Abstract
Background:
Within the past few decades, there has been an increase in the number of water-borne disease outbreaks and emergence of newly recognized waterborne parasites. Several factors which contribute to the spread of these diseases include: water, heavy rains and agricultural residues which transfer the parasites to water surface from the soil. The aim of this study was to detect the presence of parasites in the river and drinking water of Al-Wahdaa and Al-Rasheed Drinking Project and household water tanks from some regions of Baghdad.
Method:
Forty samples were collected from river and drinking water of Drinking Project. Fifty four samples of household water tanks were collected from some regions of Baghdad.
Results:
Cryptosporidium oocyst, which was founded in river water samples are more than those in drinking water. Furthermore, it was existed in Diyala Bridge &Taha Mosque from April – August and oocyst was diagnosed by using of Zheil-Neelson. Wet mount slide method was applied to detect cysts of free-living amoeba Acanthomoeba, Naegleria. The number of cysts in July and August were higher than other months for Drinking Project and the water tanks for all regions.
Conclusion:
These results emphasize the importance screening of the water to prevent possible of the spread of parasitic protozoan and that the cracks occurrence in drinking water pipes between the stations and houses led to contamination of water with the infective stage of parasites, especially in the areas that are near agricultural fields which polluted with the cows’ waste.
Keywords: Cryptosporidium, Acanthomoeba, Naegleria, Al-Wahdaa and Al-Rasheed Drinking Project
Introduction
In the past few decades, there have been steady increases in the number of water-borne disease outbreaks which have been reported and also emergence of newly-recognized waterborne parasites (Collins and Wright, 1997; Leclerc et al., 2002). There are several factors that contribute to the spread of diseases, including water, heavy rains and agricultural residues, which will transfer the parasite to surface water by soil (Mac Kenzie et al., 1994). So, the contaminated household water tanks are one of the main causes of intestinal diseases transmitted in the developing countries (Molbak et al., 1989; Sandiford et al., 1989). In the past three decades, there had been a dramatically of attention to the contamination of drinking water by pathogenic parasites, transferred by water which is causing many diseases for humans (Panagiotis et al., 2007). Free-living amoebae are one of the protozoa most prevalent in the environment including soil, air, water, dust, wastewater, and sediment (Rodriguez-Zaragoza, 1994). The genera of free-living amoeba are including: Naegleria fowleri, Acanthamoeba spp, Balamuthia mandrillari, Sappinia diploidea, that cause the dangerous infection in human (Schuster and Visvesvara, 2004; Daft et al., 2005).
Acanthamoeba is a free living amoeba founded in water (drinking water, swimming pools) and soil where are transmitted infectious stage (cyst) which is spherical diameter of 20µm to human through water. The cyst is resistant to environmental conditions) Marciano-Cabral et al., 2003(. The infection occurs by inhalation of water or dust particles containing trophozoite and cyst through its penetration to the central nervous system through the respiratory tract or may occur by penetrating through ulcerated skin or eye (Arora and Arora, 2010).
The genus of Acanthamoeba has some species including: A. culbertsoni, A. castellanii and A. polyphaga, the first species causes granulomatous amoebic encephalitis, which leads to haemorrhagic and necrotic encephalitis that is generally seen only in debilitated or immunodeficient persons. Early symptoms include drowsiness, intense headaches, nausea, vomiting, fevers and coma.
Whereas, the second and third species are associated with acanthamoebic keratitis is a painful infection of the cornea and can occur in healthy people, especially among contact lens wearers (WHO, 2011).
Naegleria fowleri is free living amoeba widely exists in the environment and it has two forms of:trophozoite and cyst. The trophozoite has two forms: amoeboid and flagellate, while the cysts are surrounded by double layer of the smooth wall and small nucleus, it is spherical and diameter 9 µm (Laura, 2012). Infection by Naegleria causes primary amoebic meningo-encephalitis (PAM). It occurs in healthy children and young adults when inhalation of dust containing the infective forms (cyst or flagellate) during swimming in fresh water lakes, ponds or swimming pools (Arora and Arora, 2010). Moreover, the infective forms enter the nose and through the olfactory neuroepithelium. The amoebae invade the nasopharyngeal mucosa and travel along the olfactory nerves where it finally invade the brain through the cribriform plate. The common symptoms of the disease are headache, stiff neck, seizures, and coma (Visvesvara et al., 2007).
Cryptosporidium parvum is a parasitic protozoan that infects epithelial cell of the stomach or intestine. Oocyst is infective stage its diameter 4-6 micron and spherical shape, which consists of four sporozoites (Kayser et al., 2005). The oocyst resistance to low temperature, high salinity and disinfectants (Ortega, 2006). Cryptosporidium causes diarrhea, nausea, vomiting and fever, the infection by Cryptosporidium in immunocompromised people can be life-threatening (Satoskar et al., 2009).
Materials and Methods
Sampling
Samples were collected by plastic bottle and the sampling was divided into including: (A) Forty samples (ten liter per sample for twice in the month) were collected from river and drinking water of Al-Wahdaa and Al-Rasheed Drinking Projectfrom March to October 2013. (B) Fifty four (five liter per sample of household water tanks for one time in the month) were collected from some regions of Baghdad (AL-Habebia, Boob AL-Sham, Diyala Bridge, Taha Mosque, AL-Zafaraniya, AL-Aalam, AL-Saydia, AL-Durra and ALGazalia) to six months since April- September 2014.
Filtration and Concentration
Samples were filtered through nitrocellulose membrane (0.45 μm pore size) by using vacuum pump. The membrane filter was washed by using phosphate-buffered saline–0.01% Tween 80and was centrifuged at 3000 × g for 15 minutes. Sediments were collected from test tubes and was re-suspended in distilled water to a volume of 20 ml. This suspension was floated by sucrose solution (53 gm sucrose+ 0.8 ml phenol+ 100 ml distilled water). 30 ml of sucrose solution was added to the deposit then was divided in test tubes (10 mL) after then centrifuge 3000 × g for 15 minutes and transmits then 5 mL of liquid surface of the sample from each tube to another test tube to be the final size of all tubes 25 mL and added 75 mL phosphate buffer saline containing Tween-80 solution concentration of 0.01%, which was divided into tubes and centrifuged again, the sediment was kept for microscopic checking of Cryptosporidium oocysts (Isaac-Renton et al., 1988).. Independently, five liter was filtered via nitrocellulose membrane (0.45 μm pore size) and washed using amount of distill water with a volume 60 ml and then centrifuged at 2000 rpm/min for 15 minutes. All pellets were used for microscopic scanning and cultivation of free living amoeba.
Staining and Identification of Cryptosporidium oocyst
Modified Ziehl- Neelsen and malachite green prepare and staining smears according to (Coles, 1986).
Examination and Enumeration of cyst and oocyst
Cyst of free-living amoeba were enumerated after the concentration of the sample size 10 liter to 1 mL by using the chamber Neubaur slide (U.S.EPA, 2001), according to the following equation:
Number of organisms/mL= number of organisms counted/number of mm2 counted × 10\1 ×dilution factor\1mm× 1 mm3\ μL.
Oocysts counted after staining by Modified Ziehlneelsen in 25 microscopic fields of the smear according to (Ortolani, 2000)
Cultivated and Identification of free living amoeba
Non-nutrient agar (N N A) was prepared by dissolving 15 g of agar in a liter of saline solution (NaCl0.12 gm, MgSO4.7H2O0.004 gm, CaCl2.2H2O0.004 g, Na2 HPO40.142 g, KH2PO4 0.136 g) and dissolved all the materials in one liter of distilled water. NNA was sterilized and then put in sterile plates, some heat-killed Escherichia coli were added approximately 10 drops on NNA plate and spread on agar. The pellets were transmitted and spread on NNA Plates. The plates were incubated at 28 C. These were daily examined for 14 days by light microscope to investigate the presence of trophozoite and cyst (Init et al., 2010).
Results
Microscopic examination showed the presence of Cryptosporidium oocyst in the river water elevated about the drinking water during March and April 2013 for Al-Wahdaa and Al-Rasheed Drinking Project (Figure 1) and (Table 1, 2). Whilst the results of household water tanks illustrated the presence of oocyst for the Diyala Bridge area and Taha Mosque since April to August 2014 and lack of its presence in September 2014, it did not exist in the rest areas during the sampling period (Figure 1) and (Table 3). Acanthomoeba and Naegleria cysts existent in all months for Al-Wahdaa and Al-Rasheed Drinking Project in 2013 as shown in the figure (2) and table (1, 2). As well, the cysts found in the household water tanks for all regions in 2014 figure (2) and table (4, 5).
Figure 1.
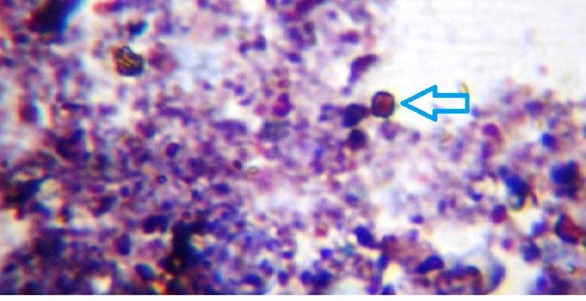
Show the oocyst of Cryptosporidium with Modified Ziehl Neelsen stain for the river water, drinking water and household water tanks.
Table 1.
Show the number of oocyst and cyst for Al-Wahdaa Drinking Project
| Month | Cryptosporidium | Free living amoeba(Naegleria +Acanthomoeba | ||
|---|---|---|---|---|
| The number of oocyst | The number of cyst | |||
| The drinking water | The river water | The drinking water | The river water | |
| March | 5 | 40 | 3.3×103 | 6×103 |
| April | 3 | 34 | 9.2×103 | 12.5×103 |
| May | 2 | 33 | 10.8×103 | 18.3×103 |
| Jun | 2 | 25 | 17.5×103 | 28.3×103 |
| July | 2 | 16 | 25×103 | 34.2×103 |
| August | 1 | 15 | 30×103 | 39.2×103 |
| September | 1 | 12 | 12.5×103 | 20×103 |
| October | 0 | 10 | 10.5×103 | 15×103 |
Table 2.
Show the number of oocyst and cyst forAl-RasheedDrinking Project
| Month | Cryptosporidium | Free living amoeba(Naegleria +Acanthomoeba | ||
|---|---|---|---|---|
| The number of oocyst | The number of cyst | |||
| The drinking water | The river water | The drinking water | The river water | |
| March | 6 | 42 | 4.2×103 | 5.8×103 |
| April | 4 | 35 | 7.5×103 | 11.6×103 |
| May | 3 | 33 | 15×103 | 19.2×103 |
| Jun | 3 | 27 | 20.8×103 | 30×103 |
| July | 2 | 18 | 26.6×103 | 35.5×103 |
| August | 1 | 16 | 35.8×103 | 40.8×103 |
| September | 1 | 13 | 17.5×103 | 25×103 |
| October | 1 | 11 | 13.3×103 | 16.7×103 |
Table 3.
Show the number of Cryptosporidium oocyst in household water tanks
| Regions | Months | |||||
|---|---|---|---|---|---|---|
| April | May | Jun | July | August | September | |
| AL-Habebia | - | - | - | - | - | - |
| Boob AL-Sham | - | - | - | - | - | - |
| DiyalaBridge | 5 | 2 | 2 | 2 | 1 | 0 |
| Taha Mosque | 4 | 3 | 1 | 1 | 1 | 0 |
| AL-Zafaraniya | - | - | - | - | - | - |
| AL-Aalam | - | - | - | - | - | - |
| AL-Saydia | - | - | - | - | - | - |
| AL-Durra | - | - | - | - | - | - |
| AL-Gazalia | - | - | - | - | - | - |
Figure 2.
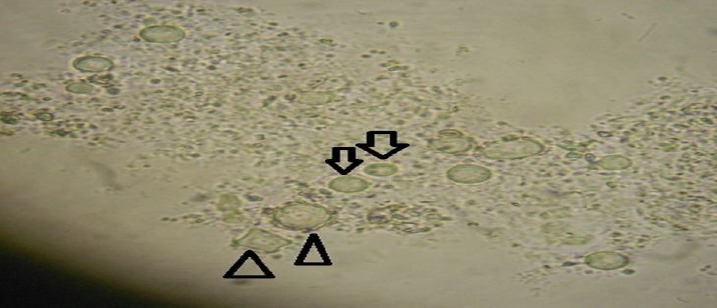
Show the cyst of Acanthomoeba (ρ) and Naegleria ([) in wet mount microscopic slides for the river water, drinking water and household water tanks
Table 4.
Show the number of Acanthomoeba cyst in household water tanks
| Regions | Months | |||||
|---|---|---|---|---|---|---|
| April | May | Jun | July | August | September | |
| AL-Habebia | 6×103 | 13.3×103 | 16.7×103 | 26.6×103 | 30×103 | 25×103 |
| Boob AL-Sham | 5.8×103 | 11.6×103 | 19.2×103 | 25.8×103 | 32.5×103 | 20×103 |
| DiyalaBridge | 9.2×103 | 18.3×103 | 26.6×103 | 32.5×103 | 37.5×103 | 30×103 |
| Taha Mosque | 10.8×103 | 17.6×103 | 27.5×103 | 35.8×103 | 39.2×103 | 34.2×103 |
| AL-Zafaraniya | 7.5×103 | 14.2×103 | 21.6×103 | 29.2×103 | 35.8×103 | 28×103 |
| AL-Aalam | 5×103 | 12.5×103 | 18.4×103 | 28.3×103 | 34.2×103 | 27.5×103 |
| AL-Saydia | 2.5×103 | 5.8×103 | 11.6×103 | 27.5×103 | 35×103 | 26.6×103 |
| AL-Durra | 3.3×103 | 8.3×103 | 15×103 | 22.5×103 | 28.3×103 | 20.8×103 |
| AL-Gazalia | 4.2×103 | 10×103 | 16.6×103 | 21.6×103 | 29.2×103 | 22.5×103 |
Table 5.
Show the number of Naegleria cyst in household water tanks
| Regions | Months | |||||
|---|---|---|---|---|---|---|
| April | May | Jun | July | August | September | |
| AL-Habebia | 5×103 | 18.4×103 | 22.5×103 | 29.2×103 | 34.2×103 | 26.6×103 |
| Boob AL-Sham | 7.5×103 | 15×103 | 21.6×103 | 28.3×103 | 30×103 | 25×103 |
| DiyalaBridge | 10×103 | 18.3×103 | 30×103 | 39.2×103 | 42.2×103 | 32.5×103 |
| Taha Mosque | 11.6×103 | 21.6×103 | 29.2×103 | 37.5×103 | 40.8×103 | 35×103 |
| AL-Zafaraniya | 9.2×103 | 16.7×103 | 20×103 | 28.3×103 | 35×103 | 29.2×103 |
| AL-Aalam | 5.8×103 | 13.3×103 | 19.2×103 | 26.6×103 | 30.5×103 | 20×103 |
| AL-Saydia | 8.3×103 | 14.2×103 | 22.5×103 | 27.5×103 | 37.5×103 | 25.8×103 |
| AL-Durra | 6×103 | 16.7×103 | 20×103 | 25.8×103 | 32.5×103 | 28.3×103 |
| AL-Gazalia | 5×103 | 12.5×103 | 21.6×103 | 26.6×103 | 30×103 | 27.5×103 |
Discussion
Cryptosporidium oocyst in drinking water existed as it is resistant to environmental conditions and penetrates the physical barriers for water treatment to its small size furthermore, it is resistant to numerous disinfectants that used in water disinfection (Moore et al., 1998). The presence of oocyst in April was rising about the other months after rainfall, particularly in the Diyala Bridge area and Taha Mosque, which are nearby agricultural areas. Also, the accumulation of cows’ waste in the fields and their flow during the rainy season, that led to pollution in the river water and drinking water was due to crack and broken in drinking water pipes between the stations and the houses.
Gennarri-Cardoso et al . (1996) demonstrated the seasonal changes have a role in the river water pollution, especially in a period of rainfall, the numbers oocyst increased in delivery season of cows and sheep, concurrent with the rainfall seasons which drifts the feces of cattle that contains oocyst into river water (Tsushima et al., 2003). Al-Baytee et al. (2012) showed the prevalence in their study of Cryptosporidium oocysts in the water sources in Mansoria city, Diala Province for the period from November 2008 to December 2009, and the presence was recorded of oocysts in tape water supplies at rate 22% in spring only, while in the tanks of houses showed that it was 32% and the rate of prevalence from 72% in spring to 16% in summer. The rivers and lakes polluted by oocyst, lead to the occurrence of borne diseases by water(Morgan-Ryan et al., 2002). The cysts of Acanthomoeba and Naegleria are more active than the rest of the months in the warm months. Also, the cysts number were observed in July and August it raised than the other months in 2013 and 2014, actually that the most of free-living amoeba are resistant to conventional disinfection processes that used for drinking water disinfection (Loret et al.,2008). The free-living amoeba was presented in the warm months because Acanthamoeba is tolerant to extreme temperature (Rodriguez-Zaragoza, 1994). Naegleria is thermotolerant up to 45, so these amoebas multiply in the summer (Visvesvara et al., 2007). The results agreed with CoGkun et al. (2013) in the prevalence of free-living amoeba in tap water at 33 (22%) of the total 150 samples for the province of Sivas, Turkey. Osman et al. (2010) demonstrate in their study, the free-living amoeba occurred with a percentage of 1.6% and 2.6% in main water reservoirs for the city of Helwan and El-Giza, Egypt. It can be found in the soil, dust, waste water, eye wash solution and dialysis units, contact lenses and dental treatment units (Dendana et al., 2008). Additionally, free-living amoebae are the vectors for some species of pathogenic bacteria to humans (Abu Kwaik et al., 1998). There are some factors contribute to raise risk of microbial pollution in tank water, such as high levels of suspended particles, temperature, some animals (birds, insects) and other factors that reach the water reservoirs (Donald et al., 2006; Hinton and Holser, 2009).
Conclusion
These results emphasize of the importance screening the water to prevent possible of the spread of parasitic protozoan and that the cracks occurrence in drinking water pipes between the stations and houses led to contamination of water with the infective stage of parasites, especially in the areas that are near agricultural fields which polluted with the cows’ waste.
Acknowledgment
We thank the Ministry of Science and Technology, Directorate of Water and Environment, Iraq.
Footnotes
Conflict of Interest: The authors have not declared any conflict of interest.
References
- 1.Abu Kwaik Y, Gao L.Y, Stone B.J, Venkataraman C, Harb O.S. Invasion of protozoa byLegionella pneumophilaand its role in bacterial ecology and pathogenesis. Applied and environmental microbiology. 1998;64:3127–33. doi: 10.1128/aem.64.9.3127-3133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Baytee A.J, Jawad S.Q, Mehdi H.S. Prevalence of Cryptosporidium Oocysts In Different Types of Water in Al-Mansoria Diala Province. Journal of Al-Nahrain University. 2012;15(1):103–107. [Google Scholar]
- 3.Arora D.R, Arora B.B. Medical parasitology. 3rd ed. Vol. 17. CBS Publishers and Distributors PVT. LTD; New Delhi: 2010. p. 95,119,192-194. [Google Scholar]
- 4.CoGkun K.A, Özçelik S, Tutar L, Elald J.N, Tutar Y. Isolation and Identification of Free-Living Amoebae fromTap Water in Sivas. Turkey Bio Med Research International. 2013:1–8. doi: 10.1155/2013/675145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coles E.H. Veterinary clinical pathology. 4th ed. WB Saunders company; Philadelphia: 1986. pp. 374–453. [Google Scholar]
- 6.Collins P.A, Wright M.S. Emerging intestinal protozoa:A diagnostic dilemma. Clinical laboratory science. 1997;10(5):273–8. [PubMed] [Google Scholar]
- 7.Daft B.M, Visvesvara G.S, Read D.H, Kinde H, Uzal F.A, Manzer M.D. Seasonal meningoencephalitis in Holstein cattle caused by Naegleria fowleri. Journal of Veterinary Diagnostic Investigation. 2005;17:605–609. doi: 10.1177/104063870501700617. [DOI] [PubMed] [Google Scholar]
- 8.Dendana F, Sellami H, Jarrayaf Sellami A, Makni F, Cheikhrouhou F, Hachicha J, Ayadi A. Free-living amoebae (FLA) Detection Morphological and molecular identification of Acanthomoeba genus in the hydraulic system of an haemo dialysis unit in Tunisia. Parasite. 2008;15:137–142. doi: 10.1051/parasite/2008152137. [DOI] [PubMed] [Google Scholar]
- 9.Donald D.J, Yi-Chen T, Miao-Chi T, Mei-Man H, Yu-Lan L, Lien-Ching C.C, Jar-Fun L, Kuo-Shi Y. Investigation of a collective diarrhea outbreak among cadets of a certain training unit located in Neipu Township, Pingtung County. Epidemiology Bulletin. 2006;25:269–279. [Google Scholar]
- 10.Gennarri-Cardoso M.L, Gosta-Cruz J.M, de-Castro E, Lima L.M, Prudent D.V. Cryptosporidiumsp. In children suffering from acute diarrhea at Ulberlandia city State of, Minas Gerais, Brazil. Memórias do Instituto Oswaldo Cruz. 1996;91(5):551–554. doi: 10.1590/s0074-02761996000500003. [DOI] [PubMed] [Google Scholar]
- 11.Hinton A, Jr, Holser R. Role of water hardness in rinsing bacteria from the skin of processed broiler chickens. Journal of International Poultry Science. 2009;2:112–115. [Google Scholar]
- 12.Init I, Lau Y.L, ArinFadzlun A, Foead A.I, Neilson R.S, Nissapatorn V. Detection of free living amoebaeAcanthamoebaandNaegleriain swimming pools. Malaysi Tropical Biomedicine. 2010;27(3):566–577. [PubMed] [Google Scholar]
- 13.Isaac-Renton J, Bowie WR, King A, Irwin G.S, Ong C.S, Fung C.P, Shokeir M.O, Dubey J.P. Detection ofToxoplasma gondiioocysts in drinking water. Applied and environmental microbiology. 1998;64:2278–2280. doi: 10.1128/aem.64.6.2278-2280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayser F.H, Beinz K.A, Eckret J, Zinkernagel R.M. Medical microbiology published by George ThiemeVerlag. 2005:517–518. [Google Scholar]
- 15.Laura Y. S. The occurrence of free living amoebae in water In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy In the Graduate College The University of Arizona 1-163 2012 [Google Scholar]
- 16.Leclerc H, Schwartzbrod L, Dei-Cas E. Microbial agents associated with waterborne diseases. Critical Reviews in Microbiology. 2002;28(4):371–409. doi: 10.1080/1040-840291046768. [DOI] [PubMed] [Google Scholar]
- 17.Mac Kenzie W.R, Hoxie N.J, Proctor M.E, Stephen Gradus M, Blair K.A, Peterson D.E, Kazmierczak J.J, Addiss D.G, Fox K. R, Rose J.B, Davis J.P. A massive outbreak in Milwaukee ofCryptosporidiuminfection transmitted through the public water supply. New England Journal of Medicine. 1994;331(3):161–7. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 18.Marciano-Cabral F, MacLean R, Mensah A, LaPat-Polasko L. Identification ofNaegleriafowleriin domestic water sources by nested PCR. Applied and environmental microbiology. 2003;69(10):5864–5869. doi: 10.1128/AEM.69.10.5864-5869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molbak K, Hojlyng N, Jepsen S, Gaarslev K. Bacterial contamination of stored water and stored food:a potential source of diarrhoea in West Africa. Epidemiological Infection. 1989;102:309–316. doi: 10.1017/s0950268800029988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore A.G, Vesey G, Champion A, Scandizzo P, Deere D, Veal D, Williams K.L. Viable Cryptosporidium parvum oocysts exposed to chlorine or other oxidizing conditions may lack identifying epitopes. International Journal for Parasitology. 1998;28(8):1205–12012. doi: 10.1016/s0020-7519(98)00070-8. [DOI] [PubMed] [Google Scholar]
- 21.Morgan-Ryan U.M, Fall A, Ward L.A, Hijjawi N, Sulaiman I, Fayer R, Thompson R.C, Olson M, Lal A, Xiao L. Cryptosporidium hominisn. sp. (Apicomplexa:Cryptosporidiidae) from Homo sapiens. Journal Eukaryotic Microbiology. 2002;49(6):433–40. doi: 10.1111/j.1550-7408.2002.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 22.Ortega Y.R. Food borne parasites published by Spring Science. USA. 2006:60. [Google Scholar]
- 23.Ortolani E.L. Standardization of the modified ziehl- neelsen technique to stain oocysts ofCryptosporidiumsp Brazilian. Journal of Veterinary Parasitology. 2000;9(1):29–31. [Google Scholar]
- 24.Osman G.A, Kamel M.M, Al-Herrawy A.Z. Microbiological criteria of tap water passed through some storage water tanks in Greater Cairo. Environmental Biotechnology. 2010;6(2):61–65. [Google Scholar]
- 25.Panagiotis K, Christina K, Huw S. Waterborne transmission of protozoan parasites:A worldwide review of outbreaks and lessons learnt. Journal of Water and Health. 2007;5(1):1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Zaragoza S. Ecology of free-living amoebae Critical Reviews in Microbiology. 1994;20(3):225–241. doi: 10.3109/10408419409114556. [DOI] [PubMed] [Google Scholar]
- 27.Satoskar A.R, Simon G.L, Hotez J.P, Tsuji M. Medical parasitology published by Landes Bioscience. 2009:216–218. [Google Scholar]
- 28.Schuster F.L, Visvesvara G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. International Journal for Parasitology. 2004;34:1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Tsushima Y, Karanis P, Kamada T, Makala L, Xuan X, Tohya Y, Akashi H, Nagasawa H. Seasonal change in the number ofCryptosporidium parvumoocysts in water samples from the rivers in Hokkaido, Japan, detected by the ferric sulfate flocculation method. Journal Veterinary Medical Science. 2003;65(1):121–3. doi: 10.1292/jvms.65.121. [DOI] [PubMed] [Google Scholar]
- 30.United State Environmental Protection Agency (U.S.EPA.) Method 1623CryptosporidiumandGiardiain water by Filtration /IMS/FA. EPA 815-R-05-002. 45268-1320. Office of Water (4607); Cincinnati, OH: 2001. p. 24. [Google Scholar]
- 31.Visvesvara G. S, Moura H, Schuster F. L. Pathogenic and opportunistic free-living amoebae:Acanthamoeba sppBalamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunology & Medical Microbiology. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 32.WHO. Guidelines for drinking–water quality. 4th ed. Published by World Health Organization; Geneva: 2011. pp. 268–269. [Google Scholar]


