Abstract
Objectives:
Surgical resection of enlarged cardiophrenic lymph nodes (CPLNs) in primary treatment of advanced ovarian cancer has not been widely studied. We report on a cohort of patients undergoing CPLN resection during primary cytoreductive surgery (CRS), examining its feasibility, safety, and potential impact on clinical outcomes.
Methods:
We identified all patients undergoing primary CRS/CPLN resection for Stages IIIB-IV high-grade epithelial ovarian cancer at our institution from 1/2001–12/2013. Clinical and pathological data were collected. Statistical tests were performed.
Results:
54 patients underwent CPLN resection. All had enlarged CPLNs on preoperative imaging. Median diameter of an enlarged CPLN: 1.3cm (range 0.6–2.9). Median patient age: 59y (range 41–74). 48 (88.9%) underwent transdiaphragmatic resection; 6 (11.1%) underwent video-assisted thoracic surgery. A median of 3 nodes (range 1–23) were resected. A median of 2 nodes (range 0–22) were positive for metastasis. 51/54 (94.4%) had positive nodes. 51 (94.4%) had chest tube placement; median time to removal: 4d (range 2–12). 44 (81.4%) had peritoneal carcinomatosis. 19 (35%) experienced major postoperative complications; 4 of these (7%) were surgery-related. Median time to adjuvant chemotherapy: 40d (range 19–205). All patients were optimally cytoreduced, 30 (55.6%) without visible residual disease. Median progression-free survival: 17.2mos (95% CI 12.6–21.8); median overall survival: 70.1mos (95% CI 51.2–89.0).
Conclusions:
Enlarged CPLNs can be identified on preoperative imaging and may indicate metastases. Resection can identify extra-abdominal disease, confirm Stage IV disease, obtain optimal cytoreduction. In the proper setting it is feasible, safe, and does not delay chemotherapy. In select patients, it may improve survival.
Keywords: Ovarian cancer, Cardiophrenic lymph nodes, Supradiaphragmatic lymph nodes, Paracardiac lymph nodes, Primary cytoreductive surgery, Optimal tumor debulking, Radical surgery
BACKGROUND
Women with suspected advanced epithelial ovarian cancer (EOC) should be evaluated by a gynecologic oncologist to determine the appropriate initial treatment modality [1]. This evaluation usually includes imaging of the chest, abdomen, and pelvis, to determine the extent of disease and the feasibility of optimal surgical resection [1, 2]. A 2001 survey of gynecologic oncologists revealed that the presence of upper abdominal disease and/or disease in “hazardous locations” were the most common reasons given for deciding against a primary cytoreductive surgery (CRS) [3]. The authors also reported that surgeons who were familiar with diverse surgical approaches were more likely to perform riskier procedures, and had more optimal outcomes [3]. Numerous studies have noted that gynecologic oncologists are expanding the surgical armamentarium for EOC, diversifying their skill sets and participating in a multidisciplinary team approach to treatment [4–6]. Several studies have also reported significant increases in survival, as well as a minimal but acceptable increase in morbidity, as a result of this shift in the surgical paradigm [7–10].
Cardiophrenic lymph nodes (CPLNs), also referred to as paracardiac and supradiaphragmatic lymph nodes, are located just above the diaphragm. The region anterior to the pericardium normally houses 0–2 visible lymph nodes, each usually measuring less than 5 mm in diameter [11]. In diseases such as advanced EOC, where there is often a large peritoneal and abdominal tumor burden, these lymph nodes can serve as a common site for metastases [12, 13].
Suspicious nodes are generally larger than 5 mm in diameter and are readily identified on computed tomography (CT) scans. In women with ovarian cancer, these enlarged nodes are usually indicative of metastatic disease [13]. Positive CPLN is an independent negative prognostic factor and indicates that the patient has Stage IVB disease. However, the evidence suggests that optimal debulking surgery (resulting in minimal or no visible residual disease)—even if it requires a “radical” surgical procedure—is associated with an increased median overall survival (OS) in the setting of Stage IV disease [14–16].
Surgical resection of enlarged CPLNs in the primary treatment of advanced ovarian cancer has not been widely studied. In this study we report on a cohort of patients that underwent CPLN resection at the time of primary CRS at a single institution. We discuss the feasibility and safety of the procedure, and assess outcomes.
METHODS
After Institutional Review Board approval, we identified patients with Stage IIIB-IV ovarian cancer who underwent primary CRS for high-grade EOC at our institution (Memorial Sloan Kettering Cancer Center) from January 2001 to December 2013. Patients were included if they had undergone a supradiaphragmatic resection with the intent of removing one or more CPLN(s) for diagnosis and/or treatment of ovarian cancer. Patients were excluded if they received neoadjuvant chemotherapy (NACT). Patients were also excluded if their primary surgery was not performed with the intent of optimal cytoreduction, (e.g., surgery for bowel obstruction or palliative indications). CPLN resection was performed either by a thoracic surgeon via video-assisted thoracic surgery (VATS), or by a gynecologic oncologist or other consulting surgeon via a transdiaphragmatic or subxiphoid approach. Transdiaphragmatic resection is accomplished by opening the diaphragm directly to enter the thoracic cavity and access the pericardial fat pad containing the CPLNs. Alternatively, the subxiphoid approach utilizes the upper portion of the laparotomy incision and leaves the diaphragm intact, entering the pleural space anterior to the diaphragm and pericardium.
We collected pertinent clinical, surgical, pathologic, outcome, and follow-up data for this cohort. Descriptive analyses were performed. Complications were graded according to an institutional surgical complication grading system [17]. Perioperative complications were defined as any adverse event related to operative treatment occurring within 30 days of surgery, including death. All patients had routine preoperative radiologic imaging. All radiologic reports and images were reviewed by the surgical team. The size (cm), location, quantity, and tumor burden of the CPLNs, and the presence of pleural effusions, were collected from radiologic, pathologic, and operative reports. Peritoneal carcinomatosis was defined as the presence of ≥ 20 tumor nodules, as noted at the time of surgery or as documented on preoperative CT scans. Residual disease was defined as the greatest dimension in diameter (cm) of the largest residual tumor nodule, as documented in the operative report. Optimal cytoreduction was defined as ≤ 1 cm of residual disease on completion of CRS. Time to adjuvant chemotherapy was defined as the number of days from postoperative day 1 to the first day of chemotherapy.
Progression-free survival (PFS) was calculated from the date of CRS to the date of first progression, last follow-up, or death. OS was defined as time elapsed (in months) from the date of CRS to the date of last follow-up, or death. Follow-up data were collected until September 2016. The Kaplan-Meier method was used to generate survival curves [18].
RESULTS
Of the 985 patients at our institution who underwent primary CRS for Stage IIIB-IV high-grade EOC from January 2001 to December 2013, we identified 54 patients who underwent surgical resection of the CPLN(s). The median age in this group was 59 years (range 41–74). In all 54 cases, enlarged or suspicious CPLNs were documented on preoperative CT reports. The median diameter of an enlarged CPLN was 1.3 cm (range 0.6–2.9). Preoperative pleural effusion was noted in 13 (24%) patients. Peritoneal carcinomatosis was documented in 44 (81%) patients. Patient and tumor characteristics are shown in Table 1.
Table 1.
Patient and Tumor Characteristics
| Patient and Tumor Characteristics (n=54) | |
|---|---|
| Variable | No. of Patients (%) |
| Median age (range) | 59 years (41–74) |
| Median BMI (range) | 27 (19–45) |
| Median pre-operative albumin (range) | 4.2 (2.7–4.9) |
| BRCA status | |
| Negative | 26 (48) |
| BRCA 1 positive | 4 (7) |
| BRCA 2 positive | 4 (7) |
| Status Unknown | 20 (37) |
| ASA | |
| 2 | 21 (39) |
| 3 | 33 (61) |
| Primary site of disease | |
| Ovary | 34 (63) |
| Fallopian Tube | 16 (29) |
| Peritoneum | 4 (7) |
| Tumor Histology | |
| High grade serous | 51 (94) |
| Carcinosarcoma | 2 (4) |
| Clear cell | 1 (2) |
| Preoperative serum CA-125 | |
| 0–500 | 19 (35) |
| 501–1000 | 7 (48) |
| 1001–2000 | 10 (19) |
| 2001–3000 | 4 (26) |
| 3001–4000 | 2 (4) |
| 4001–5000 | 5 (9) |
| > 5000 | 1 (2) |
| Ascites | |
| ≤500 | 29 (54) |
| > 500 | 25 (46) |
An intraoperative consulting surgeon performed transdiaphragmatic CPLN resection in 31 (57%) patients; a gynecologic oncologist performed the procedure in 17 (31%) patients. The other 6 (11%) patients underwent a VATS procedure by a thoracic surgeon prior to their abdominal CRS. A median of 3 nodes (range 1–23) were resected. Upon histopathologic review, a median of 2 nodes (range 0–22) were positive for metastatic disease. Fifty-one of the 54 (94%) patients had nodes that were positive for metastatic disease. In 51 (94%) patients, a chest tube was placed at the time of the procedure; the median time to removal of the chest tube was 4 days (range 2–12). Fifty-three (98%) patients underwent one or more extensive upper abdominal procedures at the time of CRS. This included diaphragmatic stripping/resection in 49 (91%) patients, resection of tumor from the porta hepatis in 19 (35%), splenectomy in 14 (26%), cholecystectomy in 12 (22%), liver resection in 10 (19%), and distal pancreatectomy in 3 (6%) patients. Median estimated blood loss (EBL) was 1000mL. Median operative time was 450 minutes. Forty-eight (88%) patients had an epidural catheter placed preoperatively for pain control. All patients were optimally cytoreduced to at least ≤ 1 cm residual disease; 30 (55.6%) had no visible residual disease at the conclusion of their primary surgeries.
There were no postoperative deaths. Nineteen (35%) major postoperative complications (Grade 3 and above) were noted. However, only 4 (7%) were of a pulmonary nature and could possibly be related to the CPLN resection. These included 1 pulmonary embolism, 1 chylothorax, 1 pleural effusion, and 1 case of acute respiratory distress syndrome (ARDS) requiring intensive medical therapy. The patient with the chylothorax was treated with re-insertion of a chest tube. The chylothorax resolved and she was able to initiate chemotherapy on postoperative day 19. The patient with ARDS was treated with supportive therapy in the ICU, where it was determined that the cause of her ARDS was aspiration; she was able to initiate chemotherapy on postoperative day 53. The other two patients experienced pulmonary complications as well as other complications related to their large abdominal surgeries. The patient with the pulmonary embolism was managed with an IVC filter and anticoagulation; however, she suffered a surgical site infection and the wound was left to heal via secondary intention, delaying initiation of her chemotherapy. Lastly, the patient with the pleural effusion was treated in the ICU with supportive therapy, but required a re-operation for a large recto-vaginal fistula; this significantly delayed initiation of her chemotherapy.
The median length of stay (LOS) for the entire cohort was 11 days. The median time to adjuvant chemotherapy was 40 days (range 19–205). All patients received a platinum doublet regimen. Twelve (22%) patients received intravenous and intraperitoneal chemotherapy, while all of the remaining 42 (78%) patients received intravenous chemotherapy.
During this study period, 45 of the 54 patients experienced a recurrence (83%). Most recurrences were multifocal, with 82 sites of recurrence in these 45 patients. A majority of patients recurred in the peritoneum/mesentery/bowel (62%) or in the abdominopelvic lymph nodes (53%). Additional sites of recurrence are noted in Table 2. Median PFS for the cohort was 17.2 months (95% CI 12.6–21.8). Median OS was 70.1 months (95% CI 51.2–89.0). Patients who obtained a complete gross resection (CGR) had a median OS of 72.3 months (95% CI 56.5–88.0); patients who obtained optimal debulking had a median OS of 68.9 months (95% CI 19.3–118.4) (Figure 1).
Table 2.
Sites of Recurrence
| Site of Recurrence (n=45) | N (%) |
| Peritoneum/mesentery/bowel | 28 (62) |
| Abdominopelvic Nodes | 24 (53) |
| Hepatobiliary | 9 (33) |
| Thoracic Nodes | 6 (13) |
| Pleura/pericardium | 4 (9) |
| Spleen | 4 (42) |
| Pelvic Organs | 4 (9) |
| Bones/soft tissues | 2 (4) |
| Lungs | 1 (2) |
Figure 1:
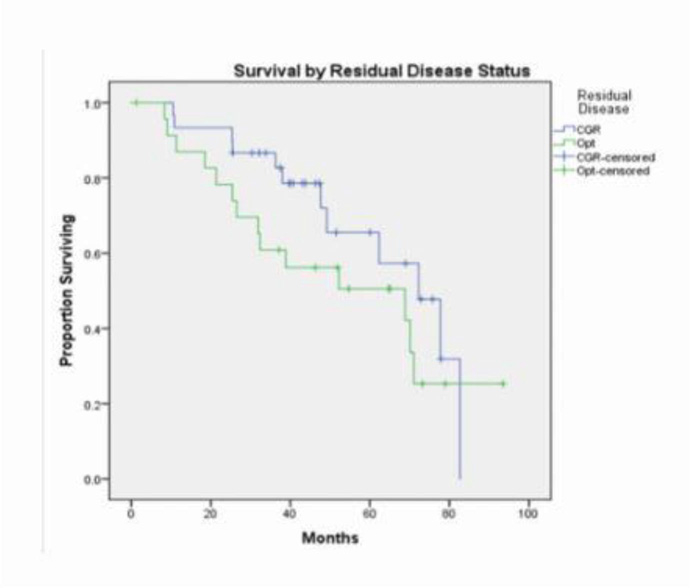
Survival by Residual Disease Status Overall survival by status of residual disease Complete gross resection [CGR] and optimal debulking [Opt]
DISCUSSION
While there is ongoing debate about whether NACT or CRS constitutes the best treatment approach for advanced EOC in the primary setting, we believe that a combination surgical/systemic therapeutic intervention provides the best opportunity for potential cure of this disease. At our institution we offer CRS as primary treatment to medically fit patients with resectable disease, at the time of diagnosis. Data have shown that CRS resulting in minimal (< 1 cm) or microscopic residual disease provides a survival benefit [19]. It has consistently been demonstrated that the only potentially modifiable factor impacting outcome and survival after primary CRS is the amount of residual disease. Therefore, many clinicians now aim for CGR [14–16, 20, 21].
If the disease burden in the abdomen and pelvis is resectable, the presence of isolated enlarged CPLN(s) should not preclude a potentially life-lengthening treatment. This approach increases the treatment options available to some patients. However, there have been few reports on the utilization of extra-abdominal resection to minimize residual disease. In 2011 we reported our initial experience with a smaller patient cohort, demonstrating the feasibility and safety of mediastinal lymphadenectomy [22]. To our knowledge, the current study reports on the largest cohort of patients undergoing primary CRS with CPLN resection aimed at optimal cytoreduction or CGR.
Over the past two decades there have been a handful of reports on CPLN resection in the setting of primary and recurrent ovarian cancer. In 2009, Lim et al described an approach using VATS in 9 patients who evidenced CPLN metastases [23]. The authors reported that they were able to complete the procedure in approximately 1 hour, with minimal blood loss, complications, or delay in adjuvant treatment. More recent reports have proposed a transdiaphragmatic approach performed by gynecologic oncologists, with similar outcomes [24–27]. In this series, we included both VATS and transdiaphragmatic approaches performed by gynecologic oncologists and consulting surgeons. Nearly all of the patients had one or more extensive surgical procedure(s) at the time of primary CRS. As a result, it is difficult to assign individual complications to the specific procedures that comprise a large debulking surgery. Prior reports suggest that there is minimal significant morbidity associated with CPLN resection, the most common being pleural effusion, pneumothorax, and pneumonia [25, 27, 28]. In this study, we took any cardiothoracic postoperative complication into consideration, recognizing that we cannot be certain all were directly related to CPLN resection. Despite this, we found a low and acceptable morbidity rate.
A recent report from the European Institute of Oncology in Milan describes a cohort of 22 women with high grade serous ovarian cancer who underwent cytoreductive surgeries involving transdiaphragmatic resection of enlarged CPLNs [29]. In concurrence with this and prior reports, we found that the additional resection of suspicious CPLN(s) did not significantly increase EBL, operative time, morbidity, or time to adjuvant therapy; furthermore, it detected and removed disease in 94% of patients. This procedure rendered all cases amenable to optimal debulking (to < 1.0 cm), with over 50% obtaining CGR. This not only demonstrates the feasibility and safety of both the mediastinal approach and extensive upper abdominal surgery, but highlights the diversity of surgical techniques available at specialty centers. The ability of the gynecologic oncologist to partner with other surgeons and specialists increases treatment options.
A 2016 study of pathologic and radiologic evaluation in 31 patients undergoing CPLN resection found a correlation between the radiographically-identified presence of peritoneal metastasis, abdominopelvic adenopathy, and CPLN adenopathy [30]. All of the enlarged CPLNs in our series were identified on preoperative CT scans. In accordance with the previous literature, we found that the majority of our patients had peritoneal carcinomatosis [30, 31]. This highlights the importance of preoperative chest imaging and attention to the mediastinum in patients with suspected advanced EOC.
A post-trial ad hoc analysis of GOG 218 described a cohort of 81 women with Stage IV disease who underwent complete resection of their disease and initiated adjuvant chemotherapy [32]. The authors reported that initiation of adjuvant chemotherapy greater than 25 days after surgery was associated with increased risk of death. Our cohort had a median time to chemotherapy of 40 days, which is within what has historically been described as an appropriate length of time to initiation of adjuvant therapy, but greater than that described by Tewari [33]. The time to chemotherapy does not appear to have affected median survival in our cohort; however, the question cannot be answered based on our retrospective study.
In 2010, Kolev et al retrospectively reviewed more than 200 patients with advanced ovarian cancer who underwent primary CRS during a 7-year period; they observed a trend towards worse survival in patients whose chest imaging studies showed enlarged CPLNs [31]. Though the numbers did not reach statistical significance, this suggests that some patients who appeared to be optimally debulked may have had residual disease in the mediastinum. Wimberger et al analyzed surgical and survival data from three prospective, randomized phase 3 trials conducted by the AGO-OVAR group, and found that CGR was associated with significantly improved OS in 70 patients with Stage IV disease [16]. They reported a median OS of 54.6 months for patients who had CGR, 25.8 months for patients with an optimal (≤ 1 cm) debulking, and 23.9 months for patients with a suboptimal (> 1 cm) debulking. While our data cannot serve as a direct comparison, our small cohort of patients—including those who obtained both optimal outcomes and CGR—had an OS of 70 months; when CGR was achieved, the median OS was 72 months. We cannot conclude that these survival times are directly related to the CPLN resection. However, this does support the safety and feasibility of a procedure that increases the ability of the surgeon to reduce a patient’s disease burden.
One of the limitations of this retrospective review is the potential for selection bias. This is due to the fact that we were unable to report specific information pertaining to the outcomes of comparable, concurrent patients with enlarged CPLNs who were not managed with primary CRS and CPLN resection. The CPLN approach may not be appropriate for all patients at all institutions. We recognize that the high true positive rate for suspicious CPLNs identified on preoperative imaging in this study may encourage gynecologic oncologists to reconsider who is appropriate for NACT. All of the patients in this series were treated at a cancer center by multidisciplinary teams trained to manage and deliver this progressive, specialized care. This should be the goal whenever possible for patients with advanced ovarian cancer. Nevertheless, the current study does demonstrate the feasibility and safety of this progressive surgical approach. Furthermore, we report improved survival data for these patients, who have historically had a poor prognosis. Future studies should include a comparative analysis of patients with enlarged CPLNs who were and were not treated with resection.
HIGHLIGHTS.
CPLN resection during PCS for ovarian cancer is feasible and safe
CPLN resection may be associated with improved surgical and survival outcomes
CPLN should be done at an expert center by a multidisciplinary team of specialists
Acknowledgments
Funding support: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Conflict of interest statement: None of the authors declare any conflicts of interest.
Dr. Chi serves on the Medical Advisory Board of Bovie Medical Corporation, and on the Medical Advisory Board of Verthermia. He has no conflicts of interest pertinent to this work.
REFERENCES
- 1.Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:3460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Mironov S, Iyer RB, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol 2014;134:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenkop SM, Spirtos NM. What are the current surgical objectives, strategies, and technical capabilities of gynecologic oncologists treating advanced epithelial ovarian cancer? Gynecol Oncol 2001;82:489–97. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Levine DA, Poynor EA, Aghajanian C, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol 2006;103:1083–90. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman MS, Tebes SJ, Sayer RA, Lockhart J. Extended cytoreduction of intraabdominal metastatic ovarian cancer in the left upper quadrant utilizing en bloc resection. Am J Obstet Gynecol 2007;197:209 e1–5. [DOI] [PubMed] [Google Scholar]
- 6.Kehoe SM, Eisenhauer EL, Chi DS. Upper abdominal surgical procedures: liver mobilization and diaphragm peritonectomy/resection, splenectomy, and distal pancreatectomy. Gynecol Oncol 2008;111(2 Suppl):S51–5. [DOI] [PubMed] [Google Scholar]
- 7.Bogani G, Ditto A, Martinelli F, Lorusso D, Chiappa V, Donfrancesco C, et al. Surgical Techniques for Diaphragmatic Resection During Cytoreduction in Advanced or Recurrent Ovarian Carcinoma: A Systematic Review and Meta-analysis. Int J Gynecol Cancer 2016;26:371–80. [DOI] [PubMed] [Google Scholar]
- 8.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 2009;114:26–31. [DOI] [PubMed] [Google Scholar]
- 9.Chi DS, Zivanovic O, Levinson KL, Kolev V, Huh J, Dottino J, et al. The incidence of major complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal, and peritoneal carcinomas. Gynecol Oncol 2010;119:38–42. [DOI] [PubMed] [Google Scholar]
- 10.Saitou M, Iida Y, Komazaki H, Narui C, Matsuno K, Kawabata A, et al. Success rate and safety of tumor debulking with diaphragmatic surgery for advanced epithelial ovarian cancer and peritoneal cancer. Arch Gynecol Obstet 2015;291:641–6. [DOI] [PubMed] [Google Scholar]
- 11.Aronberg DJ, Peterson RR, Glazer HS, Sagel SS. Superior diaphragmatic lymph nodes: CT assessment. J Comput Assist Tomogr 1986;10:937–41. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Hijleh MF, Habbal OA, Moqattash ST. The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J Anat 1995;186( Pt 3):453–67. [PMC free article] [PubMed] [Google Scholar]
- 13.Holloway BJ, Gore ME, A’Hern RP, Parsons C. The significance of paracardiac lymph node enlargement in ovarian cancer. Clin Radiol 1997;52:692–7. [DOI] [PubMed] [Google Scholar]
- 14.Chang SJ, Bristow RE, Ryu HS. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann Surg Oncol 2012;19:4059–67. [DOI] [PubMed] [Google Scholar]
- 15.Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol 2013;130:493–8. [DOI] [PubMed] [Google Scholar]
- 16.Wimberger P, Wehling M, Lehmann N, Kimmig R, Schmalfeldt B, Burges A, et al. Influence of residual tumor on outcome in ovarian cancer patients with FIGO stage IV disease: an exploratory analysis of the AGO-OVAR (Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group). Ann Surg Oncol 2010;17:1642–8. [DOI] [PubMed] [Google Scholar]
- 17.Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol 2004;94:650–4. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 19.Sioulas VD, Schiavone MB, Kadouri D, Zivanovic O, Roche KL, O’Cearbhaill R, et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol Oncol 2017;145:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol 2006;107:77–85. [DOI] [PubMed] [Google Scholar]
- 21.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009;115:1234–44. [DOI] [PubMed] [Google Scholar]
- 22.Diaz J, Park B, Stine J, Flores R, Sonoda Y, Abu-Rustum N, et al. The feasibility of mediastinal lymphadenectomy in the management of advanced and recurrent ovarian carcinoma. Gynecol Oncol 2011;120(Suppl 1):S80. [Google Scholar]
- 23.Lim MC, Lee HS, Jung DC, Choi JY, Seo SS, Park SY. Pathological diagnosis and cytoreduction of cardiophrenic lymph node and pleural metastasis in ovarian cancer patients using video-assisted thoracic surgery. Ann Surg Oncol 2009;16:1990–6. [DOI] [PubMed] [Google Scholar]
- 24.LaFargue CJ, Bristow RE. Transdiaphragmatic cardiophrenic lymph node resection for Stage IV ovarian cancer. Gynecol Oncol 2015;138:762–3. [DOI] [PubMed] [Google Scholar]
- 25.LaFargue CJ, Sawyer BT, Bristow RE. Short-term morbidity in transdiaphragmatic cardiophrenic lymph node resection for advanced stage gynecologic cancer. Gynecol Oncol Rep 2016;17:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prader S, Harter P, Grimm C, Traut A, Waltering KU, Alesina PF, et al. Surgical management of cardiophrenic lymph nodes in patients with advanced ovarian cancer. Gynecol Oncol 2016;141:271–5. [DOI] [PubMed] [Google Scholar]
- 27.Yoo HJ, Lim MC, Song YJ, Jung YS, Kim SH, Yoo CW, et al. Transabdominal cardiophrenic lymph node dissection (CPLND) via incised diaphragm replace conventional video-assisted thoracic surgery for cytoreductive surgery in advanced ovarian cancer. Gynecol Oncol 2013;129:341–5. [DOI] [PubMed] [Google Scholar]
- 28.Bashir S, Gerardi MA, Giuntoli RL 2nd, Montes TP, Bristow RE. Surgical technique of diaphragm full-thickness resection and trans-diaphragmatic decompression of pneumothorax during cytoreductive surgery for ovarian cancer. Gynecol Oncol 2010;119:255–8. [DOI] [PubMed] [Google Scholar]
- 29.Garbi A, Zanagnolo V, Colombo N, Aletti G, Achilarre MT, Bocciolone L, et al. Feasibility of Transabdominal Cardiophrenic Lymphnode Dissection in Advanced Ovarian Cancer: Initial Experience at a Tertiary Center. Int J Gynecol Cancer 2017;27:1268–73. [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Lim MC, Kim SI, Seo SS, Kim SH, Park SY. Preoperative Prediction of Cardiophrenic Lymph Node Metastasis in Advanced Ovarian Cancer Using Computed Tomography. Ann Surg Oncol 2016;23:1302–8. [DOI] [PubMed] [Google Scholar]
- 31.Kolev V, Mironov S, Mironov O, Ishill N, Moskowitz CS, Gardner GJ, et al. Prognostic significance of supradiaphragmatic lymphadenopathy identified on preoperative computed tomography scan in patients undergoing primary cytoreduction for advanced epithelial ovarian cancer. Int J Gynecol Cancer 2010;20: 979–84. [DOI] [PubMed] [Google Scholar]
- 32.Tewari KS, Java JJ, Eskander RN, Monk BJ, Burger RA. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Ann Oncol 2016;27:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu YS, Lewin SN, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstet Gynecol 2012;120:871–81. [DOI] [PubMed] [Google Scholar]


