Abstract.
Staphylococcus aureus is a common cause of bloodstream infection and methicillin-resistant S. aureus (MRSA) is a growing threat worldwide. We evaluated the incidence rate of S. aureus bacteremia (SAB) and MRSA from population-based surveillance in all hospitals from two Thai provinces. Infections were classified as community-onset (CO) when blood cultures were obtained ≤ 2 days after hospital admission and as hospital-onset (HO) thereafter. The incidence rate of HO-SAB could only be calculated for 2009–2014 when hospitalization denominator data were available. Among 147,524 blood cultures, 919 SAB cases were identified. Community-onset S. aureus bacteremia incidence rate doubled from 4.4 (95% confidence interval [CI]: 3.3–5.8) in 2006 to 9.3 per 100,000 persons per year (95% CI: 7.6–11.2) in 2014. The highest CO-SAB incidence rate was among adults aged 50 years and older. Children less than 5 years old had the next highest incidence rate, with most cases occurring among neonates. During 2009–2014, there were 89 HO-SAB cases at a rate of 0.13 per 1,000 hospitalizations per year (95% CI: 0.10–0.16). Overall, MRSA prevalence among SAB cases was 10% (90/911) and constituted 7% (55/736) of CO-SAB and 20% (22/111) of HO-SAB without a clear temporal trend in incidence rate. In conclusion, CO-SAB incidence rate has increased, whereas MRSA incidence rate remained stable. The increasing CO-SAB incidence rate, especially the burden on older adults and neonates, underscores the importance of strong SAB surveillance to identify and respond to changes in bacteremia trends and antimicrobial resistance.
INTRODUCTION
Staphylococcus aureus infections are one of the most common bloodstream infections worldwide.1,2 Antimicrobial resistant strains, namely, methicillin-resistant S. aureus (MRSA), are associated with increased mortality, length of hospitalization, and health-care costs3–5; yet, the burden of disease is poorly understood in many countries.
A 2014 World Health Organization (WHO) report emphasized the critical need for greater surveillance to more rapidly detect, respond, and control public health threats and therefore enhance global health security. World Health Organization also highlighted major gaps in surveillance in Southeast Asia.6 The few available studies suggest that S. aureus is one of the top causes of bloodstream infections in Thailand and Southeast Asia.7–13 A 2012 review found that methicillin resistance among S. aureus in Southeast Asia varied from 0–39%.14 In two cross-sectional studies in tertiary hospitals in Bangkok and northeast Thailand from 2005 and 2006, MRSA was documented in > 20% of S. aureus isolates.10,11 Since then, significant changes in the epidemiology of S. aureus and methicillin resistance in Thailand and the region may have occurred. The 2011 Jaipur Declaration emphasized the importance of antimicrobial resistance and the need for immediate regional action,15 and infection control efforts in tertiary care centers in Thailand have been intensified.16–18 A better understanding of current trends in S. aureus and MRSA bacteremia is needed to monitor trends and guide prevention and control efforts in Thailand.
In 2005, the Thailand Ministry of Public Health, in collaboration with the U.S. Centers for Disease Control and Prevention (CDC), initiated population-based surveillance of bloodstream infections in two rural provinces in Thailand to create a sustainable system to monitor changes in pathogenesis and resistance patterns in rural border provinces where emerging epidemics could occur. This included implementation of automated blood culturing systems and sustained laboratory capacity building activities that were not as developed as in a metropolitan center such as Bangkok. We report incidence rates and trends in S. aureus bacteremia and methicillin resistance from 2006–2014.
MATERIALS AND METHODS
Surveillance population.
Sa Kaeo, located in eastern Thailand near the Cambodian border, had an estimated 2010 population of 543,700. Nakhon Phanom had a 2010 population of 577,300 and is located in northeast Thailand near the Laos border.19 Both provinces are primarily agrarian.20
Surveillance included all 20 hospitals in these provinces: eight from Sa Kaeo and 12 from Nakhon Phanom. There were two provincial hospitals, 16 district hospitals, and two military hospitals. A provincial hospital (225–327 beds) is a referral center and first-line hospital for the central district in the province, whereas district and military hospitals (10–140 beds) serve the local community. Most of the Thai population has at least one kind of health insurance with minimal out-of-pocket costs for services. The CDC Human Subjects Review Office reviewed this protocol and judged that this study constituted routine public health activities and therefore did not involve human subject research (Center for Global Health Determination and Approval number 2014-273).
Data collection.
Nurses who were regularly trained in phlebotomy to reduce contamination collected blood cultures from hospitalized patients at the clinician’s discretion. The samples were inoculated into an aerobic bottle: BacT/ALERT® FA (BioMérieux, Marcy l’Etoile, France) (target volume 10 mL) for patients aged ≥ 5 years and BacT/ALERT® PF (BioMérieux, Marcy l’Etoile, France) (target volume 4 mL) for children aged less than 5 years. If more sample was available, we also inoculated a BacT/ALERT® MB bottle (BioMérieux, Marcy l’Etoile, France) (target volume 3 mL) that enhanced growth for mycobacteria and fastidious organisms. Culture bottles were processed in provincial hospital laboratories. For district and military hospitals, the samples were transported at 15–30°C within 24 hours of collection. Nurses collected information from the medical record or the patient on admission date, age, and current antibiotic use.
Laboratory methods.
Bottles that signaled positive growth (alarm-positive) by automated blood culture (BacT/ALERT® 3D, BioMérieux, Marcy l’Etoile, France ) were subcultured and identified using standard methods.21 Antimicrobial resistance testing was performed according to Clinical and Laboratory Standards Institute guidelines,22 together with local considerations such as available antibiotics and observed resistance patterns. Consequently, testing for specific antibiotics was not uniform between the two provinces. For S. aureus isolates, susceptibility testing was performed via disk diffusion for cefoxitin, oxacillin, erythromycin, gentamicin, clindamycin, trimethoprim-sulfamethoxazole (cotrimoxazole), and fosfomycin. Before 2007, methicillin resistance was defined by oxacillin disk diffusion with a zone of inhibition < 10 mm.22 In 2007, cefoxitin disk diffusion with a zone of inhibition < 21 mm was introduced.23 Vancomycin minimum inhibitory concentration (MIC) and susceptibility (≤ 2 µg/mL) was determined by E-test (Oxoid [ThermoFisher Scientific, Waltham, MA] initially and then BioMérieux). Because of possible concerns over assay quality,24 confirmatory retesting was performed of all S. aureus isolates with vancomycin MIC > 1 µg/mL (E-test, BioMérieux).
Definitions.
Patients were considered to have a case of SAB if at least one culture bottle grew S. aureus alone or together with another likely pathogen. S. aureus–positive blood cultures that had one of the following pathogens were considered contaminants and excluded: Aerococcus species, Bacillus species excluding Bacillus anthracis or Bacillus cereus, coagulase-negative staphylococci, Corynebacterium species, other Staphylococcus species, and Streptococcus viridans group. If an individual had multiple S. aureus–positive blood cultures, the first positive culture was included and repeat isolates within 30 days were excluded.25 Current antibiotic use was defined by serum disc assay positivity,26 or if not available, per nurse review of the medical record or by patient self-report.
S. aureus infections were classified as hospital-onset (HO) or community-onset (CO), using the following definitions: HO cases were defined as SAB in patients with blood culture collected > 2 days after the admission date and CO cases occurred in patients with positive blood cultures collected ≤ 2 calendar days after admission.
Statistical analysis.
We calculated CO incidence rates from 2006–2014. For CO cases, Sa Kaeo and Nakhon Phanom provincial population projections for 2010–2014 were available from the 2010 National Economic and Social Development Board of Thailand (NESDB).19 For the period 2006–2009, the 2010 NESBD age distribution was applied to the 2006–2009 NESDB overall provincial population estimates27 as official intercensal estimates were not available.
To calculate HO incidence rate, we used the number of hospitalizations from 2009–2014 as the denominator, as found in hospital administrative databases (S. Naorat, B. Piralam, personal communication). In Sa Kaeo, 2009 hospitalization data were missing from four district hospitals. After careful examination of hospitalization trends, we used 2010 data to impute 2009 hospitalizations for these four hospitals. Together, these hospitals accounted for ≤ 15% of annual hospitalizations in our surveillance system for 2010–2014. Military hospitals in Sa Kaeo and Nakhon Phanom provinces were also excluded from HO incidence rate calculations, as military hospitalization data were not available for Nakhon Phanom and only available for 2 years in Sa Kaeo (2013–2014, < 1% of all hospitalizations in each year). Of note, there were no S. aureus HO cases identified at military hospitals.
Ninety-five percent confidence intervals (CIs) on incidence rate estimates were calculated based on a Poisson distribution using the exact method. Demographic and clinical characteristics of MRSA and methicillin-sensitive S. aureus (MSSA) bacteremia cases were compared via Pearson’s χ2, Fisher’s exact, or Wilcoxon rank-sum tests as appropriate. Individuals with missing data were excluded from the denominator and totals have been noted accordingly. Analyses were conducted using Stata version 12 (StataCorp LP, College Station, TX).
RESULTS
Surveillance population characteristics.
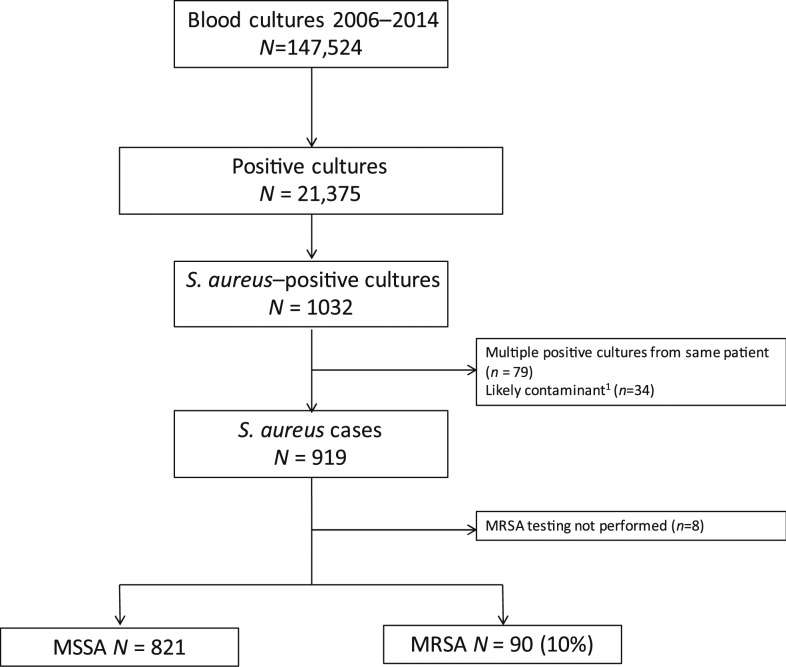
From 2006 through 2014, 147,524 blood cultures were performed in Sa Kaeo and Nakhon Phanom provinces (Figure 1), representing 141,399 individuals with cultures performed. The annual number of patients with blood cultures increased from 12,900 in 2006 to 17,892 in 2014, with greater increase in Sa Kaeo (90% increase, from 4,846 to 9,134) compared with Nakhon Phanom (8% increase, from 8,084 to 8,758). Hospitalizations ranged from 113,299 to 121,887 per year from 2009–2014.
Figure 1.
Identification of S. aureus and MRSA bacteremia cases from population-based bloodstream infection surveillance, Sa Kaeo and Nakhon Phanom provinces, Thailand, 2006–2014.1One or more of the following likely contaminants (in order of frequency) grew: coagulase-negative staphylococci (N = 27), Bacillus species excluding B. anthracis or B. cereus (N = 8), or S. viridans group (N = 4).
Staphylococcus aureus was isolated from 1,032 blood cultures from 2006–2014. After exclusion of cultures with potential contaminants (N = 34) and of repeat isolates (N = 79), we found 919 cases of SAB from 897 individuals, with 911 cases (99%) with available MRSA testing (Table 1).
Table 1.
Comparison of methicillin-susceptible (MSSA) and methicillin-resistant S. aureus (MRSA) bacteremia cases, Sa Kaeo and Nakhon Phanom provinces, Thailand 2006–2014
| Characteristic | MSSA* | MRSA* | Total* | P value† |
|---|---|---|---|---|
| Age, n (%) | ||||
| Neonate (0–28 days) | 57 (7.0) | 6 (6.7) | 63 (7.0) | 0.06 |
| 29 days–14 years | 90 (11) | 3 (3.3) | 93 (10) | |
| 15–49 years | 246 (30) | 22 (24) | 268 (29) | |
| 50–69 years | 275 (34) | 36 (40) | 311 (34) | |
| ≥ 70 years | 153 (19) | 23 (26) | 176 (19) | |
| Sa Kaeo Province, n (%) | 376 (46) | 60 (67) | 436 (48) | < 0.001 |
| Provincial Hospital, n (%) | 514 (63) | 71 (79) | 585 (64) | 0.01 |
| Community-onset, n (%) | 681/770 (88) | 55/77 (71) | 736/847 (87) | < 0.001 |
| Current antibiotic exposure, n (%) | 60/773 (7.8) | 25/85 (29) | 85/858 (9.9) | < 0.001 |
| Antibiotic resistance,‡ n (%) | ||||
| Erythromycin | 39/785 (5.0) | 73/87 (84) | 112/872 (13) | < 0.001 |
| Gentamicin | 0/505 (0) | 33/40 (83) | 33/545 (6.1) | < 0.001 |
| Clindamycin | 28/800 (3.5) | 66/88 (75) | 94/888 (11) | < 0.001 |
| Cotrimoxazole | 7/797 (0.9) | 54/83 (65) | 61/880 (6.9) | < 0.001 |
| Fosfomycin | 4/361 (1.1) | 21/65 (33) | 25/426 (5.9) | < 0.001 |
| Vancomycin | – | 0/35 | – | – |
| Resistance to at least two antibiotics other than methicillin | 24 (2.9) | 72 (80) | 96 (11) | < 0.001 |
MSSA denominator 821, MRSA denominator 90, and total 911 unless missing data, and then a different denominator noted.
χ2 or Fisher exact testing, as appropriate comparing MSSA vs. MRSA.
Antibiotic testing algorithm differed over time and between provinces. Disk diffusion method was used for all antibiotics except for vancomycin, for which E-test was used.
Staphylococcus aureus bacteremia incidence rate.
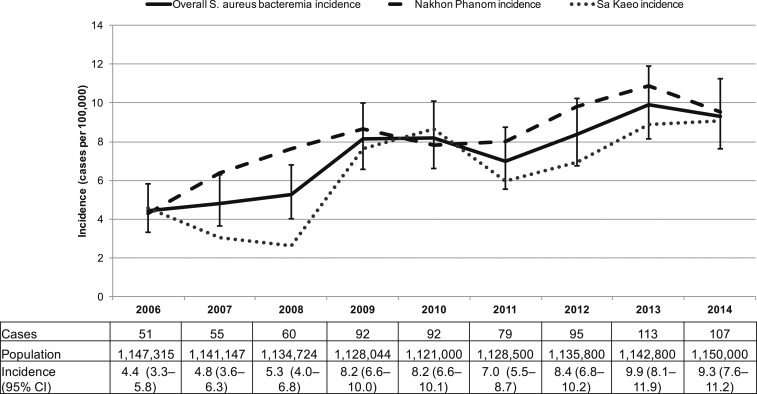
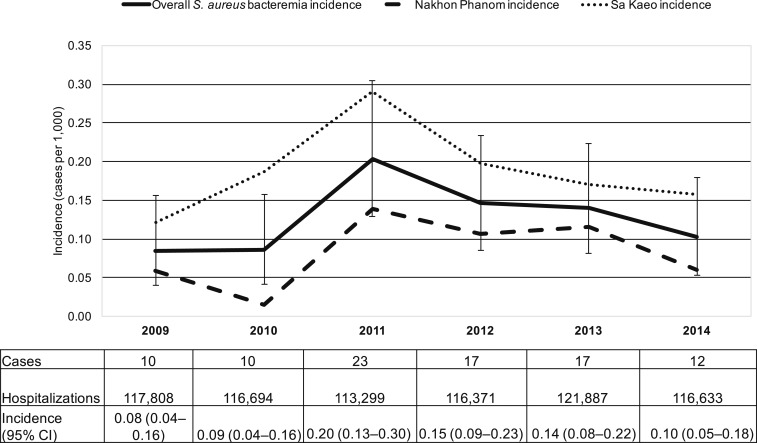
The CO-SAB incidence rate during 2006–2014 was 7.3 per 100,000 persons per year (95% CI: 6.8–7.8). The incidence rate increased from 4.4 (95% CI: 3.3–5.8) in 2006 to 9.3 per 100,000 persons per year (95% CI: 7.6–11.2) in 2014 (Figure 2). The incidence rate increased in both provinces (Table 2). The HO-SAB incidence rate from 2009–2014 (N = 89) was 0.13 per 1,000 hospitalizations per year (95% CI: 0.10–0.16), from 0.08 (95% CI: 0.04–0.16) in 2009 to 0.10 (95% CI: 0.05–0.18) in 2014 (Figure 3). Hospital-onset S. aureus bacteremia incidence rate was higher in provincial hospitals compared with district hospitals (Supplemental Table 1).
Figure 2.
Community-onset S. aureus bacteremia incidence rate,1 Sa Kaeo and Nakhon Phanom, Thailand, 2006–2014. 1Incidence rate in cases per 100,000 persons. Whiskers represent 95% confidence intervals (CIs).
Table 2.
Age- and province-stratified community-onset S. aureus bacteremia incidence rate, Sa Kaeo and Nakhon Phanom provinces, Thailand 2006–2014
| Age (year) | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Age-stratified | ||||||||||
| < 5 | ||||||||||
| Cases | 5 | 8 | 6 | 5 | 9 | 5 | 13 | 7 | 11 | 69 |
| Population | 89,774 | 89,313 | 88,832 | 88,330 | 87,800 | 87,000 | 86,600 | 86,200 | 85,800 | 789,649 |
| Incidence rate* (95% CI) | 5.6 (1.8–13.0) | 9.0 (3.9–17.7) | 6.8 (2.5–14.7) | 5.7 (1.8–13.2) | 10.3 (4.7–19.5) | 5.7 (1.9–13.4) | 15.0 (8.0–25.7) | 8.1 (3.3–16.7) | 12.8 (6.4–22.9) | 8.7 (6.8–11.1) |
| 5–14 | ||||||||||
| Cases | 5 | 7 | 9 | 4 | 6 | 5 | 6 | 7 | 8 | 57 |
| Population | 202,931 | 201,795 | 200,614 | 199,389 | 198,100 | 195,900 | 193,200 | 190,400 | 187,500 | 1,769,829 |
| Incidence rate (95% CI) | 2.5 (0.8–5.8) | 3.5 (1.4–7.2) | 4.5 (2.1–8.5) | 2.0 (0.6–5.1) | 3.0 (1.1–6.6) | 2.6 (0.8–6.0) | 3.1 (1.1–6.8) | 3.7 (1.5–7.6) | 4.3 (1.8–8.4) | 3.2 (2.4–4.2) |
| 15–49 | ||||||||||
| Cases | 18 | 13 | 13 | 37 | 33 | 28 | 22 | 36 | 23 | 223 |
| Population | 560,380 | 557,386 | 554,268 | 551,023 | 547,600 | 547,200 | 546,800 | 545,500 | 544,200 | 4,954,357 |
| Incidence rate (95% CI) | 3.2 (1.9–5.1) | 2.3 (1.2–4.0) | 2.4 (1.3–4.0) | 6.7 (4.7–9.3) | 6.0 (4.2–8.5) | 5.1 (3.4–7.4) | 4.0 (2.5–6.1) | 6.6 (4.6–9.1) | 4.2 (2.7–6.3) | 4.5 (3.9–5.1) |
| 50–69 | ||||||||||
| Cases | 16 | 16 | 22 | 27 | 24 | 26 | 35 | 39 | 47 | 252 |
| Population | 231,608 | 230,338 | 229,018 | 227,646 | 226,200 | 234,500 | 242,900 | 252,000 | 260,700 | 2,134,910 |
| Incidence rate (95% CI) | 6.9 (3.9–11.2) | 6.9 (4.0–11.3) | 9.6 (6.0–14.5) | 11.9 (7.8–17.3) | 10.6 (6.8–15.8) | 11.1 (7.2–16.2) | 14.4 (10.0–20.0) | 15.5 (11.0–21.2) | 18.0 (13.2–24.0) | 11.8 (10.4–13.4) |
| ≥ 70 | ||||||||||
| Cases | 7 | 11 | 10 | 19 | 20 | 15 | 19 | 24 | 18 | 143 |
| Population | 62,620 | 62,313 | 61,993 | 61,655 | 61,300 | 63,900 | 66,300 | 68,700 | 71,800 | 580,581 |
| Incidence rate (95% CI) | 11.2 (4.5–23.0) | 17.7 (8.8–31.6) | 16.1 (7.7–29.7) | 30.8 (18.6–48.1) | 32.6 (19.9–50.4) | 23.5 (13.1–38.7) | 28.7 (17.3–44.8) | 34.9 (22.4–52.0) | 25.1 (14.9–39.6) | 24.6 (20.8–29.0) |
| Province-stratified | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nakhon Phanom | ||||||||||
| Cases | 27 | 39 | 46 | 51 | 45 | 46 | 56 | 62 | 54 | 426 |
| Population | 624,931 | 613,106 | 601,222 | 589,284 | 577,300 | 574,900 | 572,100 | 569,300 | 566,800 | 5,288,943 |
| Incidence rate (95% CI) | 4.3 (2.8–6.3) | 6.4 (4.5–8.7) | 7.7 (5.6–10.2) | 8.7 (6.4–11.4) | 7.8 (5.7–10.4) | 8.0 (5.9–10.7) | 9.8 (7.4–12.7) | 10.9 (8.3–14.0) | 9.5 (7.2–12.4) | 8.1 (7.3–8.9) |
| Sa Kaeo | ||||||||||
| Cases | 24 | 16 | 14 | 41 | 47 | 33 | 39 | 51 | 53 | 318 |
| Population | 522,384 | 528,040 | 533,502 | 538,760 | 543,700 | 553,600 | 563,700 | 573,500 | 583,200 | 4,940,386 |
| Incidence rate (95% CI) | 4.6 (2.9–6.8) | 3.0 (1.7–4.9) | 2.6 (1.4–4.4) | 7.6 (5.5–10.3) | 8.6 (6.4–11.5) | 6.0 (4.1–8.4) | 6.9 (4.9–9.5) | 8.9 (6.6–11.7) | 9.1 (6.8–11.9) | 6.4 (5.7–7.2) |
CI = confidence interval.
Incidence rate and 95% CI are in cases per 100,000 persons per year.
Figure 3.
Hospital-onset (HO)1,2 S. aureus bacteremia incidence rate,3 Sa Kaeo and Nakhon Phanom, Thailand, 2009–2014. 1Military hospitals in Sa Kaeo and Nakhon Phanom provinces were excluded from HO incidence rate calculations as hospitalization data were not available in Nakhon Phanom and for only 2 years in Sa Kaeo (2013–2014, < 1% of all hospitalizations in each year). No S. aureus HO cases were identified at military hospitals. 2Hospitalization data for the year 2010 were imputed for four hospitals with missing 2009 hospitalization data (see methods). 3Incidence rate in cases per 1,000 hospitalizations. Whiskers represent 95% confidence intervals.
Community-onset S. aureus bacteremia incidence rate from 2006–2014 was highest among adults aged ≥ 70 years and 50–69 years and showed an increase over time (Table 2). Of note, the census indicated 23% and 5% increases in the population aged 50 years and older in Sa Kaeo and Nakhon Phanom provinces, respectively, from 2006 to 2014.
The next highest CO-SAB incidence rate was among children < 5 years old and also increased over time (Table 2). Of the 92 CO- or HO-SAB cases in this age group, 63 (68%) were in neonates, of which 34 (54%) had positive cultures on the day of birth.
Methicillin-resistant S. aureus bacteremia prevalence, incidence rate, and characteristics.
Among 911 SAB cases with methicillin-resistance testing, 90 (10%) were MRSA. Methicillin-resistant S. aureus bacteremia prevalence among SAB in 2006 (when detected by oxacillin disk diffusion) was 20% (15/74), and during 2007–2014 (when tested by cefoxitin disk diffusion) ranged from 4% to 13%. Methicillin-resistant S. aureus incidence rate did not show a clear pattern over time (Table 3).
Table 3.
Community-onset (CO) and hospital-onset (HO) methicillin-resistant S. aureus bacteremia incidence rate, Sa Kaeo and Nakhon Phanom provinces, Thailand
| CO | |||||
|---|---|---|---|---|---|
| 2006–2008 | 2009–2010 | 2011–2012 | 2013–2014 | Total | |
| Cases | 13 | 20 | 8 | 14 | 55 |
| Person-years | 3,423,186 | 2,249,044 | 2,264,300 | 2,292,800 | 10,229,330 |
| Incidence rate* (95% CI) | 0.4 (0.2–0.7) | 0.9 (0.6–1.4) | 0.4 (0.2–0.7) | 0.6 (0.3–1.0) | 0.5 (0.4–0.7) |
| HO† | |||||
|---|---|---|---|---|---|
| 2009–2010 | 2011–2012 | 2013–2014 | Total | ||
| Cases | 3 | 7 | 7 | 17 | |
| Hospitalization years | 234,502 | 229,670 | 238,520 | 702,692 | |
| Incidence rate‡ (95% CI) | 0.01 (0.003–0.04) | 0.03 (0.01–0.06) | 0.03 (0.01–0.06) | 0.02 (0.01–0.04) | |
Community-onset incidence rate and 95% confidence interval (CI) are in cases per 100,000 person-years.
Military hospitals in Sa Kaeo and Nakhon Phanom provinces were excluded from HO incidence rate calculations as hospitalization data were not available in Nakhon Phanom and for only 2 years in Sa Kaeo (2013–2014, < 1% of all hospitalizations in each year). No S. aureus HO cases were identified at military hospitals. Hospitalization data for the year 2010 were imputed for four hospitals with missing 2009 hospitalization data (see methods).
Hospital-onset incidence rate and 95% CI are in cases per 1,000 hospitalizations per year.
Age distribution was similar between MRSA and MSSA (Table 1); of note, 13% (23/176) of SAB cases from adults aged 70 years and older had MRSA and 10% of neonatal cases (6/63). Methicillin-resistant S. aureus prevalence in Sa Kaeo (14% [60/436]) exceeded that of Nakhon Phanom (6% [30/475], P < 0.001). The majority (64%, 585/911) of SAB cases and an even higher percentage of MRSA cases (79%, 71/90) were identified at the provincial-level hospitals. Overall, 64 SAB cases were missing admission dates and could not be classified as CO or HO. Among SAB cases with admission dates available (N = 847), MRSA prevalence was higher among HO-SAB cases (22/111, 20%) than in CO-SAB (55/736, 7%, P < 0.001).
There were data on antibiotic exposure for 858/911 cases (94%) with methicillin testing, of which 56% (480/858) was based on serum disk assay. Current antibiotic use before blood collection was higher among MRSA cases (29%) compared with SAB cases overall (9.9%). Methicillin-resistant S. aureus isolates were more likely than MSSA isolates to be resistant to erythromycin, gentamicin, clindamycin, cotrimoxazole, and fosfomycin (all, P < 0.001) and resistant to at least two antibiotics besides methicillin (P < 0.001). Among 90 S. aureus isolates (including repeat blood cultures from the same case) with vancomycin susceptibility testing, 34 isolates had MICs > 1 µg/mL and four had MICs > 2 µg/mL on initial testing; repeat testing of these 38 isolates demonstrated susceptibility (≤ 2 µg/mL) for all.
Community-onset MRSA cases, compared with HO-MRSA cases, were similar in terms of age, province, resistance patterns, and current antibiotic use but were more likely to occur in a district hospital (15 cases, 27.3%, versus 0 cases, P = 0.01). The median time to S. aureus identification for HO-MRSA cases was 12 days (interquartile range: 6–51 days), with 35% (6/17) occurring in less than 7 days.
DISCUSSION
Through hospital-based surveillance in two rural provinces in Thailand, we found that CO-SAB incidence rate increased during 2006–2014. Incidence rates were highest among individuals aged ≥ 50 years followed by young children < 5 years. There was no clear trend in HO-SAB incidence rates. Methicillin-resistant S. aureus accounted for 10% of SAB without a clear difference in incidence rates over time. Community-onset MRSA cases were similar to HO-MRSA cases, except the latter was more prevalent in provincial hospitals.
We are aware of no other population-based investigations focused on SAB incidence rate in Thailand. Our observed CO incidence rate (7.3 cases per 100,000 of the population per year) was notably lower than that in other countries. A 9-year prospective study in Australia, Denmark, Finland, Sweden, and Canada had an annual CO-MSSA bacteremia incidence rate of 15.0 per 100,000.28 Similar to our findings, however, the authors noted an increase in CO-SAB infections over time.28 Although the increase in CO-SAB incidence rate in our analysis may be due in part to increased use of blood cultures, two studies in northeast Thailand have also noted an increased incidence rate of bacteremia in general. First, a retrospective study of provincial hospitals in northeast Thailand found increasing overall community-acquired bacteremia incidence rate from 2004 to 2010 with S. aureus being the third most frequent pathogen.9 The same group also found increasing incidence rate of health-care–associated and HO bacteremia in provincial hospitals during this same time period and noted S. aureus as the second and third most common pathogen among health-care–associated and HO bacteremias.12
One possible reason for the increased trend in CO-SAB may be a shift in population age-structure toward an older population. The census data revealed that the population of adults aged 50 years and older is increasing (Table 2), in particular in Sa Kaeo. We found that the CO-SAB incidence rate greater than doubled in adults aged 50–69 years and 70 years and older. This is consistent with a 1-year, single-hospital study in Thailand that found S. aureus bacteremia rates and associated mortality was highest in individuals aged > 50 years.11 Other countries have reported a large burden of S. aureus and MRSA bacteremia in the elderly primarily associated with health-care exposure.29,30 Further studies are needed to explore the factors associated with greater risk of SAB in this age group in our setting, given the increased risk of mortality, length of hospital stay, and health-care costs in SAB in older adults.31,32 It should be noted that other age groups also had increases in CO-SAB (Table 2), suggesting that there are likely other contributing factors that need to be further explored.
The next highest incidence rate in CO-SAB was in children < 5 years. Neonates, although only a small percentage of the overall population, accounted for most SAB among children < 5 years. This agrees with previously reported high incidence rates of SAB in neonates in Thailand.9,11 In considering how to prevent neonatal SAB, it is important to evaluate if there is community (maternal) versus hospital exposure. In Laos, S. aureus was the most common cause of community-acquired bacteremia in infants.8 In our analysis, we found that a slight majority (54%) of positive cultures were drawn on the day of birth. As neonates are a vulnerable population who have poor outcomes from bacteremia,33 further work is required to characterize their acquisition of colonization and increase prevention measures.
We found that MRSA bacteremia incidence rate was stable during this period. This differs from findings in western and northern Europe and Canada, where MRSA bacteremia has increased,28 or in the United States and the United Kingdom where invasive MRSA infections have decreased.34,35 However, two recent studies of provincial hospitals in northeast Thailand similarly did not find a trend in the prevalence of MRSA bacteremia from 2004 to 2010 regardless of CO or HO.12,36 Most HO-MRSA cases were in the second week of admission; declines in MRSA bacteremia in the United Kingdom have in part been due to infection control efforts that have reduced MRSA incidence rate after day 6 of admission.35 This suggests that strengthened infection control programs in Thailand may be able to further reduce the MRSA incidence rate.
Our MRSA prevalence among SAB cases (10%) is lower than the prevalence in other regions; a pooled analysis of SAB in Europe and the United States found a prevalence of 20.6%, ranging from 12% to 54.7%.30 However, the prevalence falls within the wide range of MRSA estimates (regardless of specimen type) seen in other Southeast Asian countries. A 2012 review found that the proportion of MRSA among S. aureus isolates varied from 5–8% in the Philippines to 0–40% in Malaysia and more than 50% in Singapore.14 A 1-year cross-sectional study of two tertiary hospitals in south Thailand found a similar prevalence of 8.1%.37 Recent WHO surveillance data found an MRSA prevalence of 13% among 132 blood isolates in Thailand.38
These more recent estimates and our prevalence of 10% are lower than MRSA estimates from studies in Thailand before 2007. A 1-year observational study in 2006 found MRSA in 28% of 98 SAB isolates in a regional hospital in northeast Thailand.11 Similarly, a 2005 study at a tertiary hospital in Bangkok noted methicillin resistance in 17 of 53 (32%) patients with SAB.10 There are several possibilities for the differences in our findings. First, the prior studies occurred in exclusively tertiary-level hospitals over short periods with a limited number of patients, as compared with our 8-year assessment that included all blood cultures across provincial, district, and military hospitals in these two provinces. We found that a higher proportion of MRSA cases occurred in provincial hospitals as compared with district or military hospitals, suggesting a possible overestimation if only including higher level tertiary centers. Past evaluations primarily also used oxacillin testing for determining methicillin resistance rather than cefoxitin, which has greater specificity to determine mecA-mediated resistance.39 We found that MRSA prevalence among SAB isolates was higher in 2006 compared with when we used cefoxitin in subsequent years. Our prevalence could also be an underestimation if prior antibiotic exposure reduced yield. Last, there have been recent efforts to reduce antimicrobial resistance and nosocomial infections in Thailand.40 For example, a national survey of tertiary hospitals found that 71% had an antimicrobial stewardship program, of which the majority had a monitoring system for multidrug-resistant organisms.41
Similar to past studies, MRSA isolates were significantly more likely to be resistant to other antibiotics compared with MSSA isolates and more likely to be from patients who had received antibiotics.11 We did not have any cases of vancomycin resistance, and current guidelines support its use for suspected MRSA bacteremia infections.42 Our findings underscore the consideration of empiric coverage for MRSA in suspected bacteremia cases and the necessity to develop laboratory capacity to effectively identify resistant strains and initiate the correct regimen.
Our investigation had several strengths. This report represents one of the largest assessments of SAB and antimicrobial resistance in Thailand and Southeast Asia. We evaluated > 140,000 blood cultures over 8 years and included all referral and community hospitals in two provinces. This allowed for improved representativeness and greater generalizability of the results than studies conducted only at tertiary care hospitals. We further excluded multiple cultures taken within a single episode of illness, which more accurately classified cases and prevented overestimation of the SAB and MRSA disease burden. However, there were limitations to our assessment. We could only evaluate hospitalized cases that were identified by clinician-initiated blood cultures, which may underestimate the overall incidence rate of S. aureus and MRSA bacteremia. Future surveillance systems would benefit from additional clinical information to better determine true bacteremia from contaminants and assessment of the source of bacteremia and risk factors to define CO-SAB as health-care associated. About half of data on antibiotic exposure was through self-report and could have been an underestimation. Because admission hour was not recorded, CO-SAB was determined by the number of days rather than hours and may have been underestimated. There were no available population estimates for neonates, which prevented calculation of neonatal SAB incidence rate. Small case counts of HO-SAB and MRSA-SAB cases limited interpretation of time trends for these subgroups and detailed age- or hospital type stratification. Without data on specific district hospital catchment populations, we could not calculate provincial versus district hospital CO-SAB or MRSA incidence rates. Outcome data were not routinely collected to allow estimation of staphylococcal-related mortality or hospital transfer. Future studies will need to expand on the risk factors, patient outcomes, and health-care exposures associated with SAB in Thailand.
The rising incidence rate of SAB in association with an aging population and continued burden on neonates highlights the need to expand surveillance and laboratory capacity in Thailand and the Southeast Asian region to better understand the underlying factors contributing to this trend and design appropriate interventions to halt its progression. In our system, 36% of cases were in district or military hospitals where diagnosis may have been delayed or missed without appropriate microbiological laboratory capacity and reporting. Increased surveillance and laboratory capacity enable rapid pathogen detection leading to improved patient care, including appropriate antibiotic therapy. This prevents further microbial resistance and, ultimately, enhances global health security.
Supplementary Material
Acknowledgments:
We thank Prasong Srisaengchai, Pattraporn Klanjatturat, Barameht Piralam, Sathapana Naorat, Ying Lu, Anek Kaewpan, Pongpun Sawatwong, Duangkamon Siludjai, and Apiwat Lapamnouysup (Global Disease Detection Regional Center, Thailand Ministry of Public Health–U.S. Centers for Disease Control and Prevention [CDC] Collaboration) for their contributions to this work. We also acknowledge the assistance of Dr. Wantana Paveenkittiporn and the Thailand National Institute of Health and thank all our colleagues, collaborators, and partners at the Nakhon Phanom and Sa Kaeo Provincial Health Offices. We appreciate the input of Dr. Brandi Limbago on our evaluation for vancomycin resistance.
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.Keynan Y, Rubinstein E, 2013. Staphylococcus aureus bacteremia, risk factors, complications, and management. Crit Care Clin 29: 547–562. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Corey GR, 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46 (Suppl 5): S344–S349. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y, 2005. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 26: 166–174. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y, 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36: 53–59. [DOI] [PubMed] [Google Scholar]
- 5.Yaw LK, Robinson JO, Ho KM, 2014. A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: an observational cohort study. Lancet Infect Dis 14: 967–975. [DOI] [PubMed] [Google Scholar]
- 6.WHO , 2014. Antimicrobial Resistance: Global Report on Surveillance. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 7.Deen J, von Seidlein L, Andersen F, Elle N, White NJ, Lubell Y, 2012. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis 12: 480–487. [DOI] [PubMed] [Google Scholar]
- 8.Phetsouvanh R, Phongmany S, Soukaloun D, Rasachak B, Soukhaseum V, Soukhaseum S, Frichithavong K, Khounnorath S, Pengdee B, Phiasakha K, 2006. Causes of community-acquired bacteremia and patterns of antimicrobial resistance in Vientiane, Laos. Am J Trop Med Hyg 75: 978. [PMC free article] [PubMed] [Google Scholar]
- 9.Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D, 2013. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One 8: e54714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mekviwattanawong S, Srifuengfung S, Chokepaibulkit K, Lohsiriwat D, Thamlikitkul V, 2006. Epidemiology of Staphylococcus aureus infections and the prevalence of infection caused by community-acquired methicillin-resistant Staphylococcus aureus in hospitalized patients at Siriraj Hospital. J Med Assoc Thai 89 (Suppl 5): S106–S117. [PubMed] [Google Scholar]
- 11.Nickerson EK, et al. 2009. Staphylococcus aureus bacteraemia in a tropical setting: patient outcome and impact of antibiotic resistance. PLoS One 4: e4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hongsuwan M, Srisamang P, Kanoksil M, Luangasanatip N, Jatapai A, Day NP, Peacock SJ, Cooper BS, Limmathurotsakul D, 2014. Increasing incidence of hospital-acquired and healthcare-associated bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One 9: e109324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nickerson EK, West TE, Day NP, Peacock SJ, 2009. Staphylococcus aureus disease and drug resistance in resource-limited countries in south and east Asia. Lancet Infect Dis 9: 130–135. [DOI] [PubMed] [Google Scholar]
- 14.Lestari ES, Severin Jt A, Verbrugh HA, 2012. Antimicrobial resistance among pathogenic bacteria in southeast Asia. Southeast Asian J Trop Med Public Health 43: 385–422. [PubMed] [Google Scholar]
- 15.WHO SEARO , 2011. Jaipur Declaration on Antimicrobial Resistance New Delhi, India: WHO Regional Office for South-East Asia.Available at: http://www.searo.who.int/entity/world_health_day/media/2011/whd-11_amr_jaipur_declaration_.pdf. Accessed July 24, 2016.
- 16.Apisarnthanarak A, Danchaivijitr S, Khawcharoenporn T, Limsrivilai J, Warachan B, Bailey TC, Fraser VJ, 2006. Effectiveness of education and an antibiotic-control program in a tertiary care hospital in Thailand. Clin Infect Dis 42: 768–775. [DOI] [PubMed] [Google Scholar]
- 17.Apisarnthanarak A, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ, 2010. Effectiveness of a catheter-associated bloodstream infection bundle in a Thai tertiary care center: a 3-year study. Am J Infect Control 38: 449–455. [DOI] [PubMed] [Google Scholar]
- 18.Korbkitjaroen M, Vaithayapichet S, Kachintorn K, Jintanothaitavorn D, Wiruchkul N, Thamlikitkul V, 2011. Effectiveness of comprehensive implementation of individualized bundling infection control measures for prevention of health care-associated infections in general medical wards. Am J Infect Control 39: 471–476. [DOI] [PubMed] [Google Scholar]
- 19.National Economic and Social Development Board of Thailand , 2012. Population Projections of Thailand 2010–2040. Bangkok, Thailand: Office of the National Economic and Social Development Board. [Google Scholar]
- 20.National Economic and Social Development Board Table of Gross Regional and Provincial Product, 1995–2012. Bangkok, Thailand: Office of the National Economic and Social Development Board.
- 21.Jorgensen JH, Carroll KC, Pfaller MA, 2015. Manual of Clinical Microbiology. Washington, DC: ASM Press. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute , 2014. Performance Standards for Antimicrobial Susceptibility Testing; 24th Informational Supplement. CLSI document M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 23.Clinical and Laboratory Standards Institute , 2007. Performance Standards for Antimicrobial Susceptibility Testing; 17th Informational Supplement. CLSI document M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute.
- 24.Cohen D, Swift G, 2013. Laboratories and regulator misled over antibiotic susceptibility test discs: patients may have received inappropriate antibiotic treatment as a result of quality problems with one company’s test discs, Deborah Cohen and Glenn Swift report. BMJ 346: f837. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Ayers TL, Park SY, Miller FD, MacFadden R, Nakata M, Lee MC, Efflert PV, 2005. Isolate removal methods and methicillin-resistant Staphylococcus aureus surveillance. Emerg Infect Dis 11: 1552–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driscoll AJ, Bhat N, Karron RA, O’Brien KL, Murdoch DR, 2012. Disk diffusion bioassays for the detection of antibiotic activity in body fluids: applications for the pneumonia etiology research for child health project. Clin Infect Dis 54 (Suppl 2): S159–S164. [DOI] [PubMed] [Google Scholar]
- 27.National Economic and Social Development Board of Thailand , 2007. Population Projections of Thailand 2000–2030. Bangkok: Office of the National Economic and Social Development Board. [Google Scholar]
- 28.Laupland KB, et al. The International Bacteremia Surveillance Collaborative , 2013. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 19: 465–471. [DOI] [PubMed] [Google Scholar]
- 29.Klevens RM, et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 30.Kaasch AJ, et al. 2014. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 68: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB, 2012. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 25: 362–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaye KS, Marchaim D, Chen TY, Baures T, Anderson DJ, Choi Y, Sloane R, Schmader KE, 2014. Effect of nosocomial bloodstream infections on mortality, length of stay, and hospital costs in older adults. J Am Geriatr Soc 62: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoesser N, et al. 2013. Pediatric bloodstream infections in Cambodia, 2007 to 2011. Pediatr Infect Dis J 32: e272–e276. [DOI] [PubMed] [Google Scholar]
- 34.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, 2013. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 173: 1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thelwall S, Nsonwu O, Bhattacharya A, Wasti S, Gerver S, Davies J, Hope R, 2017. Annual Epidemiological Commentary: Mandatory MRSA, MSSA and E. coli bacteraemia and C. difficile infection data 2016/17. London, United Kingdom: Public Health England. [Google Scholar]
- 36.Lim C, Takahashi E, Hongsuwan M, Wuthiekanun V, Thamlikitkul V, Hinjoy S, Day NP, Peacock SJ, Limmathurotsakul D, 2016. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife 5: e18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunnueang N, Kongpheng S, Yadrak P, Rattanachuay P, Khianngam S, Sukhumungoon P, 2016. Methilcillin-resistant Staphylococcus aureus: 1-year collection and characterization from patients in two tertiary hospitals, southern Thailand. Southeast Asian J Trop Med Public Health 47: 234–244. [PubMed] [Google Scholar]
- 38.WHO , 2017. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–2017. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 39.Broekema NM, Van TT, Monson TA, Marshall SA, Warshauer DM, 2009. Comparison of cefoxitin and oxacillin disk diffusion methods for detection of mecA-mediated resistance in Staphylococcus aureus in a large-scale study. J Clin Microbiol 47: 217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO , 2017. Situational Analysis on Antimicrobial Resistance in the South-East Asia Region. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 41.Khawcharoenporn T, Apisarnthanarak A, Mundy LM, 2013. National survey of antimicrobial stewardship programs in Thailand. Am J Infect Control 41: 86–88. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52: e18–e55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.