Abstract.
Babesia is a tick-borne intraerythrocytic parasite that is clinically and diagnostically similar to malaria parasite, conferring risk of misdiagnosis in areas where both parasites are endemic. Data on Babesia in humans in Africa are lacking, despite evidence that it is present in regional animal populations. Samples that were collected in November 2014 to July 2015 in Kilosa district, Tanzania, were evaluated for evidence of malaria and Babesia infection. Clinical data and laboratory samples (i.e., hemoglobin, rapid diagnostic testing [RDT] for malaria, peripheral blood smear, and dried blood spots) from a routine survey were available for analysis. Dried blood spots were tested using an investigational enzyme linked immunosorbent assay (ELISA) against Babesia microti. A total of 1,030 children aged 1 month to < 5 years were evaluated; 186 (18.1%) were malaria RDT positive, 180 (96.8%) of whom had peripheral smears reviewed; 70/180 (38.9%) were smear positive for parasites. The median (inter quartile range) and range of B. microti ELISA signal to cutoff (S/C) ratio was 0.10 (0.06–0.15) and 0.01–1.65, respectively; the S/C ratios were significantly higher in subjects ≥ 1 year as compared with those < 1 year old (P < 0.001). There was also a statistically significant association between a positive RDT for malaria and the Babesia S/C (median 0.09 versus 0.13 in RDT negative versus RDT positive, respectively; P < 0.001). The highest S/C ratios were disproportionately clustered in a few hamlets. The findings suggest that Babesia may be present in Kilosa district, Tanzania. However, serological cross-reactivity and false positivity, notably between Babesia and Plasmodium spp., cannot be definitively excluded and have implications for testing in other settings.
INTRODUCTION
Babesia is a genus of tick-borne intraerythrocytic apicomplexan parasites that is responsible for a range of zoonotic diseases in animals worldwide, and includes several species that are known to infect humans. Babesia infection in immunocompetent human hosts is often subclinical or mild; by contrast, individuals with asplenia, at extremes of age, and/or who are immunocompromised are at risk of severe disease, which includes hemolytic anemia, cardiorespiratory-failure, renal failure, and even death.1 Although natural acquisition of Babesia is via tick bite (Ixodes or hard bodied ticks), Babesia is also transfusion transmissible, thus posing a risk to the blood supply given the ability to establish chronic asymptomatic infection that goes unnoticed at the time of donation.2,3
Babesia is endemic in the United States. Babesia microti, which has been responsible for most of the reported cases of human babesiosis, is widely distributed in the Northeastern and Upper Midwestern United States.1 By contrast, Babesia duncani, which has only rarely been implicated in cases of human infection, is thought to be endemic in California and the Pacific Northwest.4 Although most cases of human babesiosis have been reported in the United States, Babesia is the most ubiquitous genus of vertebrate parasites worldwide.5,6 It is plausible that lack of awareness, in part because of historically limited diagnostic tools, has impeded greater recognition of its role in human disease. Although still a nascent emerging infectious disease, new reports of human babesiosis have been described in South America,7 Europe,8–10 Asia,11–13 and Australia.14 Furthermore, the risk to the US blood supply has spurred development of highly sensitive and specific, automated screening assays that offer an opportunity for broadening epidemiological surveillance of Babesia.
There is a paucity of human surveillance data on Babesia in Africa, although its presence in ticks and its role as a significant veterinary pathogen is well established.15–20 Of particular interest, B. microti18 and B. microti–like parasites21 have been recovered from nonhuman primates in Africa. Babesia overlaps clinically and diagnostically with Plasmodium spp. likely contributing to underreporting and/or misdiagnosis, particularly in areas where both infections are endemic.12 Babesia is almost indistinguishable from Plasmodium on peripheral blood smear; the latter remains the most common means of diagnosis for both parasites, particularly in low resource settings where laboratory capacity is suboptimal. The limited experience with new assays in use for blood screening in the United States2,22 raises questions surrounding the assay’s performance in malaria-endemic settings.
Given a lack of human data on Babesia in Africa, particularly in areas that are malaria-endemic, we sought to conduct a pilot seroprevalence study in Kilosa district, Tanzania. Selection of Kilosa district was informed by the rural setting in which there is close contact between the residents and livestock, placing them at potential risk of tick-borne illness. Importantly, Kilosa district has a high prevalence of malaria and has regional proximity to areas where B. microti and B. microti–like organisms have been reported.18,21
MATERIALS AND METHODS
Setting and population.
Mortality reduction after oral azithromycin (MORDOR) is a multinational, cluster-randomized clinical trial to evaluate the impact of mass distribution of azithromycin on mortality in children aged less than 5 years. Our study represents those participants in the Tanzanian arm of the MORDOR study, representing 32 randomly selected communities in Kilosa District, Tanzania (sampled from a total of 646 communities). A complete census was conducted of each community, after which 40 children (aged between 1 and 59 months) who had a guardian capable of providing consent were randomly selected to participate in the survey. In communities with < 40 eligible children, all of the children were invited to participate. Samples for this study were collected between November 2014 and July 2015 as part of the initial assessment for the MORDOR clinical trial. Given the random sampling of the communities, those children who participated were not necessarily febrile or ill at the time of assessment.
Data collection.
A trained field team collected data including whether the subjects had current symptoms of malaria and any recent or ongoing treatment at the time of evaluation.
Laboratory procedures.
A finger-stick test was performed on each of the participants; testing included a point of care hemoglobin evaluation using a Hemocue instrument, rapid diagnostic testing (RDT) for malaria, preparation of thick and thin peripheral blood smears, and dried blood spots (DBS). The RDTs were performed as per the manufacturer’s instructions; a trained technologist reviewed Giemsa-stained peripheral blood smears on all RDT positive samples at the MORDOR study laboratory in Tanzania; a random sample of RDT-negative smears was also examined. The DBS were stored refrigerated with a desiccant, pending testing.
Laboratory testing.
Dried blood spots were shipped to Immunetics, Inc. (Boston, MA) for testing. Each blood spot was eluted with 300 μL of kit sample buffer overnight at 4°C. The samples were subsequently tested using a commercial B. microti ELISA.22 For the purposes of this study, the cutoff defined in the ELISA kit, which is intended for use on serum samples, was modified to equal the mean A450 of replicate-negative dried blood spot controls plus 3 standard deviations (SDs).
Outcomes.
The outcome of interest is the distribution of seroreactivity as measured by the ELISA signal to cutoff (S/C) ratio, calculated as the ELISA absorbance of the sample at 450 nm divided by the cutoff.
Statistical analyses.
Mean (SD), Median (inter quartile range [IQR]), and the range of the S/C signal are presented for the overall sample and stratified by factors of interest such as age and RDT positivity. To test for differences in the S/C signal between groups the Wilcoxon rank sum test was used. Three levels of seroreactivity were created using 95 and 99 percentiles of the S/C distribution as cutoffs. Contingency table analysis was used to examine factors related to high levels of seroreactivity; Fisher’s exact test was used to test for differences. The intraclass correlation coefficient (ICC) with 95% confidence intervals (CIs) was used to describe the level of clustering of high seroreactivity at the community level. Data were analyzed with SAS software (SAS, Raleigh, NC).
Ethical review.
Ethical approval for MORDOR was obtained from the Tanzanian National Institute for Medical Research and the Institutional Review Board of the Johns Hopkins School of Medicine. Written informed consent was obtained from guardians for all children who participated in the MORDOR study.
RESULTS
A total of 1,049 subjects participated in the study of whom 527 (51%) were male; 856 (83.1%) were aged ≥ 1 to 5 years.
Babesia ELISA.
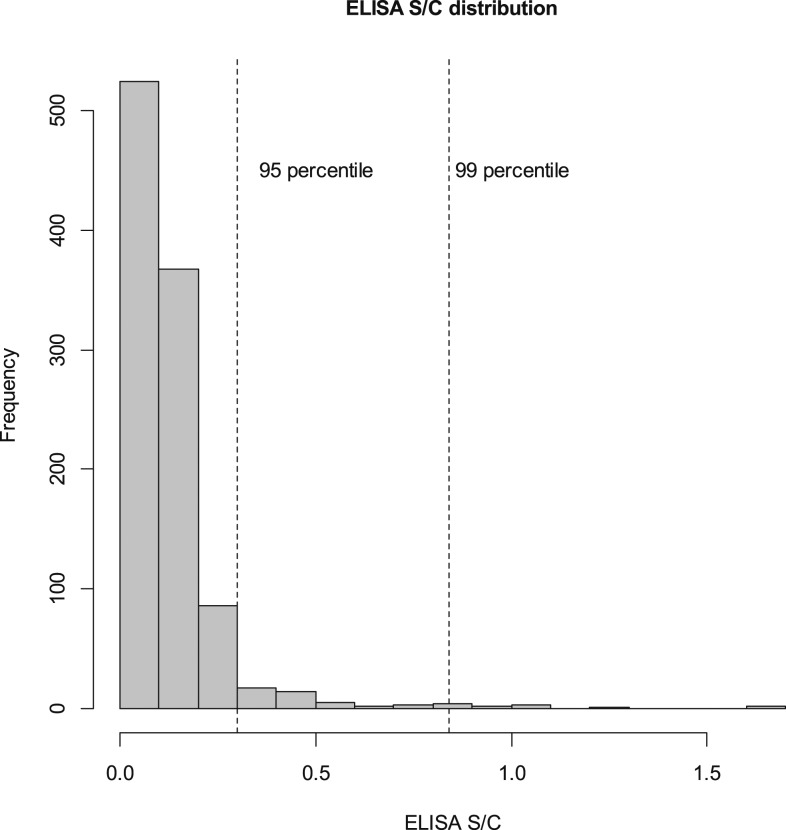
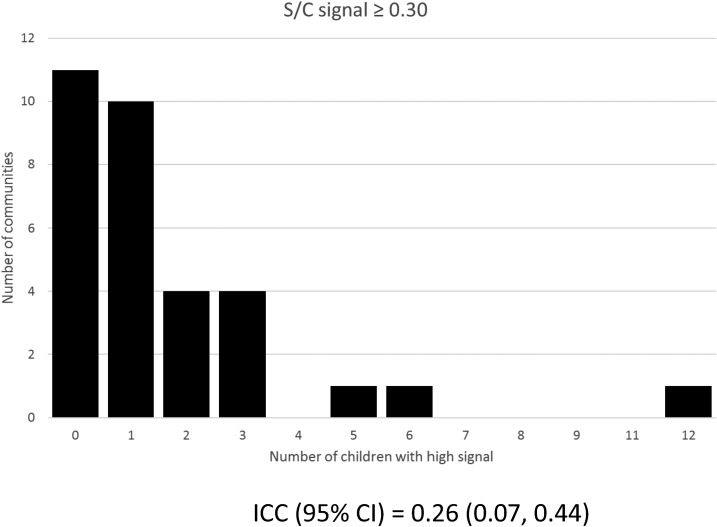
A total of 1,030 (98.2%) subjects had DBS available for Babesia evaluation (Table 1). The overall distribution of the ELISA S/C ratio is presented in Figure 1. The S/C ratios increased by age: the median (IQR) and range were 0.06 (0.04, 0.10), 0.01–1.65, in children less than a year, and 0.11 (0.07, 0.17), 0.02–0.89 in children 3 years or older with a statistically significant difference between those < 1 year and those ≥ 1 year old (P < 0.001) (Table 2); consistent with this finding, high S/C values were more likely to be present in older children (Table 3). There was also a statistically significant association between a positive RDT result for malaria and Babesia S/C (median [IQR] 0.13 [0.08, 0.18]) versus RDT negative (median [IQR] 0.09 [0.06, 0.14]; P < 0.001). Although those subjects who displayed very high S/C values (≥ 30) were rare (Figure 1), they were disproportionately clustered in a few hamlets ICC (95% CI) 0.26 (0.07, 0.44) (Figure 2).
Table 1.
Population overview
| Number of children | 1,030 |
|---|---|
| Age group (year) | |
| < 1 | 174 (16.9) |
| 1–3 | 426 (41.4) |
| 3–5 | 430 (41.7) |
| Gender | |
| Females | 503 (49.0) |
| Proportion (n/%) of children with malaria | |
| RDT+ | 186 (18.1) |
| Smear+/given RDT+ | 70/180 (38.9)* |
| Fever | 66 (6.4) |
| Hemoglobin levels (g/dL) | |
| < 10 (anemia) | 316 (30.7) |
| 10–11 | 321 (31.2) |
| > 11 | 391 (38.0) |
RDT = rapid diagnostic testing.
Missing results for six RDT positives.
Figure 1.
Distribution of signal to cutoff (S/C).
Table 2.
Factors associated with ELISA S/C
| N | ELISA S/C (mean (SD), median (IQR), range | Test | |
|---|---|---|---|
| Age group (year) | Comparing < 1 year vs. 1 year or older Wilcoxon rank-sum test P value < 0.001 |
||
| < 1 | 174 | 0.10 (0.17), 0.06 (0.04, 0.10), 0.01–1.65 | |
| 1–3 | 426 | 0.14 (0.16), 0.10 (0.07, 0.15), 0.01–1.60 | |
| 3–5 | 430 | 0.14 (0.10), 0.11 (0.07, 0.17), 0.02–0.89 | |
| Fever | Wilcoxon rank-sum test P value 0.47 | ||
| Yes | 66 | 0.11 (0.08), 0.09 (0.05, 0.14), 0.02–0.44 | |
| No | 960 | 0.13 (0.15), 0.10 (0.06, 0.15), 0.01–1.65 | |
| Hemoglobin (g/dL) | Comparing anemia vs. no anemia | ||
| < 10 (anemia) | 316 | 0.14 (0.16), 0.10 (0.05, 0.16), 0.01–1.60 | Wilcoxon rank-sum test P value 0.85 |
| 10–11 | 321 | 0.12 (0.14), 0.09 (0.06, 0.14), 0.01–1.65 | |
| > 11 | 391 | 0.13 (0.13), 0.10 (0.06, 0.15), 0.02–1.28 | |
| RDT status | Wilcoxon rank-sum test P value < 0.001 | ||
| Negative | 840 | 0.12 (0.14), 0.09 (0.06, 0.14), 0.01–1.65 | |
| Positive | 186 | 0.16 (0.15), 0.13 (0.08, 0.18), 0.02–1.01 | |
| Malaria smear*/RDT+ | Wilcoxon rank-sum test P value 0.62 | ||
| Positive | 70 | 0.14 (0.11), 0.13 (0.09, 0.17), 0.02–0.82 | |
| Negative | 110 | 0.17 (0.17), 0.13 (0.07, 0.18), 0.02–1.01 | |
| Overall | 1,030 | 0.13 (0.14), 0.10 (0.06, 0.15), 0.01–1.65 | – |
IQR = inter quartile range; RDT = rapid diagnostic testing; S/C = signal to cutoff; SD = standard deviation. Bold text highlights statistical significance.
Malaria smear: 194 slides were read, 180 RDT positives and 14 RDT negatives: 70/180 (38.9%) of the RDT positives were positive for malaria, and 4/14 (28.6) of the RDT negative were positive for malaria. Results presented in Table 2 are for RDT-positive children.
Table 3.
Factors associated with high signal-to-cutoff levels
| Characteristics | SC < 0.30 n (%) | SC 0.30–0.83 n (%) | SC ≥ 0.84 n (%) | P value Fisher’s exact |
|---|---|---|---|---|
| Age group (year) | 0.027 | |||
| < 1 | 168 (96.6) | 3 (1.7) | 3 (1.7) | |
| 1–3 | 404 (94.8) | 15 (3.5) | 7 (1.6) | |
| 3–5 | 405 (94.2) | 24 (5.6) | 1 (0.2) | |
| Fever | 0.88 | |||
| Yes | 63 (95.5) | 3 (4.6) | 0 (0.0) | |
| No | 910 (94.8) | 39 (4.1) | 11 (1.1) | |
| Hemoglobin (g/dL) | 0.38 | |||
| < 10 (anemia) | 294 (93.0) | 18 (5.7) | 4 (1.3) | |
| 10–11 | 308 (96.0) | 9 (2.8) | 4 (1.2) | |
| > 11 | 373 (95.4) | 15 (3.8) | 3 (0.8) | |
| RDT status | 0.004 | |||
| Negative | 805 (95.8) | 26 (3.1) | 9 (1.1) | |
| Positive | 168 (90.3) | 16 (8.6) | 2 (1.1) | |
| Malaria smear/RDT+ | 0.38 | |||
| Positive | 66 (94.3) | 4 (5.7) | 0 (0.0) | |
| Negative | 97 (88.2) | 11 (10.0) | 2 (1.8) | |
| Malaria smear/RDT− | – | |||
| Positive | 4 (100.0) | 0 (0.00) | 0 (0.00) | |
| Negative | 10 (100.0) | 0 (0.00) | 0 (0.00) | |
| Overall | 977 (94.9) | 42 (4.1) | 11 (1.0) | – |
RDT = rapid diagnostic testing.
Figure 2.
Distribution of subjects with a high S/C ratio by community.
Plasmodium assessment.
A total of 186 (18.1%) subjects were malaria RDT positive at the time of evaluation; 194 peripheral blood smears were reviewed, representing 180 (97%) RDT-positive cases and a random sample of RDT-negative results (N = 14). A total of 70/180 (38.9%) RDT-positive cases were smear positive. Of the 14 RDT-negative cases, four (28.6%) were smear positive for malaria. A total of 316 (30.7%) subjects were anemic (hemoglobin < 10 g/dL), and 66 (6.4%) were febrile at time of evaluation.
DISCUSSION
There is a paucity of Babesia surveillance data in humans in Africa, despite evidence that Babesia is present in the region.16–18 A newly developed B. microti ELISA was used to conduct pilot surveillance in Kilosa district where the presence of Babesia is plausible, and high prevalence of malaria poses a risk of misdiagnosis and underreporting. The study findings are mixed. The finding of high S/C ratios in a small percentage of subjects suggests that B. microti may well be present. Furthermore, the disproportionate clustering of subjects with high S/C ratios in a relatively small number of hamlets is in keeping with a localized exposure, whereas an increase in seroreactivity with age is reflective of a progressive increase in exposure. Follow-up and repeat sampling are needed to confirm the findings.
The results highlight the challenges of Interpretation when conducting pilot surveillance. There are several possible explanations for the findings. Ecological clustering, as reflected by the observation of the highest S/C values in a relatively small group of hamlets, is in keeping with tick-borne illness and could suggest local tick exposure. On the other hand, although malaria is generalized across the hamlets, including those hamlets where high S/C ratios are not observed, an association between B. microti seroreactivity and a positive malaria RDT result was found. Cross-reactivity with antibodies to Plasmodium in the B. microti ELISA may be one possible explanation. The B. microti ELISA that was used in the study contains four immunodominant peptide antigens that were shown to be highly specific to B. microti and did not exhibit reactivity with sera from Plasmodium falciparum or Plasmodium vivax infections in validation studies,22 although the numbers tested were relatively small. However, an earlier study of B. microti peptides derived from the same gene family as those used in the ELISA found varying levels of cross-reactivity depending on the peptide sequence.23 In addition, in the setting of malaria (P. falciparum) infection, (i.e., as reflected by RDT positivity) there is stimulation of a low avidity, polyclonal antibody response against the individual’s past microbial exposures.24,25 If there were to be past exposure to B. microti, it is plausible that there would be an increase in antibody titers, accounting for seroreactivity even in the absence of active infection with B. microti. Another possibility is that of cross-reactivity with other pathogens that are encountered locally. This could include Babesia species other than B. microti such as Babesia bigemina and Babesia bovis that are regionally endemic,19,20 as well as Entopolypoides macaci, a parasite of baboons which is genetically highly homologous to B. microti.26 Babesia are also phylogenetically related to Theileria, which have been reported in the region.27,28 Babesia bigemina, B. bovis, and Theileria infect cattle and wild buffalo primarily and have little evidence of zoonotic potential; nonetheless, their effect on serologic testing for B. microti is unknown.
This study has several limitations. First, it was conceived as a pilot serosurvey to take advantage of an existing parent study. As such, the available samples (i.e., DBS) were quantitatively insufficient to conduct confirmatory and ancillary testing (e.g., indirect fluorescent antibody testing, molecular evaluation). As such, our findings are preliminary and lack comprehensive evaluation for Babesia. Similarly, treatment data for malaria were lacking, thus detracting from the interpretation of cases where a malaria RDT was positive yet blood smear was negative. Second, the B. microti ELISA had not been previously evaluated in this setting. It is uncertain to what extent performance is affected by a different population and microbiome, such that the contribution of false positivity and/or cross-reactivity with other local pathogens cannot be excluded. In a pilot study such as this, in the absence of true prevalence data obtained by other methods, the positive predictive value of the assay is unknown. The validation experiments for the ELISA did not include other Babesia species, such that potential cross-reactivities are likewise not known. Third, the assay in use was designed for serum or plasma samples, rather than DBS. The assay methods and procedures were adapted as a research endeavor specifically for this study and may not be optimal for the intended purpose. The ELISA has been authorized under investigational new drug for specific use on US blood donors; the latter typically selects for a low-risk, healthy subset of the population. It is uncertain as to how this impacts evaluation in a population of young children in rural Tanzania. Given that this was an exploratory study, a provisional ELISA cutoff was applied with little objective clinical basis, highlighting the need for further study to categorize samples as true positive or true negative. Unfortunately, the absence of results from an accepted gold standard method such as PCR for Babesia renders interpretation of the test results a challenge that is not unique to this study. Well-characterized Babesia samples (i.e., from known parasitemic donors in the setting of symptomatic infection) are rarely available even in known endemic areas (e.g., Northeastern USA) let alone in an entirely novel study population as in our study. Without an objective way of ascertaining positivity, the ELISA cutoff can only be provisional. Finally, evidence of animal infection alone should not be construed as implying human risk, although discovery of enzootic B. microti in the region would be highly significant, as would evidence of anthropophilic tick vectors for the parasite. Such underscores the need for an entomological survey whereby the finding of competent tick vectors infected with B. microti would fill in a key missing element that would complement the study’s findings.
Thus, the data offer a compelling reason to perform a comprehensive evaluation of Babesia, and specifically, to revisit those hamlets that displayed the highest rates of seroreactivity. It will be particularly important to characterize the performance of the described tests and conduct an environmental assessment. Rather than a deficiency, cross-reactivity could be exploited diagnostically. For example, tests that are capable of targeting both parasites could prove useful. In the context of blood donor screening, both genera of parasites (Babesia and Plasmodium) are transfusion transmissible, such that a combined platform would be advantageous.3,29 Nonetheless, discrimination between Babesia and Plasmodium is important, given differences in therapy, risking treatment failure in the case of misdiagnosis.
In conclusion, our study offers serological evidence for B. microti infection and preliminary population-based estimates of seroprevalence in Kilosa, Tanzania. Broadly, the study underscores the need to further characterize the global epidemiology of Babesia, and likewise to better understand the potential for assay cross-reactivity in applications both within and outside the intended use, including blood donor screening in the United States.
Acknowledgments:
The authors thank the members of the MORDOR study team in Tanzania for their seminal contribution to logistical support and sample collection, without which the described study would not have been possible.
REFERENCES
- 1.Vannier E, Krause PJ, 2012. Human babesiosis. N Engl J Med 366: 2397–2407. [DOI] [PubMed] [Google Scholar]
- 2.Moritz ED, Winton CS, Tonnetti L, Townsend RL, Berardi VP, Hewins ME, Weeks KE, Dodd RY, Stramer SL, 2016. Screening for Babesia microti in the U.S. blood supply. N Engl J Med 375: 2236–2245. [DOI] [PubMed] [Google Scholar]
- 3.Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M, 2011. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 155: 509–519. [DOI] [PubMed] [Google Scholar]
- 4.Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford SR, 3rd, Herwaldt BL, 2006. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol 36: 779–789. [DOI] [PubMed] [Google Scholar]
- 5.Telford SRI, Gorenflot A, Brasseur P, Spielman A, 1993. Babesial infections in humans and wildlife. Kreier JP, ed. Parasitic Protozoa: Babesia and Plasmodia. San Diego, CA: Academic Press, 1–47. [Google Scholar]
- 6.Homer MJ, Aguilar-Delfin I, Telford SR, 3rd, Krause PJ, Persing DH, 2000. Babesiosis. Clin Microbiol Rev 13: 451–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrielli S, Totino V, Macchioni F, Zuniga F, Rojas P, Lara Y, Roselli M, Bartoloni A, Cancrini G, 2016. Human babesiosis, Bolivia, 2013. Emerg Infect Dis 22: 1445–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welc-Faleciak R, Pawelczyk A, Radkowski M, Pancewicz SA, Zajkowska J, Sinski E, 2015. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann Agric Environ Med 22: 51–54. [DOI] [PubMed] [Google Scholar]
- 9.Rigaud E, Jaulhac B, Garcia-Bonnet N, Hunfeld KP, Femenia F, Huet D, Goulvestre C, Vaillant V, Deffontaines G, Abadia-Benoist G, 2016. Seroprevalence of seven pathogens transmitted by the Ixodes ricinus tick in forestry workers in France. Clin Microbiol Infect 22: 735.e1–735.e9. [DOI] [PubMed] [Google Scholar]
- 10.Lempereur L, Shiels B, Heyman P, Moreau E, Saegerman C, Losson B, Malandrin L, 2015. A retrospective serological survey on human babesiosis in Belgium. Clin Microbiol Infect 21: 96.e1–96.e7. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Xia S, Huang JL, Tambo E, Zhuge HX, Zhou XN, 2014. Human babesiosis, an emerging tick-borne disease in the People’s Republic of China. Parasit Vectors 7: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Li SG, Wang JZ, Huang JL, Zhou HJ, Chen JH, Zhou XN, 2014. Emergence of human babesiosis along the border of China with Myanmar: detection by PCR and confirmation by sequencing. Emerg Microbes Infect 3: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong SH, Anu D, Jeong YI, Abmed D, Cho SH, Lee WJ, Lee SE, 2014. Molecular detection and seroprevalence of Babesia microti among stock farmers in Khutul city, Selenge Province, Mongolia. Korean J Parasitol 52: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paparini A, Senanayake SN, Ryan UM, Irwin PJ, 2014. Molecular confirmation of the first autochthonous case of human babesiosis in Australia using a novel primer set for the beta-tubulin gene. Exp Parasitol 141: 93–97. [DOI] [PubMed] [Google Scholar]
- 15.Ogo NI, et al. 2012. Molecular identification of tick-borne pathogens in Nigerian ticks. Vet Parasitol 187: 572–577. [DOI] [PubMed] [Google Scholar]
- 16.Lolli C, Marenzoni ML, Strona P, Lappo PG, Etiang P, Diverio S, 2016. Infections and risk factors for livestock with species of Anaplasma, Babesia and Brucella under semi-nomadic rearing in Karamoja region, Uganda. Trop Anim Health Prod 48: 603–611. [DOI] [PubMed] [Google Scholar]
- 17.Mtshali PS, Tsotetsi AM, Thekisoe MM, Mtshali MS, 2014. Nested PCR detection and phylogenetic analysis of Babesia bovis and Babesia bigemina in cattle from peri-urban localities in Gauteng province, South Africa. J Vet Med Sci 76: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maamun JM, Suleman MA, Akinyi M, Ozwara H, Kariuki T, Carlsson HE, 2011. Prevalence of Babesia microti in free-ranging baboons and African green monkeys. J Parasitol 97: 63–67. [DOI] [PubMed] [Google Scholar]
- 19.Swai ES, Karimuribo ED, French NP, Fitzpatrick JL, Bryant MJ, Kambarage DM, Ogden NH, 2007. Seroprevalence of Babesia bigemina in smallholder dairy cattle in Tanzania and associated risk factors. J S Afr Vet Assoc 78: 15–20. [DOI] [PubMed] [Google Scholar]
- 20.Swai ES, French NP, Karimuribo ED, Fitzpatrick JL, Bryant MJ, Brown PE, Ogden NH, 2005. Spatial and management factors associated with exposure of smallholder dairy cattle in Tanzania to tick-borne pathogens. Int J Parasitol 35: 1085–1096. [DOI] [PubMed] [Google Scholar]
- 21.Nakayima J, et al. 2014. Detection and characterization of zoonotic pathogens of free-ranging non-human primates from Zambia. Parasit Vectors 7: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin AE, et al. 2016. Serologic screening of United States blood donors for Babesia microti using an investigational enzyme immunoassay. Transfusion 56: 1866–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houghton RL, Homer MJ, Reynolds LD, Sleath PR, Lodes MJ, Berardi V, Leiby DA, Persing DH, 2002. Identification of Babesia microti-specific immunodominant epitopes and development of a peptide EIA for detection of antibodies in serum. Transfusion 42: 1488–1496. [DOI] [PubMed] [Google Scholar]
- 24.Fesel C, Goulart LF, Silva Neto A, Coelho A, Fontes CJ, Braga EM, Vaz NM, 2005. Increased polyclonal immunoglobulin reactivity toward human and bacterial proteins is associated with clinical protection in human Plasmodium infection. Malar J 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donati D, Zhang LP, Chene A, Chen Q, Flick K, Nystrom M, Wahlgren M, Bejarano MT, 2004. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun 72: 5412–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bronsdon MA, Homer MJ, Magera JM, Harrison C, Andrews RG, Bielitzki JT, Emerson CL, Persing DH, Fritsche TR, 1999. Detection of enzootic babesiosis in baboons (Papio cynocephalus) and phylogenetic evidence supporting synonymy of the genera Entopolypoides and Babesia. J Clin Microbiol 37: 1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomford JW, Conrad PA, Telford SR, 3rd, Mathiesen D, Bowman BH, Spielman A, Eberhard ML, Herwaldt BL, Quick RE, Persing DH, 1994. Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J Infect Dis 169: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin CL, et al. 1988. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect Immun 56: 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mungai M, Tegtmeier G, Chamberland M, Parise M, 2001. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med 344: 1973–1978. [DOI] [PubMed] [Google Scholar]