. These patients may present as a quandary to clinicians as they may exhibit symptoms of psychosis that are indistinguishable from those of primary disorders (Mashaphu et al., 2007). Although the presence of psychotic symptoms during the course of HIV infection is a well-known phenomenon, few studies have found psychosis to be a relatively rare complication (Mashaphu et al., 2007; Doyle et al., 1997). Psychosis that is found in HIV positive patients could be new onset psychosis or an exacerbation of a previous psychiatric illness (Uys, 2013).
Psychotic symptoms in HIV positive patients may be facilitated by the direct neuropathic effect of the virus on the brain or may be secondary due to the effect of medication or substance abuse; secondary due to opportunistic infections or from the psychological impact of the knowledge of an HIV diagnosis (Uys, 2013). Psychotic disorders in HIV positive patients have mostly been reported in smaller studies. In our sample of 105 patients, just over half (53.33%) of patients were diagnosed with a psychotic disorder. Most of the patients in our study (65%) had previously been admitted or diagnosed with psychiatric disorders, but their previous HIV status is uncertain. Most other studies have reported few first episode psychosis patients. First episode psychosis was reported in five out of 19 female psychiatric patients who were HIV positive (Uys, 2013). Susser et al. (1997) assumed that HIV was responsible for psychosis in twelve cases of new onset psychosis where HIV infection was antecedent to the onset of the psychotic symptoms. Another study examined the clinical information of 124 HIV infected patients followed up over a six year period and of those who participated, 57 were referred for psychiatric assessment and twelve had acute or sub-acute symptoms of psychosis (Harris et al., 1991). Similarly, new onset psychosis was seen in 20 patients in a study conducted in 1994 (Sewell et al., 1994). The evidence thus suggests that HIV infection may play an important role in the etiology of psychotic symptoms, which may be worse if patients are not on antiretroviral treatment.
Mashaphu et al. (2007) reported a high seroprevalence of 23.8% among patients presenting with first episode psychosis, suggesting that HIV infection may play a direct role in causing psychosis. These patients presented mainly with schizophreniform psychosis (Mashaphu et al., 2007). Psychotic symptoms also presented in five out of 38 untreated HIV infected mine workers (Sall et al., 2009). In another study, patients who were HIV positive were commonly diagnosed with psychosis and manic symptoms with a significant amount of substance use (Van Rensburg et al., 2007).
Our study had 27 patients with a mood disorder presenting either in a manic or a depressive phase. Similarly, 27 patients (25.71%) were found to exhibit symptoms of a mood disorder in another study (Uys, 2013), which reportedly increases as HIV infection progresses (Uys, 2013). In early HIV infection, 1 – 2% of patients may experience manic episodes, whereas after the onset of AIDS 4 – 8% may experience manic episodes (Uys, 2013). Affective symptoms have been reported in other studies (Sall et al., 2009; Van Rensburg et al., 2007) but to a lesser extent than in patients at Weskoppies hospital. Of the patients who were HIV positive in our study, 15 (14.29%) were diagnosed with a cognitive disorder, either on its own or in combination with psychotic or mood disorders.
The demographic profile of patients at Weskoppies hospital aligned with national demographic and HIV trends. In South Africa, male to female transmission of HIV is higher than the female to male transmission rate (Boily et al., 2009). The vulnerability of women can be attributed to both biological and social risk factors (Uys, 2013; Muula, 2008). Most of the patients in our study were female (65%) and unemployed (79%). In South Africa, poverty plays a crucial role in the spread of HIV (Drimie, 2002). People who are HIV positive are often unable to maintain employment with frequent absenteeism owing to the repeated clinic visits and the physical impact of the disease. The progression and spread of the disease is also exacerbated when people avoid essential health care services to avoid the perceived stigma (Kinsler et al., 2007).
Although our study did not find a significant association between level of education and HIV infection, together with poverty and gender, it is also regarded as a risk factor. Forty percent of our patients had a low level of education (less than Grade 12). In a recent study, it was demonstrated that for every additional year of schooling among the youth this was associated with an 8% reduction in the risk of HIV infection (De Neve et al., 2015). This emphasizes that improving retention within the education system may have a protective effect against HIV acquisition. If progression of schooling is reinforced, HIV may be cost effectively prevented, whilst generating other societal benefits (De Neve et al., 2015).
Patients in our study had an elevated prevalence of substance abuse, which is known to increase the risk of contracting HIV as well as non-adherence to antiretroviral therapy, and ultimately, decreased virological suppression (Chander et al., 2006). Conversely, HIV infection may increase the likelihood of depression, anxiety and mental illness which may lead to further illicit drug and alcohol use, which are high risk behaviors (Chander et al., 2006). Whilst this same study described illicit drug and alcohol use disorders to be well known co-morbidities in depression and anxiety, our study found substance use to be most prevalent in the psychotic disorders group (Chander et al., 2006).
The baseline investigations identified patients who were medically unwell with abnormal blood tests and clinical measurements. Baseline tests from our study revealed that one of the patients had elevated infective markers that necessitated a lumbar puncture, which subsequently confirmed a diagnosis of TB meningitis. Psychiatric patients are often neglected in emergency settings as their psychiatric symptoms are often emphasized and inadequate attention is given to other medical concerns. It is clear from the multitude of abnormal results found in this study that baseline investigations form an integral part of overall management. There would be a profound positive impact on the prognosis leading to improved quality of life if co-morbidities are detected and addressed early (Lorenc et al., 2014).
There is a need for routine HIV screening over targeted, risk based testing in psychiatric settings. The routine screening that is offered to patients in mental health settings provides an opportunity for counselling and enhancing patients’ knowledge of HIV risk factors and prevention. It also aids in possibly identifying many seropositive patients who may be overlooked in a system reliant on self-identified risk (Popiel et al., 2016). Staff at HIV treatment clinics should also have sufficient expertise in assessing the signs and symptoms of mental illness and realize the importance of referrals to specialists when necessary.
Conclusion
Patients with severe mental illness who are HIV positive may have more than one psychiatric diagnosis, as well as an elevated prevalence of co-morbid medical disease such as TB and hypertension. This highlights the extreme susceptibility of these patients, and underscores the need for clinicians to be vigilant in detecting various neuropsychiatric manifestations of HIV infection. If detected timeously, co-morbidities addressed; and antiretroviral therapy and psychotropics initiated early, this would have a positive impact on the prognosis of a patient resulting in an improved quality of life.
Lundberg et al profoundly stated: “…mental health services in Uganda, and other countries with generalized HIV epidemics, need to take into account the frequent co-morbidity of SMI with HIV. HIV risk assessment, HIV counselling and testing, and HIV treatment and care should be integrated with mental health services. Conversely, HIV treatment programs should take into account the high burden of mental illness among persons with HIV, and integrate mental health assessments and interventions with routine HIV care.” (Lundberg et al., 2013)
Our findings have re-affirmed the need for mental health services and HIV treatment programs to investigate these dual diagnoses.
Acknowledgements
The authors are grateful to Ms T Nkwenika and Ms N Abdelatif of the Biostatistics unit, South African Medical Research Council for their assistance with statistical analyses and interpretation of the data; Mrs M.A. Mabena, CEO of Weskoppies Hospital for enabling the research at the hospital; and to Dr C Tosh, Science Editor at the Faculty of Health Sciences, University of Pretoria, for role as science and language editor.
Footnotes
Conflict of interest: The authors affirm that this study and its interpretations were not under any conflict of interest.
References
- 1.Boily M.-C, Baggaley R.F, Wang L, Masse B, White R.G, Hayes R.J, Alary M. Heterosexual risk of HIV-1 infection per sexual act:systematic review and meta-analysis of observational studies. The Lancet Infectious Diseases. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chander G, Himelhoch S, Moore R.D. Substance abuse and psychiatric disorders in HIV-positive patients:epidemiology and impact on antiretroviral therapy. Drugs. 2006;66:769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 3.De Neve J.-W, Fink G, Subramanian S, Moyo S, Bor J. Length of secondary schooling and risk of HIV infection in Botswana:evidence from a natural experiment. The Lancet Global Health. 2015;3:e470–e477. doi: 10.1016/S2214-109X(15)00087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle M.E, Labbate L.A. Incidence of HIV infection among patients with new-onset psychosis, Psychiatric Services. 1997:237–238. doi: 10.1176/ps.48.2.237. [DOI] [PubMed] [Google Scholar]
- 5.Drimie S, editor. The impact of HIV/AIDS on rural households and land issues in Southern and Eastern Africa. Human Sciences Research Council Pretoria; South Africa: 2002. [Google Scholar]
- 6.Freeman M, Nkomo N, Kafaar Z, Kelly K. Mental Disorder in People Living with HIV/Aids in South Africa. South African Journal of Psychology. 2008;38:489–500. doi: 10.1080/09540120701426482. [DOI] [PubMed] [Google Scholar]
- 7.Hammond E. HIV, psychiatric illness. Psychiatric Times. 2007;24:57–57. [Google Scholar]
- 8.Harris M.J, Jeste D.V, Gleghorn A, Sewell D.D. New-onset psychosis in HIV-infected patients. Journal of Clinical Psychiatry. 1991;52:369–376. [PubMed] [Google Scholar]
- 9.Joska J.A, Kaliski S.Z, Benatar S.R. Patients with severe mental illness:A new approach to testing for HIV. South African Medical Journal. 2008;98:213–217. [PubMed] [Google Scholar]
- 10.Kagee A, Martin L. Symptoms of depression and anxiety among a sample of South African patients living with HIV. AIDS Care. 2010;22:159–165. doi: 10.1080/09540120903111445. [DOI] [PubMed] [Google Scholar]
- 11.Kinsler J.J, Wong M.D, Sayles J.N, Davis C, Cunningham W.E. The effect of perceived stigma from a health care provider on access to care among a low-income HIV-positive population. AIDS Patient Care and STDs. 2007;21:584–592. doi: 10.1089/apc.2006.0202. [DOI] [PubMed] [Google Scholar]
- 12.Lorenc A, Ananthavarathan P, Lorigan J, Banarsee R, Jowata M, Brook G. The prevalence of comorbidities among people living with HIV in Brent:a diverse London Borough. London Journal of Primary Care. 2014;6:84–90. doi: 10.1080/17571472.2014.11493422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg P, Nakasujja N, Musisi S, Thorson A.E, Cantor-Graae E, Allebeck P. HIV prevalence in persons with severe mental illness in Uganda:a cross-sectional hospital-based study. International Journal of Mental Health Systems. 2013;7:20. doi: 10.1186/1752-4458-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mashaphu S, Mkize D. HIV seropositivity in patients with first-episode psychosis. South African Journal of Psychiatry. 2007;13:90–94. [Google Scholar]
- 15.Muula A.S. HIV infection and AIDS among young women in South Africa. Croatian Medical Journal. 2008;49:423–435. doi: 10.3325/cmj.2008.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myer L, Smit J, Roux L.L, Parker S, Stein D.J, Seedat S. Common mental disorders among HIV-infected individuals in South Africa:prevalence, predictors, validation of brief psychiatric rating scales. AIDS Patient Care and STDs. 2008;22:147–158. doi: 10.1089/apc.2007.0102. [DOI] [PubMed] [Google Scholar]
- 17.Popiel M, Duvvi V, Turkieh A, Cowan E, Calderon Y, Umfrid C, Chacón K, Krauss J, Rao A, Zahn J. Rapid routine HIV testing for psychiatric inpatients. Journal of AIDS Clinical Research. 2016;7 [Google Scholar]
- 18.Sall L, Salamon E, Allgulander C, Owe-Larsson B. Psychiatric symptoms and disorders in HIV infected mine workers in South Africa. A retrospective descriptive study of acute first admissions. African Journal of Psychiatry. 2009;12:206–212. doi: 10.4314/ajpsy.v12i3.48495. [DOI] [PubMed] [Google Scholar]
- 19.Sewell D.D, Jeste D.V, Atkinson J.H, Heaton R.K, Hesselink J.R, Wiley C, Thal L, Chandler J.L, Grant I. HIV-associated psychosis:A study of 20 cases. American Journal of Psychiatry. 1994;151:237–242. doi: 10.1176/ajp.151.2.237. [DOI] [PubMed] [Google Scholar]
- 20.Singh D, Berkman A, Bresnahan M. Seroprevalence and HIV-associated factors among adults with severe mental illness:a vulnerable population. South African Medical Journal. 2009;99:523–527. [PMC free article] [PubMed] [Google Scholar]
- 21.Sorsdahl K.R, Mall S, Stein D.J, Joska J.A. Perspectives towards mental illness in people living with HIV/AIDS in South Africa. AIDS Care. 2010;22:1418–1427. doi: 10.1080/09540121003758655. [DOI] [PubMed] [Google Scholar]
- 22.Susser E, Colson P, Jandorf L, Berkman A, Lavelle J, Fennig S, Waniek C, Bromet E. HIV infection among young adults with psychotic disorders. American Journal of Psychiatry. 1997;154:864–866. doi: 10.1176/ajp.154.6.864. [DOI] [PubMed] [Google Scholar]
- 23.UNAIDS. Global AIDS Update-2016, UN Joint Programme on HIV/AIDS 2016 [Google Scholar]
- 24.Uys H. Prevalence and clinical presentation of HIV positive female psychiatric inpatients. African Journal of Psychiatry. 2013;16:23–28. doi: 10.4314/ajpsy.v16i1.4. [DOI] [PubMed] [Google Scholar]
- 25.Van Rensburg B.J, Bracken C. Acute psychiatric in-patients tested for HIV status:a clinical profile. South African Journal of Psychiatry. 2007;10:83–85. [Google Scholar]


