Abstract
Background:
Dietary phytochemical index (DPI) has introduced as an inexpensive method for quantifying the phytochemicals in foods. For the first time, this study was conducted to investigate the relationship between DPI and the risk of prediabetes.
Methods:
Three hundred participants were assigned to 150 prediabetics (cases) and 150 healthy (controls) groups. Anthropometric values, fasting blood glucose, and 2-h oral glucose tolerance test (OGTT) were measured. The DPI was calculated based on data collected from 168-item validated food frequency questionnaire.
Results:
The sex-specific energy-adjusted DPI was inversely related to fasting blood glucose (FBG) and OGTT (P < 0.001). The odds ratio (OR) of prediabetes was assessed across sex-specific energy-adjusted DPI quartiles. After adjusting for body mass index, physical activity, education, dietary intake of energy, fiber, carbohydrate (% of energy), fat (% of energy), and protein (% of energy), the OR of prediabetes across the sex-specific energy-adjusted DPI quartiles decreased significantly (P-trend < 0.001).
Conclusions:
We found that higher DPI score is related to lower prediabetes OR. This simple method may be used for the improvement of dietary intake to prevent prediabetes.
Keywords: Blood glucose, fruit, insulin resistance, phytochemical, prediabetic, vegetables
Introduction
Prediabetes has been defined as a progression from normal range of glucose tolerance to clinical type 2 diabetes mellitus (T2DM). It represents a stage of impaired glucose tolerance ([IGT], 2-h oral glucose tolerance test [OGTT] 140–199 mg/dl), and impaired fasting glucose (110–125 mg/dl).[1] Five to ten percent of people with prediabetes will progress to T2DM/year.[2] The prevalence of prediabetes has increased worldwide during the past decades.[3] The National survey of Tehran Lipid and Glucose Study reported that from each thousand men and women, 46 men and 38 women became prediabetic after 9-year follow-up.[4]
Previous studies linked diet rich in fresh fruits and vegetables, whole grains, nuts, legumes, and plant-based foods, which are high in phytochemicals, fiber, and antioxidants with chronic diseases risk reduction.[5,6] Three-year follow-up in Tehran adults showed higher intake of phytochemical-rich foods was negatively associated with occurrence of hyperinsulinemia and insulin resistance.[7] Data from other prospective and meta-analysis studies have shown an inverse relationship between consumption of fruits and vegetables and the risk of obesity and abdominal obesity as main predictors of glucose intolerance and prediabetes.[8,9]
Chemical compounds found abundantly in plant foods including polyphenols, phytoestrogens, and organosulfur and plant sterols by acting as antioxidant and balancing the inflammation, provide protection against the development of insulin resistance, abnormal glucose, and lipid disturbances.[10,11,12]
For the first time, health promotional effects of phytochemicals proposed by McCarty as the phytochemical index (PI), which defined as the percent of dietary calories derived from foods rich in phytochemicals. Quantification of phytochemicals in dietary intake or in biological samples is expensive. Hence, PI could be an alternative for quantifying the phytochemicals in consumed foods.[13] This index is a simple method for assessment of phytochemical intake and despite its limitations could provide important background for diet quality and may have high practical and clinical use for food selection improvement.[14]
Several studies have been conducted to investigate the association between dietary PI (DPI) and the risk of chronic diseases. Several prospective studies investigated the relation of DPI with cardiometabolic risk factors, lipid profile changes, and visceral obesity in healthy subjects.[15,16,17] A study in overweight young adult showed inverse association between DPI with weight gain and blood biomarkers of oxidative stress.[14] However, association of DPI and risk of prediabetes has not been determined yet. This time, we aimed to investigate the relationship between DPI and prediabetes morbidity in a case–control study.
Methods
Study design
Three hundred men and women including 150 healthy and 150 prediabetic participants from diabetes screening center in Shahreza, Iran were invited for this case–control study from May to October 2014. The aim of the study was to determine the relationship between dietary intake and prediabetes using a matched case–control study design. The methods of study were described previously[18] and are briefly described here. Participants above 30 years with at least one of the following criteria: Having body mass index (BMI) ≥25 kg/m2, a family history of diabetes, or reporting of at least two symptoms of diabetes were considered at risk of diabetes and were referred to this center. We recruited 150 participants with prediabetes (cases) and 150 healthy individuals with normal fasting blood glucose (FBG) (control) at this center. The inclusion criteria for case group were age 35–65 years, and FBG 100–125 mg/dl or 2-h OGTT 140–199 mg/dl, diagnosed <3 months before enrollment to the study. The inclusion criteria for the control group were age 35–65 years and FBG <100 mg/dl and 2 h OGTT <140 mg/dl during screening. Participants were not enrolled into the study if they were using alcohol, drug, and any tobacco products, having BMI ≥40 kg/m2. In addition, pregnant or lactation women, participants with special diet during the last year, participants with a history of heart disease, diabetes, hypertension, dyslipidemia, renal or hepatic failure, and multiple sclerosis were not included in the study. We used the frequency matching method and matched the two groups by age and sex. The age-frame for matching was 35–44, 45–54, and 55–65 years. This study was approved by Ethics Committee of Tehran University of Medical Sciences. All participants read and signed a written informed consent before participation.
Physical activity, anthropometric, and biochemical assessment
Height was measured to the nearest 0.1 cm while standing barefoot using the Seca 216 stadiometer. Body weight was determined while participants wore undergarments, using a Seca scale to the nearest 0.1 kg. Waist circumference (WC) was measured using tape at the midpoint between the lowest rib and the iliac crest. WC ≥94 for men and ≥80 for women were considered as the cut points for central obesity.[19] Physical activity was assessed with the short form of the International Physical Activity Questionnaire.[20] This measure was selected to assess the physical activity during the previous week by multiplying the duration and frequency of activity days to the metabolic equivalent task value of the activity. The total of the scores was considered as the total physical activity per week.
Blood samples were taken after at least 8 h fasting for FBG measurement. Moreover, the participants underwent a 2-h OGTT. The plasma glucose level was measured at 546 nm using the photometric method (glucose oxidase method).
Food intake assessment
Dietary data were collected using a validated semi-quantitative food frequency questionnaire (FFQ) with 168 food items.[21] Trained dietitian asked participants to report their consumption frequency for each food items consumed during the previous year on a daily, weekly, or monthly basis. Portion sizes of foods were reported in household measures then converted to grams. The Excel program analyzed the nutrients of each food item. Food items were analyzed for energy content using Nutritionist 4 software ( First Databank Inc., Hearst Corp, San Bruno, CA, USA) modified for Iranian foods.
The DPI was calculated based on the assessment method proposed by McCarty[13] as the percent of dietary calorie derived from foods rich in phytochemicals; (PI = [daily energy derived from phytochemical-rich foods [kcal]/total daily energy derived from food intake [kcal] ×100). Fruits and vegetables, (except potatoes), legumes, whole grains, nuts, seeds, olive and olive oil, were considered as the photochemical-rich foods categories.
Statistical analysis
The Kolmogorov–Smirnov test was used to evaluate the normality of the data. The qualitative variables were compared in case and control groups using Chi-square test. The quantitative variables with nonnormal distribution were compared using Mann–Whitney test. Energy-adjusted DPI was determined using the residual method.[22] Energy-adjusted DPI was categorized based on sex-specific quartiles. The mean values of quantitative variables were compared across the sex-specific energy-adjusted DPI quartiles categories using the ANOVA test. To assess the overall trends of odds ratio (OR) across the sex-specific energy-adjusted DPI quartiles categories, the simple logistic regression models were used: Model 1 = crude and Model 2 = adjusted for BMI, physical activity, education, dietary intake of energy, fiber, carbohydrate (% of energy), fat (% of energy), and protein (% of energy). Statistical analysis was conducted using SPSS Version 16.0; (SPSS Inc., Chicago, IL) and P < 0.05 were considered statistically significant.
Results
Characteristics, daily intakes of various phytochemical-rich foods groups, and energy-adjusted DPI of the participants in the case and control groups are shown in Table 1. Years of education were significantly higher in prediabetic participants (P = 0.002), they also had higher BMI value (P = 0.001) and lower physical activity (P < 0.001). Prediabetic participants consumed fewer phytochemical-rich foods from all the food groups, and subsequently, had lower DPI score than their control counterparts (P < 0.001). Furthermore, other characteristics such as energy intake (2433.1 ± 290.7 vs. 2231.4 ± 297.5 kcal/d), fasting blood glucose (FBG) (109.2 ± 6.5 vs. 82.1 ± 7.1 mg/dl), and OGTT (143.6 ± 18.3 vs. 120.4 ± 9.5 mg/dl) were also higher in prediabetic participants in comparison with control group (P < 0.001).
Table 1.
Characteristics, daily intake of phytochemical-rich food groups, and phytochemical index in control and prediabetic participants
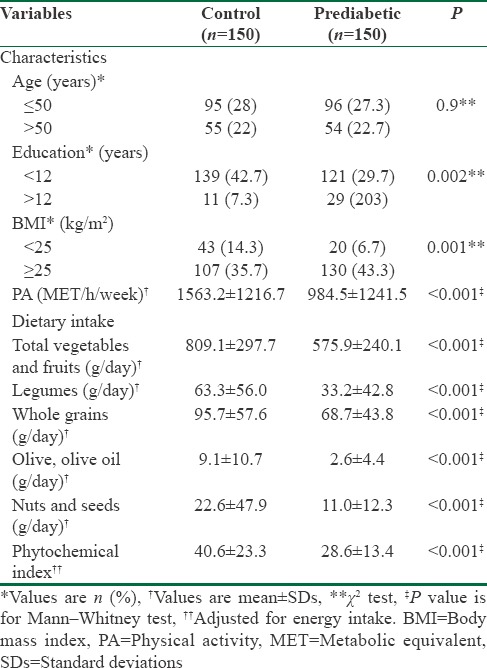
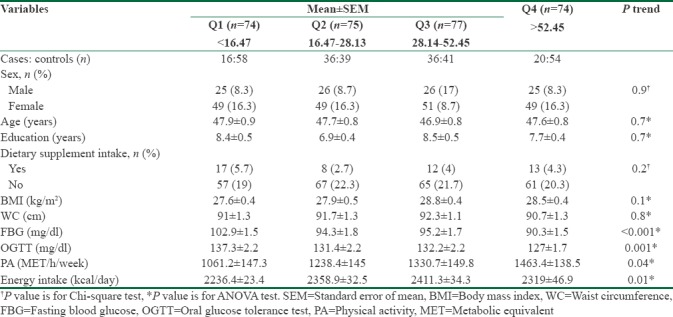
Table 2 presents the characteristics of participants across sex-specific quartiles of energy-adjusted DPI. Participants in the upper quartiles had lower FBG, OGTT (P-trend < 0.001), and higher physical activity level (P-trend = 0.04).
Table 2.
Characteristics of participants across the sex-specific quartiles of energy-adjusted phytochemical index
The relative contributions of different phytochemical-rich food groups to DPI were relatively similar in men and women as shown in Table 3. Our participants consumed a higher percentage of calories from fruits, legumes, vegetables, nut and seeds, olive, olive oil, and whole grains, respectively.
Table 3.
Contribution of food groups to energy-adjusted dietary phytochemical index (%)

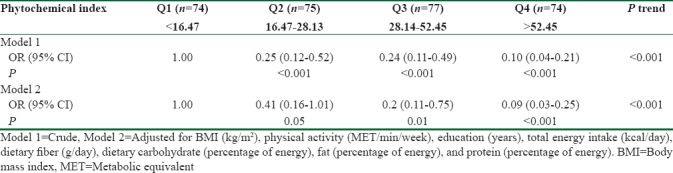
The DPI score was inversely related to prediabetes. Compared with participants in the lowest quartile of DPI, those in the highest quartile had significantly lower OR for prediabetes (Model 1: OR 0.1, 95% confidence interval [CI] 0.04–0.21), which remained significant after further adjustment for BMI, physical activity, education, dietary intake of energy, fiber, carbohydrate (% of energy), fat (% of energy), and protein (% of energy) (Model 2: OR 0.09, 95% CI 0.03–0.25) (P-trend < 0.001) [Table 4].
Table 4.
Odds ratio and 95% confidence intervals for prediabetes across sex-specific quartiles of energy-adjusted dietary phytochemical index
Discussion
In this case–control study, we determined the association of DPI with chance of prediabetes. Our results showed that compared to the control group, prediabetic participants had fewer daily intake of fruits, vegetables, whole grains, nuts and seeds, legumes, olive, and olive oil. In the present study, mean score of DPI in control group were significantly higher than prediabetic group. After adjustment for BMI, physical activity, education, intake of energy, fiber, carbohydrate (% of energy), fat (% of energy), and protein (% of energy), participants with higher sex-specific energy-adjusted DPI had significantly lower OR of prediabetes.
The association and potential effects of dietary phytochemicals on prevention of T2DM and hyperinsulinemia have been confirmed in previous studies.[23,24] However, the relationship of DPI and prediabetes morbidity has not been determined yet. To the best of our knowledge, the present study is the first.
Dietary phytochemicals have been shown to lower postprandial glycemia and development of glucose intolerance.[25] Prior studies have confirmed that intake of plant-based foods, rich in phytochemicals, was associated with improvement in glucose tolerance and insulin sensitivity in overweight participants.[25,26] Data from Tehran Lipid and Glucose Study found higher DPI was associated with lower FBG in healthy individuals. However, this study had lack of data on the postprandial glucose level.[7]
In the present study, the result showed a decreasing trend of OR of prediabetes across the increasing quartiles of DPI. This result is supported by the report from a controlled cross-over study which showed consuming whole grains, fruits, and vegetables had beneficial effects on FBG and insulin resistance in obese participants with elevated FBG.[27] However, DPI did not show significant association with HbA1c in another study in normal and overweight participants.[14]
We previously on the present study population reported that participants who had the higher adherence to the fruits, vegetables, and legumes dietary pattern had lower FBG and OGTT.[18] Regarding the sources of DPI, fruits (approximately 46%) were the main contributor to DPI, followed by the legumes, vegetables, nuts and seeds, olive, olive oil, and whole grains. Therefore, increased consumption of these high nutrient, lower energy foods could be a beneficial dietary target for prevention of IGT as well as attenuating the development of chronic diseases.
A prospective study in healthy women demonstrated that higher intake of green leafy vegetables and whole fruits was associated with a lower risk of T2DM, whereas increase in fruit juice intake did not appear to be beneficial for reducing the risk.[28] Polyphenols, found abundantly in berry, apple, and cherry, are among the main phytochemicals compounds with antihyperglycemic properties.[25] Evidence from large prospective cohort studies supported this fact that high consumption of whole fruits, especially blueberries, grapes, and apples, is strongly related to a reduced risk of developing T2DM.[29] There are several mechanisms through which the dietary phytochemicals could improve postprandial glucose as well as long-term glucose metabolism.[11] Phytochemicals derived from plant foods have shown to possess beneficial effects including improvement of pancreatic β-cell function by boosting antioxidant system, increasing insulin sensitivity, and enhancing uptake of insulin-dependent glucose transporter 4.[30,31]
Our results were in conflict with the previous studies and showed no decreasing trend in BMI, WC, and energy intake with increasing sex-specific energy-adjusted DPI quartiles. A cross-sectional study reported that DPI score was negatively associated with BMI, WC, waist-to-hip ratio, and body fat in overweight adults. Furthermore, this study showed a significant inverse association between DPI and weight gain over the previous year of study.[14] Data from a large longitudinal study on healthy adults revealed DPI assessed at baseline of the study was inversely associated with body weight and WC and 3-year weight gain. Therefore, high DPI and consumption of phytochemical-rich foods could be beneficial for prevention of obesity and maintenance of body weight which are linked to imbalance glucose homeostasis and T2DM. However, in this study, DPI was not adjusted for energy.[32]
Plant-based foods and vegetarian diet are high in many dietary phytochemicals which appear to be related to the lower body weight and risk of metabolic disorders.[33,34,35] Phytochemical-rich foods have lower calorie and glycemic index which explain the reduced risk of obesity.[36,37] Moreover, plant phytochemical have strong anti-obesity effects by targeting adipocyte lifecycle in different ways, including inhibiting proliferation and increasing apoptosis in adipocytes.[34]
Our study has some limitations including the case–control nature of the study that does not allow cause and effect conclusions. On the other hand, using the retrospective dietary data by FFQ which largely depends on recall may cause measurement error. In addition, prediabetic participants were aware of their blood glucose disturbances, so it may affect their dietary intake reporting. Other limitations of our study was not considering the non-calorie phytochemical-rich foods such as green and black tea, spices, type and source of phytochmical and food processing. Despite these limitations, this is the first study to examine the relationship of DPI and prediabetes in a case–control design.
Conclusions
Our study showed that lower PI score is related to higher prediabetes OR. This inexpensive index might have utility as a dietary target for intake of phytochemical-rich foods in prediabetes prevention. Nevertheless, it would be important to investigate these findings in other groups and prospective studies as well.
Financial support and sponsorship
This research project was supported by Tehran University of Medical Sciences (TUMS) (grant no. 93-454-76).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This research project was supported by Tehran University of Medical Sciences (TUMS).
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: A high-risk state for diabetes development. Lancet. 2012;379:2279–90. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. Control CfD Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. [Google Scholar]
- 4.Hadaegh F, Shafiee G, Ghasemi A, Sarbakhsh P, Azizi F. Impact of metabolic syndrome, diabetes and prediabetes on cardiovascular events: Tehran lipid and glucose study. Diabetes Res Clin Pract. 2010;87:342–7. doi: 10.1016/j.diabres.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Rajaram S. The effect of vegetarian diet, plant foods, and phytochemicals on hemostasis and thrombosis. Am J Clin Nutr. 2003;78:552S–558S. doi: 10.1093/ajcn/78.3.552S. [DOI] [PubMed] [Google Scholar]
- 6.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahadoran Z, Mirmiran P, Tohidi M, Azizi F. Dietary phytochemical index and the risk of insulin resistance and β-cell dysfunction: A prospective approach in Tehran lipid and glucose study. Int J Food Sci Nutr. 2015;66:950–5. doi: 10.3109/09637486.2015.1111867. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Serdula M, Janket SJ, Cook NR, Sesso HD, Willett WC, et al. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care. 2004;27:2993–6. doi: 10.2337/diacare.27.12.2993. [DOI] [PubMed] [Google Scholar]
- 9.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the framingham study. Arterioscler Thromb Vasc Biol. 2003;23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 11.Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365–402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahleova H, Matoulek M, Malinska H, Oliyarnik O, Kazdova L, Neskudla T, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med. 2011;28:549–59. doi: 10.1111/j.1464-5491.2010.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarty MF. Proposal for a dietary “phytochemical index”. Med Hypotheses. 2004;63:813–7. doi: 10.1016/j.mehy.2002.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Vincent HK, Bourguignon CM, Taylor AG. Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J Hum Nutr Diet. 2010;23:20–9. doi: 10.1111/j.1365-277X.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahadoran Z, Golzarand M, Mirmiran P, Saadati N, Azizi F. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran lipid and glucose study. J Hum Nutr Diet. 2013;26(Suppl 1):145–53. doi: 10.1111/jhn.12048. [DOI] [PubMed] [Google Scholar]
- 16.Golzarand M, Mirmiran P, Bahadoran Z, Alamdari S, Azizi F. Dietary phytochemical index and subsequent changes of lipid profile: A 3-year follow-up in Tehran lipid and glucose study in Iran. ARYA Atheroscler. 2014;10:203–10. [PMC free article] [PubMed] [Google Scholar]
- 17.Mottaghi A, Bahadoran Z, Mirmiran P, Azizi F. Assessment of relationship between dietary phytochemical intake and visceral obesity: Tehran Lipid and Glucose Study. Pajoohandeh J. 2015;20:258–65. [Google Scholar]
- 18.Bagheri F, Siassi F, Koohdani F, Mahaki B, Qorbani M, Yavari P, et al. Healthy and unhealthy dietary patterns are related to pre-diabetes: A case-control study. Br J Nutr. 2016;116:874–81. doi: 10.1017/S0007114516002634. [DOI] [PubMed] [Google Scholar]
- 19.Alberti G, Zimmet P, Shaw J. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels: Diabetes Research and Clinical Practice. 2006:1–23. [Google Scholar]
- 20.Committee IR. Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ)–Short and long forms. 2005. [Last retrieved 2008 Sep 17]. Available from: http://www.ipaq.ki.se .
- 21.Azadbakht L, Esmaillzadeh A. Macro and micro-nutrients intake, food groups consumption and dietary habits among female students in Isfahan university of medical sciences. Iran Red Crescent Med J. 2012;14:204–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 23.Belobrajdic DP, Bird AR. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr J. 2013;12:62. doi: 10.1186/1475-2891-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leiherer A, Mündlein A, Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul Pharmacol. 2013;58:3–20. doi: 10.1016/j.vph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J Diabetes Metab Disord. 2013;12:43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnard ND, Scialli AR, Turner-McGrievy G, Lanou AJ, Glass J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am J Med. 2005;118:991–7. doi: 10.1016/j.amjmed.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Rave K, Roggen K, Dellweg S, Heise T, tom Dieck H. Improvement of insulin resistance after diet with a whole-grain based dietary product: Results of a randomized, controlled cross-over study in obese subjects with elevated fasting blood glucose. Br J Nutr. 2007;98:929–36. doi: 10.1017/S0007114507749267. [DOI] [PubMed] [Google Scholar]
- 28.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31:1311–7. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, et al. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y, Keogh JB, Clifton PM. Polyphenols and glycemic control. Nutrients. 2016;8 doi: 10.3390/nu8010017. pii: E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–6. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 32.Mirmiran P, Bahadoran Z, Golzarand M, Shiva N, Azizi F. Association between dietary phytochemical index and 3-year changes in weight, waist circumference and body adiposity index in adults: Tehran lipid and glucose study. Nutr Metab (Lond) 2012;9:108. doi: 10.1186/1743-7075-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith CF, Burke LE, Wing RR. Vegetarian and weight-loss diets among young adults. Obes Res. 2000;8:123–9. doi: 10.1038/oby.2000.13. [DOI] [PubMed] [Google Scholar]
- 34.Williams DJ, Edwards D, Hamernig I, Jian L, James AP, Johnson SK, et al. Vegetables containing phytochemicals with potential anti-obesity properties: A review. Food Res Int. 2013;52:323–33. [Google Scholar]
- 35.Howes MJ, Simmonds MS. The role of phytochemicals as micronutrients in health and disease. Curr Opin Clin Nutr Metab Care. 2014;17:558–66. doi: 10.1097/MCO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 36.Bertoia ML, Mukamal KJ, Cahill LE, Hou T, Ludwig DS, Mozaffarian D, et al. Correction: Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: Analysis from three prospective cohort studies. PLoS Med. 2016;13:e1001956. doi: 10.1371/journal.pmed.1001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rautiainen S, Wang L, Lee IM, Manson JE, Buring JE, Sesso HD, et al. Higher intake of fruit, but not vegetables or fiber, at baseline is associated with lower risk of becoming overweight or obese in middle-aged and older women of normal BMI at baseline. J Nutr. 2015;145:960–8. doi: 10.3945/jn.114.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]


