Abstract.
Ivermectin treatment can cause central nervous system adverse events (CNS-AEs) in persons with very high-density Loa loa microfilaremia (≥ 30,000 mf/mL blood). Hypoendemic onchocerciasis areas where L. loa is endemic have been excluded from ivermectin mass drug administration programs (MDA) because of the concern for CNS AEs. The rapid assessment procedure for L. loa (RAPLOA) is a questionnaire survey to assess history of eye worm. If ≥ 40% of respondents report eye worm, this correlates with ≥ 2% prevalence of very high-density loiasis microfilaremia, posing an unacceptable risk of CNS-AEs after MDA. In 2016, we conducted a L. loa study in 110 ivermectin-naïve, suspected onchocerciasis hypoendemic villages in southern Nigeria. In previous RAPLOA surveys these villages had prevalences between 10% and 67%. We examined 10,605 residents using the LoaScope, a cell phone–based imaging device for rapidly determining the microfilaria (mf) density of L. loa infections. The mean L. loa village mf prevalence was 6.3% (range 0–29%) and the mean individual mf count among positives was 326 mf/mL. The maximum individual mf count was only 11,429 mf/mL, and among 2,748 persons sampled from the 28 villages with ≥ 40% RAPLOA, the ≥ 2% threshold of very high Loa mf density could be excluded with high statistical confidence (P < 0.01). These findings indicate that ivermectin MDA can be delivered in this area with extremely low risk of L. loa–related CNS-AEs. We also concluded that in Nigeria the RAPLOA survey methodology is not predictive of ≥ 2% prevalence of very high-density L. loa microfilaremia.
INTRODUCTION
Onchocerciasis, commonly known as river blindness, is a filarial nematode infection caused by Onchocerca volvulus, transmitted by certain insect vector species of the genus Simulium.1 This disease is of public health importance because of its associated visual impairment, blindness, stigmatizing skin disease, and debilitating itching. Human disease results from inflammation around microfilaria (mf) released from fertilized adult female worms residing in fibrous subcutaneous “nodules.” Disease is more severe in individuals who have high numbers (“intensities”) of mf. The Simulium black fly vectors breed in rapidly flowing rivers and streams and become infected when they ingest mf during a blood meal; mf develop into third stage larvae that can infect humans when the vector takes subsequent blood meals. The World Health Organization (WHO) estimates that about 198.2 million people are at risk of infection and that 99% of them reside in Africa.1–3 Nigeria is among the countries with the highest burden of onchocerciasis.4
Mass drug administration (MDA) with ivermectin (Mectizan®, an oral medication donated by Merck, Kenilworth, NJ) kills the mf stage of the parasite and prevents onchocerciasis-associated eye and skin disease. A global partnership against river blindness, which includes the endemic countries, nongovernmental organizations, the Mectizan® Donation Program, and the WHO, has given more than 1 billion treatments for onchocerciasis since 1987.5 If the coverage and duration of ivermectin MDA programs are sufficient, mf densities can be kept sufficiently low to prevent the vectors from transmitting the infection. Because there are no important animal reservoirs of O. volvulus to maintain the transmission cycle independent of the human population, permanent elimination of transmission of onchocerciasis can be achieved, such as in four countries in the Americas and in some parts of Africa.6,7 In 2014 the African Program for Onchocerciasis Control called for a new goal of onchocerciasis transmission elimination for Africa. As part of that policy, an expansion of ivermectin MDA into previously untreated areas was proposed. These areas (the so-called “hypoendemic” areas) are those with sufficient O. volvulus transmission to maintain the adult parasite population but very little morbidity due to the near absence of high mf density infections. Untreated areas bordering ivermectin MDA programs are those most likely to be hypoendemic and therefore newly targeted for MDA.8
Loa loa, another filarial parasite prevalent in central Africa, is complicating the MDA expansion plan under the new onchocerciasis elimination paradigm. Loa loa is transmitted by deerflies (Chrysops species) that breed in high canopy-forested areas in Africa. Adult L. loa worms may migrate under the eye’s conjunctiva and be recognized by the infected individual.9–11 Adult female L. loa worms produce mf that (unlike in onchocerciasis) enter the blood stream; circulating L. loa mf can reach extremely high densities in the blood. The abrupt death of mf after the administration of a microfilaricidal agent such as ivermectin can rarely result in central nervous system adverse events (CNS-AEs) shortly after treatment that include changes in consciousness and, rarely, coma. Deaths have resulted from complications arising from prolonged coma events.12 Only individuals with very high L. loa mf densities (≥ 30,000/mL of blood) are at risk of these CNS-AEs.13–15
A technique called the Rapid Assessment Procedure for L. loa (RAPLOA) was developed over a decade ago to quickly and noninvasively assess an area for the risk of L. loa–related CNS-AEs after ivermectin MDA. A sample of 80 residents aged 15 years and older are individually asked if they at some point in the past experienced a worm moving across the surface of their eye. During the interview the respondents are shown a photograph of a L. loa worm in the eye. A multi-country study showed a strong correlation with ≥ 40% of residents answering “yes” (e.g., a RAPLOA prevalence of ≥ 40%), a village prevalence of L. loa microfilaremia ≥ 20%, and the village prevalence of very high-density L. loa ≥ 2%.16–20 These critical and correlated thresholds (RAPLOA ≥ 40%, L. loa microfilaremia prevalence ≥ 20% and very high-density L. loa ≥ 2%) define an area at high risk for L. loa CNS-AEs. The magnitude of this risk is poorly defined.14
High RAPLOA determinations in onchocerciasis hypoendemic areas are roadblocks to the onchocerciasis elimination agenda in L. loa–endemic countries such as Nigeria. Expansion of MDA into these hypoendemic areas is difficult to justify because the benefit from MDA in reducing morbidity from onchocerciasis is low compared with the risk of CNS-AEs from L. loa treatment. We report a survey in just such an area in Nigeria where there is presumed hypoendemic onchocerciasis and hyperendemic L. loa. Our purpose was to reevaluate the relationships among RAPLOA, L. loa microfilaremia prevalence, and most importantly, very high-density L. loa. We also assessed for onchocerciasis endemicity using a rapid diagnostic test for OV16 IgG4 antibodies; the results of that study will be reported elsewhere.
MATERIALS AND METHODS
Study area.
The survey was conducted in five Nigerian states assisted by The Carter Center in Abia, Anambra, Delta, Ebonyi, and Imo states, which are located in the forested South-South and Southeast geopolitical zones of Nigeria. The economic activities in these states are mainly agricultural subsistence farming and fishing. The climate is characterized by a rainy season from March to October and a dry season from November to February.
Study design.
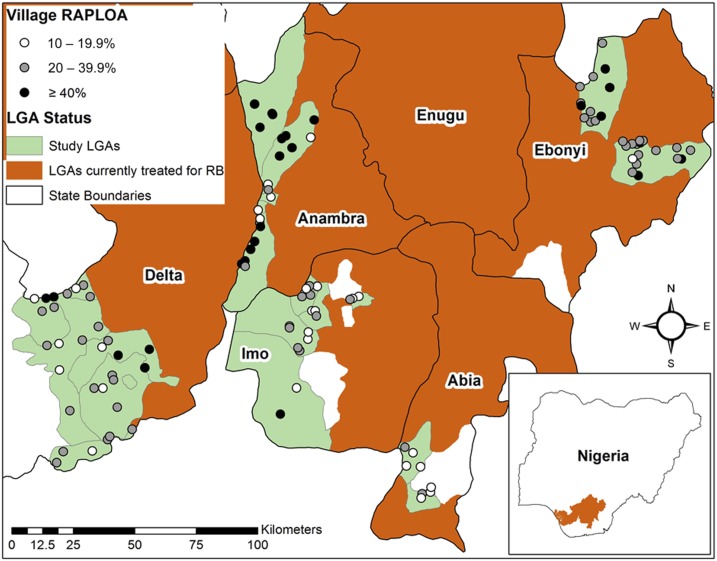
The survey was built in a way to purposefully identify villages likely to have both (hypoendemic) onchocerciasis and individuals with very high-density L. loa infections. First, twenty ivermectin untreated districts (in Nigeria called local government areas, LGAs) were selected that bordered LGAs already under ivermectin MDA for meso- or hyperendemic onchocerciasis. It was assumed that due to this proximity the selected LGAs would likely have hypoendemic onchocerciasis. Second, villages within those LGAs were selected for survey that had the highest historical RAPLOA prevalence in studies conducted in 2012–2013 by The Carter Center or in 2014–2015 by the Federal Ministry of Health (FMOH) (Figure 1, Table 1). Villages were sorted by RAPLOA prevalence in descending order. All villages having ≥ 20% RAPLOA were selected for survey, followed by a portion of villages with 10–19.9%, until the target of 110 villages was met. Five villages were excluded due to insecurity in their LGA and were replaced by five villages with the next highest RAPLOA prevalence. In surveyed villages, we determined L. loa mf prevalence and density using a revised version of the original LoaScope.21 This is an innovative cell phone–based microscope reader that gives a reading of L. loa mf density within 3 minutes of placing a special cubical capillary tube containing uncoagulated blood (obtained by finger stick) under the lens. From the same finger stick another 10 μL of blood was obtained for a thin smear and10 μL for an OV16 RDT test for onchocerciasis IgG4 antibodies.
Figure 1.
Map of study area: Location of surveyed villages and their historical Rapid Assessment Procedure for Loa loa (RAPLOA) prevalence. LGA = local government area. This figure appears in color at www.ajtmh.org.
Table 1.
Village sample size, Rapid Assessment Procedure for Loa loa (RAPLOA) information, max and average cellScope counts, and LoaScope prevalence
| State | LGA | Village | Number surveyed | Max of RAPLOA (%) | Source of max RAPLOA value | Year of RAPLOA survey | Max of LoaScope mf/mL | Average LoaScope mf/mL among positives | Prevalence of LoaScope positives (%) |
|---|---|---|---|---|---|---|---|---|---|
| Abia | Osisioma | Amapu Ife | 96 | 15 | FMOH | 2015 | 439 | 207 | 7 |
| Umuakpara | 99 | 14 | FMOH | 2015 | 592 | 229 | 9 | ||
| Umule | 101 | 19 | FMOH | 2015 | 282 | 179 | 7 | ||
| Umumba | 77 | 23 | FMOH | 2015 | 526 | 209 | 6 | ||
| Ugwunagbo | Owerri Aba | 100 | 14 | FMOH | 2015 | 1,049 | 602 | 2 | |
| Umule Osoamadi | 100 | 11 | FMOH | 2015 | 92 | 92 | 1 | ||
| Umuode | 100 | 13 | TCC | 2013 | 461 | 370 | 4 | ||
| Umuodo | 87 | 24 | FMOH | 2015 | 921 | 271 | 8 | ||
| Anambra | Anambra east | Agbudu Nando | 98 | 65 | TCC | 2012 | 921 | 264 | 14 |
| Nneyi Umueri | 98 | 67 | TCC | 2012 | 877 | 263 | 26 | ||
| Ogwari Nsugbe | 100 | 62 | TCC | 2012 | 680 | 237 | 21 | ||
| Otuocha | 100 | 49 | TCC | 2012 | 263 | 197 | 2 | ||
| Ubaru Ugwuoji | 104 | 55 | TCC | 2012 | 1,259 | 313 | 19 | ||
| Anambra west | Mmiata Anam | 101 | 46 | TCC | 2012 | 1,254 | 292 | 11 | |
| Nzam Assa | 99 | 56 | TCC | 2012 | 197 | 121 | 5 | ||
| Umuenwelum | 100 | 47 | TCC | 2012 | 856 | 179 | 11 | ||
| Umueze Anam | 100 | 59 | TCC | 2012 | 461 | 197 | 18 | ||
| Umuoba Abegbu | 101 | 47 | TCC | 2012 | 1,930 | 301 | 15 | ||
| Ogbaru | Atani | 100 | 20 | FMOH | 2015 | 486 | 166 | 18 | |
| Isiolu Ugalo | 97 | 61 | TCC | 2012 | 307 | 143 | 10 | ||
| Odekpe | 102 | 14 | FMOH | 2015 | 2,632 | 297 | 15 | ||
| Ohita | 99 | 13 | FMOH | 2015 | 614 | 298 | 6 | ||
| Okpoko | 99 | 18 | FMOH | 2015 | 1,290 | 208 | 22 | ||
| Onyili/Ibelenta | 101 | 67 | TCC | 2012 | 128 | 59 | 12 | ||
| Umudashi/Esielle | 100 | 65 | TCC | 2012 | 329 | 128 | 10 | ||
| Umuezegbo | 100 | 50 | TCC | 2012 | 1,100 | 182 | 20 | ||
| Umunankwo | 100 | 26 | FMOH | 2015 | 1,290 | 184 | 21 | ||
| Umuokoloigbo | 101 | 49 | TCC | 2012 | 351 | 120 | 13 | ||
| Onitsha north | American Quarters | 100 | 16 | FMOH | 2015 | 61 | 61 | 1 | |
| Onitsha south | Fegge | 100 | 21 | FMOH | 2015 | 154 | 137 | 3 | |
| Delta | Ethiope east | Ekrejeta | 69 | 28 | TCC | 2013 | 1,791 | 591 | 6 |
| Eku (Emure) | 99 | 43 | TCC | 2012 | 2,610 | 569 | 7 | ||
| Igun | 100 | 48 | TCC | 2012 | 563 | 216 | 9 | ||
| Okpara Inland | 100 | 11 | TCC | 2013 | 11,429 | 4,047 | 3 | ||
| Okurekpo | 99 | 35 | TCC | 2012 | 1,177 | 405 | 6 | ||
| Orhoakpo | 100 | 25 | TCC | 2012 | 461 | 217 | 3 | ||
| Oria Abraka | 100 | 29 | TCC | 2013 | 921 | 368 | 3 | ||
| Otorho Abraka | 100 | 29 | TCC | 2013 | 307 | 165 | 5 | ||
| Samagidi | 100 | 34 | TCC | 2012 | 31 | 31 | 1 | ||
| Urhuovie Igun | 99 | 13 | FMOH | 2015 | 522 | 522 | 1 | ||
| Isoko north | Ofagbe | 100 | 40 | TCC | 2012 | 338 | 338 | 1 | |
| Okpe Isoko | 100 | 60 | TCC | 2012 | 184 | 107 | 5 | ||
| Otor-Igho/Emevor | 100 | 13 | TCC | 2013 | 7,568 | 1,281 | 8 | ||
| Owhelogbo | 100 | 20 | TCC | 2012 | 338 | 148 | 5 | ||
| Ozoro | 100 | 40 | TCC | 2012 | 706 | 231 | 9 | ||
| Isoko south | Emede | 101 | 15 | TCC | 2012 | 329 | 142 | 6 | |
| Emore | 99 | 20 | TCC | 2012 | 746 | 172 | 7 | ||
| Irri | 100 | 21 | TCC | 2012 | 998 | 363 | 5 | ||
| Olomoro | 100 | 21 | TCC | 2012 | 526 | 287 | 4 | ||
| Uzere | 100 | 29 | TCC | 2012 | 307 | 149 | 4 | ||
| Patani | Abari | 97 | 36 | TCC | 2012 | 0 | 0 | 0 | |
| Bolu Angiama | 97 | 16 | TCC | 2013 | 465 | 465 | 1 | ||
| Bulu Aperebiri | 100 | 39 | TCC | 2012 | 154 | 92 | 2 | ||
| Odorubu | 100 | 35 | TCC | 2012 | 369 | 138 | 4 | ||
| Patani II | 100 | 34 | TCC | 2012 | 0 | 0 | 0 | ||
| Uduophri | 100 | 35 | TCC | 2012 | 0 | 0 | 0 | ||
| Ugheli north | Odovie | 100 | 13 | TCC | 2013 | 44 | 44 | 1 | |
| Oghara Agharha | 101 | 20 | TCC | 2013 | 397 | 229 | 2 | ||
| Onidjor Uwheru | 100 | 25 | TCC | 2013 | 92 | 51 | 3 | ||
| Orogun | 97 | 21 | TCC | 2013 | 0 | 0 | 0 | ||
| Otovwodo | 102 | 18 | TCC | 2013 | 0 | 0 | 0 | ||
| Ebonyi | Abakaliki | Abofia (Unagbo Oke) | 100 | 33 | TCC | 2012 | 439 | 126 | 5 |
| Amachi Unuhu | 100 | 25 | FMOH | 2015 | 491 | 321 | 2 | ||
| Amagu Onicha | 100 | 28 | TCC | 2012 | 526 | 396 | 5 | ||
| Ametta Amachi | 100 | 25 | FMOH | 2015 | 483 | 228 | 5 | ||
| Azugwu | 98 | 21 | FMOH | 2015 | 768 | 257 | 8 | ||
| Azuiyiokwu | 100 | 28 | FMOH | 2015 | 230 | 99 | 4 | ||
| Egwudinagu | 100 | 39 | FMOH | 2015 | 0 | 0 | 0 | ||
| Enyigba | 100 | 40 | FMOH | 2015 | 263 | 175 | 2 | ||
| Igbegu Unuhu | 100 | 14 | TCC | 2012 | 276 | 146 | 4 | ||
| Ndiaja ndiagu | 100 | 44 | FMOH | 2015 | 483 | 285 | 3 | ||
| Ndigboke | 100 | 24 | FMOH | 2015 | 197 | 137 | 3 | ||
| Nkaliki | 100 | 25 | FMOH | 2015 | 307 | 307 | 1 | ||
| Onuebonyi | 100 | 44 | TCC | 2012 | 0 | 0 | 0 | ||
| Orizor ndiuruku | 100 | 24 | FMOH | 2015 | 0 | 0 | 0 | ||
| Uburu Amachi | 100 | 38 | TCC | 2012 | 505 | 505 | 1 | ||
| Ugwuachara | 100 | 26 | FMOH | 2015 | 0 | 0 | 0 | ||
| Unagboke | 100 | 31 | FMOH | 2015 | 51 | 51 | 1 | ||
| Agodo | 100 | 43 | FMOH | 2015 | 2,874 | 487 | 9 | ||
| Enyimagu | 100 | 36 | TCC | 2012 | 1,259 | 334 | 7 | ||
| Ezzamgbo | 100 | 25 | FMOH | 2015 | 246 | 127 | 4 | ||
| Iniki ri | 87 | 33 | FMOH | 2015 | 7,875 | 1,559 | 8 | ||
| Ishi-izhia | 89 | 35 | FMOH | 2015 | 829 | 829 | 1 | ||
| Ohaukwu | Ndi agu obu okposhi ehaku | 89 | 40 | FMOH | 2015 | 505 | 505 | 1 | |
| Ndiaguonwe | 82 | 38 | TCC | 2012 | 154 | 153 | 4 | ||
| Ndiakparata Ugwuagba | 84 | 31 | FMOH | 2015 | 614 | 399 | 6 | ||
| Obodo Ogbani | 88 | 39 | TCC | 2012 | 505 | 188 | 5 | ||
| Omocha | 76 | 48 | FMOH | 2015 | 1,750 | 721 | 9 | ||
| Otaku Umuagara | 84 | 40 | TCC | 2012 | 1,228 | 595 | 7 | ||
| Ufoboto | 77 | 33 | TCC | 2012 | 215 | 138 | 5 | ||
| Imo | Nkwerre | Ezemeneha | 100 | 30 | TCC | 2013 | 395 | 395 | 1 |
| Umuakuma | 100 | 18 | TCC | 2013 | 706 | 263 | 5 | ||
| Umunaga | 100 | 19 | TCC | 2013 | 790 | 246 | 4 | ||
| Oguta | Abosi Izombe | 100 | 20 | TCC | 2013 | 276 | 88 | 7 | |
| Ejemekwuru | 97 | 19 | TCC | 2013 | 486 | 293 | 3 | ||
| Obujuju Akabor | 99 | 10 | TCC | 2013 | 92 | 92 | 1 | ||
| Ugbele Izombe | 100 | 28 | TCC | 2013 | 491 | 204 | 7 | ||
| Umuoba Agwa | 100 | 30 | TCC | 2013 | 102 | 102 | 1 | ||
| Umuofeke Agwa | 96 | 25 | TCC | 2013 | 0 | 0 | 0 | ||
| Ohaji/Egbema | Obitti | 100 | 41 | TCC | 2013 | 2,815 | 1,076 | 4 | |
| Umuogologo | 100 | 19 | TCC | 2013 | 31 | 31 | 1 | ||
| Oru east | Abia Omuma | 63 | 30 | TCC | 2013 | 1,106 | 614 | 5 | |
| Amagu Awo | 54 | 15 | TCC | 2013 | 435 | 182 | 7 | ||
| Awo-omama | 71 | 13 | FMOH | 2015 | 592 | 167 | 27 | ||
| Eziawo | 81 | 14 | TCC | 2013 | 5,042 | 953 | 21 | ||
| Ihitte Akata | 120 | 14 | TCC | 2013 | 4,212 | 795 | 6 | ||
| Ubachima Awo | 92 | 29 | TCC | 2012 | 150 | 150 | 2 | ||
| Oru west | Ihite Oha | 91 | 15 | TCC | 2013 | 150 | 150 | 2 | |
| Nempi | 66 | 21 | TCC | 2013 | 4,936 | 640 | 29 | ||
| Umuduru Ezigbidi | 101 | 20 | TCC | 2013 | 972 | 972 | 1 | ||
| Total/Max/Average/Overall | 10,605 | NA | NA | NA | 11,429 | 326 | 6 | ||
FMOH = federal ministry of health surveys; LGA = local government area; TCC = the Carter Center surveys.
Procedures.
In each village, we aimed to test 50 adults (more than 18 years of age) and 50 children (≥ 5 and < 10 years of age). We excluded anyone who was ill or who might not tolerate finger-stick blood collection. Village leaders met with the research team who explained the study and then obtained verbal permission for the study to take place. Village leaders selected one of their common meeting areas as the survey venue for adults. For children, the venue was the village primary school. On the day of the study, written consent was obtained from each individual (assent from children) and a unique ID number was assigned. The ID and general information (name, age, gender, occupation, and duration of time living in the village) was recorded in tablets and on paper forms.
Sampling took place from 10:00 am to 4:00 pm when the maximum number of L. loa mf circulate.22 Whole blood was obtained using standard finger-lancing procedures by gloved technicians working in an enclosed private area. After cleaning the puncture site with isopropyl alcohol, a sterile single-use lancet was used to obtain blood from the index finger of the subject. A glass capillary tube with a rectangular cross section (Vitrocom Inc., Mountain Lakes, NJ) was filled first and immediately passed on to another technician who operated the LoaScope and read the number of L. loa mf in 15 μL of whole blood. A 10-μL capillary tube was used to make a thin smear blood slide. A final sample of 10 μL of blood was obtained by capillary tube and immediately placed on a Bioline Ov16 rapid test card (Standard Diagnostics, Inc., Gyeonggi-do, Republic of Korea) and then read per directions. LoaScope and RDT results were recorded in the tablets and on paper. The LoaScope automatically recorded in its memory the mf results along with the participant’s ID number; the team was later able to verify the LoaScope results uploaded by the tablets using the stored data uploaded directly from the LoaScopes. The entire registration, blood draw and testing procedure took approximately 10–15 minutes for each participant; the LoaScope portion of this process took about 5 minutes. It should be noted that the LoaScope is calibrated to capture high-density infections accurately, and as a result, a single capillary per person in the standard settings for number of fields of view per capillary is accurate to within 150 mf/mL.
The 10 μL blood smears were labeled with the participant’s ID, air-dried and returned to The Carter Center laboratory in Owerri. All individuals with LoaScope readings ≥ 20,000 mf/mL and a random selection of 10% of slides from those with readings < 20,000 (“negatives”) were stained with Giemsa and examined under light microscopy by an experienced technician. All L. loa mf on the slide were counted and recorded with their corresponding ID on paper forms.
Data analysis.
Paper data forms with personal identifiers were kept locked in a file cabinet at The Carter Center Owerri office. Data were uploaded from the tablets and LoaScopes using only ID number identifiers, combined in a Microsoft Excel spreadsheet, cleaned, and then analyzed using IBM SPSS 24, Excel pivot tables, Epinfo7, and OpenEpi statistical packages. Crude (age unadjusted) prevalence of L. loa mf, overall and by village, were calculated by dividing the number of mf positive persons by the number of persons examined. Density among LoaScope-positive readings was expressed as arithmetic means and medians. Very high-density L. loa infection was defined as ≥ 20,000 mf/mL because the lower 95% confidence intervals from LoaScope readings of ≥ 30,000 included 20,000 mf/mL.23 Prevalence of very high-density infection was calculated as the number of persons with ≥ 20,000 mf/mL divided by the total number of persons examined overall, by age group (adult or children), and in the subset of villages having RAPLOA prevalence ≥ 40%. Two percent very high-density infection was assumed to be at a CNS-AE risk threshold at or above which ivermectin MDA should not be given. The categorical χ2 test was used to determine if the observed percent positive with high-density infection differed from the expected result of 2%. We used the Spearman’s rho to test the correlation between RAPLOA versus L. loa village mf prevalence, and RAPLOA versus peak village L. loa mf density.
The 10 μL-thin blood films from all persons with LoaScope readings of ≥ 20,000 mf/mL were examined by light microscopy to confirm those results. In addition, 10% of negatives (e.g., < 20,000 mf/mL) were also examined to determine if high-density carriers might have been missed by LoaScope. Results however were not used to check the range of LoaScope density readings because the volume of blood used in the thin smear was 50% less than that examined by the LoaScope, and so less sensitive.
RESULTS
One of the twenty selected ivermectin-naive LGAs could not be visited due to insecurity. In the remaining 19 LGAs, 110 villages were surveyed: 82 of these (75%) had RAPLOA prevalence of ≥ 20% and 28 villages (25%) were ≥ 40% (range 40–67%). A total of 10,605 persons were tested by LoaScope, an average of 96 persons per village; 5,776 (54.5%) were females and 5,282 (49.8%) were children. The sample included 2,748 residents of the highest risk villages (RAPLOA ≥ 40%).
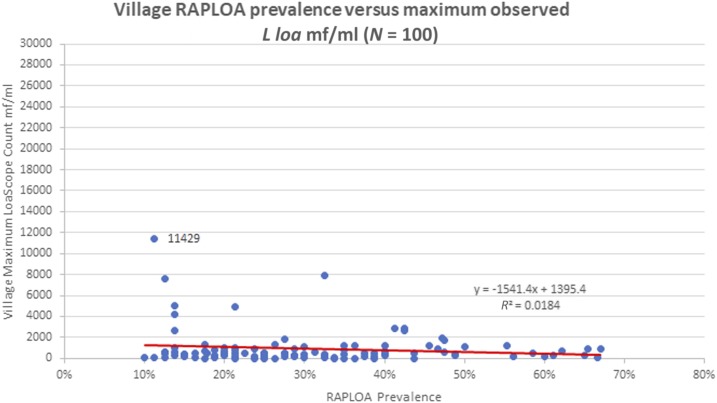
Only 661 (6.2%) persons were positive for mf by LoaScope. The mean village L. loa prevalence was 6.3% (median 4.9%, range 0–28.8%). The mean mf count among positive individuals was 326 mf/mL (median 150 mf/mL) and the maximum individual mf count was 11,429 mf/mL. Results for villages with RAPLOA ≥ 40% showed a mean village prevalence of 7.1% (median 5.0%, range 0.0–25.5%), a mean mf count among positives of 267 mf/mL (median 150 mf/mL), and the highest individual mf count 2,874 mf/mL. There was no correlation between L. loa mf prevalence and RAPLOA results (Figure 2). Peak village microfilaremia also did not correlate with RAPLOA results (Figure 3).
Figure 2.
Village Rapid Assessment Procedure for Loa loa (RAPLOA) prevalence vs. L. loa microfilaria (mf) prevalence (N = 110). This figure appears in color at www.ajtmh.org.
Figure 3.
Village Rapid Assessment Procedure for Loa loa (RAPLOA) prevalence vs. maximum observed L. loa microfilaremia. This figure appears in color at www.ajtmh.org.
No participants were detected with high-density L. loa microfilaremia. The highest count in the study (11,429 mf/mL) was in a resident of the low risk RAPLOA village of Okpara Inland (RAPLOA 11%) of Ethiope East LGA in Delta State. The second (7,875 mf/mL) and third highest (7,568 mf/mL) mf densities were in residents of villages with RAPLOAs of 33% and 13%, respectively.
The 2% prevalence of very high-density microfilaremia did not occur in the overall sample (P < 0.01) and the subsample of 2,748 persons resident in ≥ 40% RAPLOA villages (P < 0.01). Similarly, the 2% threshold was not exceeded when only adults were considered in the analysis for all villages (N = 5,323, P < 0.01) or villages with RAPLOA ≥ 40% (N = 1,380, P < 0.05).
A random selection of 1,342 (12.7%) of the 10-μL blood slides were stained and read by light microscopy. The maximum L. loa density was 11,800 mf/mL, from the same individual with a LoaScope reading of 11,429. No very high-density L. loa infections were observed in the blood slide reading.
DISCUSSION
In 2013 the FMOH of Nigeria released a master plan for neglected tropical diseases that included a new policy for using ivermectin MDA to interrupt onchocerciasis transmission throughout the country. Soon thereafter the minister of health launched the Nigeria Onchocerciasis Elimination Committee (NOEC) to provide technical advice to the FMOH on how to accomplish this goal by 2025. In 2016 the NOEC completed a “National Guideline for Onchocerciasis Elimination in Nigeria” that called for the MDA program to expand treatment into hypoendemic areas.24 That expansion had to address concerns about possible CNS-AEs in L. loa endemic states, especially where there were villages with RAPLOA survey results ≥ 40%. The purpose of our study was to access whether MDA expansion into hypoendemic areas in L. loa endemic states assisted by The Carter Center could be done with minimal risk. Using the new LoaScope technology we sampled residents from all villages in these states with > 20% RAPLOA prevalence located in ivermectin-naïve and likely onchocerciasis hypoendemic LGAs. Our purpose was to determine the prevalence of very high-density L. loa microfilaremia. A key outcome was to identify how many of these villages had ≥ 2% residents with microfilaremia ≥ 30,000 mf/mL.
In 110 villages sampled we found no LoaScope readings more than 20,000 mf/mL in 10,605 LoaScope examinations. In residents of villages with RAPLOA ≥ 40%, where the prevalence of very high-density microfilaremia should equal or exceed 2%, we could exclude the 2% threshold with > 99% statistical confidence. We concluded that ivermectin MDA for treatment of hypoendemic onchocerciasis could be deployed in these areas with very low risk of CNS-AEs.
In our study L. loa prevalence was lower than expected based on the RAPLOA findings. We found only 661 participants (6.2%) to be positive for mf and a mean village prevalence of 6.3% (median 4.9%, 0–28.8% range). Only eight villages had a L. loa prevalence ≥ 20%, compared with 28 villages having RAPLOA > 40%. Loa loa prevalence did not increase with RAPLOA as other studies has shown.25 We also were unable to demonstrate a correlation between RAPLOA and L. loa mf density, in contrast to Takougang et al.’s study that showed this relationship in Cameroon’s Southwest and Northwest provinces. Although Takougang et al.25 detected no persons with very high-density microfilaremia in Nigeria’s Cross River State, the RAPLOA prevalence of all villages in his study was below 40%. An important contribution of our study is that we were able to assess residents of 28 Nigerian villages where RAPLOA results were above the 40% threshold, and found no very high-density infections there. Our results are consistent with a report by Hassan et al.,26 who studied a village in southwest Nigeria (Ikpakodo) with 54% eye worm history where the highest L. loa mf count was only 420 mf/mL. Our village sample included an expanded range of RAPLOA prevalence (10–67%) that mitigated our risk of missing very high-density infections if they occurred in villages with RAPLOA < 40% that are not believed to be at risk of CNS-AEs.
The lack of reported CNS-AEs after more than 70 million ivermectin MDA treatments in known L. loa areas in Nigeria is further evidence that very high-density L. loa infections are exceedingly rare in the country. Of 758 CNS-AEs reported to the Mectizan Donation Program between 1990 and 2014, 99% of these were from Democratic Republic of Congo and Cameroon (Y. Sodahlon, personal email communication). Only one case (in 1991) in this series (0.1%) was reported from Nigeria.15 The NOEC has concluded that the lack of CNS-AEs despite millions of ivermectin treatments is evidence that the risk of these events in Nigeria is essentially nonexistent.27
The reasons that L. loa mf densities rarely, if ever, reach very high levels in Nigeria are unknown but could include the following: 1) The Nigerian strain of the L. loa parasite is not prone to produce very high-density microfilaremia; 2) the Nigerian human population may have genetic or other host factors that limit mf circulation; 3) deforestation or other recent environmental changes may have reduced Chrysops breeding and biting rates, leading to fewer adult L. loa adult parasites resulting in a decreasing mf prevalence and density; 4) unrecognized ivermectin treatment in the study population that has lowered mf prevalence and density. This last possibility is unlikely considering that in a questionnaire survey administered to 5,910 (56%) of our study participants, 98% stated that they had not taken ivermectin.
In Cameroon, the LoaScope has been successfully used to determine each individual’s treatment eligibility based on his/her L. loa density, excluding those with microfilaremia exceeding 20,000 mf/mL.21,23 This strategy, called “Test and not Treat,” is proposed as a possible way forward for onchocerciasis programs in L. loa–endemic areas. In this report we used the LoaScope as a survey technology to judge village (rather than individual) risk for CNS-AEs. We demonstrated that the LoaScope can be used to rapidly delineate areas where high-density L. loa infections do not occur and so allow ivermectin MDA using approaches that can be implemented more quickly and cheaply than “Test and not Treat.” This same survey approach could be used for lymphatic filariasis (LF) elimination programs in areas in Africa coendemic for L. loa. In this case, LF MDA programs could use a strategy of ivermectin and albendazole once per year rather than the costlier WHO-recommended strategy of albendazole alone every 6 months.28 Our recommendation, therefore, is that the LoaScope should be the primary tool in surveys needed to rapid expand ivermectin use in onchocerciasis and LF elimination programs operating in Africa where RAPLOA findings suggest that L. Loa microfilaremia densities could be dangerously high.
This study had a number of limitations: 1) We selected study villages based on historical RAPLOA results from surveys conducted between 2012 and 2015. This was justified because these villages were not receiving ivermectin treatment so that the prevalence and density of L. loa infections were unlikely to change dramatically in the 1–4 years between the RAPLOA assessment and the LoaScope survey; 2) We assumed that a convenience sample of 50 adults (aged ≥ 18 years) and 50 children (aged ≥ 5 and < 10 years) accurately reflected village L. loa prevalence. The sample was skewed toward younger children to assess OV16 antibody in an age group where positive results would indicate recent transmission of O. volvulus in the area. It is likely that having 50% of our overall sample consisting of children under 10 years of age resulted in an underestimate of the village L. loa prevalence and density, assuming adults are more likely than children to have very high-density L. loa infections. However, in a subanalysis of our data we could still statistically rule out the ≥ 2% very high-density prevalence threshold among all adults in the study, and among adults resident in high risk (RAPLOA ≥ 40%) villages.
CONCLUSION
We conducted a LoaScope-based blood survey in 10,605 residents of 110 ivermectin-naïve villages located in L. loa–endemic areas in southern Nigeria. The villages likely have hypoendemic onchocerciasis transmission and need to be treated with ivermectin MDA. Twenty-five percent of the villages surveyed had RAPLOA values of ≥ 40%. We determined that there is minimum risk for CNS-AEs because no very high-density L. loa infections could be detected, and the LoaScope prevalence of very high-density infections was significantly below 2%. The LoaScope is a useful tool for use in village surveys designed to assess risk of ivermectin MDA in L. loa–endemic areas.
Acknowledgments:
We would like to thank the State and Local Government Area Ministries of Health, including the field teams who made this study possible. We would also like to thank the village members who assisted us in village mobilization and organization, and the village members who participated in the study. We express our gratitude to Josephine Obiezu for her excellent and tireless data management. The study was funded by The Carter Center and the Sir Emeka Offor Foundation. The Bill and Melinda Gates Foundation and USAID supported LoaScope development.
REFERENCES
- 1.Cupp EW, Sauerbrey M, Richards F, 2011. Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Trop 120 (Suppl 1): S100–S108. [DOI] [PubMed] [Google Scholar]
- 2.Boatin BA, Richards FO, Jr., 2006. Control of onchocerciasis. Adv Parasitol 61: 349–394. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization , 2017. Summary of global update on preventive chemotherapy implementation in 2016: crossing the billion. Wkly Epidemiol Rec 92: 589–593. [PubMed] [Google Scholar]
- 4.Herricks JR, et al. 2017. The global burden of disease study 2013: what does it mean for the NTDs? PLoS Negl Trop Dis 11: e0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mectizan Donation Program , 2017. Available at: https://www.mectizan.org/achievements. Accessed December 21, 2017.
- 6.Katabarwa M, Richards F, 2014. Twice-yearly ivermectin for onchocerciasis: the time is now. Lancet Infect Dis 14: 373–374. [DOI] [PubMed] [Google Scholar]
- 7.Diawara L, et al. 2009. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl Trop Dis 3: e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly-Hope LA, Unnasch TR, Stanton MC, Molyneux DH, 2015. Hypo-endemic onchocerciasis hotspots: defining areas of high risk through micro-mapping and environmental delineation. Infect Dis Poverty 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mectizan Donation Program , 1996. Central Nervous System (CNS) Complications of Loiasis and Adverse CNS Events Following Treatment: Report of an Invited Consultation. October 2–3, 1995, Atlanta, GA: Mectizan Donation Program. [Google Scholar]
- 10.Boussinesq M, Gardon J, 1997. Prevalences of Loa loa microfilaraemia throughout the area endemic for the infection. Ann Trop Med Parasitol 91: 573–589. [DOI] [PubMed] [Google Scholar]
- 11.Boussinesq M, Gardon J, Gardon-Wendel N, Kamgno J, Ngoumou P, Chippaux JP, 1998. Three probable cases of Loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am J Trop Med Hyg 58: 461–469. [DOI] [PubMed] [Google Scholar]
- 12.Kamgno J, Boussinesq M, Labrousse F, Nkegoum B, Thylefors BI, Mackenzie CD, 2008. Encephalopathy after ivermectin treatment in a patient infected with Loa loa and Plasmodium spp. Am J Trop Med Hyg 78: 546–551. [PubMed] [Google Scholar]
- 13.Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M, 1997. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 350: 18–22. [DOI] [PubMed] [Google Scholar]
- 14.Addiss DG, Rheingans R, Twum-Danso NA, Richards FO, 2003. A framework for decision-making for mass distribution of Mectizan(R) in areas endemic for Loa loa. Filaria J 2 (Suppl 1): S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twum-Danso NA, 2003. Loa loa encephalopathy temporally related to ivermectin administration reported from onchocerciasis mass treatment programs from 1989 to 2001: implications for the future. Filaria J 2 (Suppl 1): S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diggle PJ, et al. 2007. Spatial modelling and the prediction of Loa loa risk: decision making under uncertainty. Ann Trop Med Parasitol 101: 499–509. [DOI] [PubMed] [Google Scholar]
- 17.Tekle AH, Zoure H, Wanji S, Leak S, Noma M, Remme JH, Amazigo U, 2011. Integrated rapid mapping of onchocerciasis and loiasis in the Democratic Republic of Congo: impact on control strategies. Acta Trop 120 (Suppl 1): S81–S90. [DOI] [PubMed] [Google Scholar]
- 18.Wanji S, 2001. Rapid Assessment Procedures for Loiasis: Report of a Multi-centre Study. Geneva, Switzerland: UNDP/World Bank/World Health Organization Special Programme for Research & Training in Tropical Diseases. [Google Scholar]
- 19.Wanji S, Akotshi DO, Mutro MN, Tepage F, Ukety TO, Diggle PJ, Remme JH, 2012. Validation of the rapid assessment procedure for loiasis (RAPLOA) in the Democratic Republic of Congo. Parasit Vectors 5: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoure HG, Wanji S, Noma M, Amazigo UV, Diggle PJ, Tekle AH, Remme JH, 2011. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA). PLoS Negl Trop Dis 5: e1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Ambrosio MV, et al. 2015. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci Transl Med 7: 286re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamgno J, Pion SD, Mackenzie CD, Thylefors B, Boussinesq M, 2009. Loa loa microfilarial periodicity in ivermectin-treated patients: comparison between those developing and those free of serious adverse events. Am J Trop Med Hyg 81: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 23.Kamgno J, et al. 2017. A test-and-not-treat strategy for onchocerciasis in Loa loa-endemic areas. N Engl J Med 377: 2044–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Federal Ministry of Health, Nigeria , 2017. Nigeria Onchocerciasis Elimination Plan. Abuja, Nigeria: Federal Ministry of Health. [Google Scholar]
- 25.Takougang I, et al. 2002. Rapid assessment method for prevalence and intensity of Loa loa infection. Bull World Health Organ 80: 852–858. [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan AA, Akinsanya B, Iyase N, Owagboriaye FO, 2011. Assessment of loiasis and outcomes of ivermectin masstreatment in Ijebu-North, Nigeria. Korean J Parasitol 49: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anonymous , 2016. Communiqué of the 5th Onchocerciasis Elimination Committee (NOEC). December 14–16, 2016, Barcelona Hotel, Abuja, Nigeria. [Google Scholar]
- 28.World Health Organization/Department of Control of Neglected Tropical Diseases , 2012. Provisional Strategy for Interrupting Lymphatic Filariasis Transmission in Loiasis-endemic Countries: Report of the Meeting on Lymphatic Filariasis, Malaria and Integrated Vector Management. Accra, Ghana: WHO. [Google Scholar]