Abstract.
This study evaluated a newly developed paper analytical device (PAD) for screening amoxicillin samples in Blantyre urban townships. Covert shoppers attempted to buy amoxicillin from a geographically stratified selection of private pharmacies (N = 22 out of 26) and drug stores (N = 23 out of 103) in the township area. According to the PAD results, all 42 samples obtained by the shoppers contained amoxicillin and none contained suspicious filler materials. Next, the products were assayed using high-performance liquid chromatography. Consistent with the PAD results, all samples contained the correct amount of amoxicillin with no unexpected ingredients. However, one sample was purchased as amoxicillin and contained that ingredient, but was packaged in capsules that are normally used to package ampicillin. Almost every sample failed a simple packaging analysis. Nine in 10 samples were missing their original packaging and/or inserts (52.4% repackaged capsules and 35.7% repackaged blister packs). Only 33.3% of the packages had expiry dates, 16.7% had batch numbers, and 47.6% had the manufacturer’s name. Dispensing practices were likewise unsatisfactory. Ninety-five percentage of the sellers sold the amoxicillin without a prescription, even though this medicine is regulated as prescription-only in Malawi. Although the chemical analysis showed that amoxicillin quality was good, our market survey revealed poor adherence to prescription-only medicine dispensing of antibiotics, which threatens antimicrobial stewardship efforts. Furthermore, the wide prevalence of repackaging deprives medicines of important information needed during patient’s use, regulatory investigations, and pharmacovigilance reporting.
INTRODUCTION
The circulation of substandard and falsified medical products1 poses a serious threat to the quality, safety, and efficacy of medicines, and this is becoming a major public health concern worldwide.2 The problem is more serious and detrimental in lower- and middle-income countries (LMICs) of the Southeast Asia and sub-Saharan African regions. This is attributed to weaknesses in the regulatory systems as a result of limited funding, personnel, expertise, and resources needed for routine quality control activities. The shortage of funding and trained personnel limits many countries and institutions from accessing and effectively using most of the analytical systems and assays because expensive equipment and specialist training are needed to effectively execute the activities.3 Hence, development of cheap, reliable, and user-friendly assays has been recommended so that LMICs can carry out routine field screening within their means.4,5 In this study, we evaluated a new tool, the paper analytical device (PAD), which can detect falsified amoxicillin and other antibiotics.6 We also analyzed how antibiotics are packaged and sold by different medicine shops in Blantyre urban townships. In Malawi, “pharmacies” must have a full-time or part-time qualified and registered pharmacist, and they are allowed to stock antibiotics, whereas “drug stores” can be managed by “pharmacy personnel” (Pharmacy, Medicines and Poisons Board [PMPB] registration forms on website), and they are not designated to stock antibiotics.
METHODOLOGY
Medicine samples were collected between June 8, 2017, and July 27, 2017, by two of the investigators from private pharmacies and medicine shops in Blantyre urban areas. The team used geographically stratified sampling based on the total number of registered pharmacies (26) and drug stores (103) at the time of the study (PMPB website, accessed on June 30, 2017) and visited at least three shops in Chilomoni, Ndirande, central business district, Chemusa, Mpemba, Machinjiri, Kachere, Nancholi, Bangwe, Chigumula, Chileka, Chatha, Limbe, and all areas along these roads. They selected the most visible facilities within each township but avoided sampling neighboring facilities where the repeated buying activities might be noticed by the shopkeepers. Some of the facilities listed on the PMPB web site were not sampled because they had closed or were difficult to locate.
The samples were collected by covert shoppers because covert shopping is recognized as the best practice for prevalence studies.7 The team members told the shopkeepers that they were patients (or guardians of patients) on self-medication and requested amoxicillin. When asked the disease they wanted medication for, they mentioned flu and cold, the diseases for which antibiotics are often misused. The U.S. shopper spoke in American-accented English and the Malawi native shopper spoke in Chichewa.
After the purchase had been made, a global position system (GPS)-enabled phone was used for entering the pharmacy name and the GPS coordinates this into an excel spreadsheet with other sample metadata. The samples were transported to the College of Pharmacy laboratories and subjected to packaging analysis. One tablet was removed for screening using the PADs, and the samples were shipped to the University of Notre Dame where another tablet was used for confirmatory analysis via high-performance liquid chromatography (HPLC).
Packaging analysis.
The packaging of the samples was analyzed based on World Health Organization (WHO) International Pharmacopoeia guidelines.8 In addition to checking the capsules, blister pack, and box for defects, the following metadata were sought: the name of the pharmaceutical product; the name(s) of the active pharmaceutical ingredient (API), International Nonproprietary Names where possible, amount of API in each tablet, the number of tablets in a container, the batch or lot number assigned by the manufacturer, expiry date, date of manufacture, special storage conditions or handling precautions, directions for use, warnings, precautions, and the name and address of the manufacturer or the person responsible for placing the product on the market. A sample was identified as “failed packaging analysis” if it lacked any of the prescribed metadata.8
Paper analytical device screening.
A capsule was selected for analysis and a portion (20–50 mg) of the powder within was spread with a spatula across the PAD at the swipe line, indicated by arrows on the side of the card. The sample was pressed firmly onto the paper using the spatula and the sample number was written at the top of the PAD. The bottom of the PAD was dipped into 0.5-cm depth of tap water in a shallow dish and the card was leaned against a beaker or bottle, so it would stand upright. After 3 minutes, the PAD was removed and laid flat on the table for three more minutes of color development. Each PAD was photographed using a cell phone to collect a 1.4–2.0 megabytes image, and the image was uploaded to a Dropbox folder for evaluation. The PAD image was then compared with a set of “typical” amoxicillin PAD images. Each PAD image was evaluated by a reader in Malawi and a reader in the United States. If the image was judged to be substantially different from the typical amoxicillin images, the sample was recorded as “failed PAD analysis.”
High-performance liquid chromatography analysis.
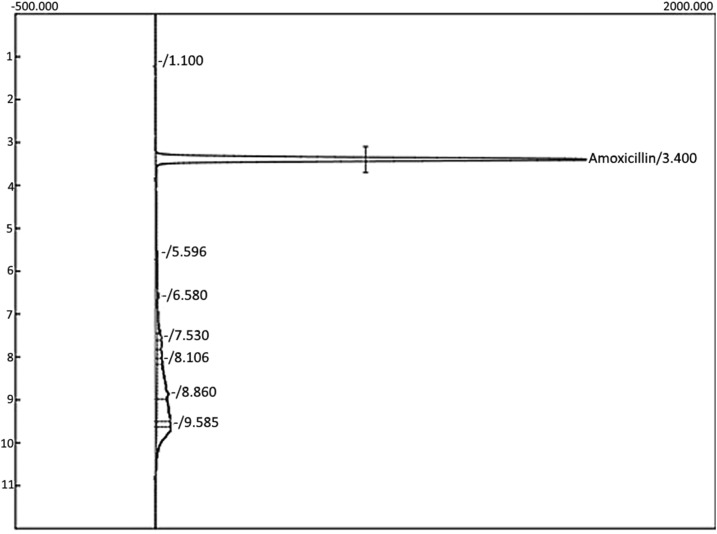
The United States Pharmacopeia (USP) method for “amoxicillin capsules” requires combining the contents of 20 capsules of each product for assay. Because of the small number of pills sold to each shopper (15–30 per product) and the need to preserve pills for independent analysis by the Malawi PMPB in case a bad-quality product was identified, HPLC analysis was performed on one pill taken from each of the 42 samples. These 42 amoxicillin samples were each prepared at nominal concentration of 0.5 mg/mL in deionized water, and then analyzed on a Waters 2695 HPLC instrument with a Waters 2487 dual-λ absorbance detector set at 220 nm. A complete system suitability assessment (USP method 621) was performed to ensure that the assay met USP standards for peak metrics, linearity, precision, accuracy, resolution, and recovery of amoxicillin from a degraded pill matrix. Each batch of unknowns was analyzed only after five replicate injections of the reference standard (amoxicillin trihydrate from TCI, catalog number A2099, lot #Y5FND-HE, based on certificate of analysis 87.98% pure as anhydrous amoxicillin) produced less than a 2% relative standard deviation for reproducibility. The column used was a Symmetry C18 5 μm, 4.6 × 100 mm column. Eighteen microliters of the sample was injected via an auto-injector at a flow rate of 1.00 mL/min. The gradient is shown in Table 1. Amoxicillin was eluted at 3.3–3.4 minutes into this 12-minutes gradient (Figure 1). A calibration check sample (freshly prepared each day from amoxicillin trihydrate from TCI, lot #Y5FND-HE) was run after every five unknowns, and these five runs were rejected if the integrated intensity of the calibration check peak fell outside the 2% relative standard deviation established at the start of the run. The standard operating procedure for any pill that failed to meet USP standards (90–120% API content)9 was to prepare another sample from the original pill material, and if that also failed, to analyze two additional pills. None of the pills failed, so only one tablet from each package was analyzed.
Table 1.
Mobile-phase gradient for high-performance liquid chromatography analysis of amoxicillin samples
| Time (minutes) | Methanol (%) | 20 mM, Phosphate buffer pH = 4.4 ± 0.1 (%) | Change |
|---|---|---|---|
| 0.0 | 5.0 | 95.0 | Hold |
| 0.50 | 5.0 | 95.0 | Hold |
| 5.00 | 30.0 | 70.0 | Linear |
| 7.00 | 90.0 | 10.0 | Linear |
| 8.00 | 90.0 | 10.0 | Hold |
| 8.50 | 25.0 | 75.0 | Linear |
| 10.00 | 10.0 | 90.0 | Linear |
| 11.00 | 5.0 | 95.0 | Linear |
| 12.00 | 5.0 | 95.0 | Hold |
Figure 1.
Sample high-performance liquid chromatography chromatogram for amoxicillin.
The samples were rated pass or fail according to whether single-tablet HPLC analysis met USP requirements for active ingredient content of 90–120% of the stated content on the label.10
RESULTS
The total number of amoxicillin samples bought was 42. Shoppers attempted to buy amoxicillin from a total of 56 shops, evenly split between pharmacies and drug stores. Twenty-two pharmacies and 23 drug stores had amoxicillin in stock. At two pharmacies, the sellers requested a prescription and then refused to sell because the covert shoppers did not present one. At one pharmacy, the pharmacist authorized to sell this drug was out, so there was no sale. Table 2 shows a summary of the number of samples collected and their characteristics. Observations about the selling and buying process and the results of the packaging analyses are also shown.
Table 2.
Sample characteristics and purchasing process
| Set | Elements | Available | Total (n) | Percent (%) |
|---|---|---|---|---|
| Visited shops | Amoxicillin in stock | 45 | 56 | 80.4 |
| Amoxicillin out of stock | 11 | 56 | 19.6 | |
| Type of shop | Drug store | 23 | 45 | 51.1 |
| Pharmacy | 22 | 45 | 48.9 | |
| Dosage | 250 mg | 35 | 42 | 83.3 |
| 500 mg | 7 | 42 | 16.7 | |
| Number of capsules per sample | 15 capsules | 2 (500 mg) | 42 | 4.8 |
| 21 capsules | 5 (500 mg) | 42 | 11.9 | |
| 30 capsules | 35 (250 mg) | 42 | 83.3 | |
| Packaging/insert | Capsules repackaged in plastic bag | 22 | 42 | 52.4 |
| Blister pack repacked in plastic bag | 15 | 42 | 35.7 | |
| Blister pack in original box | 5 | 42 | 11.9 | |
| Prescription request | Requested/no sell | 2 | 45 | 4.4 |
| No request | 43 | 45 | 95.6 | |
| Pharmacies that requested | 2 | 22 | 9.1 | |
| Drug stores that requested | 0 | 23 | 0.0 | |
| PAD analysis | Pass | 42 | 42 | 100.0 |
| Fail | 0 | 42 | 0.0 | |
| HPLC analysis | Pass (90–120% API content) | 42 | 42 | 100.0 |
| Fail | 0 | 42 | 0.0 | |
| Expiry date | Available | 14 | 42 | 33.3 |
| Missing | 28 | 42 | 66.7 | |
| Batch number | With batch | 7 | 42 | 16.7 |
| Without batch | 35 | 42 | 83.3 | |
| Manufacturer | Name | 20 | 42 | 47.6 |
| No names | 22 | 42 | 52.4 | |
| Cost | Range (MK2400–MK2500) | 5 (500 mg) | – | – |
| Mean cost MK2440 | 5 (500 mg) | – | – | |
| Range (MK700–MK1500) | 37 (250 mg) | – | – | |
| Mean cost MK919 | 37 (250 mg) | – | – |
API = active pharmaceutical ingredient; HPLC = high-performance liquid chromatography; MK = Malawi Kwacha; PAD = paper analytical device.
All of the samples failed to meet at least one of the requirements for proper packaging and labeling. Nearly all (37/42) of the samples were repackaged, relabeled, and had no package inserts.
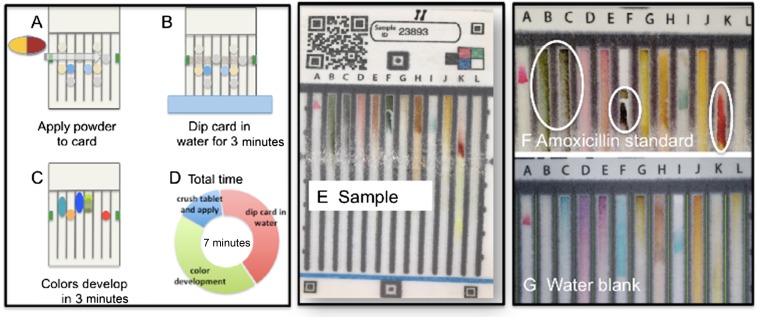
The samples were screened using PADs to determine if the product contained amoxicillin. Figure 2 shows a schematic for how the PAD is used to screen a sample, an image of a typical amoxicillin capsule run on a PAD in Malawi, and reference images of the “bar codes” for a known good amoxicillin dosage form and a water blank. The characteristic lane colors expected from amoxicillin include green in Lanes B and C, dark green in Lane F, and red in Lane K. All of the samples were read as containing amoxicillin by both a new user and an expert user. There were no samples that were interpreted as suspicious.
Figure 2.
Paper analytical device (PAD) operation and results. (A) Powder from capsule is applied to paper. (B) PAD is activated by dipping in water. (C) Colors develop. (D) Total time required is 7 minutes. (E) Image of PAD sample run in Malawi. (F) PAD standard image or “bar code” for good amoxicillin dosage form; critical lanes are B and C (green), F (dark green), and K (bright red). (G) PAD standard image for water blank. This figure appears in color at www.ajtmh.org.
To verify the presence of amoxicillin found by the PAD and quantify the amount of the API, an HPLC assay was performed for each sample at the University of Notre Dame. A typical chromatogram for one of the samples of amoxicillin is shown in Figure 1. Using the peak area on the chromatogram, the concentration of the API was determined by comparing to the concentration and peak area of a USP primary standard. All samples passed within 90–120% of the claimed API content as required by the USP guidelines for amoxicillin capsules.
One sample of amoxicillin was packaged in red/black capsules, which are typically used to contain ampicillin, shown in Figure 3. It was sold as amoxicillin and when analyzed by PAD, was found to be amoxicillin. The HPLC assay confirmed that amoxicillin was present in the correct dosage with no traces of ampicillin.
Figure 3.
Repackaging of antibiotics was common. Almost all samples of amoxicillin were dispensed either as loose capsules in a plastic bag or as blister-packed capsules with no box or insert. The rightmost bag shows a product that was sold as amoxicillin but is packaged in red and black capsules that are normally used for ampicillin. This product was confirmed to be amoxicillin, so it is not a falsified product. This figure appears in color at www.ajtmh.org.
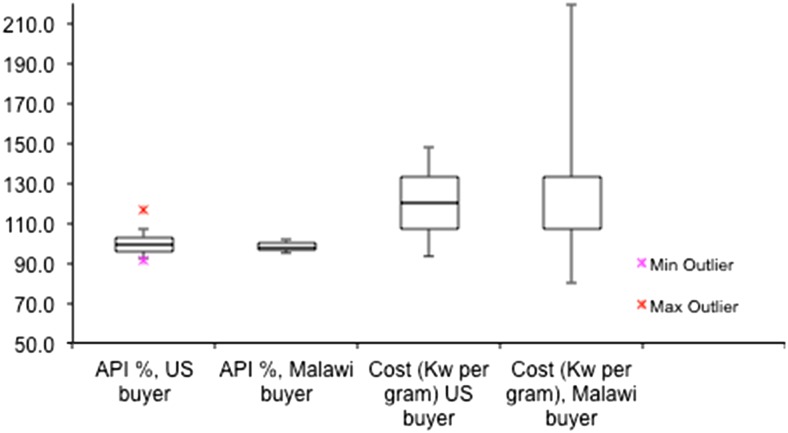
Another area of interest in this study was to look at trends between how drug sellers treated the two shoppers, one a native of Malawi and the other a visiting Caucasian student from the University of Notre Dame in the United States. Figure 4 shows the box-and-whisker plot for the API content of the products sold to the two shoppers and the cost per gram of the amoxicillin. There was no significant difference for the quality of the amoxicillin or the average cost of the products (significance determined using Student’s t test at 95% confidence level).
Figure 4.
Amoxicillin purity (90–120% active pharmaceutical ingredient [API] content meets United States Pharmacopeia requirement for purity) and cost (Malawi Kwacha per gram). U.S. buyer (Caucasian and therefore easily identifiable as non-Malawian customer) and Malawi buyer received the same drug quality at the same price from 42 vendors in the Blantyre metro region. Box shows middle two quartiles, whiskers show range of points within (1.5 × interquartile range), and outliers are shown as crosses. This figure appears in color at www.ajtmh.org.
DISCUSSION
The PADs reliably identified amoxicillin samples, as all samples with the PAD “bar code” characteristic of amoxicillin were confirmed by the HPLC analyses. The results are consistent with earlier studies6,10,11 that PADs can be used for field screening of amoxicillin samples. There were no false negatives in which a good-quality product was identified as suspicious. The sample pool did not include any falsified amoxicillin, so the rate of false positives (cases in which a falsified product is identified as good) could not be determined. As indicated by the ability of a new user to generate useful PAD images and the good agreement between independent evaluations of the PAD images by a new user in Malawi and an experienced user in the United States, it is easy to learn to use and practical for field screening in Malawi.
The results of the HPLC analysis show that all of the 42 samples met the USP requirements for API content. A study that included 19 samples of amoxicillin collected from four districts in Malawi during December 2014 through May 2015 found that this product was also of good quality.12 It is most unlikely that 30% of amoxicillin capsules available in private pharmacies and drug stores of Blantyre urban areas could be substandard, contrary to figures widely reported in the literature about antibiotics.13
The PADs have been shown to be a useful tool for medicine field screening in Malawi and other countries with similar regulatory status as they can be easily carried across the country and they are stable in Malawian field conditions. They could be used to assist in post-market surveillance programs. Paper analytical devices can also be easily scaled up to the clinic and potentially to household-level analysis of sample quality as the only supplies needed are the PAD cards and ordinary water from the tap or borehole. This can expand medicine quality testing services, which is currently centrally controlled and could lead to increased levels of spontaneous case reporting and prevent harm from consumption of poor-quality products. A more decentralized approach has been taken by many testing programs including HIV and is proving effective.14 The PAD system being developed can also be easily integrated into the pharmacovigilance system so that health-care providers and pharmacists can bring suspect drugs more rapidly to the attention of the PMPB.
The study exposed irregularities in dispensing practices in the private medicine markets in Malawi. The high proportion of medicines being sold without prescription could lead to inappropriate use of antibiotics, as reported by Nga et al.,15 in a community where pharmacies and drug stores readily dispense antibiotics for self-limiting infections without a prescription.In Malawi and many other countries, regulations state that the sale of antibiotics is done only on production of medical prescription. Antibiotics are technically known as prescription-only medicines (POM)13,16 as opposed to over-the-counter (OTC) medicines that are sold without prescription. This requirement arises from the fact that patients or customers may not be able to understand the difference between “POM” and “OTC,” and they may treat them inappropriately. Although sometimes, patients may use the POM correctly, getting doctor’s prescription and complying with the indicated treatment regimen and dose completion, the POMs can be misused through self-medication or medicine sharing with family and friends. Also, improper storage under adverse conditions or long periods that may exceed expiry dates may make them ineffective or even toxic, as in the case of tetracycline.14 However, sale of antibiotics without medical prescription has been reported widely in most medicine selling points in many countries, including Nigeria,17 the Phillipines,18 Saudi Arabia,19 Spain,20 Syria,21 Tanzania,22 Vietnam,15 and many others.13 Our results show that there is limited adherence to the prescription requirement in Malawi. In our study, only two sellers refused to provide the POM product without a valid prescription. Even pharmacies that were managed by pharmacists sold amoxicillin without prescription, and these POM antibiotics were readily available in regular drug stores that are not designated to stock antibiotics. It was also noted that except for one individual, the sellers did not give the buyer any instructions or precautions for how to take the medicine.
A separate problem is the widespread repackaging of amoxicillin. The practice of dispensing amoxicillin capsules in a paper or plastic bag renders the API more vulnerable to degradation, as the manufacturer’s original packaging is intended to protect the sensitive beta-lactam from atmospheric moisture. The drug sellers and pharmacists must write information by hand on the bags, and most did not include the name of the shop where the drug was purchased, the expiration date, brand, lot number, instructions for taking the product, or warnings about storage or adverse effects. Sale of loose capsules or pills cut from blister packs also makes it difficult for regulatory agencies to track problem products because these products often lack the manufacturer’s name, batch number, or expiration date, and may use nonstandard capsule color coding or pill markings. The lack of information about the identity, source, and provenance of the pills makes tracing difficult in case there is a claim for counterfeiting or an adverse medical reaction.
Repackaging violates WHO guidelines on good manufacturing practices for pharmaceutical products, which must be followed to prevent mix-up or contamination of the drugs during packaging.8 For example, there is a risk of mix-up already reported in this study for the amoxicillin samples that were packaged in capsules normally used for ampicillin. This risk is exacerbated by the observation that some pharmacies and drug stores are managed by nonmedical personnel. In addition, although some repackaged samples had expiry dates, the dates were those of the original packs, contrary to good manufacturing practices requirements of an independent stability study for any repackaged product. Furthermore, the lack of package insert deprives patients of information about physician and/or pharmacist instructions for how to take the product, adverse drug reactions, interactions and storage, and may contribute to poor adherence.21
With growing global concern about antibiotic resistance and antimicrobial stewardship, it is imperative to promote the rational use of these medicines.23 Antibiotic resistance in Malawi has also been reported in Malawi. Musicha et al.,24 conducted a sentinel surveillance of bacteremia since 1998 to 2016 and reported long-term trends in bloodstream infection and antimicrobial resistance from the surveillance on blood cultures from adult and pediatric patients with fever or suspicion of sepsis admitted to Queen Elizabeth Central Hospital, Blantyre, Malawi. The results showed that 51.1% of bacterial isolates were resistant to the Malawian first-line antibiotics amoxicillin or penicillin, chloramphenicol, and co-trimoxazole. In addition, 68.3% of Gram-negative and 6.6% of Gram-positive pathogens were also resistant.24
As health seeking behavior of many people worldwide has changed toward acceptance of pharmaceuticals, public health-care systems need to ensure that patients do not contribute to development of antimicrobial resistance through self-medication with antibiotics such as amoxicillin.13 In this study, the symptoms and complaints of the covert shoppers should not have resulted in sale of an antibiotic, yet almost all of the shops sold this product without prescription. According to the Malawi Standard Treatment Guidelines,25 the recommended treatment is pain killers. Over-the-counter drugs, including antitussive agents, antihistamines, nasal decongestants, cough suppressants, and expectorants, are sometimes prescribed in syrup or tablet forms. However, their use is surrounded with controversy with respect to efficacy. Antibiotics may sometimes be added if one presents with coughing and signs of upper respiratory tract infection, but the patient should be provided with a prescription for the antibiotic.26
CONCLUSION
Paper analytical devices are a promising tool that can be used for the rapid screening of common medicines such as amoxicillin. To determine whether this would be a practical system for monitoring the quality of medicines in Malawi, the PAD needs to be expanded to other samples available in Malawi and pilot implementation scaled up to cover more regions, including rural drug shops and clinics. It would be helpful to test the PAD device with samples that include both good-quality and bad-quality formulations, and such studies are under way in neighboring countries.
Our results in this study show that amoxicillin was readily sold to patients in Blantyre urban district in cases where they are probably not needed. Overuse and misuse of antibiotics are significant factors contributing to antibiotic resistance, which has become a big problem and focus of attention. Therefore, in addition to designing health-care strategies to reduce antibiotic misuse by focusing on physicians’ prescription practices, more attention should be put on how patients, drug sellers, and pharmacists circumvent the prescription-only medication regulations for antibiotics.15
Acknowledgments:
This publication was made possible, in part, with support from the Indiana Clinical and Translational Sciences Institute funded in part by Grant Number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. S. L. acknowledges support from the Global Development Lab program of USAID through grant AID-OAA-A-14-00073.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the United States National Institutes of Health.
REFERENCES
- 1.World Health Organization , 2017. Definitions of Substandard and Falsified (SF) Medical Products Available at: http://www.who.int/medicines/regulation/ssffc/defintions/en/. Accessed January 22, 2018.
- 2.Trapsida JM, Desta AT, Kasilo O, 2012. Preventing and Controlling Substandard and Counterfeit Medical Products in the Who African Region. World Health Organization, Regional Office for Africa. Brazzaville, Republic of Congo: African Health Monitor.
- 3.Almuzaini T, Choonara I, Sammons H, 2013. Substandard and counterfeit medicines: a systematic review of the literature. BMJ Open 3: e002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osei-Safo D, Harrison JJEK, Addae-Mensah I, 2010. Validation and application of quality assurance methods developed for artemisinin-based anti-malarial drugs to assess the quality of a selection of such drugs distributed in Accra, Ghana. Afr J Pharm Sci Pharm 1: 1–25. [Google Scholar]
- 5.Derda R, et al. 2015. Enabling the development and deployment of next generation point-of-care diagnostics. PLoS Neg Trop Dis 9: e0003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver A, Reiser H, Barstis T, Benvenuti M, Ghosh D, Hunkler M, Joy B, Koenig L, Raddell K, Lieberman M, 2013. Paper analytical devices for fast field screening of beta lactam antibiotics and antituberculosis pharmaceuticals. Anal Chem 85: 6453–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton PN, et al. 2009. Guidelines for field surveys of the quality of medicines: a proposal. PLoS Med 6: e1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Expert Committee on Specifications for Pharmaceutical Preparations , 2002. WHO Expert Committee on Specifications for Pharmaceutical Preparations: Thirty-Sixth Report. WHO Technical Report Series 902. Geneva, Switzerland: WHO. [PubMed]
- 9.USP , 2018. Amoxicillin Capsules. Pharmacopeial Forum 30: 1583. [Google Scholar]
- 10.Weaver A, Lieberman M, 2015. Paper test cards for presumptive testing of very low quality antimalarial medications. Am J Trop Med Hyg 92 (Suppl): 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chikowe I, Lieberman M, 2016. Presented: Evaluation of a New Tool for Assessing Pharmaceutical Quality at College of Medicine (CoM). 20th Annual Research Dissemination Conference. September 2016, Blantyre, Malawi. [Google Scholar]
- 12.Khuluza F, Kigera S, Heidi L, 2017. Low prevalence of substandard and falsified antimalarial and antibiotic medicines in public and faith-based health facilities of southern Malawi. Am J Trop Med Hyg 96: 1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelesidis T, Falagas ME, 2015. Substandard/counterfeit antimicrobial drugs. Clin Microbiol Rev 28: 443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low C, Pop-Eleches C, Rono W, Plous E, Kirk A, Ndege S, Goldstein M, Thirumurthy H, 2013. The effects of home-based HIV counseling and testing on HIV/AIDS stigma among individuals and community leaders in western Kenya: evidence from a cluster-randomized trial. AIDS Care 25 (Suppl 1): S97–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nga DTT, et al. 2014. Antibiotic sales in rural and urban pharmacies in northern Vietnam: an observational study. BMC Pharmacol Toxicol 15: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster EK, Bandawe CR, 2014. How much do patients in Blantyre, Malawi know about antibiotics and other prescription only medicines? Malawi Med J 26: 12–15. [PMC free article] [PubMed] [Google Scholar]
- 17.Akinyandenu O, Akinyandenu A, 2014. Irrational use and non-prescription sale of antibiotics in Nigeria: a need for change. J Sci Innov Res 3: 251–257. [Google Scholar]
- 18.Crisostomo S, 2013. FDA: Report Drugstores Selling Antibiotics without Prescription. Taguig, Philippines: The Philippine Star. [Google Scholar]
- 19.Bin Abdulhak AA, et al. 2011. Non prescribed sale of antibiotics in Riyadh, Saudi Arabia: a cross sectional study. BMC Public Health 11: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llor C, Cots JM, 2009. The sale of antibiotics without prescription in pharmacies in Catalonia, Spain. Clin Infect Dis 48: 1345–1349. [DOI] [PubMed] [Google Scholar]
- 21.Al-Faham Z, Habboub G, Takriti F, 2011. The sale of antibiotics without prescription in pharmacies in Damascus, Syria. J Infect Dev Ctries 5: 396–399. [DOI] [PubMed] [Google Scholar]
- 22.Goodman CA, Kachur SP, Abdulla S, Bloland P, Mills A, 2007. Drug shop regulation and malaria treatment in Tanzania—why do shops break the rules, and does it matter? Health Policy Plan 22: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima SIVC, Diniz RS, Egito EST, Azevedo PRM, Oliveira AG, Araujo IB, 2015. Rationality of antimicrobial prescriptions in community pharmacy users. PLoS One 10: e0141615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musicha P, et al. 2017. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. Lancet Infect Dis 17: 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Health , 2015. Malawi Standard Treatment Guidelines, 5th edition. Lilongwe, Malawi: Government of Malawi. [Google Scholar]
- 26.Mossad SB, 2013. Upper Respiratory Tract Infections, Disease Management. Lyndhurst, Ohio: Center for Continuing Education, Cleveland Clinic. [Google Scholar]