Abstract.
Spotted fever group rickettsioses (SFGRs), such as African tick bite fever (ATBF), are among the most commonly diagnosed diseases for ill travelers returning from southern Africa. We summarized demographic, clinical, and diagnostic features of imported SFGR cases in U.S. travelers returning from Africa who had laboratory specimens submitted to the Centers for Disease Control and Prevention. Diagnosis of SFGR was performed by indirect immunofluorescence antibody assay, immunohistochemical staining, polymerase chain reaction (PCR), or culture. Cases were defined as probable SFGR, confirmed SFGR, or confirmed ATBF. Clinical and epidemiological categorical variables were described as counts and proportions; continuous variables were described using geometric mean titers, median, and range. One hundred and twenty-seven patients satisfied laboratory criteria for confirmed or probable SFGR. Fever was the most common symptom (N = 88; 69%), followed by ≥ 1 eschars (N = 70; 55%). Paired serums were submitted for 36 patients (28%); 12 patients (33%) had nonreactive initial serum sample but converted to a titer ≥ 64 with the convalescent sample. Twenty-seven patients (21%) had infection with Rickettsia africae based on PCR analysis of eschar swab (N = 8) or biopsy (N = 23). Fifteen patients had eschar biopsy or swab samples and serum sample(s) submitted together; 9 (60%) had PCR-positive eschar results and nonreactive acute serology. Health-care providers should consider SFGR when evaluating patients for a febrile illness with eschar and compatible foreign travel history. Polymerase chain reaction testing of eschar biopsies or swabs provides a confirmed diagnosis in early stages of disease; eschar swabs or biopsies are an underutilized diagnostic technique.
INTRODUCTION
African tick bite fever (ATBF), caused by infection with the bacterium Rickettsia africae and transmitted by Amblyomma hebraeum or Amblyomma variegatum ticks (Figure 1), is the most commonly diagnosed rickettsial disease among international travelers.1,2 Although the disease is primarily associated with travel to Africa, R. africae has been identified in A. variegatum ticks in other parts of the world, such as the Caribbean.3 Nearly 14% of all returned travelers from sub-Saharan Africa seeking health care are diagnosed with a spotted fever group rickettsiosis (SFGR), and of these infections, most are attributable to R. africae.1 Other tick-borne rickettsial pathogens endemic to the African continent include Rickettsia conorii, Rickettsia aeschlimannii, Rickettsia sibirica mongolitimonae, and Rickettsia monacensis.4 Among patients presenting to GeoSentinel clinics, SFGR is the most common individual diagnosis for ill travelers returning from South Africa and the third most frequently reported diagnosis for travelers returning from Botswana, Lesotho, Mozambique, Namibia, Swaziland, Zimbabwe, or Zambia.5 African tick-bite fever may exceed malaria as the most common vector-borne infection among short-term visitors to southern Africa.6,7 Unlike other well-known tick-borne rickettsioses, such as Rocky Mountain spotted fever (caused by Rickettsia rickettsii) and Mediterranean spotted fever (caused by R. conorii), ATBF is typically a self-limited disease and there are no known deaths attributable to infection with R. africae.2,4 Nonetheless, some patients who do not receive appropriate antibiotic therapy may remain febrile for 1–3 weeks, and in rare cases, the illness may be associated with more severe manifestations that include convalescent-phase asthenia, reactive arthritis, peripheral neuropathy, or myocarditis.2,8,9
Figure 1.
Principal tick vectors of Rickettsia africae. Amblyomma hebraeum female (top left) and male (bottom left). Amblyomma variegatum female (top right) and male (bottom right). All feeding stages (larvae, nymphs, and adults) of both species demonstrate highly aggressive host-seeking behavior and a host is often attacked simultaneously by multiple ticks. Both species are distributed across much of southern Africa, including Botswana, Mozambique, South Africa, Swaziland, and Zimbabwe. The distribution of A. variegatum is even broader and includes most countries of sub-Saharan Africa and eight islands of the Caribbean.2,3 Tick specimens were provided by Lorenza Beati, MD, PhD; images courtesy of James Gathany. This figure appears in color at www.ajtmh.org.
Current clinical knowledge about ATBF and other African tick-borne rickettsioses derives predominantly from several large European case series.1,10,11 There are relatively fewer case series summaries that describe ATBF in U.S. travelers, and among those, the largest series documented 53 SFGR cases from the African continent, although none of these were confirmed as ATBF by molecular methods.12–15 Advances in laboratory techniques, such as polymerase chain reaction (PCR) testing of eschar swabs and biopsies, have enhanced the capacity for confirming infections with R. africae. In addition, the numbers of U.S. travelers to Africa have increased steadily during the last decade and during 2015, almost 1 million persons from the United States traveled to Africa,16 representing a 74% increase compared with 2004.17 This report summarizes various demographic and clinical features of imported SFGR in U.S. travelers returning from Africa during 2007–2016 and provides data for up-to-date recommendations for detection and treatment.
METHODS
Patients and samples.
We reviewed laboratory test requests submitted to the Rickettsial Zoonoses Branch and Infectious Diseases Pathology Branch at the Centers for Disease Control and Prevention (CDC) during January 1, 2007, through July 31, 2016, for those requesting confirmatory serologic, molecular, or immunohistochemistry (IHC) assays for suspected SFGR in a specimen from a traveler returning from Africa. We defined a probable case of imported SFGR as a single reciprocal immunoglobulin G (IgG) antibody titer ≥ 64 to antigens of R. africae or R. conorii when tested by an indirect immunofluorescence antibody (IFA) assay. We defined a confirmed imported SFGR infection as a 4-fold increase in IgG antibody titers between paired serum samples, or a positive IHC staining result for SFGR, or detection of rickettsial DNA by PCR that was not further characterized by sequence analysis. We defined a confirmed case of ATBF as sequence identification of R. africae using the PCR-positive products tested from nucleic acid extraction of the source specimen or from cultured isolates. The Centers for Disease Control and Prevention reviewed this study for human subjects’ protection and deemed it to be nonresearch.
Data management and analysis.
Medical providers submitting diagnostic specimens to CDC laboratories are required to complete a general laboratory submission form (https://www.cdc.gov/laboratory/specimen-submission/pdf/form-50-34.pdf) that contains a text box for a brief clinical summary and information on travel and exposure history. These data varied in completeness. The data were entered into an Epi Info 7.2.0.1 (Centers for Disease Control and Prevention, Atlanta, GA) database and analyzed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Categorical variables were described as counts and proportions; continuous variables were described using geometric mean titers (GMTs), median, and range. Responses were not provided for all fields, and missing data were not reported.
Serological testing.
Rickettsia africae and R. conorii were cultivated in Vero E6 cells. Infected cells were irradiated, dotted onto glass slides, and fixed in absolute acetone. A fluorescein isothiocyanate–labeled, goat antihuman IgG (heavy and light chain–specific) conjugate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) was used at a dilution of 1:200. Serum samples were screened at consecutive 2-fold dilutions starting at 1:32 and titers were represented as the reciprocal of the last dilution exhibiting reactivity with a specific antigen. Reciprocal IgG antibody titers ≥ 64 were considered positive for data analysis.
Molecular analysis.
DNA was extracted from skin and eschar swabs using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA) and eluted in a final volume of 200 μL. Extracted DNA was tested using a Rickettsia genus–specific real-time PCR assay.18 Threshold cycle values < 40 were considered positive. For species identification, a nested PCR assay was used to amplify a segment of the ompA gene, using 3 μL of purified DNA template and 0.8 μM each of primers 190-70 and 190-701 in the primary reaction, and 1 μL of the completed primary PCR and 0.8 μM each of primers 190-FN1 and 190-RN1 in the nested reaction.19 The amplified DNA fragment was sequenced using a 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequence alignments were made using SeqMan Pro in the DNASTAR® Lasergene 12 suite (DNASTAR, Inc., Madison, WI) and evaluated using the National Center for Biotechnology Information nucleotide Basic Local Alignment Search Tool.
DNA was extracted from formalin-fixed, paraffin-embedded tissues and its integrity evaluated as previously described.20 Testing before the year 2015 was performed by conventional PCR to the ompA gene followed by Sanger sequencing and analysis as mentioned earlier.19 Beginning in 2015, and for older extracts for which sequence data were not obtained, extracts were tested by a real-time PCR for R. africae using the Qiagen Quantitect Multiplex PCR kit (Qiagen, Germantown, MD) following the primer and probe concentrations and cycling conditions (45 total cycles with a diagnostic cutoff of 40 cycles) stipulated in the manual for duplex PCR using an Mx3005P (Agilent Technologies, Santa Clara, CA) thermal cycler. The primers/probes used correspond to an R. africae intergenic sequence21; the probe was labeled on the 5′ end with FAM (6-carboxyfluorescein) and on the 3′ end with Black Hole Quencher-1. Reactions were performed in a 25-μL volume with 2.5 μL of DNA extract.
Immunohistochemistry.
Skin biopsy specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections cut at 3 μm were stained with hematoxylin–eosin and by an immunoalkaline phosphatase technique with a polyclonal anti-R. rickettsii antiserum, diluted at 1:500.22
Cell culture isolation.
Vero E6 cells were inoculated with minced eschar biopsy tissues and incubated at 34.5°C in a 5% CO2-enriched atmosphere.23 Cultures were screened for infection with rickettsiae using acridine orange stain and confirmed by previously described molecular assays.
RESULTS
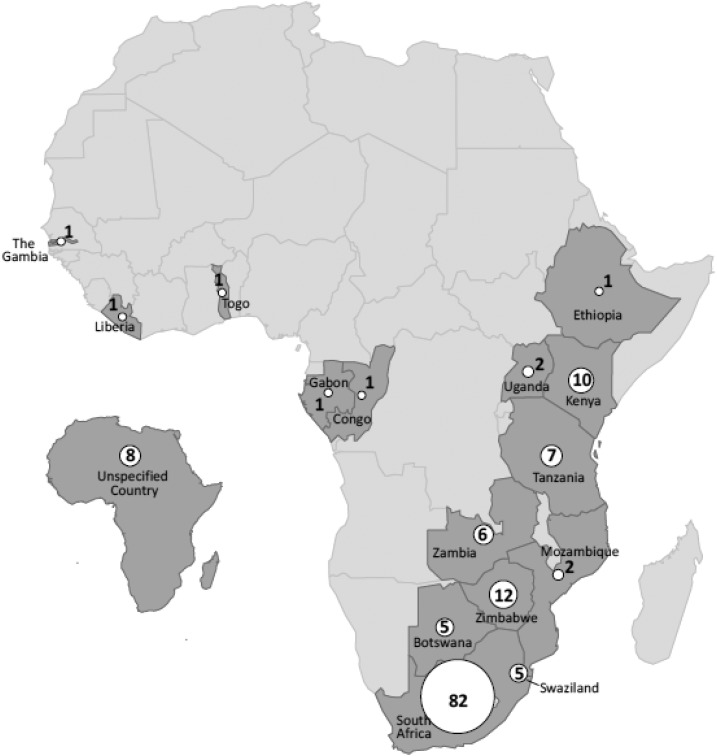
During the period of evaluation, the Rickettsial Zoonoses Branch received clinical samples from 223 U.S. travelers returning from Africa for evaluation of possible infection with a SFGR. An additional seven patients were identified from the Infectious Diseases Pathology Branch. From these, 127 (55%) satisfied one or more laboratory criteria for a confirmed or probable SFGR submitted from 31 states and the District of Columbia. Approximately 59% of all submissions originated from six states: Massachusetts (12%), New York (11%), Florida (11%), California (9%), Wisconsin (8%), and Washington (8%). Travel was reported to 15 African countries; South Africa was the most frequently visited country, reported by 82 patients (65%) (Figure 2). Fifteen travelers visited two African countries and three travelers visited three African countries during their trip. The majority of the patients were male (N = 67; 53%) and the median age for all patients was 53 years (range: 10–86 years). Fewer than half of patients (N = 54; 44%) reported a history of tick bite. Onset of symptoms was reported across all months, with the majority (N = 79; 62%) of patients reporting symptoms from March to August. Fever was the most commonly reported symptom (N = 88; 69%). One or more eschars (Figure 3) or skin lesions were described in 70 patients (55%), including 24 (34%) who reported multiple eschars. Rash was reported in 46 patients (36%); 24 patients (19%) reported having both a rash and an eschar. Other frequently reported symptoms included headache (N = 54; 43%) and myalgia (N = 42; 33%).
Figure 2.
Reported African travel before symptom onset among 127 U.S. travelers with imported spotted fever group rickettsioses diagnosed at the Centers for Diseases Control and Prevention, 2007–2016. Counts represent number of travelers who reported travel to the country before illness onset. Eighteen travelers reported travel to multiple African countries. Travel listed only as “Africa” is displayed on the map as “Unspecified Country.”
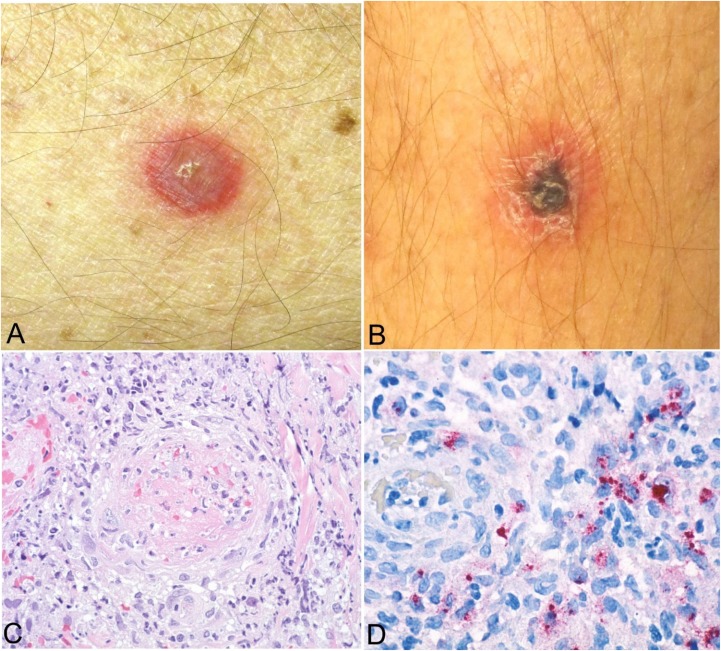
Figure 3.
Clinical and histopathological appearance of primary lesions of African tick bite fever (ATBF). Lesions occur at the tick-bite site and begin as an erythematous plaque or vesicle (A) that enlarges and develops into an inoculation eschar, characterized by a central, 0.5–3.0-cm ulcer covered by a brown-black crust and typically surrounded by an annular red halo (B). Eschars of ATBF occur most commonly on the lower extremities and frequently as multiple lesions.10 The histological appearance of eschars includes dermal and epidermal necrosis with predominantly lymphohistocytic perivascular inflammatory cell infiltrates in the superficial and deep dermis, often associated with thrombosis and mural necrosis of inflamed small vessels (hematoxylin and eosin stain, original magnification ×100) (C). Immunohistochemical staining typically reveals abundant rickettsial antigens (red) in the infiltrates that surround the vessels (immunoalkaline phosphatase with naphthol fast-red and hematoxylin counterstain, original magnification ×158) (D). Images (A) and (B) courtesy of Charles Thurston, MD, and Lester Libow, MD; Images (C) and (D) courtesy of Sherif Zaki, MD, PhD.
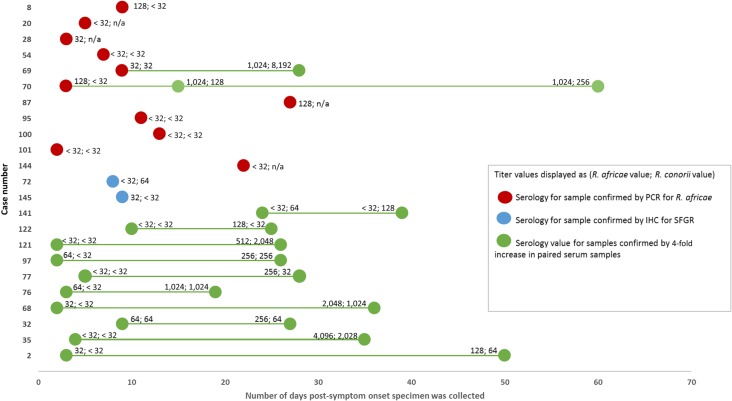
For 59 (46%) patients, a single serum sample was the only specimen submitted for testing. Paired serum samples were submitted for 36 patients (28%). Median days from symptom onset to first serum collection was 10 days (range: 1–1,461 days). Median days from onset to second serum sample was 35 days (range: 12–89 days). The GMT for all seropositive serum samples (N = 124) tested for R. africae was 219 (range: 64–8,192). The GMT of the first positive serum samples for R. africae (N = 95) was 203 (range: 64–8,192) and 223 for the second samples (N = 20) (range: 64–1,024). The GMT for all seropositive serum samples (N = 58) tested for R. conorii was 353 (range: 64–65,536). The GMT of the first positive serum samples for R. conorii (N = 45) was 338 (range: 64–65,536) and 210 for the second serum samples (N = 7) (range: 64–1,024). Sixty-eight serum samples (45%) were positive to both antigens. Thirty serum samples (24%) had a titer that was 4-fold greater to antigens of R. africae than to those of R. conorii. Only two serum samples (2%) had 4-fold greater titers to antigens of R. conorii than to R. africae. Among the 36 patients with paired serum samples, 12 (33%) patients had a nonreactive initial serum sample but converted to a titer ≥ 64 with the convalescent sample (Figure 4). The median number of days between first and second serum sample collection dates was 23 days (range: 5–73 days). By using serological criteria, IFA assays identified 85 probable cases of SFGR and 10 cases of a confirmed but unspecified SFGR (Table 1).
Figure 4.
Rickettsia africae and Rickettsia conorii titer values by days post-onset of symptoms for all confirmed spotted fever group rickettsiosis (SFGR) cases and all confirmed R. africae cases. The first titer value listed is against R. africae; the second titer value is against R. conorii. Red circles are serology titers for patients confirmed as R. africae by polymerase chain reaction (PCR) of tissue/eschar biopsy or swab. Blue circles are serology titers for patients confirmed for SFGR by immunohistochemistry (IHC) on tissue biopsies. Green circles are serology values for patients confirmed for SFGR by a 4-fold increase in titer values by paired serum samples.
Table 1.
Specimen sample combinations submitted for imported spotted fever group Rickettsia infections in travelers by disease case classification—United States, 2007–2016
| Specimen samples submitted per patient | Probable SFGR | Confirmed SFGR by paired serology* | Confirmed SFGR by IHC† | Confirmed Rickettsia africae | Total |
|---|---|---|---|---|---|
| Only one serum sample | 59 | 0 | 0 | 0 | 59 |
| Paired serum samples | 26 | 10 | 0 | 0 | 36 |
| Tissue/eschar sample only | 0 | 0 | 3 | 14 | 17 |
| Tissue/eschar sample plus serum sample(s) | 0 | 0 | 2 | 13 | 15 |
| Total | 85 | 10 | 5 | 27 | 127 |
IgG = immunoglobulin G; IHC = immunohistochemistry; SFGR = spotted fever group rickettsiosis.
Confirmed SFGR infection through 4-fold increase in IgG antibody titers between paired serum samples.
Confirmed SFGR infection through positive IHC staining.
Thirty-one eschar biopsy and eight eschar swab samples were submitted from 32 (25%) patients. One or more serum samples were also submitted for 15 of these patients (Table 1). Eschar swabs or skin biopsy specimens were obtained a median of 7 days from symptom onset (range: 0–27 days). Of the 15 patients who had eschar biopsy or swab samples and serum sample(s) submitted together, nine (60%) had PCR-positive eschar results associated with nonreactive acute serology. Twenty-seven patients (21%) had a laboratory-confirmed infection with R. africae based on PCR analysis of an eschar swab (N = 8) or skin biopsy specimen (N = 23). From two of these patients, isolates of R. africae were obtained in cell culture from skin biopsy specimens. Five patients were diagnosed with a confirmed but unspecified SFGR by IHC.
DISCUSSION
These data describe the largest series of cases of imported SFGR in U.S. travelers returning from Africa, comprising 27 confirmed cases of ATBF, 15 confirmed but unspecified cases of SFGR, and 85 probable cases of SFGR. In 2015, 53.5 million international tourists traveled to Africa24, with 951,000 of those tourists arriving from the United States.16 With the rapid expansion of international ecotourism, ATBF has emerged as a common cause of imported fever.2 Similar to previously described case series from Europe, most affected patients in the United States are middle-aged males who traveled to southern Africa.1,10,11 There are few reports of ATBF in children25,26; indeed, only two cases in this series occurred in children aged less than 18 years. In this series, the percentages of patients with fever (69%), headache (43%), myalgia (33%), and eschars or skin lesion (55%) were lower than those reported from most European series, which described fever in 81–100%, headache in 42–83%, myalgia in 63–87%, and eschar(s) in 53–95% of cases.10,11,27 The CDC laboratory submission form does not collect detailed clinical information uniquely associated with SFGRs. The data that accompany each specimen are not obtained systematically, but rather passively, and therefore vary greatly in completeness. In this context, the frequencies of clinical findings described in this evaluation likely underestimate the true frequencies of signs and symptoms of these diseases.
Eschars are an important clinical feature of ATBF, but also occur in SFGRs caused by other Rickettsia species endemic to sub-Saharan Africa, including R. conorii, R. aeschlimannii, R. sibirica mongolitimonae, and R. monacensis.4 Because of the antigenic cross-reactivity among species of SFG Rickettsia, it is possible that some of the 100 confirmed or probable cases of SFGR were caused by one or more of these pathogens. In samples where molecular or culture-based methods were applied, R. africae was identified as the infecting Rickettsia species, suggesting that most non-speciated cases were indeed ATBF. Multiple eschars are described in approximately 20–50% of patients with ATBF, as A. variegatum and A. hebraeum are highly aggressive and patients are often bitten by multiple infected ticks simultaneously.2,28 Rickettsia africae infection rates in these ticks are characteristically very high and can range from 87% to 100%.4,29–32 Clustered cases of ATBF occur often among groups of game hunters, bush hikers, and other tourist groups engaging in outdoor activities in sub-Saharan Africa.2 Previous case series have suggested that as many as 74% of travel-associated ATBF cases occurred in clusters.2,10,11 Persons traveling to habitats infested with A. variegatum and A. hebraeum should be educated about the risk of contracting ATBF and the importance of tick-bite prevention methods.2 Clinicians should be aware that clustered cases of ATBF occur commonly among families or other tourist groups and that establishing a diagnosis of ATBF in an index patient can alert similarly exposed persons in the group to seek appropriate medical care if they develop signs or symptoms compatible with ATBF.
For 77% of probable or confirmed patients in this dataset, only serum was submitted for laboratory testing. A single serum specimen was the only diagnostic specimen provided to the laboratory for 46% of the SFGR patients in this series, making single serum samples the most common diagnostic submission. The reported median time to IgG seroconversion to R. africae is 28 days after the onset of symptoms,33 and the use of single serum samples collected during the first 2 weeks of illness is often inadequate for the laboratory diagnosis of ATBF.12 Seven patients’ specimens were collected > 90 days after symptom onset (range: 117–1,461 days). Although antibody titers to SFG Rickettsia can persist for many months after the acute illness,34 the clinical relevance of a single reactive sample collected many months after the onset of the primary illness could be confounded by a subsequent exposure to a SFG Rickettsia species endemic to the United States. For suspected ATBF cases, two serum samples should be collected, including one sample during the acute illness and a second sample collected at least 4 weeks after symptom onset.33,35 Antigenic cross-reactivity among SFG Rickettsia species can prevent the identification of the infecting species when only serological methods are used.36 Although most cases were classified generically as a SFGR, exposure histories placed most individuals in countries where ATBF is highly prevalent, suggesting that most of these patients were likely infected with R. africae.1,10,11 Occasionally, a patient infected with a SFG or typhus group Rickettsia species can develop heterotypic antibodies that react with Rickettsia species of the other group, although characteristically at a much lower titer.37 Only seven patients evaluated in this study were tested for antibodies to typhus group rickettsiae, and all were nonreactive. In addition, a history of tick bite or the occurrence of an eschar, findings not typically associated with typhus group rickettsioses, was described for many of the patients. Nonetheless, clinicians should consider testing for typhus group rickettsiae in patients returning from Africa with a febrile rash-associated illness for whom a history of tick bite or clinical evidence of an inoculation eschar is lacking.
Although serology remains the most common diagnostic method for SFGRs, PCR of eschar biopsies or swabs provides a confirmed diagnosis in the early stages of the disease.36,38,39 In recent years, eschar swabs and eschar crust samples have proven to be a useful diagnostic method for many tick-borne SFGRs.36,38,39 The eschar swab procedure is generally preferred over eschar biopsy by clinicians and patients because of the ease of sample collection and limited invasiveness of the procedure.36 To collect an eschar swab, the eschar first should be cleansed with a standard disinfectant solution. The eschar scab is then removed in part or in entirety with a sterile tweezer. If the scab is removed completely, it can be placed in a sterile container and submitted with the swab. Using a dry, sterile cotton swab, rotate the swab vigorously at a 50°–60° angle, while applying steady gentle pressure. The swab is then placed in a sterile specimen container. Eschar swabs do not allow for IHC or cell culture evaluation.38 Few commercial laboratories provide this testing in the United States. Clinicians interested in submitting eschar swabs or biopsy samples to CDC for diagnostic testing should contact their local or state public health department to coordinate specimen submission to CDC.
Appropriate antibiotic therapy should not be delayed while awaiting laboratory confirmation of a rickettsial disease.35 Doxycycline (100 mg twice daily) is the recommended dose for adults; 2.2 mg/kg body weight twice daily is the recommended dose for children weighing less than 100 lb.35 Treatment should continue until at least 3 days after defervescence and evidence of clinical improvement.35 Symptoms in most cases of ATBF improve within 24–48 hours after antibiotic administration.2 Rifampin has been used to successfully treat ATBF in a patient allergic to tetracyclines.40
Data from this case series were derived from persons who received medical care in the United States and had diagnostic samples sent to CDC. The CDC laboratory submission form is a standardized and relatively generic collection document that obtains limited clinical and epidemiological information pertinent to ATBF and there is marked variability in the completeness of data provided. More detailed clinical data, such as those compiled from clinic or hospital records, were not captured by this collection tool. In this context, the frequency of specific clinical features reported from this passive mechanism is not directly comparable with that of other case series with greater access to patient data. Travel history was required to be included in this analysis, so incomplete submission forms could have prevented inclusion of certain cases. This case series likely underestimates the true number of returned U.S. travelers with imported SFGRs.
Increases in international travel necessitate enhancing awareness by health-care providers of diseases, such as ATBF, that are highly endemic in regions far beyond their geographical area of practice. Health-care providers should be aware of internationally recognized rickettsial diseases such as ATBF when evaluating returning travelers with a febrile, eschar-associated illness and compatible travel history.12 Diagnostic sampling techniques for ATBF should include the collection of appropriately timed acute and convalescent serum specimens and PCR testing of eschar biopsies or swabs, when available.
Acknowledgments:
We thank Joseph Singleton, Marah Condit, and Ida Chung (CDC) for their assistance with confirmatory diagnostic tests; Lorenza Beati (Georgia Southern University) for providing specimens of Amblyomma variegatum and Amblyomma hebraeum; James Gathany (CDC) for the photographic images presented in Figure 1; Elaine Hallisey (CDC) for assistance with creating Figure 2; Charles Thurston and Lester Libow (Aurora Diagnostics) and Sherif Zaki (CDC) for the images used in Figure 3; and Anne Straily (CDC) for assistance with creating Figure 4.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Jensenius M, et al. 2009. Multicenter GeoSentinel analysis of rickettsial diseases in international travelers, 1996–2008. Emerg Infect Dis 15: 1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensenius M, Fournier PE, Kelly P, Myrvang B, Raoult D, 2003. African tick bite fever. Lancet Infect Dis 3: 557–564. [DOI] [PubMed] [Google Scholar]
- 3.Kelly P, Lucas H, Beati L, Yowell C, Mahan S, Dame J, 2010. Rickettsia africae in Amblyomma variegatum and domestic ruminants on eight Caribbean islands. J Parasitol 96: 1086–1088. [DOI] [PubMed] [Google Scholar]
- 4.Parola P, et al. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26: 657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendelson M, Davis XM, Jensenius M, Keystone JS, von Sonnenburg F, Hale DC, Burchard GD, Field V, Vincent P, Freedman DO; GeoSentinel Surveillance Network , 2010. Health risks in travelers to South Africa: the GeoSentinel experience and implications for the 2010 FIFA world cup. Am J Trop Med Hyg 82: 991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensenius M, Parola P, Raoult D, 2006. Threats to international travellers posed by tick-borne diseases. Travel Med Infect Dis 4: 4–13. [DOI] [PubMed] [Google Scholar]
- 7.Rahman A, Tegnell A, Vene S, Giesecke J, 2003. Rickettsioses in Swedish travellers, 1997–2001. Scand J Infect Dis 35: 247–250. [DOI] [PubMed] [Google Scholar]
- 8.Roch N, Epaulard O, Pelloux I, Pavese P, Brion JP, Raoult D, Maurin M, 2008. African tick bite fever in elderly patients: 8 cases in French tourists returning from South Africa. Clin Infect Dis 47: e28–e35. [DOI] [PubMed] [Google Scholar]
- 9.Bellini C, Monti M, Potin M, Dalle Ave A, Bille J, Greub G, 2005. Cardiac involvement in a patient with clinical and serological evidence of African tick-bite fever. BMC Infect Dis 5: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raoult D, et al. 2001. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N Engl J Med 344: 1504–1510. [DOI] [PubMed] [Google Scholar]
- 11.Jensenius M, Fournier PE, Vene S, Hoel T, Hasle G, Henriksen AZ, Hellum KB, Raoult D, Myrvang B; Norwegian African Tick Bite Fever Study Group , 2003. African tick bite fever in travelers to rural sub-Equatorial Africa. Clin Infect Dis 36: 1411–1417. [DOI] [PubMed] [Google Scholar]
- 12.McQuiston JH, Paddock CD, Singleton J, Jr., Wheeling JT, Zaki SR, Childs JE, 2004. Imported spotted fever rickettsioses in United States travelers returning from Africa: a summary of cases confirmed by laboratory testing at the Centers for Disease Control and Prevention, 1999–2002. Am J Trop Med Hyg 70: 98–101. [PubMed] [Google Scholar]
- 13.McDonald JC, MacLean JD, McDade JE, 1988. Imported rickettsial disease: clinical and epidemiologic features. Am J Med 85: 799–805. [DOI] [PubMed] [Google Scholar]
- 14.Neal S, Cieslak P, Hedberg K, Fleming D, 1998. African tick-bite fever among international travelers–Oregon, 1998. MMWR Morb Mortal Wkly Rep 47: 950–952. [PubMed] [Google Scholar]
- 15.Smoak BL, McClain JB, Brundage JF, Broadhurst L, Kelly DJ, Dasch GA, Miller RN, 1996. An outbreak of spotted fever rickettsiosis in U.S. Army troops deployed to Botswana. Emerg Infect Dis 2: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Departmenf of Commerce , 2016. 2015 United States Resident Travel Abroad Available at: http://tinet.ita.doc.gov/outreachpages/download_data_table/2015_US_Travel_Abroad.pdf. Accessed April 4, 2018.
- 17.U.S. Department of Commerce , 2005. 2004 Profile of U.S. Resident Traveler Visiting Overseas Destinations Reported from: Survey of International Air Travelers Available at: http://tinet.ita.doc.gov/view/f-2004-101-001/index.html. Accessed April 4, 2018.
- 18.Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF, 2013. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol 51: 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD, 2007. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis 13: 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paddock CD, et al. 2014. Phylogeography of Rickettsia rickettsii genotypes associated with fatal Rocky Mountain spotted fever. Am J Trop Med Hyg 91: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renvoise A, Rolain JM, Socolovschi C, Raoult D, 2012. Widespread use of real-time PCR for rickettsial diagnosis. FEMS Immunol Med Microbiol 64: 126–129. [DOI] [PubMed] [Google Scholar]
- 22.Paddock CD, et al. 1999. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J Infect Dis 179: 1469–1476. [DOI] [PubMed] [Google Scholar]
- 23.Paddock CD, Koss T, Eremeeva ME, Dasch GA, Zaki SR, Sumner JW, 2006. Isolation of Rickettsia akari from eschars of patients with rickettsialpox. Am J Trop Med Hyg 75: 732–738. [PubMed] [Google Scholar]
- 24.UNWTO , 2016. UNTWO Tourism Highlights, 2016 Edition. Madrid, Spain: World Tourism Organization.
- 25.Bohaty BR, Hebert AA, 2015. Images in clinical medicine: African tick-bite fever after a game-hunting expedition. N Engl J Med 372: e14. [DOI] [PubMed] [Google Scholar]
- 26.Albizuri Prado F, Sanchez A, Feito M, Mayor A, Rodriguez A, de Lucas R, 2017. Fever and multiple eschars after an African safari: report of three cases. Pediatr Dermatol 34: e179–e181. [DOI] [PubMed] [Google Scholar]
- 27.Jelinek T, Loscher T, 2001. Clinical features and epidemiology of tick typhus in travelers. J Travel Med 8: 57–59. [DOI] [PubMed] [Google Scholar]
- 28.Rolain JM, Jensenius M, Raoult D, 2004. Rickettsial infections—a threat to travellers? Curr Opin Infect Dis 17: 433–437. [DOI] [PubMed] [Google Scholar]
- 29.Maina AN, et al. 2014. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural western Kenya: implications for human health. Vector Borne Zoonotic Dis 14: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Socolovschi C, Huynh TP, Davoust B, Gomez J, Raoult D, Parola P, 2009. Transovarial and trans-stadial transmission of Rickettsiae africae in Amblyomma variegatum ticks. Clin Microbiol Infect 15 (Suppl 2): 317–318. [DOI] [PubMed] [Google Scholar]
- 31.Keller C, et al. 2016. High detection rate of Rickettsia africae in Amblyomma variegatum but low prevalence of anti-rickettsial antibodies in healthy pregnant women in Madagascar. Ticks Tick Borne Dis 7: 60–65. [DOI] [PubMed] [Google Scholar]
- 32.Nakao R, Qiu Y, Igarashi M, Magona JW, Zhou L, Ito K, Sugimoto C, 2013. High prevalence of spotted fever group rickettsiae in Amblyomma variegatum from Uganda and their identification using sizes of intergenic spacers. Ticks Tick Borne Dis 4: 506–512. [DOI] [PubMed] [Google Scholar]
- 33.Fournier PE, Jensenius M, Laferl H, Vene S, Raoult D, 2002. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin Diagn Lab Immunol 9: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clements ML, Dumler JS, Fiset P, Wisseman CL, Jr., Snyder MJ, Levine MM, 1983. Serodiagnosis of Rocky Mountain spotted fever: comparison of IgM and IgG enzyme-linked immunosorbent assays and indirect fluorescent antibody test. J Infect Dis 148: 876–880. [DOI] [PubMed] [Google Scholar]
- 35.Biggs HM, et al. 2016. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 65: 1–44. [DOI] [PubMed] [Google Scholar]
- 36.Socolovschi C, Renvoise A, Brouqui P, Parola P, Raoult D, 2012. The use of eschar swabs for the diagnosis of African tick-bite fever. Ticks Tick Borne Dis 3: 361–363. [DOI] [PubMed] [Google Scholar]
- 37.Philip RN, Casper EA, Ormsbee RA, Peacock MG, Burgdorfer W, 1976. Microimmunofluorescence test for the serological study of Rocky Mountain spotted fever and typhus. J Clin Microbiol 3: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouffok N, Socolovschi C, Benabdellah A, Renvoise A, Parola P, Raoult D, 2011. Diagnosis of rickettsioses from eschar swab samples, Algeria. Emerg Infect Dis 17: 1968–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bechah Y, Socolovschi C, Raoult D, 2011. Identification of rickettsial infections by using cutaneous swab specimens and PCR. Emerg Infect Dis 17: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strand A, Paddock CD, Rinehart AR, Condit ME, Marus JR, Gilliani S, Chung IH, Fowler VG, Jr., 2017. African tick bite fever treated successfully with rifampin in a patient with doxycycline intolerance. Clin Infect Dis 65: 1582–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]