Abstract
Background:
Influenza sentinel surveillance in Cote d’Ivoire showed that 70% of Acute Respiratory Infections (ARI) cases remained without etiology. This work aims to describe the epidemiological, clinical, and virological pattern of ARI that tested negative for influenza virus, in children under five years old.
Materials and Methods:
one thousand and fifty nine samples of patients presenting influenza Like Illness (ILI) or Severe Acute Respiratory Infections (SARI) symptoms were tested for other respiratory viruses using multiplex RT-PCR assays targeting 10 respiratory viruses.
Results:
The following pathogens were detected as follows, hRV 31,92% (98/307), hRSV 24.4% (75/329), PIV 20.5% (63/307), HCoV 229E 12,05% (37/307), hMPV 6.2% (19/307), HCoVOC43 1.0% (3/307) and EnV 1.0% (3/307). Among the 1,059 specimens analyzed, 917 (86.6%) were ILI samples and 142 (23.4%) were SARI samples. The proportion of children infected with at least one virus was 29.8% (273/917) in ILI cases and 23.9% (34/142) in SARI cases. The most prevalent viruses, responsible for ILI cases were hRV with 35.89% (98/273) and hRSV in SARI cases with 41.2% (14/34) of cases. Among the 1,059 patients, only 22 (2.1%) children presented risk factors related to the severity of influenza virus infection.
Conclusion:
This study showed that respiratory viruses play an important role in the etiology of ARI in children. For a better understanding of the epidemiology of ARI and improved case management, it would be interesting in this context to expand the surveillance of influenza to other respiratory viruses.
Keywords: Detection, Non influenza viruses, Acute respiratory infections, Children
Introduction
Acute respiratory infections (ARI) are a real public health problem because of their high morbidity and mortality (Black et al., 2010). The World Health Organization (WHO) classifies respiratory infections as the second leading cause of death worldwide in children under the age of five (Bryce et al., 2005). Approximately 2.2 million children worldwide die each year of ARI and nearly 40% of these deaths occur in Africa because of limited diagnostic capacities (Williams et al., 2002). It has been shown that viral infections play a major role in the occurrence of ARI in developed countries. In sub-Saharan Africa some work on specific viruses such as influenza (Inf) and human respiratory syncytial viruses (hRSV) have been realized but data on the occurrence of ARI remain insufficient (Tiveljung-Lindell et al., 2009, Brittain-Long et al., 2008). Indeed, the responsibility of viruses other than influenza in the occurrence of ARI is not yet sufficiently documented.
An international multicenter study coordinated by the Board of Science and Technology for International Development of the National Academy of Sciences of the United States found that viral etiology of ARI in developing countries is present in a higher proportion than the bacterial etiology (1990). Recent studies done in Kenya, Cameroon and Senegal have highlighted the high prevalence of these pathogens in ARI (Berkley et al., 2010, Njouom et al., 2012, Niang et al., 2010) especially in children. Respiratory viruses frequently associated with ARI include Inf A and B, hRSV, Enteroviruses (EnV), human Metapneumovirus (hMPV), Rhinovirus (hRV), Adenovirus, Parainfluenza virus (PIV) type 1-3 (Pretorius et al., 2012, Razanajatovo et al., 2011). These viruses are responsible for severe clinical forms of common viral infections (bronchiolitis, pneumonia, laryngitis, asthma)(Puzelli et al., 2009). However these etiologic pathogens are not sought in practice due to the underestimation of their involvement in ARI, the lack of an adapted laboratory diagnosis capacities, and limited treatment options. The control of children morbidity and mortality is based on a better understanding of the epidemiology of the disease and the availability of adequate laboratory diagnosis capacity.
In Côte d’Ivoire, a sentinel surveillance system for influenza viruses was established in 2003 at the initiative of the Institut Pasteur of Cote d’Ivoire. This surveillance has helped to identify and characterize circulating seasonal influenza A(H1N1), A(H3N2), A(H1N1) pdm09 and influenza B viruses. These viruses circulate throughout the year with a peak that varies according to the occurrence of the rainy season between the months of May and June. This surveillance system has contributed to identify age groups at high risk of severe influenza disease (Kadjo et al., 2013). Along with the circulation of influenza viruses, other respiratory viruses were detected in patients presenting with ILI. Given the limited resources of the laboratory, the diagnosis of other respiratory viruses was retrospective and limited to children under five years old. From 2006, as part of the preparation for influenza pandemic, the technical capacities of the laboratory have been strengthened by the tools for molecular diagnosis, staff training and regular supply of reagents and consumables through a cooperative agreement with the U.S. CDC Influenza Division in Atlanta and the Institut Pasteur in Paris. Therefore, it seemed appropriate in this context to add the surveillance of other respiratory viruses to the influenza surveillance network. This study aims to describe the epidemiological and virological patterns of ARI due to non-influenza respiratory viruses in children under five years of age in Côte d’Ivoire.
Materials and Methods
Enrollment of patients
Patients have been enrolled in the influenza sentinel surveillance network (figure 1). This network this network is composed of 9 sentinel sites distributed in the different geographical regions of the country. Every day, doctors, nurses and other trained healthcare staff identified children meeting the ILI or SARI case definition. At the time of the study and according to the WHO, an ILI case was defined as any patient with a fever (temperature ≥ 38°C) of sudden onset with cough lasting for less than ten days. A SARI case was defined as any patient with acute respiratory infection with a history of fever or fever with a temperature ≥ 38°C and cough with onset of symptoms within ten days whose condition requires hospitalization (WHO, 2014).
Figure 1.
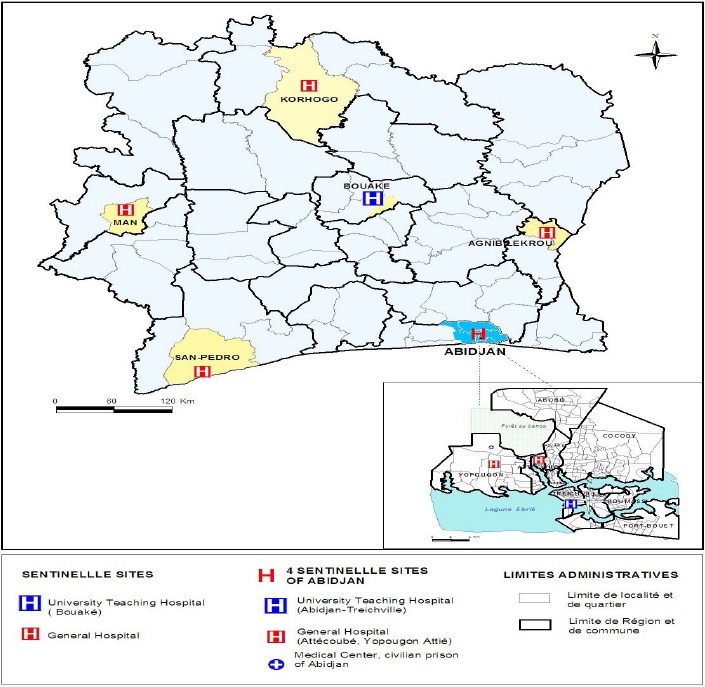
Influenza sentinel surveillance network
All cases of SARI were eligible for inclusion if a parent or legal guardian gave his or her verbal consent. However for ILI, only the first three cases of the day were enrolled at each site after obtaining verbal consent from a parent or legal guardian of the child. After collecting specimens from the first three cases each day, all subsequent cases meeting the case definition for ILI were counted, recorded and reported in a weekly summary. Patients who did not meet the case definitions or for which the verbal consent of the parent or legal guardian was not obtained were excluded from the study. A structured and standardized questionnaire to collect socio-demographic, epidemiological and clinical data was developed and used. The questionnaire also included the collection of outcome data, risk factors related to the severity of the influenza virus infection such as asthma, chronic lung disease, hepatitis, diabetes, malnutrition, obesity, heart disease, chronic kidney disease, blood disorders, and immune deficiencies (e.g. HIV infection) (Mertz et al., 2013, Gill et al., 2015). Samples from each patient included in the study were collected using sterile nasopharyngeal swabs that were then placed in a viral transport medium, VTM (MEM, Gibco-BRL, life Technologies, Paisley, Scotland, Penicillin 200 U / ml, streptomycin 200 mg / ml, BioWhittaker, MA; mycostatin 200U/ml, Sigma, bovine serum albumin 0.25%, Merck, Darmstadt, Germany). Once collected, samples were placed into coolers and transported immediatly to the national influenza center (NIC) located at the Institut Pasteur of Côte d’Ivoire for sites located in Abidjan or stored in a refrigerator at the sentinel site between 2°C - 8°C until they’re transported to the NIC twice a week. The maximum storage period at each site did not exceed 3 days.
Molecular diagnostic
RNA extraction
RNA was extracted from 200 µl of the collected nasopharyngeal secretions and put in VTM. Extraction was performed using the QIAamp Viral RNA Mini kit (QIAGEN®, Hilden, Germany) with RNA elution into a final volume of 80 µl., Nuclease free water were included for each extraction run as a negative control. Two aliquots of each extracted RNA were made; one the aliquot is used for PCR and the second stored at -20°C for future analyses.
Influenza detection
Influenza viruses A(H1N1) pdm09, A(H3N2), B Yamagata and Victoria lineages were detected by real time RT-PCR using primers and probes provided by the CDC International Reagent Resources (IRR). For the detection of viruses mentioned, three different amplification kits were used; CDC influenza virus real time RT-PCR influenza A/B typing panel (RUO), CDC influenza virus real-time RT-PCR influenza A(H1, H3/H1pdm09) subtyping panel (RUO) and CDC influenza B Lineage genotyping panel (RUO), according to the CDC real time RT-PCR (qRT-PCR) protocol for detection and characterization of influenza. All negative specimens were tested for other respiratory viruses.
Detection of other respiratory viruses
Three multiplex conventional RT-PCR assays were used as described by Bellau-Pujol S et al. (Bellau-Pujol et al., 2005) to test specimens negative for influenza diagnostic. The multiplex 1-targeted human hRSV andhMPV. The multiplex 2 targeted parainfluenza viruses 1-4 and the multiplex 3 targeted human coronaviruses (HCoV) OC43 and 229E, influenza C, rhinovirus (hRV), enterovirus (Env).An hemi-nested singleplex PCR was used to detect hRV. The differents primers used for amplification are listed on the table 1. For master mix reagents, QIAGEN OneStep RT-PCR Cat No.210212 has been used according to the manufacturer’s recommendation. All heminested have been done with Amplitaq Recombinant + 10x buffer (Applied Biosystem/Applera Ref 8080152) and dNTPs 2mM (Set deoxynucleotides PCR grade - Roche - Ref 11969064). For each multiplex, positive controls provided by the virology laboratory of Clemenceau University Teaching Hospital (Caen, France) were used. The RT-PCR cycling condition were 30 min at 50°C and 15 min at 94°C followed by 40 cycles of 95°C for 30 sec at 94°C, 30 sec at 55°C and 1mn at 72°C following by 72°C for 10mn and final at 4) °C in GeneAmp® PCR System 9700 (Applied Biosystems).
Table 1.
List of primers for respiratory virus detection
| Virus | Primers | Sequence (5’→3’) | |
|---|---|---|---|
| RT-PCR | hRSV | vrs P1 | GGA ACA AGT TGT TGA GGT TTA TGA ATA TGC |
| vrs P2 | CTT CTG CTG TCA AGT CTA GTA CAC TGT AGT | ||
| hMPV | hmpv 1 | CCC TTT GTT TCA GGC CAA C | |
| hmpv 2 | GCA GCT TCA ACA GTA GCT G | ||
| SEMI-NESTED | hRSV | vrs i | GGT GTA CCT CTG TAC TCT C |
| hMPV | hmpv 3 | AGG CCA ACA CAC CAC CAG | |
| PRIMERS MULTIPLEX 2 | |||
| Virus | Primers | Sequence(5’→3’) | |
| RT-PCR | parainfluenza virus 1 | PIS1+ | CCG GTA ATT TCT CAT ACC TAT G |
| PIS1- | CCT TGG AGC GGA GTT GTT AAG | ||
| parainfluenza virus 2 | PIP2+ | AAC AAT CTG CTG CAGCAT TT | |
| PIP2- | ATG TCA GAC AAT GGG CAA AT | ||
| parainfluenza virus 3 | Para 3.1 | CTC GAG GTT GTC AGG ATA TAG | |
| Para 3.2 | CTT TGG GAG TTG AAC ACA GTT | ||
| parainfluenza virus 4 | PIP4+ | CTG AAC GGT TGC ATT CAG GT | |
| PIP4- | TTG CAT CAA GAA TGA GTC CT | ||
| internal control | GAPDH1 | TCA TCC ATG ACA ACT TTG GTA TCG TG | |
| GAPDH2 | CTC TTC CTC TTG TGC TCT TG | ||
| SEMI-NESTED | parainfluenza virus 1 | PiS1i | AGC TGC AGG AAC AAG GGG |
| parainfluenza virus 2c | CTA GCT GAA CTG AGA CTT G | ||
| parainfluenza virus 3 | Para 3i | GCT AGA GAA CAT GAC TTC C | |
| parainfluenza virus 4 | Pi4i | GTC TGA TCC CAT AAG CAG C | |
| PRIMERS MULTIPLEX 3 | |||
| Virus | Primers | Sequence(5’→3’) | |
| RT-PCR | hRV/HEnV | SRHI1 | GCA TCI GGY ARY TTC CAC CAC CAN CC |
| SRHI2 | GGG ACC AAC TAC TTT GGG TGT CCG TGT | ||
| HCoV 229E | MD1 | TGG CCC CAT TAA AAA TGT GT | |
| MD3 | CCT GAA CAC CTG AAG CCA AT | ||
| HCoV OC43 | MF1 | GGC TTA TGT GGC CCC TTA CT | |
| MF3 | GGC AAA TCT GCC CAA GAA TA | ||
| SEMI-NESTED | hRV | Nestrhi1 | ATG GGN GCW CAN GTN TCH ANH CA |
| HCoV 229E | MD2i | CCG TAT CAA CAC TCG TTA TGT GG | |
| HCoV OC43 | MF2i | CTC CAA AAA CTT CCA GTT C | |
All amplified products were visualized after electrophoresis on an ethidium bromide - stained 2% agarose gel (Agarose, Electrophoresis Grade, Ultra pure, Invitrogen).
Data analysis
The data collected were stored in a generated database using the Epi Info 3.5.1 software. We analyzed the epidemiological and clinical ILI and SARI data as well as associated comorbidities data. The statistical data analysis was performed using the R software version 2.15.2. The averages were compared using the t-Student test or using analysis of variance (ANOVA) when we had more than two averages; otherwise the non-parametric Kruskal Wallis test was used when variances for studied variable were not homogeneous across subgroups. Proportions were compared using the Fisher Exact test in univariate analysis. Logistic regression was used for multivariate analysis. The type I error α was set at 0.05.
Results
Demographic characteristics of Patients
During the period from January through December 2013, 1,340 specimens from patients under five years of age have been collected from the sentinel surveillance network. Among the 1,340 specimens, 281 (21.0%) were positive for influenza. Our study focused on the 1059 specimens that were negative for influenza. The average age of children in the study population was 17.8 ± 15.8 months with a median age equal to 12 months. Among this population, 53.6% of these children were male (sex ratio = 1.2). The most represented age group was children 0-12 months (67%). The average age was not significantly different according to gender (17.6 months vs. 17.9 for men) with a p-value p = 0.76. More than three-quarters (798/1,059) of the patients came from sentinel sites located in the capital city, Abidjan. Among the 1,059 samples analyzed, 29.0% (307/1,059) were positive for at least one of the pathogens tested. More than sixty percent (62.89%) of positive cases were found in children aged 0-12 months.
Patients with samples that tested positive were significantly younger than those that tested negative (15.8 months vs. 18.5 months) with p-value p = 0.009. The probability of a child 0-12 months of developing a respiratory infection other than influenza is 1.77 times higher than in a 37-60-month-old child (p <0.05) (Table 2).
Table 2.
Demographic and clinical characteristics for children under five years, Influenza sentinel surveillance Network, CIV, January to December 2013
| Characteristics | Other viruses result | OR (95% CI)a | p value | |
|---|---|---|---|---|
| Positive | Negative | |||
| n=307 | n=752 | |||
| Age Group in month n (%) | ||||
| [0 - 12] | 193(62.86) | 514 (68.35) | 1.00 (0.64 – 1.56) | |
| [13 - 24] | 57 (18.56) | 86 (11.43) | 1.77 (1.04 – 3.01) | <0.05 |
| [25 - 36] | 26(8.46) | 69 (9.17) | 1.00 (0.54 – 1.85) | |
| [37 - 60] | 31 (9.12) | 83 (11.46) | 1 | |
| Sexe n (%) | ||||
| Female | 134 (43.6) | 357 (47.5) | 1.2 (0.9 –1.5) | 0.3 |
| Temperature (°C) | ||||
| Mean ± sd | 38.4 ± 0.7 | 38.5 ± 0.9 | - | 0.18 |
| Hospitalization n (%) | ||||
| Yes | 34 (11.1) | 108 (14.4) | 1.3 (0.9 – 2.0) | 0.15 |
| Risk Factors (at least one) n (%) | ||||
| Yes | 5 (1.63) | 17 (2.26) | 0.72 (0.20 – 2.05) | 0.64 |
| Residence (Abidjan vs other location) n (%) | ||||
| Abidjan | 220 (71.7) | 578 (76.9) | 1.3 (1.0 – 1.8) | 0.07 |
Respiratory virus detection
Among the positive cases the following agents were detected in order of frequency HRhV 31,92% (98/307), hRSV 24.75% (76/307), PIV 20.5% (63/307), HCoV229E 12,05% (37/307), hMPV 6.2% (19/307), HCoV0C43 1.0% (3/307) and EnV 1.0% (3/307). Some Co-detections (two viruses) were observed in 2.9% (9/307) of cases Parainfluenza viruses were frequently detected in co-detection cases. The 9 cases were distributed as follow: 0.33 % (1/307) PIV3/hRSV, 0.65% (2/307) hMPV/PIV3, 0.33% (1/307) PIV3/ HCoV229E, 0.65% (2/307) PIV1/hMPV, 0.33% (1/307) RSV/ HCoV229E and 0.33% (1/307) hRSV/PIV1. All viruses targeted by the three multiplex PCR were detected in the age group of 0-12 months. The detection rate of these different viruses was also highest in this age group. The HRhV, PIV hRSV and hMPV, were detected in all age groups. The EnV were detected only in the age group of 0-12 months (Table 3).
Table 3.
Distribution of other respiratory viruses involved in ARI among children under five years by age group, Influenza sentinel surveillance network, CIV, January to December 2013
| Age Group (Months) N, (%) | |||||
|---|---|---|---|---|---|
| [0-12] | [13-24] | [25-36] | [37-60] | Total | |
| Coronavirus 229E | 0(0.0) | 29 (50.87) | 2 (7.5) | 6 (10) | 37 |
| Parainfluenza | 48 (24.87) | 4 (6.3) | 4 (6.3) | 7 (11.1) | 63 |
| VRS | 56 (29.01) | 11 (14.7) | 6 (8) | 2 (2.7) | 75 |
| Rhinovirus | 68 (35.23) | 6 | 11 (6.7) | 13(13.3) | 98 |
| Metapneumovirus | 12 (6,21) | 3 (15.8) | 3 (15.8) | 1 (5.3) | 19 |
| Coronavirus OC43 | 2 (1.03) | 0 | 0 | 1 (33.3) | 3 |
| Enterovirus | 3 (1.55) | 0 | 0 | 0 | 3 |
| Coinfection (two viruses) | 4 (2.07)) | 4 (44.4) | 0 | 1(11.1) | 9 |
Clinical characteristics
Patients were classified under two clinical presentation groups of ILI and SARI. Among the 1,059 samples analyzed; 917 (86.6%) were ILI cases 142 (23.4%) were SARI cases. The proportion of patients infected with at least one virus was 29.8% (273/917) in ILI cases and 23.9% (34/142) in SARI cases. There was no statistically significant difference between the two groups.. In the 2 groups, the highest positivity rate was observed in children with age ranging between 0 - 12 months. The most detected virus in ILI positive cases was rhinovirus with 35.89% (98/273) and hRSV in SARI positive cases with 44.11% (15/34) of cases (Table 4). Among the 1,059 patients, 2.1% (22/1,059) had infection severity risk factors related to their clinical history. The risk factor were present in 31.8% (7/22) of the ILI cases and 68.2% (15/22) in SARI cases. The proportion of SARI cases with risk factors was significantly higher than that SARI cases without risk factor (p-value p < 0.01). Asthma was the most present risk factor with 63.6% (14/22) of cases, followed by malnutrition 18.2% (4/22), chronic lung disease and obesity 9.1% (2/22). Asthma was found in 73.3% (11/15) of SARI cases with risk factors. Of the 22 children with risk factors, half (11/22) were positive for RSV, 9% (2/22) were positive for hMPV and 18.2% (4/22) presented a co-detection (RSV/HCoV229E, PIV3/RSV, PIV1/RSV hMPV/PIV3).
Table 4.
Results of Other respiratory virus screening by syndromes and age Groups
| Virus type | Infection Type and Age Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SARI | ILI | |||||||||
| [0-12] | [13-24] | [25-36] | [37-60] | Total | [0-12] | [13-24] | [25-36] | [37-60] | Total | |
| Coronavirus 229E | 0 | 12 | 0 | 0 | 12 | 0 | 17 | 0 | 8 | 25 |
| Enterovirus | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 2 |
| Metapneumovirus | 1 | 0 | 1 | 0 | 2 | 11 | 3 | 3 | 0 | 17 |
| Coronavirus OC43 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 2 |
| Parainfluenzavirus | 2 | 0 | 1 | 1 | 4 | 46 | 5 | 4 | 4 | 59 |
| Rhinovirus | 0 | 0 | 0 | 0 | 0 | 68 | 6 | 11 | 13 | 98 |
| RSV | 12 | 0 | 1 | 1 | 14 | 44 | 10 | 5 | 2 | 61 |
| Co-infections | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 1 | 9 |
| Total | 16 | 12 | 3 | 3 | 34 | 177 | 45 | 23 | 28 | 273 |
Seasonnality
The circulation of respiratory viruses was greater in the first half of the year. The proportion of positive cases was 58.5% in the first semester and 31.6% in the second half (OR = 3 IC 95% OR = [2.2; 4.2], p value < 0.001. However, the individual trends of the viruses were not the same. Cases of HRV infection occurred throughout the year. The months of January to May demonstrated the highest number of HRV infections (72,44% of detected virus). A pic was observed in the month of March. A similar trend occurred for Parainfluenza virus with 85,71% of virus detected between the months January to June. High circulation of hRSV and hMPV occurred during the months of June to December. Circulation of other viruses (coronavirus OC43 and enterovirus) occurred in the months of March, May, June and November. (Figure 2).
Figure 2.
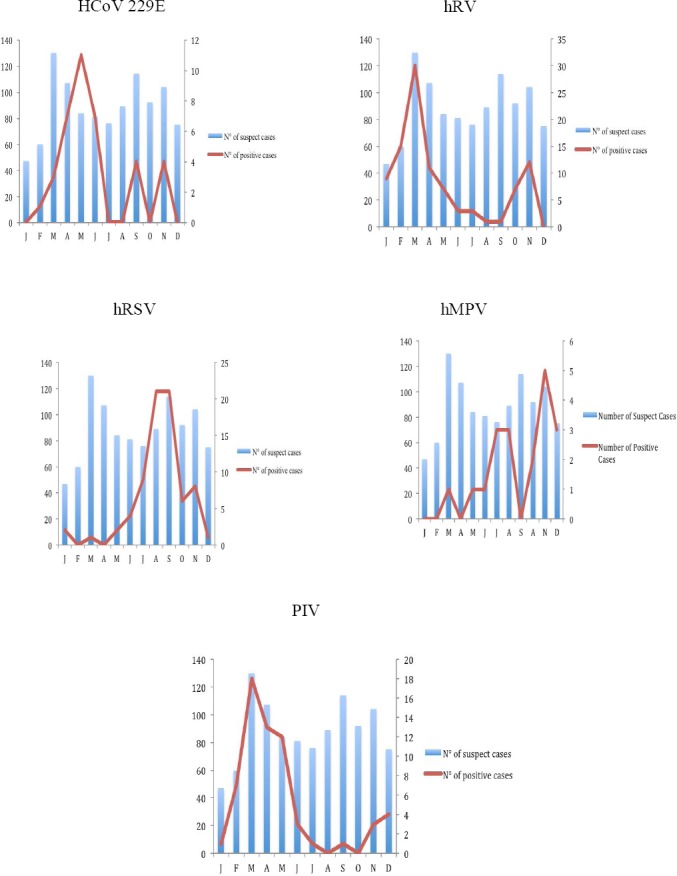
Distribution of viruses detected by month
Discussion
This is the first study describing the circulation throughout the year of respiratory viruses other than influenza associated with patients less than 5 years with ILI or SARI cases in Cote d’Ivoire and demonstrating their contribution to respiratory disease in this age group. The present study targeted eleven respiratory viruses involved in human respiratory infections. The global detection rate of viruses in our study was 29.1%. This result is consistent with the results of several previous studies that found percentages between 27–35% (Yang et al., 2012, Ren et al., 2009, Pierangeli et al., 2007, Arbefeville and Ferrieri, 2017) but it is lower than those reported by several studies in other Africa countries and other regions in the world where the percentages of virus detection were ranged between 40–60% (Pretorius et al., 2012, Lekana-Douki et al., 2014, Ahmed et al., 2012). The difference observed in rate of viral etiology could be explained by several factors related to the different study population, geographical catchment area, inclusion criteria, targeted viruses and diagnostic techniques and primers used (Choudhary et al., 2016, Ali et al., 2011). When conducting this study, conventional PCR was the only method available in the laboratory for the screening of other respiratory viruses. In 10 recent years the sensitivity of diagnostic tests for the detection of respiratory viruses has been greatly improved with the development of multiplex real time PCR protocols with high sensitivity and targeting major respiratory viruses involved in human pathology (Barratt et al., 2017, Zhao et al., 2017, Malhotra et al., 2016). The use of conventional PCR could thus be one of the main limitations of our study and explain to a large extent the overall virus detection rate reported in our study. However, it should be noted that for a better understanding of the involvement of respiratory viruses in the respiratory infections of children, it would be interesting to have standardized study protocols.
The most common viruses detected in our study were rhinovirus and hRSV accounting for almost 56% of all cases. Others reported both viruses previously as the major etiology for respiratory viral infections in children under five (Razanajatovo et al., 2011, Kumar et al., 2017, Buecher et al., 2010). The positive rate found for RSV of 24.4% in the study was consistent with our previously published results (Kadjo et al., 2013). The age distribution in our study indicated that infants whose age ranged between 0 and 1 year were more likely to be infected by Rhinovirus and hRSV. Our results also showed that 20.5% of viral pathogens detected were PIV, which implied that PIVs played an important role in children with respiratory as mentioned by Henrickson KJ and al. similar findings were obtained in the studies conducted in several countries in Africa (Lekana-Douki et al., 2014, Lagare et al., 2015). Human metapneumovirus has been proven to be one of the main viral pathogens responsible of respiratory infections. However, its prevalence in cases of acute respiratory infections in children remains low as attested by the results of this study and previous studies conducted in Cote d’Ivoire and in Sub-Saharan Africa regions (Njouom et al., 2012, Kadjo et al., 2013, Lekana-Douki et al., 2014). The results of a meta-analysis study of 75 articles on hMPV prevalence data shown 6.24% cases among ARI cases (Gill et al., 2015). The HCoV229E was with a rate of 12, 05 % of detection. Some studies have found low rates of detection of coronaviruses. According to McIntosh et al (2004), the global incidence of LRTIs due to HCoV was not higher than 3.8% However some studies have shown that the prevalence of these coronavirus in pediatric patients can be significantly higher, varying from about 5% to more than 30% and these discrepancies could be attributed to differences in the research methodologies. Parainfluenza viruses were the most frequent pathogen detected in dual detection cases. These data do not corroborate the assertion that the viruses most commonly involved in co-detection were the viruses with the highest incidence (O’Grady et al., 2016). From the clinical point of view, our study showed that respiratory viruses were found in both cases of ILI and SARI in Children with high proportions (23.4% - 29.8%). Search for respiratory viruses in cases of acute respiratory infections is not systematic in our tropics. The diagnostic approach is empirically oriented on bacterial etiologies. Today with the accessibility and widespread of PCR, this research should be integrated into the clinicians’ diagnostic approach particularly in infants in whom the viral infection can lead to a severe form of respiratory infection especially in the presence of risk factors. Indeed as attested by our study, over 68.0% of children with risk factors presented with severe acute respiratory infections related to viral infection. The most predominant viruses in these cases were hRSV with a rate of 50.0%. The hRSV were also the most involved viruses in SARI cases with 41.2% of cases. This correlated well with the results of several studies showing hRSV as a common cause of lower respiratory tract infection in children admitted to the hospital (Nair H et al., 2010) Our study showed a high proportion of asthma (63.4%) in patients with history of risk factors in According to recent systematic review, using molecular techniques, respiratory viruses are detected in around 80.0% of asthma exacerbation with highest prevalence in children (Papadopoulos and Johnston, 1998). It is obvious that preventive measures and timely management of viral infections in children including children with a history of asthma reduce exacerbation of seizures and progression to severe forms of viral infection.
Among all viruses detected, we have three seasonal profiles. The first profile is that of viruses with a high circulation during the first semester of the year it is the HCoV229E, hRV and PIV; Viruses with high circulation during the second semester of the year, RSV and hMPV and the group of viruses with sporadic circulation, HCoVOC43, EnV. Interestingly the circulation of HRhV and PIV was well correlated with the dry season, which occurred between the months of January and May (fig 2). Our results showed a significant association between the proportion of positive cases of HRV and PIV viruses and dry season with a p-value < 0.001. This has also been observed with the proportion of hRSV positive cases during the rainy season with p-value < 0.001. This association between hRSV and the rainy season has been put in evidence in several studies conducted in tropical area (Lekana-Douki et al., 2014, Do et al., 2016). The transmission pattern of hRSV and the observed promiscuity during rainy seasons play a key role in the high incidence of hRSV during the rainy season.
Conclusion
The findings presented in this study provide a better understanding of virus distribution among different pediatric ages, genders, and seasons in Cote d’Ivoire. Though influenza viruses represent a major cause of ARI, it is also appropriate to extend influenza surveillance to the other respiratory viruses so as to better understand the epidemiology and burden of viral respiratory infections, especially in children under the age of five and particularly in settings like Sub-Saharan Africa where there are still limited capacities for the molecular diagnosis of other respiratory viruses. Furthermore, such initiatives will assist policy makers to evaluate the benefit of introducing prevention and treatment strategies.
List of abbreviations
- ARI-
Acute Respiratory Infections
- EnV-
Enterovirus
- HCoV-
Human Coronavirus
- hMPV-
human Metapneumovirus
- hRSV-
human Respiratory Syncitial Virus
- hRV-
human Rhinovirus
- ILI-
Influenza Like Illness
- Inf-
Influenza
- IRR-
International Reagent Resource
- NIC-
National Influenza Center
- PIV-
Parainfluenzavirus
- SARI-
Severe Acute Respiratory Infections
- VTM-
Viral Transport Media
- WHO-
World Health Organisation
Acknowledgements
We are grateful to personnel of influenza sentinel network for sending specimens of suspected influenza cases. We would like to thank the Pasteur Institute of Cote d’Ivoire and National Institute of Public Hygien personnels; Gbahouo Jeannie, Koffi Melissa, Konan Yannick and Anderson Ngattia, for technical assistance.
Footnotes
Conflict of interest: The authors declared no conflict of interest with this study.
Funding Sources: Funding for this study was provided by the Ministry of Health of Cote D’Ivoire, the World Health Organization and the U.S. Centers for Diseases Control and Prevention.
Ethical considerations: The Cote d’Ivoire influenza and other respiratory viruses surveillance is a national program approved by the Ministry of Health and supported by WHO/AFRO as part of the Influenza pandemic preparedness. Patient information and specimen collection respected the procedures stipulated in the WHO/AFRO influenza surveillance protocol.
References
- 1.Black R.E, Cousens S, Johnson H.L, Lawn J.E, Rudan I, Bassani D.G, Jha P, Campbell H, Walker C.F, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional, and national causes of child mortality in 2008:a systematic analysis. Lancet. 2010;375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, Black R.E. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Williams B.G, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infectious Diseases. 2002;2(1):25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 4.Tiveljung-Lindell A, Rotzen-Ostlund M, Gupta S, Ullstrand R, Grillner L, Zweygberg-Wirgart B, Allander T. Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses. Journal of Medical Virology. 2009;81(1):167–75. doi: 10.1002/jmv.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brittain-Long R, Nord S, Olofsson S, Westin J, Anderson L.M, Lindh M. Multiplex real-time PCR for detection of respiratory tract infections. Journal of Clinical Virology. 2008;41(1):53–56. doi: 10.1016/j.jcv.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selwyn BJ. Etiology and epidemiology of acute respiratory tract infection in children in developing countries. Irvine, California, 20-22 October 1988. Proceedings. Reviews of Infectious Diseases. 1990;12(Suppl 8):S861–1083. [PubMed] [Google Scholar]
- 7.Berkley J.A, Munywoki P, Ngama M, Kazungu S, Abwao J, Bett A, Lassauniere R, Kresfelder T, Cane P.A, Venter M, Scott J.A, Nokes D.J. Viral etiology of severe pneumonia among Kenyan infants and children. Journal of American Medical Association. 2010;303(20):2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Njouom R, Yekwa E.L, Cappy P, Vabret A, Boisier P, Rousset D. Viral etiology of influenza-like illnesses in Cameroon, January-December 2009. Journal of Infectious Diseases. 2012;206(Suppl 1):S29–35. doi: 10.1093/infdis/jis573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niang M.N, Diop O.M, Sarr F.D, Goudiaby D, Malou-Sompy H, Ndiaye K, Vabret A, Baril L. Viral etiology of respiratory infections in children under 5 years old living in tropical rural areas of Senegal:The EVIRA project. Journal of Medical Virology. 2010;82(5):866–872. doi: 10.1002/jmv.21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pretorius M.A, Madhi S.A, Cohen C, Naidoo D, Groome M, Moyes J, Buys A, Walaza S, Dawood H, Chhagan M, Haffjee S, Kahn K, Puren A, Venter M. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness--South Africa, 2009-2010. Journal of Infectious Diseases. 2012;206(Suppl 1):S159–65. doi: 10.1093/infdis/jis538. [DOI] [PubMed] [Google Scholar]
- 11.Razanajatovo N.H, Richard V, Hoffmann J, Reynes J.M, Razafitrimo G.M, Randremanana R.V, Heraud J.M. Viral etiology of influenza-like illnesses in Antananarivo, Madagascar, July 2008 to June 2009. PloS One. 2011;6(3):e17579. doi: 10.1371/journal.pone.0017579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puzelli S, Valdarchi C, Ciotti M, Dorrucci M, Farchi F, Babakir-Mina M, Perno C.F, Donatelli I, Rezza G. Viral causes of influenza-like illness:Insight from a study during the winters 2004-2007. Journal of Medical Virology. 2009;81(12):2066–2071. doi: 10.1002/jmv.21610. [DOI] [PubMed] [Google Scholar]
- 13.Kadjo H.A, Ekaza E, Coulibaly D, Kouassi D.P, Nzussouo N.T, Kouakou B, Ouattara A, Adjogoua E.V, Akoua-Koffi C.G, Elia G.A, Victoir K, Bretin-Dosso M.C, Mott J.A. Sentinel surveillance for influenza and other respiratory viruses in Cote d'Ivoire, 2003-2010. Influenza Other Respir Viruses. 2013;7(3):296–303. doi: 10.1111/j.1750-2659.2012.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) [accessed];Global epidemiological surveillance standard for influenza. 2014 www.who.int/influenza/
- 15.Mertz D, Kim T.H, Johnstone J, Lam P.P, Science M, Kuster S.P, Fadel S.A, Tran D, Fernandez E, Bhatnagar N, Loeb M. Populations at risk for severe or complicated influenza illness:systematic review and meta-analysis. British Medical Journal. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill P.J, Ashdown H.F, Wang K, Heneghan C, Roberts N.W, Harnden A, Mallett S. Identification of children at risk of influenza-related complications in primary and ambulatory care:a systematic review and meta-analysis. Lancet Respiratory Medicine. 2015;3(2):139–149. doi: 10.1016/S2213-2600(14)70252-8. [DOI] [PubMed] [Google Scholar]
- 17.Bellau-Pujol S, Vabret A, Legrand L, Dina J, Gouarin S, Petitjean-Lecherbonnier J, Pozzetto B, Ginevra C, Freymuth F. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. Journal of Virological Methods. 2005;126(1-2):53–63. doi: 10.1016/j.jviromet.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Yao Y, Chen M, Xie Y, Liu Y, Zhao X, Gao Y, Wei L. Etiology and clinical characteristics of influenza-like illness (ILI) in outpatients in Beijing, June 2010 to May 2011. PloS One. 2012;7(1):e28786. doi: 10.1371/journal.pone.0028786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren L, Gonzalez R, Wang Z, Xiang Z, Wang Y, Zhou H, Li J, Xiao Y, Yang Q, Zhang J, Chen L, Wang W, Li Y, Li T, Meng X, Zhang Y, Vernet G, Paranhos-Baccala G, Chen J, Jin Q, Wang J. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005-2007. Clinical Microbiology and Infection. 2009;15(12):1146–53. doi: 10.1111/j.1469-0691.2009.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierangeli A, Gentile M, Di Marco P, Pagnotti P, Scagnolari C, Trombetti S, Lo Russo L, Tromba V, Moretti C, Midulla F, Antonelli G. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. Journal of Medical Virology. 2007;79(4):463–468. doi: 10.1002/jmv.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbefeville S, Ferrieri P. Epidemiologic Analysis of Respiratory Viral Infections Mainly in Hospitalized Children and Adults in a Midwest University Medical Center After the Implementation of a 14-Virus Multiplex Nucleic Acid Amplification Test. American Journal of Clinical Pathology. 2017;147(1):43–49. doi: 10.1093/ajcp/aqw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lekana-Douki S.E, Nkoghe D, Drosten C, Ngoungou E.B, Drexler J.F, Leroy E.M. Viral etiology and seasonality of influenza-like illness in Gabon, March 2010 to June 2011. BMC Infectious Diseases. 2014;14:373. doi: 10.1186/1471-2334-14-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed J.A, Katz M.A, Auko E, Njenga M.K, Weinberg M, Kapella B.K, Burke H, Nyoka R, Gichangi A, Waiboci L.W, Mahamud A, Qassim M, Swai B, Wagacha B, Mutonga D, Nguhi M, Breiman R.F, Eidex R.B. Epidemiology of respiratory viral infections in two long-term refugee camps in Kenya, 2007-2010. BMC Infectious Diseases. 2012;12:17. doi: 10.1186/1471-2334-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhary M.L, Anand S.P, Tikhe S.A, Walimbe A.M, Potdar V.A, Chadha M.S, Mishra A.C. Comparison of the conventional multiplex RT-PCR, real time RT-PCR and Luminex xTAG(R) RVP fast assay for the detection of respiratory viruses. Journal of Medical Virology. 2016;88(1):51–57. doi: 10.1002/jmv.24299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali S.A, Gern J.E, Hartert T.V, Edwards K.M, Griffin M.R, Miller E.K, Gebretsadik T, Pappas T, Lee W.M, Williams J.V. Real-world comparison of two molecular methods for detection of respiratory viruses. Virology Journal. 2011;8:332. doi: 10.1186/1743-422X-8-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barratt K, Anderson T.P, Fahey J.A, Jennings L.C, Werno A.M, Murdoch D.R. Comparison of the fast track diagnostics respiratory 21 and Seegene Allplex multiplex polymerase chain reaction assays for the detection of respiratory viruses. British Journal of Biomedical Science. 2017;74(2):85–89. doi: 10.1080/09674845.2017.1278885. [DOI] [PubMed] [Google Scholar]
- 27.Zhao M.C, Li G.X, Zhang D, Zhou H.Y, Wang H, Yang S, Wang L, Feng Z.S, Ma X.J. Clinical evaluation of a new single-tube multiplex reverse transcription PCR assay for simultaneous detection of 11 respiratory viruses, Mycoplasma pneumoniae and Chlamydia in hospitalized children with acute respiratory infections. Diagnostic Microbiology and Infectious Disease. 2017;88(2):115–119. doi: 10.1016/j.diagmicrobio.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malhotra B, Swamy M.A, Reddy P.V, Kumar N, Tiwari J.K. Evaluation of custom multiplex real - time RT - PCR in comparison to fast - track diagnostics respiratory 21 pathogens kit for detection of multiple respiratory viruses. Virology Journal. 2016;13:91. doi: 10.1186/s12985-016-0549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar P, Medigeshi G.R, Mishra V.S, Islam M, Randev S, Mukherjee A, Chaudhry R, Kapil A, Ram Jat K, Lodha R, Kabra S.K. Etiology of Acute Respiratory Infections in Infants:A Prospective Birth Cohort Study. Pediatric Infectious Disease Journal. 2017;36(1):25–30. doi: 10.1097/INF.0000000000001359. [DOI] [PubMed] [Google Scholar]
- 30.Buecher C, Mardy S, Wang W, Duong V, Vong S, Naughtin M, Vabret A, Freymuth F, Deubel V, Buchy P. Use of a multiplex PCR/RT-PCR approach to assess the viral causes of influenza-like illnesses in Cambodia during three consecutive dry seasons. Journal of Medical Virology. 2010;82(10):1762–72. doi: 10.1002/jmv.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagare A, Mainassara H.B, Issaka B, Sidiki A, Tempia S. Viral and bacterial etiology of severe acute respiratory illness among children <5 years of age without influenza in Niger. BMC Infectious Diseases. 2015;15:515. doi: 10.1186/s12879-015-1251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIntosh K. McIntosh K, Chao RK, Krause HE, Wasil R, Mocega HE, Mufson MA, editors. Coronavirus infection in acute lower respiratory tract disease of infants. Journal of Infectious Diseases, 1974; 130 502-507. Journal of Infectious Diseases. 2004;190(5):1033–1041. doi: 10.1093/infdis/130.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Grady K.F, Grimwood K, Sloots T.P, Whiley D.M, Acworth J.P, Phillips N, Goyal V, Chang A.B. Prevalence, codetection and seasonal distribution of upper airway viruses and bacteria in children with acute respiratory illnesses with cough as a symptom. Clinical Microbiology and Infection. 2016;22(6):527–534. doi: 10.1016/j.cmi.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadopoulos N.G, Johnston S.L. Viruses and asthma exacerbations. Thorax. 1998;53(11):91391–91394. doi: 10.1136/thx.53.11.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Do L.A, Bryant J.E, Tran A.T, Nguyen B.H, Tran T.T, Tran Q.H, Vo Q.B, Tran Dac N.A, Trinh H.N, Nguyen T.T, Le Binh B.T, Le K, Nguyen M.T, Thai Q.T, Vo T.V, Ngo N.Q, Dang T.K, Cao N.H, Tran T.V, Ho L.V, Farrar J, de Jong M, van Doorn H.R. Respiratory Syncytial Virus and Other Viral Infections among Children under Two Years Old in Southern Vietnam 2009-2010:Clinical Characteristics and Disease Severity. PloS One. 2016;11(8):e01606. doi: 10.1371/journal.pone.0160606. [DOI] [PMC free article] [PubMed] [Google Scholar]


