Abstract.
A total of 1,090 residents of the city of Reynosa, Tamaulipas, on the Mexico–U.S. border presented at hospitals and clinics of the Secretariat of Health, Mexico, in 2015 with symptoms characteristic of dengue. Dengue virus (DENV) antigen was detected by enzyme-linked immunosorbent assay in acute sera from 134 (12.3%) patients. Sera from select patients (N = 34) were also tested for chikungunya virus (CHIKV) RNA by quantitative reverse transcription–polymerase chain reaction. Thirteen (38.2%) patients, including five DENV antigen-positive patients, were positive. Sera from three CHIKV RNA-positive patients were further assayed by virus isolation in cell culture and CHIKV was recovered on each occasion. The genome of one isolate and structural genes of the other two isolates were sequenced. In conclusion, we present evidence of CHIKV and DENV coinfections in patients who live near the Mexico–U.S. border and provide the first genome sequence of a CHIKV isolate from northern Mexico.
Chikungunya virus ([CHIKV]; genus Alphavirus, family Togaviridae) and dengue virus ([DENV]; family Flaviviridae, genus Flavivirus) are the etiological agents of chikungunya fever (CHIKF) and dengue, respectively.1,2 Both viruses are transmitted by Aedes mosquitoes and endemic in the tropics and subtropics. The co-circulation of CHIKV and DENV presents a clinical and diagnostic challenge in endemic countries because the clinical presentations of these viruses overlap substantially. Chikungunya fever is characterized by an acute febrile illness that is often accompanied by severe and debilitating polyarthralgia that sometimes persists for years, in addition to periarticular edema, and a nonspecific macular or maculopapular rash. Complications are rare but include severe organ dysfunction and encephalitis. Dengue is usually characterized by a febrile illness accompanied by headache, arthralgia, and myalgia, sometimes with a macular rash. In some cases, DENV infection can result in vascular leakage, hemorrhagic manifestations, thrombocytopenia, and hypotensive shock, and progress to organ failure and death.
The first autochthonous case of CHIKF in Mexico occurred in October 2014 in the southern state of Chiapas.3 The first autochthonous case of CHIKV in Texas occurred in November 2015 in Cameron County on the Mexico–U.S. border.4 Chikungunya virus cases have now been reported in almost every state of Mexico but are most common in southern and central states.5 The most comprehensive study to investigate the occurrence of CHIKF in northern Mexico was performed by Gomez-Govea et al. who detected CHIKV RNA in 41 of 101 (40.6%) patients in the state of Nuevo León.6 Although the incursion of CHIKV into Mexico is recent, DENV has been circulating in the country since at least the 1800s.7 The incidence and severity of dengue outbreaks in Mexico have increased at an alarming rate in recent decades and an estimated 139,000 cases now occur nationwide each year.8 To increase our understanding of the public health consequences of the recent introduction of CHIKV into Mexico, patients sampled as part of the nationwide dengue surveillance program were tested for evidence of CHIKV and DENV infection and coinfection.
All patients were residents of Reynosa, a city in the state of Tamaulipas, northern Mexico. Reynosa is in the binational Reynosa–McAllen metropolitan area, directly across the Mexico–U.S. border from McAllen, a city in Hidalgo County, Texas (Figure 1). Patients presented at Secretariat de Health (Secretaría de Salud; [SS]) hospitals and clinics in Reynosa in January to December 2015. To be eligible for inclusion, patients had to meet the clinical criteria for dengue fever (DF) or dengue hemorrhagic fever (DHF) according to the guidelines established by the WHO in 1997.9 Although the WHO revised the guidelines in 2009, with patients now classified as dengue without warning signs, dengue with warning signs, and severe dengue, the new disease classifications were first used in Mexico in 2016.10
Figure 1.
Geographic location of the Reynosa–McAllen metropolitan area.
Demographic and clinical information were documented and acute sera were collected from patients. It is obligatory to report dengue in Mexico; thus, informed consent was not required. All identifying information was removed to protect patient identities. Sera were tested for DENV nonstructural protein 1 (NS1) by enzyme-linked immunosorbent assay (ELISA) using the Panbio Dengue Early ELISA (Inverness Medical Innovations Ltd., Australia [formerly Panbio Ltd.]) or Platelia™ Dengue NS1 Antigen ELISA (BioRad, Hercules, CA). Aliquots of select sera were sent to the National Institute for Diagnosis and Epidemiological Reference in Mexico City where total RNA was extracted using the QIAamp viral RNA extraction kit (Qiagen, Valencia, CA) and tested for CHIKV RNA by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) using a standardized protocol established by the U.S. Centers for Disease Control and Prevention.11 Sera tested by qRT-PCR were selected using an algorithm established by the SS in which 1) all patients with arthralgia are tested until an autochthonous case of CHIKF is identified in the state, after which approximately 5% are tested, and 2) approximately 2% and 5% of non-arthralgic patients without and with DENV antigen, respectively, are tested. The first autochthonous case of CHIKF in Tamaulipas occurred in July 2015 during epidemiological week (EW) 27.12 Patients were considered to have laboratory-confirmed DENV and CHIKV infections if they tested positive for DENV antigen and CHIKV RNA, respectively.
Select CHIKV RNA-positive sera were tested by virus isolation in cell culture by performing two blind passages in Aedes albopictus (C6/36) cells, followed by two blind passages in African green monkey kidney (Vero) cells. Total RNA was extracted from cell culture supernatants using the QIAamp viral RNA extraction kit (Qiagen). The complete genome or structural protein genes (capsid-E3-E2-6K-E1) of each isolate were amplified by reverse transcription–polymerase chain reaction as overlapping fragments (primer sequences available on request). Complementary DNAs were generated using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) and PCRs were performed using high-fidelity DNA Taq polymerase (Invitrogen). Negative and positive control reactions were performed using dH20 and total RNA extracted from cells infected with a CHIKV isolate from Yucatan State, respectively, as template. Polymerase chain reaction products were purified using the QIAquick PCR purification kit (Qiagen) and sequenced using a 3730 × 1 DNA sequencer (Applied Biosystems, Foster City, CA).
The study cohort consisted of 1,090 patients. Of these, 1,065 patients had suspected DF and 25 patients met the clinical criteria for DHF, including six patients who were hospitalized. Sera from 134 (12.3%) patients were positive by ELISA for DENV antigen. Other studies have also shown that most patients clinically diagnosed with dengue do not have laboratory-confirmed DENV infections.13,14 Overall, 126 patients were diagnosed with laboratory-confirmed DF and eight patients were diagnosed with laboratory-confirmed DHF. Sera from select patients (N = 34) were further tested by qRT-PCR for CHIKV RNA, including 20 patients with laboratory-confirmed DF and four patients with laboratory-confirmed DHF. Of these, seven patients presented with arthralgia and 27 patients were non-arthralgic. Thirty-one patients tested for CHIKV RNA developed symptoms in October or November. Thirteen (38.2%) patients, including four patients with laboratory-confirmed DF and one patient with laboratory-confirmed DHF, were positive for CHIKV RNA (Table 1). All patients with laboratory-confirmed CHIKF developed symptoms in October or November 2015. Polyarthralgia persisted in two CHIKF patients (R-606 and R-607) for more than 6 months and both were also positive for DENV antigen, suggesting that coinfection may result in more severe symptoms. At least six CHIKF patients had not traveled outside of Tamaulipas that year.
Table 1.
Demographic, clinical, and laboratory findings of chikungunya patients
| Patient ID | Gender | Age (years) | Illness onset (date) | Illness onset (EW*) | Travel† | DENV NS1 ELISA | Dengue diagnosis | CHIKV RNA‡ | CHIKV isolation |
|---|---|---|---|---|---|---|---|---|---|
| R-413 | F§ | 38 | October 3 | 40 | ND‖ | −¶ | − | + | NT# |
| R-462 | F | 68 | October 14 | 41 | No | − | − | + | NT |
| R-482 | F | 28 | October 19 | 42 | No | Inconclusive | − | + | NT |
| R-606 | F | 67 | October 29 | 43 | No | +** | DF†† | + | NT |
| R-607 | F | 51 | November 3 | 44 | Yes | + | DF | + | NT |
| R-615 | M‡‡ | 33 | November 3 | 44 | No | − | − | + | NT |
| R-702 | F | 52 | November 8 | 45 | ND | − | − | + | NT |
| R-703 | F | 43 | November 9 | 45 | ND | − | − | + | NT |
| R-756 | M | 18 | November 10 | 45 | ND | + | DHF§§ | + | NT |
| R-779 | F | 29 | November 12 | 45 | No | − | − | + | NT |
| R-1950 | M | 28 | November 3 | 44 | No | + | DF | + | + |
| R-1972 | F | 62 | November 4 | 44 | ND | + | DF | + | + |
| R-2295 | F | 43 | November 10 | 45 | ND | − | − | + | + |
CHIKV = chikungunya virus; DENV = dengue virus; ELISA = enzyme-linked immunosorbent assay; EW = epidemiological week; NS1 = nonstructural protein 1.
Epidemiological week.
Defined as traveling outside of Tamaulipas in 2015.
Ct values ≤ 36 were considered positive.
Female.
Not documented.
Negative.
Not tested.
Positive.
Dengue fever.
Male.
Dengue hemorrhagic fever.
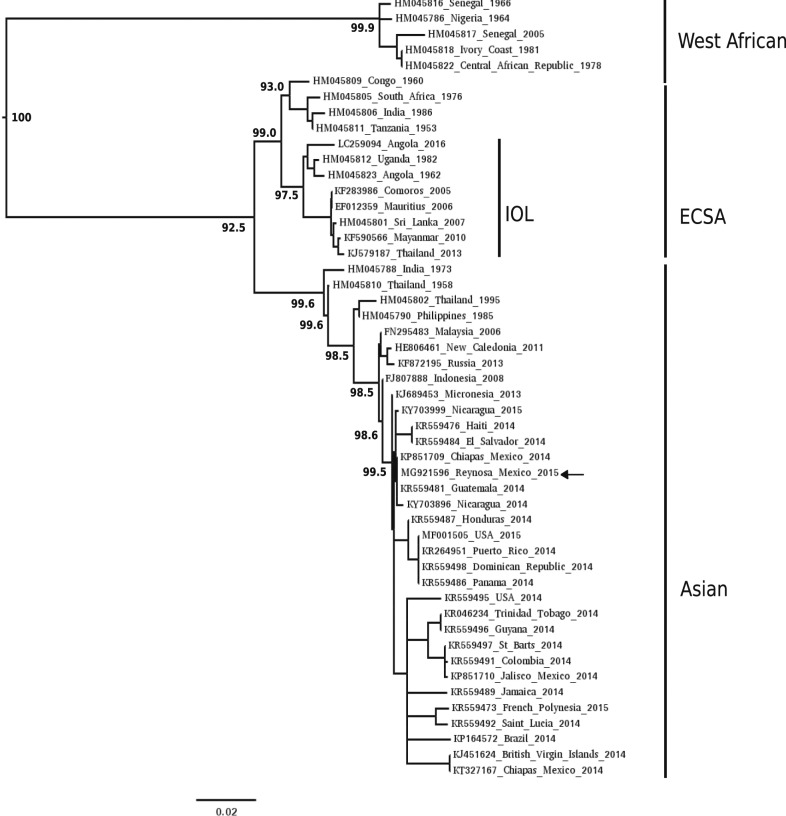
Three randomly selected CHIKV RNA-positive sera were tested for CHIKV by virus isolation in cell culture. An isolate was recovered on each occasion. The complete genome of one isolate (designated CH-R-1950) and capsid-E3-E2-6K-E1 regions of the other two isolates were sequenced (GenBank accession nos. MG921596, MG822707, and MG822708). Pairwise alignments of the nucleotide and deduced amino acid sequences of the capsid-E3-E2-6K-E1 regions of all three isolates were performed using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) and shown to have 99.9–100% and 99.8–100% identity, respectively, with each other. The genome sequence of CH-R-1950 was aligned with all other CHIKV sequences in the GenBank database and shown to have highest nucleotide identity (> 99.8%) with the genomes of isolates from Chiapas, Nicaragua, and elsewhere in the Americas. Phylogenetic inference is in agreement with these findings (Figure 2).
Figure 2.
Phylogenetic analysis of the complete genome sequences of CH-R-1950 and other select chikungunya virus (CHIKV) isolates. Nucleotide sequences were aligned using the program multiple alignment using fast Fourier transform. A Markov Chain Monte Carlo–based Bayesian analysis was performed by MrBayes using the general time reversible plus invariant sites plus gamma distribution 4 model.20 Four million iterations were used to reach convergence. Select posterior probabilities are denoted as percentages under nodes. Branch lengths are proportional to the number of nucleotide differences. The Asian, East/Central/South African (ECSA), Indian Ocean, and West African lineages of CHIKV are denoted.
This is the first study to report the clinical presentations and laboratory findings of an autonomous case of CHIKF in northern Mexico (defined as a state that borders the United States). The first case of CHIKF in northern Mexico occurred in Sonora in 2014 but it was assumed that the case was imported because the patient had recently traveled to Chiapas.15 In another study, CHIKV RNA was detected in 41 of 101 (40.6%) febrile patients in Nuevo León in 2015.6 Thirty-one CHIKV RNA-positive patients had recently traveled to southern Mexico. Travel histories of the other patients were not documented. According to the database of the SS, the first autonomous case of CHIKF in northern Mexico occurred in Sonora in EW 52 of 2014.12 As already noted, the first autochthonous case in Tamaulipas occurred in EW 27 of 2015.12
According to the Texas Department of State Health Services, 32 of the 254 counties in Texas are designated as border counties (defined as within 100 km of the U.S.–Mexico border).16 One local and three imported cases of CHIKF occurred in border counties of Texas in 2015.4 One imported case was identified in Hildago County. The number of CHIKF cases in Texas border counties in 2015 is, therefore, considerably lower than the number of cases in Reynosa alone. Two cases of dengue (both imported) also occurred in border counties of Texas in 2015 and, thus, there is also a notable difference between the numbers of dengue cases reported either side of the Reynosa–Texas border. Other studies performed in binational metropolitan areas have also reported a much higher incidence of dengue in Mexico compared with that in the United States.17,18 Environmental factors that affect contact between mosquitoes and humans (i.e., air-conditioning) were suggested to be the primary reasons for these differences.18
We report the first genome sequence of a CHIKV isolate from northern Mexico. The structural protein genes of two other isolates were also sequenced. One other CHIKV sequence from northern Mexico is available in the GenBank database: a 457-nt. region of the E2 gene detected in a patient in Nuevo León.6 None of the E2 or E1 substitutions previously shown to increase fitness in Ae. albopictus were identified.19 We also provide evidence of CHIKV and DENV coinfections in five patients in northern Mexico. Five patients from the Nuevo León study could have also been coinfected with these viruses because both CHIKV RNA and DENV IgM were detected in their sera.6 Taken together, the aforementioned data underscore the important need to continue performing differential diagnoses for CHIKV and DENV in northern Mexico.
Acknowledgments:
We thank all the nurses who participated in the study, particularly Dinorah Muñoz-Gutiérrez, Fanny B. Hernández-Castan, Karla E. Mendez-Jiménez, and Leticia Guzmán-Hernández from the Secretaría de Salud, Jurisdicción IV in Reynosa, Tamaulipas.
REFERENCES
- 1.Simmons CP, Farrar JJ, Nguyen vV, Wills B, 2012. Dengue. N Engl J Med 366: 1423–1432. [DOI] [PubMed] [Google Scholar]
- 2.Weaver SC, Charlier C, Vasilakis N, Lecuit M, 2017. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu Rev Med 69: 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz-Quinonez JA, et al. 2015. Complete genome sequences of chikungunya virus strains isolated in Mexico: first detection of imported and autochthonous cases. Genome Announc 3: e00300–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Geological Survey Chikungunya Virus Cases Available at: https://diseasemaps.usgs.gov/mapviewer/. Accessed January 1, 2018.
- 5.Garay-Moran C, Roman-Pedroza JF, Lopez-Martinez I, Rodriguez-Martinez JC, Ruiz-Matus C, Kuri-Morales P, Diaz-Quinonez JA, 2017. Clinical and epidemiological characterization of chikungunya fever in Mexico. Rev Panam Salud Publica 41: e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Govea MA, Zamudio-Osuna MD, Murillo KDT, Ponce G, Cavazos MED, Tavitas-Aguilar MI, Flores-Suarez AE, Villarreal-Perez JZ, Rodriguez-Sanchez IP, 2017. Chikungunya fever in patients from northeastern Mexico. Southwest Entomol 42: 143–152. [Google Scholar]
- 7.Brathwaite Dick O, San Martin JL, Montoya RH, del Diego J, Zambrano B, Dayan GH, 2012. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 87: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Undurraga EA, et al. 2015. Economic and disease burden of dengue in Mexico. PLoS Negl Trop Dis 9: e0003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization , 1997. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention, and Control. Geneva, Switzerland: WHO. [Google Scholar]
- 10.Special Programme for Research and Training in Tropical Diseases, World Health Organization , 2009. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control. Geneva, Switzerland: TDR, WHO.
- 11.Centers for Disease Control and Prevention , 2011. Preparedness and Response for Chikungunya Virus Introduction in the Americas Available at: http://www1.paho.org/hq/dmdocuments/CHIKV_English.pdf. Accessed January 1, 2018.
- 12.Secretaría de Salud México, Boletín Epidemiológico SNdVESÚdI , 2015. Available at: https://www.gob.mx/cms/uploads/attachment/file/10564/sem27.pdf. Accessed January 1, 2018.
- 13.Heringer M, Souza TMA, Lima M, Nunes PCG, Faria N, de Bruycker-Nogueira F, Chouin-Carneiro T, Nogueira RMR, Dos Santos FB, 2017. Dengue type 4 in Rio de Janeiro, Brazil: case characterization following its introduction in an endemic region. BMC Infect Dis 17: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorono-Pino MA, et al. 2004. Introduction of the American/Asian genotype of dengue 2 virus into the Yucatan state of Mexico. Am J Trop Med Hyg 71: 485–492. [PubMed] [Google Scholar]
- 15.Martinez-Medina MA, Canedo-Dorame IA, 2017. First case of chikungunya fever in Hermosillo, Sonora, Mexico. Rev Med Inst Mex Seguro Soc 55: 123–127. [PubMed] [Google Scholar]
- 16.Texas Department of State Health Services Available at: https://www.dshs.texas.gov/chs/hprc/counties.shtm. Accessed January 1, 2018.
- 17.Ramos MM, et al. 2008. Epidemic dengue and dengue hemorrhagic fever at the Texas–Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg 78: 364–369. [PubMed] [Google Scholar]
- 18.Reiter P, et al. 2003. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis 9: 86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsetsarkin KA, et al. 2014. Multi-peaked adaptive landscape for chikungunya virus evolution predicts continued fitness optimization in Aedes albopictus mosquitoes. Nat Commun 5: 4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronquist F, Huelsenbeck JP, 2003. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]