Abstract.
The pharmacokinetics (PK) and ex vivo activity (pharmacodynamics [PD]) of two artemisinin combination therapies (ACTs) (artemisinin–piperaquine [ARN–PPQ] [Artequick®] and artesunate–amodiaquine [ARS–AQ] [Coarsucam™]) in healthy Vietnamese volunteers were compared following 3-day courses of the ACTs for the preselection of the drugs for falciparum malaria therapy. For PK analysis, serial plasma samples were collected from two separate groups of 22 volunteers after ACT administration. Of these volunteers, ex vivo activity was assessed in plasma samples from seven volunteers who received both ACTs. The area under the concentration–time curve (AUC0–∞) was 3.6-fold higher for dihydroartemisinin (active metabolite of ARS) than that for ARN, whereas the AUC0–∞ of desethylamodiaquine (active metabolite of AQ) was 2.0-fold lower than that of PPQ. Based on the 50% inhibitory dilution values of the volunteers’ plasma samples collected from 0.25 to 3 hours after the last dose, the ex vivo activity of ARS–AQ was 2.9- to 16.2-fold more potent than that of ARN–PPQ against the drug-sensitive D6 Plasmodium falciparum line. In addition, at 1.5, 4.0, and 24 hours after the last dose, the ex vivo activity of ARS–AQ was 20.8-, 3.5-, and 8.5-fold more potent than that of ARN–PPQ against the ARN-sensitive MRA1239 line. By contrast, at 1.5 hours, the ex vivo activity of ARS–AQ was 5.4-fold more active than that of ARN–PPQ but had similar activities at 4 and 24 hours against the ARN-resistant MRA1240 line. The PK–PD data suggest that ARS–AQ possesses superior antimalarial activity than that of ARN–PPQ and would be the preferred ACT for further in vivo efficacy testing in multidrug-resistant falciparum malaria areas.
INTRODUCTION
Although morbidity and mortality from malaria has reduced over the past decade, the disease is still a serious public health problem with 214 million infections and 438,000 deaths in 2015.1,2 Of the global malaria burden, sub-Saharan Africa carries a disproportionately high share, with 88% of malaria cases and 90% of malaria deaths reported in 2015.3 Artemisinin (ARN)-based combination therapies (ACTs) are currently recommended worldwide for first-line treatment of uncomplicated Plasmodium falciparum malaria.4 Of the commercially available fixed-dose ACTs, Coartem® (artemether–lumefantrine) or Coarsucam™ (artesunate–amodiaquine [ARS–AQ], also available as ASAQ Winthrop®) are used as first-line treatment of falciparum malaria in Africa.1 In Southeast Asia, dihydroartemisinin–piperaquine (DHA–PPQ) is the ACT of choice for the treatment of falciparum malaria in several countries such as Cambodia, Indonesia, and Vietnam.1 Artequick® (ARN–PPQ) is another fixed-dose ACT, which is administered either as a 2-day or 3-day regimen with the longer regimen being more efficacious in Thailand.5–7 Artemisinin is cheaper to produce than its derivatives ARS, artemether, or DHA and, thus, Artequick, particularly a 2-day regimen, would be a more cost-effective ACT than DHA–PPQ if proven to be efficacious. However, further studies are required to determine whether Artequick is more effective against multidrug-resistant P. falciparum malaria than that of standard ACTs such as ARS–AQ.
Resistance to ACTs has been reported in the Southeast Asian countries of Cambodia, Laos, Myanmar, Thailand, and Vietnam.1 Since 2008, high failure rates to ARS–mefloquine8 and DHA–PPQ9 have been reported in Cambodia, ARS–mefloquine10 in Thailand, and reduced ARN susceptibility also observed in Vietnam.11,12 This is of immense concern, as drug options to ACTs are limited, and until new non-ACTs are developed, there is an urgent need to contain and eliminate ACT-resistant malaria parasite populations. Also, alternative ACTs need to be assessed if first-line treatment drugs start to fail. In 2012, we reported that Coarsucam and Artequick were highly efficacious with a cure rate > 98% in Vietnamese patients treated for uncomplicated falciparum malaria in south-central Vietnam.6 Because of reduced susceptibility to DHA–PPQ11,12 and a recent report of high treatment failures (PCR-corrected 26%) of the ACT in Vietnam,13 further studies of other ACTs are required to determine their potential nationwide therapeutic efficacy.
Information from ex vivo antimalarial bioassay systems that study the pharmacokinetic (PK)–pharmacodynamic (PD) interaction in sera or plasma collected from healthy volunteers or malaria patients following antimalarial drug treatment has been found to be a useful and a potentially cost-effective strategy to guide the selection and development of drugs, including ACTs.14–18 To further demonstrate the utility of such PK–PD studies, we compared the PK and ex vivo antimalarial activity of ARS–AQ (Coarsucam) and ARN–PPQ (Artequick) in plasma from healthy Vietnamese volunteers administered with the two ACTs to determine which ACT had the superior PK–PD profile for further in vivo efficacy assessments.
MATERIALS AND METHODS
Drugs.
Artemisinin, ARS, desethylamodiaquine (DAQ), DHA, and PPQ tetraphosphate were obtained from the Worldwide Antimalarial Resistance Network (WWARN QA/QC Reference Material Program, Bangkok, Thailand), and AQ hydrochloride, chloroquine disphosphate, and mefloquine hydrochloride were obtained from Sigma (St. Louis, MO).
Parasites.
Three laboratory-adapted P. falciparum lines were used in this study: D6 from Sierra Leone is chloroquine and pyrimethamine sensitive but innately less susceptible to mefloquine, MRA1239 is DHA sensitive but chloroquine and mefloquine resistant, and MRA1240 is DHA, chloroquine, and mefloquine resistant.19 Both MRA139 and MRA1240 are recent lines from Cambodia and were obtained from BEI Resources (Manassas, VA).
Volunteers and study site.
Two groups of 22 healthy male Vietnamese volunteers (Group 1 mean [± stand deviation (SD)] age: 21.01 [1.8] years; weight 59.6 [2.2] kg and Group 2: 20.5 [1.5] years; weight 64.1 [4.7] kg) participated in the study. The study was carried out in the Central Military Hospital in Hanoi (Vietnam). The volunteers were judged healthy based on medical history, normal vital signs, electrocardiogram (ECG), and laboratory testing (hematology and biochemistry) within normal range. Physical examination and laboratory blood tests were performed during screening and blood tests repeated on day 7 after commencement of the ACTs. A 12-lead ECG was recorded for each volunteer during screening and at approximately 6 hours after the last ACT dose. The corrected QT (QTc) interval for heart rate was calculated using the Fridericia’s (QTcF = QT/3√RR) formula,20 where RR is the time between the start of the Q wave and the end of the T wave in the volunteer’s heart’s electrical cycle. The ECGs were reviewed by the hospital’s cardiologist. Volunteers were not allowed to drink alcohol or caffeine-containing beverages for 1 day before and after drug administration. The Review and Scientific Board of Central Military Hospital 108 (1368/QD-BV108) and the Australian Defense Human Research Ethics Committee (ADHREC No. 562-09) gave ethical approval for the study and the volunteers gave written informed consent before entering the trial.
Study design and drug administration.
The study was an open-label design with no randomization. Artequick tablets (each tablet contains 62.5 mg ARN and 375 mg PPQ base) were obtained from Artepharm Co., Ltd., Guangdong, China. Two tablets of Artequick were administered to Group 1 volunteers once daily for 3 days. Coarsucam tablets (each tablet contains 100 mg ARS and 270 mg AQ) were obtained from Sanofi-Aventis, France. Two tablets of Coarsucam were administered to Group 2 volunteers once daily for 3 days. Drug administration took place 10–15 minutes after the volunteers had ingested a low-fat (16.7 g) breakfast of one packet of instant noodles. The Artequick and Coarsucam tablets were administered with 200 mL of water. There was a 19-week washout period for seven volunteers who received both ACTs.
Blood sampling.
Venous blood samples (7 mL at each time point) were collected with 3.5 mL transferred to a fluoride oxalate tube for drug analysis and 3.5 mL to a lithium heparin tube for ex vivo assay at 0 (pre-dose), 1, 3, and 6 hours after the first and second dose of Artequick or Coarsucam administration. Immediately before the last dose, an indwelling cannula was inserted into a forearm vein and kept patent with heparinized saline. Blood samples (7 mL each) were then collected at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 hours after drug administration. Fluoride oxalate was used as the anticoagulant for the PK samples to minimize further hydrolysis of ARS to DHA because of plasma esterases.21 Because fluoride oxalate is toxic to the malaria parasite, plasma samples for ex vivo antimalarial studies were collected in heparin, which does not inhibit parasite growth. Subsequent blood samples were collected by venipuncture at days 3, 4, 7, 14, 21, and 28 for both ACTs, with additional collections on days 35 and 42 for volunteers on Artequick. All blood samples were centrifuged at 1,400 × g for 15 minutes and the separated plasma samples were stored at −80°C before transport to Australia on dry ice for drug analysis at the Department of Drug Evaluation, Australian Army Malaria Institute. The laboratory participates in the WWARN quality control and assurance proficiency testing program with satisfactory performance.22
Drug analysis.
Plasma concentrations of AQ and its active metabolite DAQ, ARN, ARS and its active metabolite DHA, and PPQ were measured using liquid chromatography-tandem mass spectrometry (LC/MS/MS). The instrumentation used for measuring the compounds, the precision of the assays and the lower limit of quantification for each compound are outlined in Supplemental Materials. Contrary to the concern of further hydrolysis of ARS to DHA due to plasma esterases, subsequent drug analysis revealed no significant differences (P > 0.05) in the concentrations of the four drugs and two metabolites collected in fluoride oxalate and lithium heparin tubes (data not shown).
In vitro antimalarial activity of reference drugs.
For in vitro antimalarial activity assessment, AQ, ARN, ARS, DAQ, DHA, and PPQ were dissolved either in dimethyl sulfoxide, methanol, or 50% methanol/50% water. All drugs were subsequently diluted in drug-free human plasma. Evaluation of the antimalarial activity of the drugs was carried out by measuring the inhibition of the radioactive [3H] hypoxanthine uptake by parasites,23 which were cultured in RPMI-1640 media with 50% human plasma as previously described.24 Drug 50% inhibitory concentration (IC50) values (i.e., concentrations that cause 50% inhibition of parasite growth or [3H]-hypoxanthine uptake, when compared with drug-free samples) were determined using a nonlinear regression analysis (GraphPad Prism V5.0; GraphPad Software, Inc., San Diego, CA).
Ex vivo antimalarial activity (PD) of plasma samples.
Antimalarial activity of ARS–AQ and ARN–PPQ in plasma samples collected from seven volunteers at 0 (before the first dose) and at 48 (before the last dose), 48.25, 48.5, 49, 49.5, 50, 52, 54, 56, 58, 60, 72, 96, 120, and 168 hours (after the last dose) was evaluated against the D6 line. In addition, the ex vivo antimalarial activity of ARS–AQ in plasma samples collected from volunteers at 0 (before the first dose) and at 1.5, 4, and 24 hours after the last dose, was also tested against the MRA1239 and MRA1240 lines.
The ex vivo antimalarial activity was assessed using the inhibition of radioactive [3H] hypoxanthine uptake assay as described previously, except that malaria parasites were cultured in the presence of volunteers’ plasma samples (50% plasma concentration in each well) collected after the last administration of the ACTs as previously described.25 Reference drugs were run in parallel with the volunteers’ plasma samples under the same conditions.
The antimalarial activity of the volunteers’ plasma was expressed using the inhibitory dilution (ID50) as a potency parameter, defined as a dilution of the volunteer’s plasma sample required to achieve inhibition of parasite growth by 50% (ID50). The ID50s were determined using GraphPad Prism V5.0.
Pharmacokinetic analysis.
The PK parameters of maximum plasma drug concentration (Cmax), time to maximum concentrations (Tmax); the area under the drug concentration–time curve (AUC); elimination half-life (t1/2); clearance (CL/F), the apparent volume of distribution (V/F); and V/F at steady-state (Vss/F) were determined by non-compartmental analysis (PK Solutions 2.0; Summit Research Services, OH). Estimations of CL/F, V/F, and Vss/F were uncorrected for the extent of bioavailability. Both ARS and AQ act as prodrugs with complete in vivo conversion to DHA26,27 and DAQ,28 respectively, assumed.
Statistical analysis.
Pharmacokinetic and drug concentrations data are presented as median values (interquartile range [IQR]) and ex vivo assay data have been summarized as means ± SD or standard error of the mean (SEM). Statistical comparison was made using the paired t test (SigmaStat version 3.0; Jandel Scientific, San Rafael, CA). Data were accepted as significant using the 5% significance level.
RESULTS
Study participants.
In the present study, 37 Vietnamese subjects volunteered to participate. Of these, 15 subjects received ARN–PPQ and 15 subjects were administered ARS–AQ. Another seven subjects volunteered to receive both ACTs. Thus, 22 subjects were administered each ACT.
The PK of ARN–PPQ in healthy volunteers.
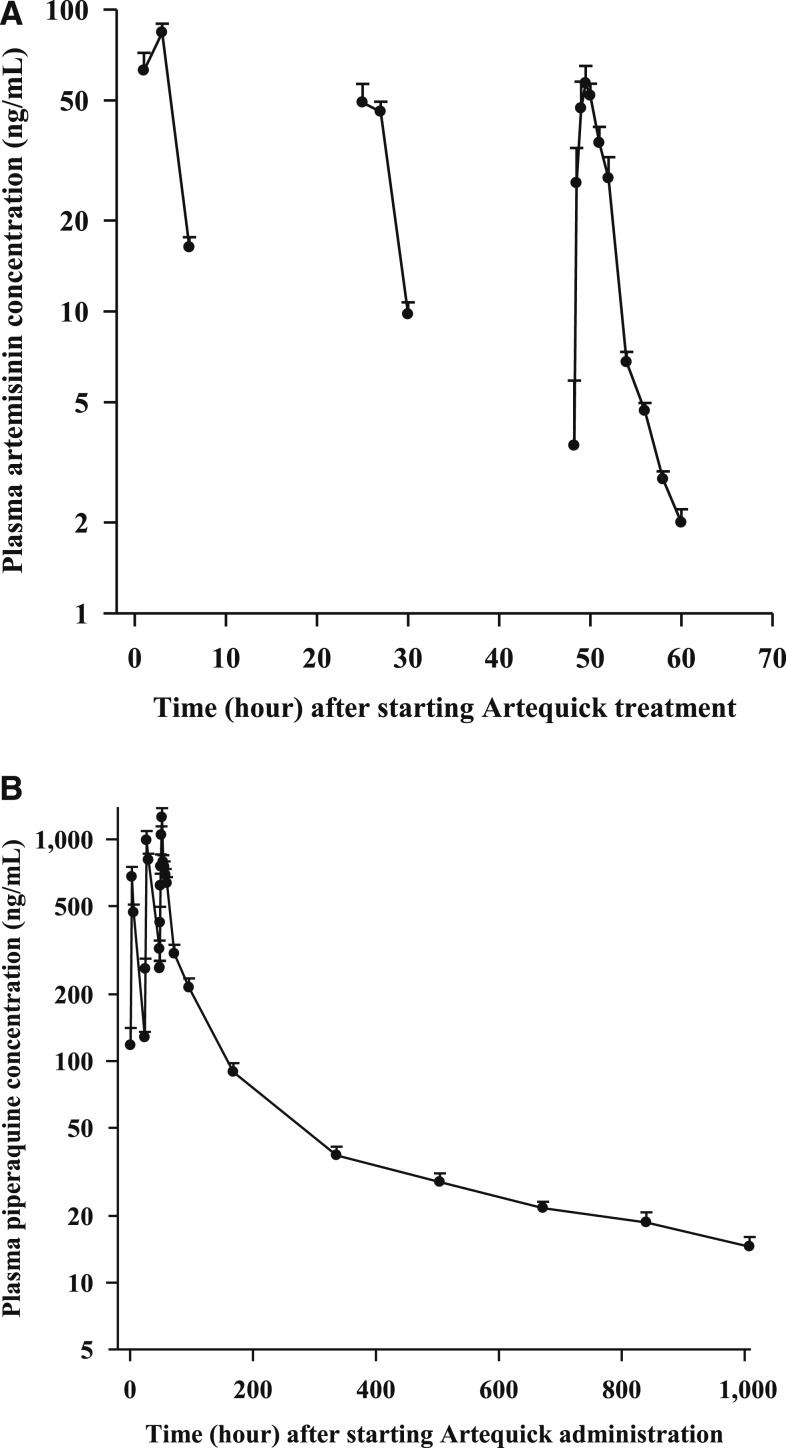
The mean (SD) plasma AUCs of ARN and PPQ after commencement of the 3-day regimen of Artequick are shown in Figure 1A and B, respectively, and PK properties are outlined in Table 1. Artemisinin was rapidly absorbed with a Cmax of 74.1 ng/mL being reached at 1.5 hours after dosing. Thereafter, plasma concentrations of ARN declined monoexponentially with a short t1/2 of 2.1 hours and a high CL/F of 9.8 L/h/kg.
Figure 1.
Mean (SD) artemisinin (ARN) (A) and piperaquine (PPQ) (B) concentration–time curves following the administration of ARN (125 mg)–PPQ base (750 mg) (Artequick) daily for 3 days in 22 healthy male Vietnamese volunteers.
Table 1.
Pharmacokinetic properties of ARN and PPQ in 22 healthy volunteers after a 3-day regimen of Artequick (ARN–PPQ)
| Pharmacokinetic parameter | ARN | PPQ | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| Total dose (mg/kg) | 6.3 | 6.3–6.5 | 37.5 | 37.5–38.8 |
| Cmax (ng/mL) | 74.1 | 50.4–97.5 | 1,259.2 | 882.5–1,821.5 |
| Tmax (hours) | 1.5 | 1.0–2.0 | 52.0 | 30.0–52.0 |
| t1/2 (hours) | 2.1 | 1.9–2.3 | 488.3 | 312.4–620.6 |
| CL/F (L/h/kg) | 9.8 | 9.3–12.8 | 0.4 | 0.4–0.5 |
| V/F (L/kg) | 32.4 | 24.0–36.9 | 268.8 | 233.5–410.5 |
| Vss/F (L/kg) | 36.9 | 28.5–47.9 | 149.6 | 121.5–218.4 |
| AUC48–t (ng·h/mL) for ARN | 206.2 | 165.3–230.6 | 78,479 | 62,013–89,544 |
| AUC0–t (ng·h/mL) for PPQ | ||||
| AUC48–∞ (ng·h/mL) for ARN | 212.8 | 168.2–237.7 | 91,804 | 74,908–101,637 |
| AUC48–∞ (ng·h/mL) for PPQ | ||||
| Extrapolated AUC (%) | 2.8 | 2.2–3.6 | 11.8 | 5.4–17.2 |
ARN = artemisinin; AUC = area under the concentration–time curve; AUC0–t or AUC48–t = area under the plasma drug AUC from either time 0 or 48 hour to the last sampling time; AUC0–∞ or AUC48–∞ = area under the plasma drug AUC from either time 0 or 48 hour to infinity; Cmax = maximum plasma concentration after the last oral administration; CL/F = clearance; extrapolated AUC = percentage of AUC0–∞ or AUC48–∞, extrapolated from the last observation to infinity; IQR = interquartile range; PPQ = piperaquine; t1/2 = terminal half-life; Tmax = observed time to reach Cmax after last dose; V/F = volume of distribution; Vss/F = volume of distribution at steady-state.
By contrast to ARN, PPQ absorption was considerably longer, with a Tmax of 4 hours and a Cmax of 1,259 ng/mL, after the last dose of the ACT. Piperaquine has a high V/F of 269 L/kg, suggesting the drug is widely distributed to tissues and has a low CL/F of 0.4 L/h/kg, which is reflected in its long t1/2 of 488 hours. Because of PPQ’s lengthy t1/2, the drug accumulates on multiple dosing (see Supplemental Materials).
The PK of ARS–AQ in healthy volunteers.
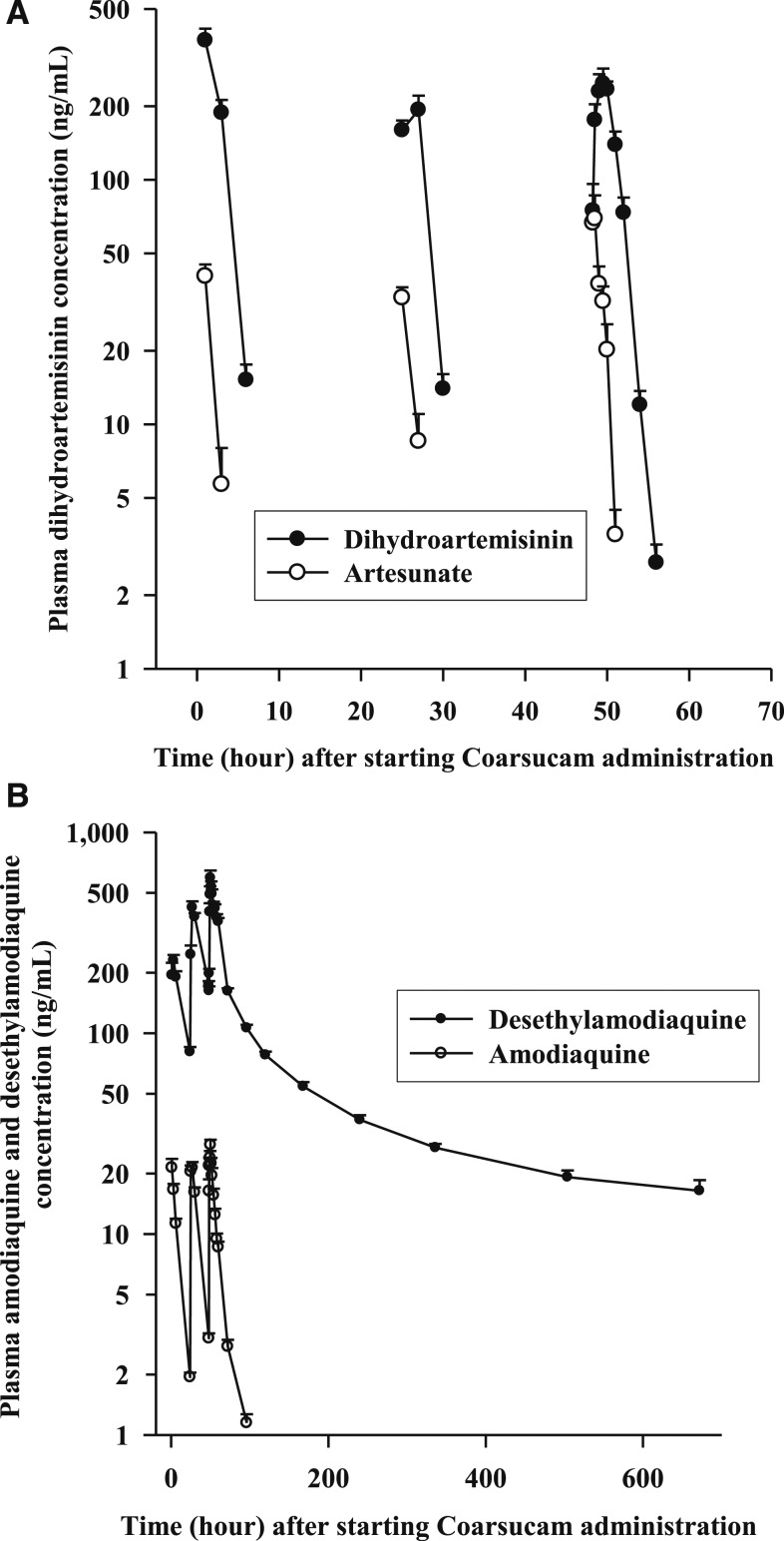
The mean (SD) plasma AUCs of ARS and AQ, including their principal metabolites DHA and DAQ, after commencement of Coarsucam are shown in Figure 2A and B, respectively. The PK properties of ARS, DHA, AQ, and DAQ are outlined in Table 2. Artesunate was rapidly and extensively hydrolyzed,26 primarily by plasma or tissue choline esterases, to DHA with marked interindividual variability in plasma concentrations of the ARN derivatives in the volunteers. The Cmax of ARS of 84 ng/mL was achieved at 0.5 hours followed by rapid elimination with a t1/2 of 0.5 hours. Corresponding PK values for DHA were 349 ng/mL, 1.5 and 0.9 hours.
Figure 2.
Mean (SD) artesunate (ARS) and dihydroartemisinin (A), and amodiaquine (AQ) and desethylamodiaquine (B) concentration–time curves following the administration of ARS (200 mg)–AQ (540 mg) (Coarsucam) daily for 3 days in 22 healthy male Vietnamese volunteers.
Table 2.
Pharmacokinetic properties of ARS, DHA, AQ, and DAQ in 22 healthy volunteers after a 3-day regimen of Coarsucam (ARS–AQ)
| Pharmacokinetic parameter | ARS | DHA | AQ | DAQ | ||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Total dose (mg/kg) | 9.7 | 9.2–10.0 | 7.2 | 6.8–7.4 | 26.1 | 24.9–27.0 | 24.1 | 23.0–24.9 |
| Cmax (ng/mL) | 84.0 | 57.8–126.4 | 349.1 | 231.6–446.7 | 35.0 | 29.0–38.7 | 670.3 | 562.6–785.5 |
| Tmax (hours) | 0.5 | 0.3–1.5 | 1.5 | 1.0–2.0 | 48.5 | 48.3–50.0 | 50.0 | 50.0–51.0 |
| t1/2 (hours) | 0.5 | 0.3–0.6 | 0.9 | 0.8–1.0 | 14.1 | 11.5–15.5 | 249.3 | 223.9–296.2 |
| CL/F (L/h/kg) | 32.7 | 23.8–43.6 | ND | ND | 31.5 | 28.1–34.3 | ND | ND |
| V/F (L/kg) | 22.0 | 12.3–32.2 | ND | ND | 633.9 | 465.6–711.8 | ND | ND |
| Vss/F (L/kg) | 35.0 | 28.0–49.8 | ND | ND | 1,185 | 1,022–1,406 | ND | ND |
| AUC48–t (ng·h/mL) | 84.4 | 68.6–121.2 | 704 | 572–1,020 | 824.6 | 728.3–906.4 | 39,180 | 33,942–44,347 |
| AUC48–∞ (ng·h/mL) | 94.0 | 70.6–129.5 | 704 | 574–1,028 | 834.6 | 736.8–916.4 | 45,607 | 38,566–53,838 |
| Extrapolated AUC (%) | 3.2 | 1.8–6.0 | 0.5 | 0.0–0.8 | 1.4 | 1.1–2.2 | 10.3 | 8.9–15.4 |
AQ = amodiaquine; AUC = area under the concentration–time curve; AUC48–t = area under the plasma drug concentration-time curve from 48 hours to the last sampling time; AUC48–∞ = area under the plasma drug concentration-time curve from 48 hours to infinity; Cmax = maximum plasma concentration after the last oral administration; CL/F = clearance; DAQ = desethylamodiaquine; DHA = dihydroartemisinin; extrapolated AUC = percentage of AUC0–∞ extrapolated from the last observation to infinity; IQR = interquartile range; ND = not determined; t1/2 = terminal half-life; Tmax = observed time to reach Cmax after last dose; V/F = volume of distribution; Vss/F = volume of distribution at steady-state.
Amodiaquine was rapidly absorbed and undergoes extensive hepatic metabolism primarily mediated by cytochrome CYP2C829 to DAQ. The Cmax of AQ was 35 ng/mL, and this was achieved at 0.5 hours after the last dose of Coarsucam. The Cmax of DAQ was 670 ng/mL, which occurred at 2 hours after the last dose of Coarsucam. With daily administration of the ACT and a t1/2 of 14.1 hours for AQ, plasma concentrations of the prodrug did not appreciably accumulate with Coarsucam administration (see Supplemental Materials). By contrast, DAQ with a t1/2 of 249 hours did markedly accumulate with the 3-day regimen of Coarsucam (see Supplemental Materials). Both AQ and DAQ have high V/F, suggesting wide tissue distribution.
Tolerability and safety of ARN–PPQ and ARS–AQ in healthy subjects.
Both 3-day regimens of ARN–PPQ and ARS–AQ were well tolerated by all participants with no adverse events reported. Hematology and biochemical indices were comparable and in the normal range before commencement and at day 7 after starting the two ACT administrations. In the present study, there was a significant (P = 0.013) increase in the QTc interval after Artequick administration from a mean of 392 ± 23 ms (range: 348–443 ms) immediately before commencement of the 3-day Artequick regimen to 412 ± 30 ms (range: 359–463 ms) at 6 hours after the last dose of the ACT. The 6-hour ECG test was associated with a median (IQR) plasma PPQ concentration of 704 (613–908) ng/mL. One volunteer on Artequick had an increase in the QT value over baseline greater than 60 ms (391–463 ms).
By contrast to Artequick, there was no significant difference (P = 0.232) in the volunteers’ QTc intervals after Coarsucam administration: from a mean of 395 ± 31 ms (range: 352–446 ms) immediately before commencement of the 3-day Coarsucam regimen to 405 ± 22 ms (range: 363–443 ms) at 6 hours after the last dose of the ACT with median (IQR) AQ and DAQ concentrations of 14.2 (11.0–17.2) ng/mL and 426 (363–467) ng/mL, respectively. None of the 22 volunteers on Coarsucam had an increase over baseline greater than 60 ms.
Ex vivo antimalarial activities of ARS–AQ and ARN–PPQ.
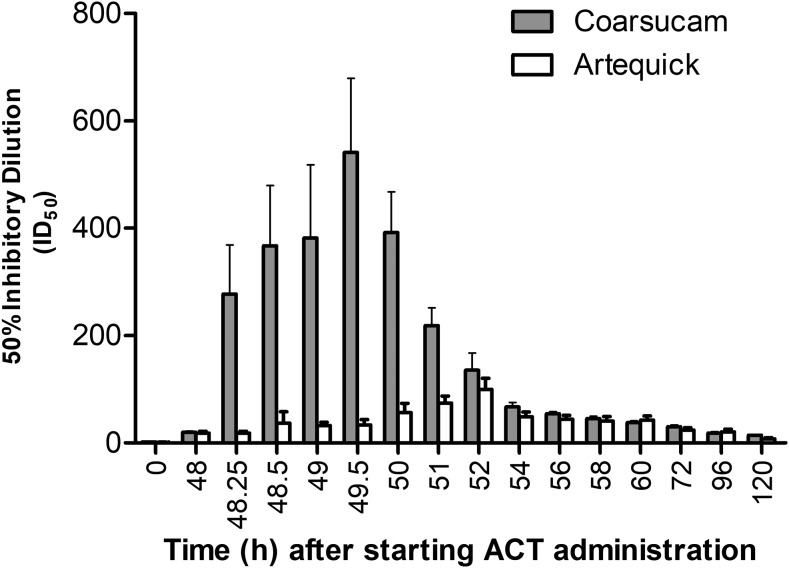
The ex vivo antimalarial activities of ARS–AQ and ARN–PPQ were assessed using plasma samples collected from seven healthy Vietnamese volunteers who had received both ACTs. The mean (SEM) ID50 of plasma samples from the volunteers collected before dosing and at various times up to 120 hours after the last dose of each ACT are shown in Figure 3 against the D6 line. Artesunate–AQ was more potent than ARN–PPQ with its antimalarial activity 2.9- to 16.2-fold higher from 0.25 to 3.0 hours after the last dose. The highest ID50 for ARS–AQ was 541 at 1.5 hours after the last dose, which was 5.4-fold higher than that of ARN–PPQ (ID50 of 100 at 4 hours after the last dose).
Figure 3.
Comparison of the mean (SEM) inhibitory dilution (ID50: number of dilutions of plasma sample that produces a 50% inhibition of hypoxanthine uptake into malaria parasites compared to drug-free plasma control samples) of plasma samples obtained from seven healthy Vietnamese volunteers who were administered on separate occasions a 3-day course of the artemisinin combination therapies (ACT) [artesunate-amodiaquine (Coarsucam) or artemisinin-piperaquine (Artequick)] against the drug-sensitive D6 Plasmodium falciparum line. The difference between the two treatments is statistically significant P < 0.05 for the time points from 48.25 to 51 hours (inclusive) and not statistically significant for all other time points.
The ex vivo potency of ARS–AQ and ARN–PPQ was further evaluated against an ARN-sensitive (MRA1239) and an ARN-resistant (MRA1240) P. falciparum line. The MRA1240 parasites carry mutation in the PfKelch 13 gene (R539T), which confers ARN resistance in clinical isolates collected from Cambodia.30 Plasma samples collected from six of seven volunteers who received both ACTs were tested at 0 (pre-first dose), 1.5, 4.0, and 24 hours after the last dose. There was insufficient plasma samples available from the seventh volunteer to perform the ex vivo potency tests. Drug concentrations in samples collected at 1.5 post-last dose were close to the volunteer’s Cmax values for both ARN and DHA.
When comparing the drug susceptibility profiles (i.e., IC50 values, Table 3) of the two lines, MRA1240 was 3.8-fold less susceptible to DHA and 2.4-fold less susceptible to DAQ compared with MRA1239. The MRA1240 line was also 4.9-fold less susceptible to ARN and 1.3-fold less susceptible to PPQ compared with MRA1239.
Table 3.
In vitro activities of antimalarial (IC50, nM) in 50% plasma against Plasmodium falciparum isolates with different drug susceptibilities
| Drug | D6 | MRA1239 | MRA1240 |
|---|---|---|---|
| Artemisinin | 13.2 ± 2.6 | 8.5 ± 2.8 | 41.6 ± 11.9 |
| Piperaquine | 33.2 ± 5.5 | 104.2 ± 12.1 | 131.2 ± 28.9 |
| Artesunate | 3.3 ± 1.1 | ND± | ND± |
| Dihydroartemisinin | 2.5 ± 0.1 | 2.4 ± 1.2 | 9.0 ± 1.9 |
| Amodiaquine | 8.54 ± 0.03 | ND± | ND± |
| Desethylamodiaquine | 8.2 ± 0.3 | 14.8 ± 6.8 | 34.8 ± 8.6 |
ND = not determined. Mean ± SD. IC50 data based on at least two independent experiments.
The mean ID50 values using the volunteers’ plasma samples against the MRA1239 and MRA1240 lines are outlined in Table 4. Based on the intrinsic in vitro activities of ARN, DHA, DAQ, and PPQ and their PK exposure, the volunteers’ plasma ID50 values were higher against the ARN-sensitive MRA1239 line than that of the multidrug-resistant MRA1240 line for both ACTs. When comparing the ex vivo antimalarial activity of the two ACTs against both MRA lines, ARS–AQ was significantly (P < 0.05) more active than ARN–PPQ by 20.8-fold against MRA1239 and by 5.4-fold against MRA1240 at 1.5 hours after dosing. However, by 24 hours after the last dose, the difference in ex vivo antimalarial activity was less between the two ACTs with ARS–AQ being 8.5-fold (P < 0.05) more active than ARN–PPQ against MRA1239. There were no significant differences between the two treatments against the MRA1240 line at 4 or 24 hours (ID50: 83.6-fold versus 96.4-fold or 9.3-fold versus 9.6-fold, respectively).
Table 4.
Comparison of the inhibitory dilution (ID50: number of dilutions of plasma sample that produces a 50% inhibition of hypoxanthine uptake into malaria parasites compared with drug-free plasma control samples) of plasma samples obtained from six healthy Vietnamese volunteers who were administered either a 3-day regimen of ARS–AQ (Coarsucam) or ARN–PPQ (Artequick) against the ARN-sensitive MRA1239 and the ARN-resistant MRA1240 Plasmodium falciparum lines
| Time (hour) | MRA1239 ID50 (fold) ARS–AQ | MRA1239 ID50 (fold) ARN–PPQ | Difference ratio* (P value) |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| D0 | 0 ± 0 | 0 ± 0 | – |
| D2—1.5 hours | 1,117.3 ± 265.5 | 53.7 ± 14.7 | 20.8 (0.0428) |
| D2—4.0 hours | 360.8 ± 98.5 | 103.1 ± 19.2 | 3.5 (0.0417) |
| D3—24 hours | 169.4 ± 15.5 | 20.0 ± 5.6 | 8.5 (0.0003) |
| Time (hours) | MRA1240 ID50 (fold) ARS–AQ | MRA1240 ID50 (fold) ARN–PPQ | Difference ratio* (P value) |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| D0 | 0 ± 0 | 0 ± 0 | – |
| D2—1.5 hours | 252.9 ± 57.2 | 46.8 ± 11.1 | 5.4 (0.0206) |
| D2—4.0 hours | 83.6 ± 17.1 | 96.4 ± 18.0 | 0.9 (0.5891) |
| D3—24 hours | 9.3 ± 0.8 | 9.6 ± 2.4 | 1.0 (0.2510) |
AQ = amodiaquine; ARN = artemisinin; ARS = artesunate; PPQ = piperaquine.
Difference: ratio of ID50 values for ARS-AQ/ARN-PPQ against MRA1239 and MRA1240.
Relationship between PK and PD of the two ACTs.
The markedly higher ex vivo antimalarial activity in the volunteers’ plasma samples after Coarsucam compared with Artequick administration is due to a composite of ARS and DHA superior intrinsic in vitro activities compared with ARN. Notably, the timing of the peak ex vivo activity of ARS–AQ coincides with the Cmax of DHA. Furthermore, DHA plasma AUC0–∞ after the last dose was 3.3-fold greater than that of ARN (704 ng·h/mL versus 213 ng·h/mL), which would provide a far greater window of rapid-acting blood schizontocidal activity after Coarsucam administration compared with that of Artequick. Although PPQ’s plasma AUC was 2.0-fold higher than that of DAQ following administration of the two ACTs (AUC0–∞: 91,804 ng·h/mL versus 45,607 ng·h/mL), PPQ is markedly less active than DAQ in vitro by 4- to 7-fold against the three P. falciparum lines suggesting that there may not be a large difference in the ex vivo potency of the two ACTs, particularly after the clearance of either DHA or ARN.
DISCUSSION
The PK of ARN–PPQ in healthy volunteers.
This is the first study to report on the PK properties of ARN–PPQ after Artequick administration in healthy volunteers and follows from our study of Artequick versus Coarsucam for uncomplicated falciparum malaria treatment.6 Although the manufacturer of Artequick recommends only a 2-day treatment regimen for falciparum malaria, we evaluated a 3-day regimen of the ACT, as studies in Thailand have shown a 3-day regimen to be far more efficacious than the 2-day regimen (cure rate: 98.2% versus 71.5%).5
After adjusting for dose differences, the PK properties of ARN derived in the present study were similar to that reported in healthy Vietnamese volunteers administered single oral doses of 250, 500, and 1,000 mg of ARN.31 Artemisinin exhibits a time-dependent autoinductive effect on drug metabolism, with AUC values declining after repetitive daily dosing of ARN alone.32 In the present study, there was evidence of autoinduction of ARN metabolism, with the AUC of ARN after the third dose of the ACT being 1.9-fold less than that after the first dose (175.5 ng·h/mL versus 328 ng·h/mL using data points of 1, 3, and 6 hours post-dose).
The PPQ PK properties are in good agreement with other studies in healthy volunteers administered the drug alone33–36 or coadministered with DHA.37,38 Similar to other studies, multiple peaks were observed in the PPQ concentration–time profiles of 82% (18 of 22) of participants. The multiple kinetics of PPQ may be because of enterohepatic recirculation33 or erratic dissolution–absorption regulated by gastric emptying or precipitation and slow redissolution as the lipophilic base passes from the acidic environment of the stomach to the more alkaline small intestine.37
The PK of ARS–AQ in healthy volunteers.
Despite Coarsucam being registered in more than 35 countries worldwide and more than 400 million treatments have been made available since its launch in 2007 as a Drugs for Neglected Diseases Initiative in partnership with Sanofi-Aventis,39 there is a paucity of information on the PK of the fixed-dose combination in healthy volunteers. This is the first study to report on the PK properties of ARS and AQ following the standard 3-day regimen of Coarsucam. Overall, the PK properties of ARS, DHA, AQ, and DAQ obtained in the present study are in broad agreement with other studies in healthy volunteers, given either ARS or AQ alone or in combination with a longer acting antimalarial drug, including AQ.40–45
Tolerability and safety of ARN–PPQ and ARS–AQ in healthy subjects.
Both ACTs were well tolerated by the healthy volunteers with no adverse events reported. The only safety concern in the group of 22 volunteers who received Artequick was a participant who had an increase in the QT value over baseline greater than 60 ms, which is considered a clinical concern.46 Piperaquine has been shown to prolong the QTc interval in a concentration-dependent way in healthy volunteers,47 and QTc prolongation is described in the clinical use of DHA–PPQ marketed as Eurartesim®, with the safety precaution that the ACT has the potential to cause pro-arrhythmias and is to be avoided in patients on other drugs that may cause QTc prolongation.48 None of the volunteers on ARS–AQ had an increase in the QT value over baseline greater than 60 ms.
Ex vivo antimalarial activities of ARS–AQ superior to ARN–PPQ.
The intrinsic value of the ex vivo bioassay is that it measures the potency of both parent drugs and their active metabolites at physiological plasma drug concentrations. In the present study, we have used parasite lines with varying drug susceptibilities, including the highly multidrug (chloroquine, DHA, and mefloquine)-resistant MRA1240 strain from Cambodia. At 1.5 hours after the last dose of ARS–AQ, the mean ex vivo antimalarial activities of the volunteer’s plasma samples was at least 5.4-fold greater than that of ARN–PPQ against the drug-sensitive P. falciparum D6 and MRA1239 lines, and the multidrug-resistant MRA1240 line. Although the ex vivo ID values obtained in the present study cannot directly predict the in vivo efficacy of ARS–AQ against P. falciparum, they do provide preliminary baseline data in comparing the ex vivo response between ARN-sensitive and ARN-resistant lines from Cambodia, the epicenter of antimalarial drug resistance.49
Comparison of PK–PD studies of ARN–PPQ and ARS–AQ in healthy subjects.
The findings in the present study show the superior ex vivo antimalarial activity in plasma samples obtained from volunteers after receiving a standard therapeutic regimen of Coarsucam compared with Artequick. The PK analysis of the partner drugs of Artequick revealed low plasma concentrations of ARN compared with higher DHA concentrations associated with Coarsucam administration. Caution needs to be exercised with the high concentrations of PPQ following a 3-day regimen of Artequick to enhance efficacy, with potential concerns of QTc prolongation cardiotoxicity.
A distinct feature of the ex vivo assay is that preliminary pharmacodynamic data can be obtained at an early stage of clinical development. Ideally, at Phase 1 of drug development, at the same time blood samples are collected for PK analysis, additional samples could be obtained for ex vivo potency analysis against multiple antimalarial drug–resistant P. falciparum lines. By studying the PK–PD relationship of new antimalarial compounds at Phase I, the drug concentration to potency data could be applied for dose optimization and be an important criterion for the go/no-go decision for Phase II studies. This cost-effective process may assist in fast tracking the selection and development of new drugs.
In conclusion, our PK study in healthy volunteers supported by the ex vivo antimalarial assay provides a useful approach in studying the PK–PD relationship of new and established ACTs or non-ACTs in the preselection process for future in vivo efficacy studies. The superior PK–PD profile of ARS–AQ compared with that of ARN–PPQ would favor Coarsucam over Artequick for clinical trials in areas of reduced susceptibility to ARNs. Furthermore, reports of increasing clinical treatment failures of dihydroartemisinin-piperaquine in western Cambodia9 and in south Vietnam,13 would suggest that the usefulness of Artequick as an alternative ACT in malaria treatment appears limited at least in Southeast Asia.
Supplementary Material
Supplemental materials, Figures, and Tables.
Acknowledgments:
This study was carried out under the auspices of the Vietnam Australia Defence Malaria Project, a defence cooperation between the Vietnam People’s Army and the Australian Defence Force. We are most grateful to the volunteers who participated in the study and to Nguyen Minh Thu for excellent technical support and her nursing colleagues at Central Military Hospital 108 for blood collection. We are grateful to the Australian Red Cross Blood Service for the provision of human red blood cells and plasma for in vitro cultivation of Plasmodium falciparum lines. We thank Valérie Lameyre from Sanofi (Access to Medicines) for assisting with the procurement of Coarsucam.
Note: Supplemental materials, figures, and tables appear at www.ajtmh.org.
Disclaimer: The opinions expressed are those of the authors and do not necessarily reflect those of the Australian Defence Organization or any extant policy.
REFERENCES
- 1.World Health Organization , 2015. World Malaria Report 2015 Geneva, Switzerland: WHO. Available at: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/. Accessed April 23, 2018.
- 2.Bhatt S, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization , 2018. Malaria Geneva, Switzerland: WHO. Available at: http://www.who.int/mediacentre/factsheets/fs094/en/. Accessed April 23, 2018.
- 4.World Health Organization , 2015. Guidelines for the Treatment of Malaria, 3rd edition. Geneva, Switzerland: WHO. Available at: http://www.who.int/malaria/publications/atoz/9789241549127/en/. Accessed April 23, 2018.
- 5.Krudsood S, Tangpukdee N, Thanchatwet V, Wilairatana P, Srivilairit S, Pothipak N, Jianping S, Guoqiao L, Brittenham GM, Looareesuwan S, 2007. Dose ranging studies of new artemisinin-piperaquine fixed combinations compared to standard regimens of artemisisnin combination therapies for acute uncomplicated falciparum malaria. Southeast Asian J Trop Med Public Health 38: 971–978. [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TX, Trieu TN, Nguyen PC, Huynh QH, Bui D, Shanks GD, Chavchich M, Edstein MD, 2012. The efficacy and tolerability of artemisinin-piperaquine (Artequick®) versus artesunate-amodiaquine (Coarsucam™) for the treatment of uncomplicated Plasmodium falciparum malaria in south–central Vietnam. Malar J 11: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salman S, Page-Sharp M, Batty KT, Kose K, Griffin S, Siba PM, Ilett KF, Mueller I, Davis TM, 2012. Pharmacokinetic comparison of two piperaquine-containing artemisinin combination therapies in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother 56: 3288–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C, 2009. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders DL, Vanachayangkul P, Lon C, 2014. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 371: 484–485. [DOI] [PubMed] [Google Scholar]
- 10.Na-Bangchang K, Ruengweerayut R, Mahamad P, Ruengweerayut K, Chaijaroenkul W, 2010. Declining in efficacy of a three-day combination regimen of mefloquine-artesunate in a multi-drug resistance area along the Thai-Myanmar border. Malar J 9: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hien TT, et al. 2012. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J 11: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thriemer K, et al. 2014. Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob Agents Chemother 58: 7049–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanh NV, et al. 2017. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin-piperaquine in the south of Vietnam. Malar J 16: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edstein MD, Yeo AE, Kyle DE, Looareesuwan S, Wilairatana P, Rieckmann KH, 1996. Proguanil polymorphism does not affect the antimalarial activity of proguanil combined with atovaquone in vitro. Trans R Soc Trop Med Hyg 90: 418–421. [DOI] [PubMed] [Google Scholar]
- 15.Na-Bangchang K, Tippawangkosol P, Thanavibul A, Ubalee R, Karbwang J, 1999. Pharmacokinetic and pharmacodynamic interactions of mefloquine and dihydroartemisinin. Int J Clin Pharmacol Res 19: 9–17. [PubMed] [Google Scholar]
- 16.Kongthaisong M, Na-Bangchang K, Mungthin M, Sinchaipanid N, Tan-Ariya P, 2004. Comparison of the bioequivalence of three oral formulations of dihydroartemisinin based on ex vivo blood schizontocidal activities against Plasmodium falciparum. Am J Trop Med Hyg 71: 703–710. [PubMed] [Google Scholar]
- 17.Teja-Isavadharm P, Peggins JO, Brewer TG, White NJ, Webster HK, Kyle DE, 2004. Plasmodium falciparum-based bioassay for measurement of artemisinin derivatives in plasma or serum. Antimicrob Agents Chemother 48: 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen DV, Nguyen QP, Nguyen ND, Le TT, Nguyen TD, Dinh DN, Nguyen TX, Bui D, Chavchich M, Edstein MD, 2009. Pharmacokinetics and ex vivo pharmacodynamic antimalarial activity of dihydroartemisinin-piperaquine in patients with uncomplicated falciparum malaria in Vietnam. Antimicrob Agents Chemother 53: 3534–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavchich M, Birrell GW, Ager AL, MacKenzie DO, Heffernan GD, Schiehser GA, Jacobus LR, Shanks DG, Jacobus DP, Edstein MD, 2016. Lead selection of the new aminomethylphenol, JPC-3210 for malaria treatment and prevention. Antimicrob Agents Chemother 60: 3115–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO , 2016. WHO Plans for Reviewing the Cardiotoxicity of Antimalarial Medicines Salle A, ed. Malaria Policy Advisory Committee (MPAC) Meeting, September 14–16, 2016. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/malaria/mpac/mpac-sep2016-cardiotoxicity-of-antimalarials.pdf?ua=1. Accessed April 23, 2018.
- 21.Lindegardh N, et al. 2008. Major pitfalls in the measurement of artemisinin derivatives in plasma in clinical studies. J Chromatogr B Analyt Technol Biomed Life Sci 876: 54–60. [DOI] [PubMed] [Google Scholar]
- 22.Lourens C, Lindegardh N, Barnes KI, Guerin PJ, Sibley CH, White NJ, Tarning J, 2014. Benefits of a pharmacology antimalarial reference standard and proficiency testing program provided by the worldwide antimalarial resistance network (WWARN). Antimicrob Agents Chemother 58: 3889–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD, 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 16: 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ovenden SP, Cobbe M, Kissell R, Birrell GW, Chavchich M, Edstein MD, 2011. Three novel phenolic glycosides with antimalarial activity from Grevillea “Poorinda Queen.” J Nat Prod 74: 74–78. [DOI] [PubMed] [Google Scholar]
- 25.Nagelschmitz J, Voith B, Wensing G, Roemer A, Fugmann B, Haynes RK, Kotecka BM, Rieckmann KH, Edstein MD, 2008. First-time-in-humans safety, tolerability, pharmacokinetics and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative, artemisone. Antimicrob Agents Chemother 52: 3085–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin C-S, Fleckenstein L, 2011. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar J 10: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis TM, Phuong HL, Ilett KF, Hung NC, Batty KT, Phuong VDB, Powell SM, Thien HV, Binh TQ, 2001. Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria. Antimicrob Agents Chemother 45: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh S, Ouedraogo JB, Goldstein JA, Rosenthal PJ, Kroetz DL, 2007. Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin Pharmacol Ther 82: 197–203. [DOI] [PubMed] [Google Scholar]
- 29.Li XQ, Björkman A, Andersson TB, Ridderström M, Masimirembwa CM, 2002. Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J Pharmacol Exp Ther 300: 399–407. [DOI] [PubMed] [Google Scholar]
- 30.Straimer J, et al. 2015. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 347: 428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashton M, Gordi T, Hai TN, Huong NV, Sy ND, Nieu NT, Huong DX, Johansson M, Cong DL, 1998. Artemisinin pharmacokinetics in healthy adults after 250, 500, and 1000 mg single oral doses. Biopharm Drug Dispos 19: 245–250. [DOI] [PubMed] [Google Scholar]
- 32.Ashton M, Hai TN, Sy ND, Huong DX, Huong NV, Niêu NT, Công LD, 1998. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos 26: 25–27. [PubMed] [Google Scholar]
- 33.Sim IK, Davis TME, Ilett KF, 2005. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob Agents Chemother 49: 2407–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed T, Sharma P, Gautam A, Varshney B, Kothari M, Ganguly S, Moehrle JJ, Paliwal J, Saha N, Batra V, 2008. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. J Clin Pharmacol 48: 166–175. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen TC, Nguyen NQ, Nguyen XT, Bui D, Travers T, Edstein MD, 2008. Pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am J Trop Med Hyg 79: 620–623. [PubMed] [Google Scholar]
- 36.Reuter SE, Evans AM, Shakib S, Lungershausen Y, Francis B, Valentini G, Bacchieri A, Ubben D, Pace S, 2015. Effect of food on the pharmacokinetics of piperaquine and dihydroartemisinin. Clin Drug Investig 35: 559–567. [DOI] [PubMed] [Google Scholar]
- 37.Hai TN, Hietala SF, Huong NV, Ashton M, 2008. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop 107: 145–149. [DOI] [PubMed] [Google Scholar]
- 38.Chinh NT, Quang NN, Thanh NX, Dai B, Travers T, Edstein MD, 2009. Pharmacokinetics and bioequivalence evaluation of two fixed tablet formulations of dihydroartemisinin and piperaquine in Vietnamese subjects. Antimicrob Agents Chemother 53: 828–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pécoul B, Sevcsik A-M, Amuasi J, Diap G, Kiechel JR, 2008. The story of ASAQ: the first antimalarial product development partnership success. Health Partnerships Review Geneva, Switzerland: Global Forum for Health Research, 77–83. Available at: https://www.dndi.org/2008/media-centre/scientific-articles/scientific-articles-malaria/the-story-of-asaq-the-first-antimalarial-product-development-partnership-success/. Accessed April 23, 2018. [Google Scholar]
- 40.Fitoussi S, Thang C, Lesauvage E, Barré J, Charron B, Filali-Ansary A, Lameyre V, 2009. Bioavailability of a co-formulated combination of amodiaquine and artesunate under fed and fasted conditions. A randomised, open-label crossover study. Arzneimittelforschung 59: 370–376. [DOI] [PubMed] [Google Scholar]
- 41.Teja-Isavadharm P, Watt G, Eamsila C, Jongsakul K, Li Q, Keeratithakul G, Sirisopana N, Luesutthiviboon L, Brewer TG, Kyle DE, 2001. Comparative pharmacokinetics and effect kinetics of orally administered artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am J Trop Med Hyg 65: 717–721. [DOI] [PubMed] [Google Scholar]
- 42.Batty KT, Ilett KF, Powell SM, Martin J, Davis TM, 2002. Relative bioavailability of artesunate and dihydroartemisinin: investigations in the isolated perfused rat liver and in healthy Caucasian volunteers. Am J Trop Med Hyg 66: 130–136. [DOI] [PubMed] [Google Scholar]
- 43.Navaratnam V, Ramanathan S, Wahab MS, Siew Hua G, Mansor SM, Kiechel JR, Vaillant M, Taylor WR, Olliaro P, 2009. Tolerability and pharmacokinetics of non-fixed and fixed combinations of artesunate and amodiaquine in Malaysian healthy normal volunteers. Eur J Clin Pharmacol 65: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orrell C, Little F, Smith P, Folb P, Taylor W, Olliaro P, Barnes KI, 2008. Pharmacokinetics and tolerability of artesunate and amodiaquine alone and in combination in healthy volunteers. Eur J Clin Pharmacol 64: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chinh NT, Quang NN, Thanh NX, Dai B, Chavchich M, Birrell G, Edstein MD, 2011. The pharmacokinetics and ex vivo antimalarial activity of artesunate plus azithromycin in healthy volunteers. Antimicrob Agents Chemother 55: 4412–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Food and Drug Administration , 2005. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-antiarrhythmic Drugs. Rockville, MD: FDA. [Google Scholar]
- 47.Darpo B, Ferber G, Siegl P, Laurijssens B, Macintyre F, Toovey S, Duparc S, 2015. Evaluation of the QT effect of a combination of piperaquine and a novel anti-malarial drug candidate OZ439, for the treatment of uncomplicated malaria. Br J Clin Pharmacol 80: 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eurartesim , 2011. EU Summary of Product Characteristics Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001199/WC500118113.pdf. Accessed April 23, 2018.
- 49.Wongsrichanalai C, Meshnick SR, 2008. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia–Thailand border. Emerg Infect Dis 14: 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental materials, Figures, and Tables.