Abstract
Background:
Follistatin-like 1 (FSTL1) is a novel profibrogenic factor that induces pulmonary fibrosis (PF) through the transforming growth factor-beta 1 (TGF-β1)/Smad signaling. Little is known about its effects on PF through the non-Smad signaling, like the mitogen-activated protein kinase (MAPK) pathway. Therefore, this study aimed to investigate the role of FSTL1 in PF through the MAPK signaling pathway and its mechanisms in lung fibrogenesis.
Methods:
PF was induced in Fstl1+/− and wild-type (WT) C57BL/6 mice with bleomycin. After 14 days, the mice were sacrificed, and lung tissues were stained with hematoxylin and eosin; the hydroxyproline content was measured to confirm PF. The mRNA and protein level of FSTL1 and the change of MAPK phosphorylation were measured by quantitative polymerase chain reaction and Western blotting. The effect of Fstl1 deficiency on fibroblasts differentiation was measured by Western blotting and cell immunofluorescence. MAPK signaling activation was measured by Western blotting in Fstl1+/− and WT fibroblasts treated with recombinant human FSTL1 protein. We pretreated mouse lung fibroblast cells with inhibitors of the extracellular signal-regulated kinase (ERK), p38, and Jun N-terminal kinase (JNK) signaling and analyzed their differentiation, proliferation, migration, and invasion by Western blotting, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide analysis, and transwell assays. The Student's t-test was used to compare the differences between two groups.
Results:
Fstl1 deficiency attenuated phosphorylation of the ERK, p38, and JNK signaling in bleomycin-induced fibrotic lung tissue 14 days after injury (0.67 ± 0.05 vs. 1.22 ± 0.03, t = 14.92, P = 0.0001; 0.41 ± 0.01 vs. 1.15 ± 0.07; t = 11.19; P = 0.0004; and 0.41 ± 0.01 vs. 1.07 ± 0.07, t = 8.92, P = 0.0009; respectively), compared with WT lungs at the same time and in primary lung fibroblasts (0.82 ± 0.01 vs. 1.01 ± 0.04, t = 4.06, P = 0.0150; 1.04 ± 0.03 vs. 1.24 ± 0.03, t = 4.44, P = 0.0100; and 0.76 ± 0.05 vs. 0.99 ± 0.05, t = 4.48, P = 0.0100; respectively), compared with TGF-β1-stimulated WT group. Recombinant human FSTL1 protein in lung fibroblasts enhanced TGF-β1-mediated phosphorylation of the ERK (1.19 ± 0.08 vs. 0.55 ± 0.04, t = 6.99, P = 0.0020), p38 (1.18 ± 0.04 vs. 0.66 ± 0.03, t = 11.20, P = 0.0020), and JNK (1.11 ± 0.01 vs. 0.84 ± 0.04, t = 6.53, P = 0.0030), compared with the TGF-β1-stimulated WT group. Fstl1-deficient fibroblasts showed reduced alpha-smooth muscle actin (α-SMA) expression (0.70 ± 0.06 vs. 1.28 ± 0.11, t = 4.65, P = 0.0035, compared with the untreated WT group; 1.40 ± 0.05 vs. 1.76 ± 0.02, t = 6.31, P = 0.0007; compared with the TGF-β1-treated WT group). Compared with the corresponding condition in the control group, the TGF-β1/FSTL1-mediated α-SMA expression was significantly suppressed by pretreatment with an inhibitor of p38 (0.73 ± 0.01 vs. 1.13 ± 0.10, t = 3.92, P = 0.0078) and JNK (0.78 ± 0.03 vs. 1.08 ± 0.06, t = 4.40, P = 0.0046) signaling. The proliferation of mouse lung fibroblast cells (MLgs) significantly decreased after treatment of an inhibitor of p38 (0.30 ± 0.01 vs. 0.46 ± 0.03, t = 4.64, P = 0.0009), JNK (0.30 ± 0.01 vs. 0.49 ± 0.01, t = 12.84, P = 0.0001), and Smad2/3 (0.18 ± 0.02 vs. 0.46 ± 0.02, t = 12.69, P = 0.0001) signaling compared with the dimethylsulfoxide group. The migration and invasion cells of MLgs significantly decreased in medium pretreated with an inhibitor of p38 (70.17 ± 3.28 vs. 116.30 ± 7.11, t = 5.89, P = 0.0042 for the migratory cells; 19.87 ± 0.84 vs. 32.70 ± 0.95, t = 10.14, P = 0.0005 for the invasive cells), JNK (72.30 ± 3.85 vs. 116.30 ± 7.11, t = 5.44, P = 0.0056 for the migratory cells; 18.03 ± 0.94 vs. 32.70 ± 0.95, t = 11.00, P = 0.0004 for the invasive cells), and Smad2/3 (64.76 ± 1.41 vs. 116.30 ± 7.11, t = 7.11, P = 0.0021 for the migratory cells; 18.03 ± 0.94 vs. 32.70 ± 0.95, t = 13.29, P = 0.0002 for the invasive cells) signaling compared with the corresponding condition in the dimethylsulfoxide group.
Conclusion:
FSTL1 affects lung fibroblast differentiation, proliferation, migration, and invasion through p38 and JNK signaling, and in this way, it might influence the development of PF.
Keywords: Follistatin-Like 1, Mitogen-Activated Protein Kinase, Pulmonary Fibrosis, Transforming Growth Factor Beta 1
摘要
背景:
卵泡抑素样蛋白1(Follistatin-like 1, FSTL1)具有促纤维化作用,能够通过转化生长因子β1(TGF-β1)/Smad信号通路诱发 肺纤维化。但FSTL1是否也能通过非Smad信号通路,如丝裂原活化蛋白激酶(MAPK)信号通路,影响肺纤维化发生发展,目 前鲜有报道。基于此,本研究的目的是探讨FSTL1是否通过TGF-β1/MAPK信号通路影响肺纤维化发生、发展的影响及其可 能的作用机制。
方法:
在Fstl1+/-和野生型C57BL/6小鼠中,气管内注入博来霉素建立小鼠肺纤维化模型;对照组气管内注入生理盐水。14天 后,取肺组织,用HE染色及羟脯氨酸含量测定方法验证纤维化模型是否构建成功。用qPCR和Western blotting的方法分别检 测小鼠肺组织内FSTL1的mRNA和蛋白水平变化,以及MAPK信号通路磷酸化水平变化。从Fstl1+/-和野生型小鼠肺组织中分 离原代肺成纤维细胞,对比两组MAPK信号通路磷酸化水平变化。用Western blotting和细胞荧光免疫的方法观察肺成纤维细 胞向肌成纤维细胞转分化情况及FSTL1缺失对其分化的影响。通过Western blotting方法观察Fstl1+/-小鼠成纤维细胞和在野生 型同窝小鼠成纤维细胞中,外源加入FSTL1蛋白后,MAPK信号通路磷酸化水平变化。在小鼠肺成纤维细胞系(MLgs)中, 分别加入ERK、p38及JNK的抑制剂U0126、SB202190及SP600125后,运用Western blotting、MTT法和Transwell小室分别观察 MLgs的分化、增殖、迁移和侵袭情况。两组数据间差异用t检验进行分析。
结果:
博来霉素处理14天后,与对照组相比,FSTL1的缺失能减弱肺组织中ERK (0.67 ± 0.05 vs 1.22 ± 0.03; t = 14.92; P = 0.0001),p38 (0.41 ± 0.01 vs 1.15 ± 0.07; t = 11.19; P = 0.0004),JNK (0.41 ± 0.01 vs 1.07 ± 0.07; t = 8.92; P = 0.0009)信号通路的 磷酸化水平。在细胞学实验中,与TGF-β1刺激的原代小鼠成纤维细胞相比,FSTL1的缺失减弱ERK,p38,JNK信号通路的 磷酸化水平(分别减弱0.82 ± 0.01 vs 1.01 ± 0.04; t = 4.06; P = 0.0150; 1.04 ± 0.03 vs 1.24 ± 0.03; t = 4.44; P = 0.0100; 0.76 ± 0.05 vs 0.99 ± 0.05; t = 4.48; P = 0.0100);与单纯使用TGF-β1刺激MLgs相比,外源加入FSTL1蛋白后能增强MLgs中ERK (1.19 ± 0.08 vs 0.55 ± 0.04; t = 6.99; P = 0.0020), p38 (1.18 ± 0.04 vs 0.66 ± 0.03; t = 11.2; P = 0.0020), JNK (1.11 ± 0.01 vs 0.84 ± 0.04; t = 6.53; P = 0.0030)信号通路的磷酸化水平。FSTL1的缺失可减少a-SMA的表达 (0.70 ± 0.06 vs 1.28 ± 0.11; t = 4.65; P = 0.0030,与对照 组相比;1.40 ± 0.05 vs 1.76 ± 0.02; t = 6.31; P = 0.0007,与TGF-β1组相比)。与DMSO组中相对应组别相比,p38及JNK信号通 路抑制剂可抑制肌成纤维细胞的分化(分别为0.73 ± 0.01 vs 1.13 ± 0.10; t = 3.92; P = 0.0078和0.78 ± 0.03 vs 1.08 ± 0.06; t = 4.40; P = 0.0046) ;还能抑制MLgs细胞系的增殖p38 (0.30 ± 0.01 vs 0.46 ± 0.03; t = 4.64; P = 0.0009), JNK (0.30 ± 0.01 vs 0.49 ± 0.01; t = 12.84; P = 0.0001), Smad2/3 (0.18 ± 0.02 vs 0.46 ± 0.02; t = 12.69; P = 0.0001)、迁移p38 (70.17 ± 3.28 vs 116.30 ± 7.11; t = 5.89; P = 0.0042), JNK (72.30 ± 3.85 vs 116.30 ± 7.11; t = 5.44; P = 0.0056), Smad2/3 (64.76 ± 1.41 vs 116.30 ± 7.11; t = 7.11; P = 0.0021) 及侵袭p38 (19.87 ± 0.84 vs 32.70 ± 0.95; t = 10.14; P = 0.0005), JNK (18.03 ± 0.94 vs 32.70 ± 0.95; t = 11.00; P = 0.0004), Smad2/3 (18.03 ± 0.94 vs 32.70 ± 0.95; t = 13.29; P = 0.0002)能力。
结论:
FSTL1可能通过TGF-β1/p38/JNK信号通路影响肺成纤维细胞的分化、增殖、迁移和侵袭能力,从而可能影响肺纤维化 的发生、发展。
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a severe chronic lung disease with progressive interstitial fibrosis, high mortality, and morbidity occurring mainly in older adults; it is a devastating disease, with poor therapeutic effect and a mean survival time of 2–3 years after diagnosis.[1,2,3] The hallmark of IPF is the formation of fibroblast foci and the accumulation of extracellular matrix (ECM), resulting in irreversible alteration of pulmonary architecture and ultimately leading to respiratory failure.[4,5,6] The effector cells in pulmonary fibrosis (PF) are the myofibroblasts, differentiated fibroblasts, which have contractile properties similar to smooth muscle cells and characterized by the presence of alpha-smooth muscle actin (α-SMA). Unlike normal lung tissue repair, where fibroblast proliferation is self-limited, PF is characterized by uncontrolled proliferation and migration/invasion of the myofibroblasts and sustained accumulation of ECM.[7] Distinct phenotypic alterations of the fibroblasts, combined with aberrant ECM production, contribute to the development of PF.[8] Despite intensive studies, reduplicative epithelial injury along with unremitting ECM deposition seems to involve partly in lung fibrogenesis; however, the precise molecular mechanisms that modulate these processes remain elusive.
Although many elements have been proposed to be involved in the development of PF, it is generally agreed that transforming growth factor-beta (TGF-β) plays a central role in this disease, acting as a crucial profibrotic cytokine that induces fibroblasts differentiation, proliferation, migration, and ECM deposition.[9,10,11] However, the mechanisms through which TGF-β1 regulates lung fibrogenesis remain mostly unclear. Both Smad-dependent and Smad-independent signaling pathways induced by TGF-β1 are involved in PF. Specifically, the mitogen-activated protein kinases (MAPKs), the main noncanonical TGF-β1 signaling pathway, including the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 signaling, has been implicated in the development of PF.[11,12,13,14,15,16]
Follistatin-like 1 (FSTL1) is a secreted extracellular glycoprotein that was first isolated in a differential screening for TGF-β1-inducible genes in the MC3T3-E1 murine osteoblastic cell line.[17] Its functions and mechanism of action are not known. Previous studies have shown that FSTL1 is increased in response to bleomycin- or silica-induced lung injuries and causes pulmonary fibrogenesis; additionally, haplodeletion of Fstl1 or blockage of FSTL1 with neutralizing antibodies in mice reduced fibrosis in vivo.[18,19] These findings indicated that FSTL1 promotes epithelial-mesenchymal communication and subsequent fibroblast activation through facilitating the TGF-β1/Smad signaling. Although many studies have focused on the role of MAPKs in inflammatory lung diseases, the role of FSTL1 on the TGF-β1/MAPK signaling in lung fibrosis has not been demonstrated. Thus, the aim of this study was to investigate the possible effect of FSTL1 on fibroblasts activation, differentiation, proliferation, migration, and invasion through the TGF-β1/MAPK signaling in PF.
METHODS
Ethical statement
All animal protocols were approved by the Institutional Animal Care and Use Committee of Capital Medical University and complied with the Regulations for the Management of Laboratory Animals announced by the Ministry of Science and Technology of the People's Republic of China.
Mice and bleomycin mouse model
All mice were housed and bred in a pathogen-free facility in Nankai University (Tianjin, China). The generation of Fstl1+/− mice has been previously described.[20] The mice have been crossed onto the C57BL/6 background for at least 12 generations before use. C57BL/6 mice (6–8 weeks old) were purchased from Vital River Laboratories (Beijing, China). For the bleomycin mouse model, 2 U/kg bleomycin (Nippon Kayaku Co Ltd., Tokyo, Japan) in 20–25 μl phosphate-buffered saline (PBS) was administrated intratracheally using a 1 ml syringe with a 25 G needle inserted between the cartilaginous rings of the trachea. Mice were sacrificed 7 or 14 days after bleomycin administration; the left lungs were then excised for histological analysis, while the right lungs were homogenized for hydroxyproline assays and Western blotting analysis.
Histology
The left lungs were washed to eliminate blood residues and dilated with 0.4 ml of 10% formalin after the trachea was cannulated. The lungs were fixed overnight and then embedded in paraffin. To evaluate the extent of fibrosis, the sections were stained with hematoxylin and eosin and photographed with an Olympus BX53 microscope digital camera system (Tokyo, Japan) at a magnification of ×100; a total of ten random fields per sample were scored.
Western blotting analysis
The proteins were extracted from cells or tissue, and Western blotting was performed as previously described.[21] Equal amounts of proteins were separated by polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). Membranes were blocked in 5% nonfat dry milk for 1 h, followed by incubation with primary antibodies against FSTL1 (1:500 dilution, Santa Cruz Biotechnology, CA, USA), Collagen I (1:200 dilution, Abcam, Cambridge, UK), β-tubulin (1:1,000 dilution, Abcam, Cambridge, UK), phosphorylated (p)-p38, p38, p-ERK, ERK, p-JNK, and JNK (1:200 dilution, Cell Signaling Technology, MA, USA), α-SMA (1:500 dilution, Sigma-Aldrich, St. Louis, MO, USA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:3,000 dilution, Sigma-Aldrich, St. Louis, MO, USA) overnight at 4°C. The protein bands were visualized using the LI-COR Odyssey infrared double-fluorescence imaging system (LI-COR, Lincoln, NE, USA). The value of the relative density of each target protein band was normalized to the density of the corresponding β-tubulin or GAPDH band.
Hydroxyproline assay
The collagen content in right lungs was measured with the conventional hydroxyproline method.[22] The right lungs were dried at 120°C overnight and then acid-hydrolyzed in sealed, oxygen-purged glass ampules containing 3 ml HCl at 120°C for 16 h. Samples were filtered through a 5.0 μm syringe-driven filter, and the pH was then adjusted to 6.5–8.0 using NaOH.
Primary pulmonary fibroblasts isolation and culture
Primary pulmonary fibroblasts isolated from the bleomycin-treated mice were cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, ScienCell Research Laboratories, Carlsbad, CA, USA) and 100 U/ml of penicillin and 100 mg/ml of streptomycin in 21% O2/5% CO2 at 37°C in a humidified atmosphere as described previously.[23] Newly isolated lung fibroblasts cultured 24 h on cover slides were used for immunofluorescence staining. Primary cells at passages 3–4 were used for myofibroblast activation and ECM production assays.
Cell line culture
Mouse lung fibroblast cells (MLgs) obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in DMEM supplemented with 10% FBS and antibiotics in 5% CO2 at 37°C in a humidified atmosphere.
Quantitative polymerase chain reaction analysis
Total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA, USA), purified with the RNeasy Mini Kit (Qiagen, Hilden, Germany) and the DNA-free kit (Ambion, Austin, TX, USA) according to the manufacture's protocol, and quantified using a NanoDrop 2000c (Thermo Scientific, Waltham, MA, USA). The Fstl1 mRNA in mice was quantified using a SYBR® Green Master Mix Kit (Roche Applied Science, Basel, Switzerland). Gene expression was measured relative to the internal reference gene β-actin using the comparative CT method as previously described.[20] The sequences of the quantitative polymerase chain reaction primers were: Fstl1: 5’-TTATGATGGGCACTGCAAAGAA-3’ and 5’-ACTGCCTTTAGAGAACCAGCC-3’. β-actin: 5’-AGGCCAACCGTGAAAAGATG-3’ and 5’-AGAGCATAGCCCTCGTAGATGG-3’.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde for 30 min, washed with PBS, permeabilized with 0.2% Triton X100 in PBS for 10 min, blocked with 5% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO, USA), and incubated with anti-α-SMA (1:100, Sigma-Aldrich, St. Louis, MO, USA) antibodies overnight at 4°C. Cells were washed and incubated with Alexa Fluor® 594-conjugated goat anti-mouse IgG (Beijing Zhong Shan-Golden Bridge Biological Technology Company, Beijing, China) for 30 min in the dark. After washing with PBS three times, the slices were mounted with 4’,6-diamidino-2-phenylindole (DAPI, Beijing Zhong Shan-Golden Bridge Biological Technology Company, Beijing, China) lysis buffer. Immunofluorescence was observed under a microscope digital camera system (Nikon, Tokyo, Japan).
Cell proliferation
Cell proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Amresco, Solon, OH, USA) assays. MLgs were cultured in 96-well plates for 24 h, and serum starved for 24 h. The cells were preincubated for 1 h with U0126 (ERK inhibitor, Cell Signaling Technology, MA, USA), SB202190 (p38 inhibitor, Cell Signaling Technology, MA, USA), SP600125 (JNK inhibitor, Cell Signaling Technology, MA, USA), and SB525334 (Smad2/3 inhibitor, R and D Systems, MN, USA) before treatment with 5 ng/ml TGF-β1 (R and D Systems, MN, USA) in the presence or absence of 100 ng/ml recombinant human FSTL1 protein (R and D Systems, MN, USA) for 24 h and finally incubated with MTT (final concentration of 0.5 mg/ml) for 4 h. The supernatant was removed, and 150 ml/well of dimethylsulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) was added to dissolve the blue formazan crystals by shaking the plates for 15 min on an orbital shaker at 25°C. Absorbance at 490 nm was read on a microplate reader (Bio-Rad, Hercules, CA, USA).
Cell migration and invasion
Cell migration assays were performed using 8.0 μm pore transwell chambers (Millipore, Billerica, MA, USA). Cells were treated with 5 ng/ml TGF-β1 or 100 ng/ml FSTL1 protein for 24 h and seeded in the upper chamber of the transwells (1 × 105 cells/chamber). FBS-containing DMEM medium was placed in the bottom chambers and used as a chemoattractant. After incubation for 6 h at 37°C, the cells that had migrated through the 8.0 μm membrane pore were counted. Specifically, the upper surface of membrane was swabbed with a cotton swab to remove noninvasive cells and washed with PBS. Invasive cells on the bottom surface were fixed in 4% paraformaldehyde for 30 min and quantified in high-power fields using a microscope digital camera system (Nikon, Tokyo, Japan), after staining the nuclei with DAPI. Invasion assays were performed using similar procedures, coating the transwell chambers with 50 μl of Matrigel (BD Biosciences, San Jose, CA, USA), and allowing it to solidify for 30 min at 37°C before performing the assay.
Statistical analysis
All data are expressed as a mean ± standard deviation (SD) and analyzed using the GraphPad Prism 5.0 software (La Jolla, CA, USA). The Student's t-test was used to compare the differences between two groups. A value of P < 0.05 was considered statistically significant.
RESULTS
Follistatin-like 1 is upregulated in mice with bleomycin-induced pulmonary fibrosis
To determine whether FSTL1 expression is aberrant in progressive lung fibrotic diseases, we induced PF in C57BL/6 mice through bleomycin intratracheal administration.[24] Lung sections showed significant tissue structural damage and inflammatory cell infiltration 14 days after bleomycin injury [Figure 1a] and concomitant collagen accumulation in lung tissues as determined by hydroxyproline content measurement [Figure 1b]. Next, we investigated the change in FSTL1 levels in PF and found that it was significantly increased 14 days after bleomycin injury, both in mRNA level (2.44 ± 0.33 vs. 0.98 ± 0.02, t = 3.45, P = 0.0400; Figure 1c) and protein expression in lung tissues (1.19 ± 0.03 vs. 0.62 ± 0.02, t = 16.51, P = 0.0001; Figure 1d), compared with control group.
Figure 1.
FSTL1 expression level in lung tissue of bleomycin-treated mice. Lung tissue of C57BL/6 mice was treated with 2.5 U/kg bleomycin. (a) Hematoxylin and eosin staining of lung sections showed significant tissue structural damage (black arrows) and inflammatory cell infiltration (red arrow) 14 days after bleomycin injury. (b) Hydroxyproline contents in lung tissues. (c) Fstl1 mRNA expression in lung tissues. (d) FSTL1 protein expression in lung tissue. n = 7; Bar = 100 μm. *P < 0.05, †P < 0.001 versus D0. FSTL1: Follistatin-like 1.
Follistatin-like 1 activates the mitogen-activated protein kinase signaling in primary lung fibroblasts
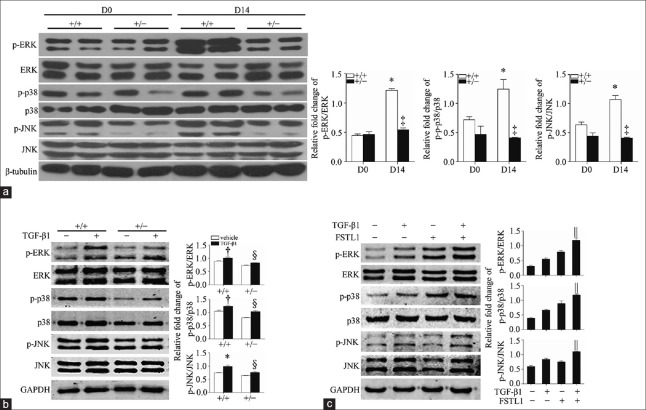
The MAPK signaling is involved in lung fibrogenesis. To determine whether FSTL1 has an effect on PF through the MAPK signaling, we first examined the activation of the TGF-β1-mediated MAPK signaling in the lungs of Fstl1+/− mice and their wild-type (WT) littermates 14 days after bleomycin injury. The phosphorylation levels of the ERK, p38, and JNK in lung tissue homogenates were significantly increased in WT lungs (1.22 ± 0.03 vs. 0.44 ± 0.03, t = 18.26, P = 0.0011; 1.15 ± 0.07 vs. 0.65 ± 0.02, t = 7.33, P = 0.0018; and 1.07 ± 0.07 vs. 0.64 ± 0.05, t = 5.01, P = 0.0070; respectively, compared with untreated WT group) and attenuated in Fstl1+/− lungs at day 14, compared with WT lungs at the same time (0.67 ± 0.05 vs. 1.22 ± 0.03, t = 14.92, P = 0.0001; 0.41 ± 0.01 vs. 1.15 ± 0.07, t = 11.19, P = 0.0004; 0.41 ± 0.01 vs. 1.07 ± 0.07, t = 8.92, P = 0.0009; respectively; Figure 2a). These findings suggest that Fstl1 deficiency attenuates the activation of the MAPK signaling in vivo. We then isolated primary fibroblasts from lungs of Fstl1+/− mice and their WT littermates 14 days after bleomycin treatment. The fibroblasts were treated with 5 ng/ml TGF-β1 for 30 min, and the TGF-β1-mediated MAPK signaling was measured by Western blotting. In the WT group, the phosphorylation levels of ERK, p38, and JNK were significantly increased after TGF-β1 stimulation (1.01 ± 0.04 vs. 0.85 ± 0.02, t = 3.31, P = 0.0300; 1.24 ± 0.03 vs. 1.05 ± 0.05, t = 3.52, P = 0.0200; and 0.99 ± 0.05 vs. 0.75 ± 0.01, t = 5.03, P = 0.0070; respectively), compared with untreated WT group, while in Fstl1+/– lung fibroblasts, the phosphorylation levels of ERK, p38, and JNK showed significantly decreased (0.82 ± 0.01 vs. 1.01 ± 0.04, t = 4.06, P = 0.0150; 1.04 ± 0.03 vs. 1.24 ± 0.03, t = 4.44, P = 0.0100; and 0.76 ± 0.05 vs. 0.99 ± 0.05, t = 4.48, P = 0.0100; respectively), compared with TGF-β1-stimulated WT group [Figure 2b]. We then performed gain of function experiments by administering recombinant human FSTL1 protein to primary lung fibroblasts. Recombinant human FSTL1 protein increased the TGF-β1-mediated phosphorylation levels of ERK (1.19 ± 0.08 vs. 0.55 ± 0.04, t = 6.99, P = 0.0020), p38 (1.18 ± 0.04 vs. 0.66 ± 0.03, t = 11.2, P = 0.0020), and JNK (1.11 ± 0.01 vs. 0.84 ± 0.04, t = 6.53, P = 0.0030), compared with the TGF-β1-stimulated WT group [Figure 2c]. These data suggest that FSTL1 modulates the activation of the MAPK signaling in primary lung fibroblasts.
Figure 2.
FSTL1 modulates the activation of MAPK signaling in primary lung fibroblast. Western blotting analysis of total and phosphorylated ERK, p38, and JNK in lung tissues (a) and primary lung fibroblasts (b and c) from Fstl1+/− and their WT littermates. Primary lung fibroblasts were treated with 5 ng/ml TGF-β1 and/or 100 ng/ml FSTL1 protein. β-Tubulin and GAPDH served as control loadings. n = 3; *P < 0.01, †P < 0.05 versus untreated WT group; ‡P < 0.001 versus WT group in D14; §P < 0.05, ||P < 0.01 versus TGF-β1 treated WT group. FSTL1: Follistatin-like 1; MAPK: Mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; JNK: Jun N-terminal kinase; WT: Wild type; TGF-β1: Transforming growth factor-β1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Follistatin-like 1 facilitates transforming growth factor-beta 1-induced fibroblast activation by modulating the transforming growth factor beta 1/p38/Jun N-terminal kinase signaling
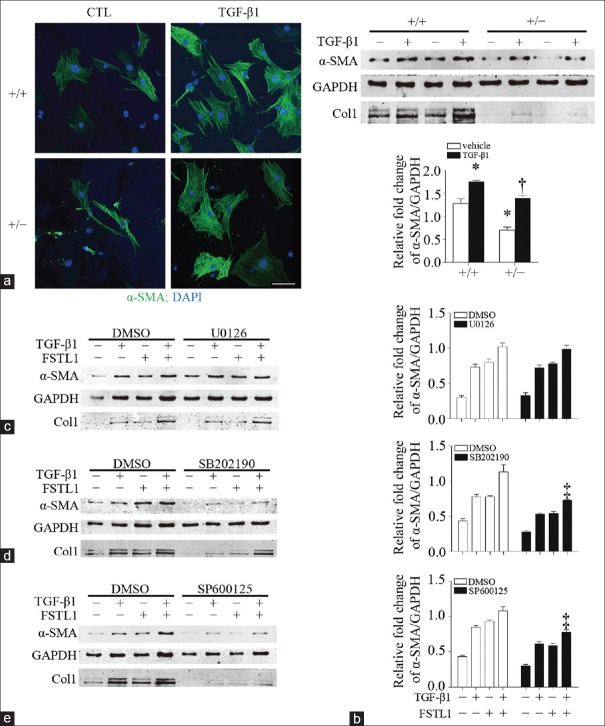
To determine the molecular mechanisms through which FSTL1 promotes the TGF-β1/MAPK signaling pathway described above, we isolated primary lung fibroblasts from Fstl1+/− and their WT littermates to investigate the expression of α-SMA, myofibroblast markers, which indicate lung fibroblast activation and myofibroblast differentiation. Primary lung fibroblasts were pretreated with 5 ng/ml TGF-β1 for 24 h. Immunofluorescence staining and Western blotting results showed that TGF-β1 treatment promoted the expression of α-SMA (1.76 ± 0.02 vs. 1.28 ± 0.11, t = 4.390, P = 0.0046) and the secretion of type I collagen in the untreated WT group, while Fstl1-deficient fibroblasts showed reduced α-SMA expression in both groups (0.70 ± 0.06 vs. 1.28 ± 0.11, t = 4.650, P = 0.0035, compared with the untreated WT group; 1.40 ± 0.05 vs. 1.76 ± 0.02, t = 6.310, P = 0.0007, compared with the TGF-β1-treated WT group) and type I collagen production [Figure 3a and 3b]. These results suggest a positive correlation between FSTL1 and TGF-β1-induced myofibroblast differentiation and subsequent ECM accumulation.
Figure 3.
FSTL1 modulates myofibroblast differentiation by facilitating p38/JNK signaling. (a and b) Primary lung fibroblasts from Fstl1+/− and their WT littermates were treated with 5 ng/ml TGF-β1. (a) Immunofluorescence staining of α-SMA in lung fibroblasts. (b) Protein expression levels of α-SMA and type I collagen. (c-e) MLgs were pretreated with U0126, SB202190, and SP600125. Protein expression levels of α-SMA and type I collagen were detected by Western blotting. n = 4; Bars = 100 μm. *P < 0.01 versus untreated WT group; †P < 0.001, versus TGF-β1 treated WT group, ‡P < 0.01 versus corresponding condition in the DMSO group. α-SMA: Alpha-smooth muscle actin; CTL: Control group; col1: Type I collagen; DAPI: 4’,6-Diamidino-2-phenylindole; DMSO: Dimethylsulfoxide; FSTL1: Follistatin-like 1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; TGF-β1: Transforming growth factor-β1; U0126: ERK inhibitor; SB202190: p38 inhibitor; SP600125: JNK inhibitor.
Therefore, to further examine whether these signaling pathways were involved in TGF-β1-induced fibroblast activation and ECM production, we used selective chemical inhibitors of MAPK signaling. MLgs were pretreated with DMSO and 10 μmm/L U0126 (ERK inhibitor), SB202190 (p38 inhibitor), or SP600125 (JNK inhibitor) for 1 h and then treated with 5 ng/ml TGF-β1 and/or 100 ng/ml FSTL1 protein for 24 h. The TGF-β1/FSTL1-mediated α-SMA expression and type I collagen production were significantly suppressed by pre-treatment with SB202190 (0.73 ± 0.01 vs. 1.13 ± 0.10, t = 3.92, P = 0.0078) and SP600125 (0.78 ± 0.03 vs. 1.08 ± 0.06, t = 4.40, P = 0.0046), compared with the corresponding condition in the DMSO group, while, surprisingly, pretreatment with U0126 did not have a significant effect (P > 0.05; Figure 3c–3e). These data suggest that the TGF-β1/p38/JNK signaling is involved in the regulation of FSTL1 on promoting fibroblasts’ differentiation and ECM production.
Follistatin-like 1 promotes fibroblasts proliferation, migration, and invasion by modulating the transforming growth factor beta 1/p38/Jun N-terminal kinase/Smad2/3 signaling
To further validate the effect of FSTL1 on pulmonary fibrogenesis, we observed the proliferation, migration, and invasion abilities of MLgs in MTT and transwell assays. MLgs were pretreated with DMSO or 10 μmm/L U0126, SB202190, SP600125, or SB525334 for 1 h and then treated with 5 ng/ml TGF-β1 and/or 100 ng/ml FSTL1 protein for 24 h. Compared with corresponding condition in DMSO groups, the proliferation of MLgs significantly decreased after treatment with an inhibitor of p38 (0.30 ± 0.01 vs. 0.46 ± 0.03, t = 4.64, P = 0.0009), JNK (0.30 ± 0.01 vs. 0.49 ± 0.01, t = 12.84, P = 0.0001), and Smad2/3 (0.18 ± 0.02 vs. 0.46 ± 0.02, t = 12.69, P = 0.0001) but not after treatment with an inhibitor of ERK (P > 0.05; Figure 4a). In transwell assays, we then evaluated the number of cells that had migrated or invaded in nine random fields per chamber. The number of cells at the bottom of the membrane significantly decreased when the medium was supplemented with an inhibitor of the p38 (70.17 ± 3.28 vs. 116.30 ± 7.11, t = 5.89, P = 0.0042 for the migratory cells; and 19.87 ± 0.84 vs. 32.70 ± 0.95, t = 10.14, P = 0.0005 for the invasive cells; respectively), JNK (72.30 ± 3.85 vs. 116.30 ± 7.11, t = 5.44, P = 0.0056 for the migratory cells; and 18.03 ± 0.94 vs. 32.70 ± 0.95, t = 11.00, P = 0.0004 for the invasive cells; respectively), and Smad2/3 (64.76 ± 1.41 vs. 116.30 ± 7.11, t = 7.11, P = 0.0021 for the migratory cells; and 18.03 ± 0.94 vs. 32.70 ± 0.95, t = 13.29, P = 0.0002 for the invasive cells; respectively) signaling but not with an inhibitor of the ERK signaling (P > 0.05; Figure 4b and 4c), compared with the corresponding condition in the DMSO group. These data suggest that FSTL1 induces proliferation, migration, and invasion of MLgs by modulating the TGF-β1/p38/JNK/Smad2/3 signaling.
Figure 4.
FSTL1 promotes fibroblasts proliferation, migration, and invasion through positively regulating p38/JNK/Smad2/3 signaling. (a) Cell proliferation was measured by MTT. MLgs migration (b) and invasion (c) were measured by transwell chambers. Representative histogram represents cells per field. n = 3, Bars = 100 μm. *P < 0.001, †P < 0.01 versus corresponding condition in the DMSO group. DMSO: Dimethylsulfoxide; FSTL1: Follistatin-like 1; TGF-β1: Transforming growth factor-β1; U0126: ERK inhibitor; SB202190: p38 inhibitor; SP600125: JNK inhibitor; SB525334: Smad2/3 inhibitor.
DISCUSSION
IPF is a chronic, fatal lung disease of unknown etiology that has few treatment options. Myofibroblasts, the primary collagen-producing cells, are the key effectors of fibrosis. Among various mediators, TGF-β1 is the key cytokine regulating the activation and differentiation of myofibroblasts. Recently, FSTL1 has been identified as a novel profibrotic factor that promotes the differentiation of myofibroblasts and consequent PF by positively regulating the TGF-β1/Smad2/3 pathway. In this study, we investigated whether FSTL1 promotes the aggressive behaviors of myofibroblasts through the TGF-β1/Smad-independent signaling. We found that knockdown of Fstl1 attenuated the TGF-β1-induced phosphorylation of components of the MAPK signaling, while recombinant human FSTL1 protein had opposite effects. Moreover, we found that, in fibroblasts, FSTL1 regulated the proliferation, migration, and invasion induced by TGF-β1 though the p38, JNK, and Smad2/3 signaling but not through the ERK signaling. These results demonstrate that FSTL1 is a novel regulator of fibroblasts, partly through the MAPK and Smad signaling, which plays an important role in the pathogenesis of IPF.
TGF-β1 plays a key role in PF, and both its downstream Smad-dependent and Smad-independent signaling pathways have been reported to be involved in PF. A previous study has shown that decreased levels of FSTL1 are associated with the attenuation of bleomycin-induced fibrosis, with limited expansion of the fibroblast foci because of the decreased accumulation and activation of myofibroblasts and reduced Smad2/3 phosphorylation. The same study demonstrated that selective blockade of Smad2/3 phosphorylation decreased the expression of fibroblast activation markers.[18] Another research has shown that FSTL1 is upregulated through the expression of the microRNA miR-21 after paraquat challenge, resulting in increased Smad2/3 and p38 phosphorylation and progressive paraquat-induced PF.[25] A recent study has demonstrated that knockdown of Fstl1 inhibits oxLDL-induced inflammation responses through the MAPK pathway.[26] Consistently, our results demonstrated that MAPK signaling pathways were activated in the lungs of bleomycin-treated mice. In primary lung fibroblasts, depletion of Fstl1 attenuated p38, JNK, and ERK phosphorylation, while administration of recombinant human FSTL1 protein had opposite effects. Therefore, FSTL1 facilitates the function of TGF-β1 in activating MAPK signaling.
Yoshida et al.[27] found that, in lung tissue of patients with IPF, the levels of activated ERK in epithelial and endothelial cells, but not in fibroblasts or smooth muscle cells, decreased; at the same time, activated JNK and p38 in epithelial and endothelial cells and in fibroblasts, were increased, accompanied by the progression of fibrosis, suggesting that the activation of ERK, p38, and JNK plays a role in a cell-type specific way. The function of these three signaling pathways in cell cycle or other processes in the same cell type may be different. In PF, FSTL1 regulates fibroblast differentiation, proliferation, migration, and invasion partly through the MAPK signaling. On the other hand, novel research indicates that PF might be considered a cancer-like disorder.[28] In addition, several studies have shown that the MAPK pathways play a role in lung cancer,[29,30] and that activating mutations in RAS are found in approximately 30% of human cancer.[31] Thus, we cannot exclude the presence of mutant RAS in IPF fibroblasts, which results in the aberrant activation of ERK. Phosphorylated ERK activates a series of transcription factors including Elk-1, SAP-1α, and C-Myc, regulating the expression of genes involved in growth, differentiation, and mitosis.[32] Our data demonstrated that, although FSTL1 can modulate the phosphorylation of ERK, activated ERK has no effect on fibroblast differentiation, proliferation, migration, and invasion. Therefore, we speculate that FSTL1 can regulate the TGF-β1-induced phosphorylation of ERK but cannot further activate ERK-related transcription factors. However, further investigation is needed to verify this assumption. Although the basic framework of the TGF-β1/MAPK pathway is well characterized, its precise and complex regulatory mechanism remains to be further studied.
The main limitation of this study is the lack of clinical samples to further verify the phenomenon we observed in animals. Another limitation is that we only tested the MAPK signaling pathway; however, the non-Smad signaling also includes the Rho-like GTPase and phosphatidylinositol-3-kinase/AKT pathways. Therefore, to fully understand the mechanism of FSTL1 on PF, we need further clarification in this regard.
In summary, we have shown that FSTL1 is a profibrotic protein: it can promote fibroblasts differentiation, proliferation, migration, and invasion through TGF-β1-induced p38, JNK, and Smad2/3 signaling. Despite the limitations of this study, we made a further insight on the function of FSTL1 in IPF and demonstrated that FSTL1 regulates the TGF-β1/MAPK signaling in bleomycin-induced PF.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81430001, 31471373, and 31600939).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells AU. The revised ATS/ERS/JRS/ALAT diagnostic criteria for idiopathic pulmonary fibrosis (IPF) – Practical implications. Respir Res. 2013;14(Suppl 1):S2. doi: 10.1186/1465-9921-14-S1-S2. doi: 10.1186/1465-9921-14-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai M, Zhu M, Ban C, Su J, Ye Q, Liu Y, et al. Clinical features and outcomes of 210 patients with idiopathic pulmonary fibrosis. Chin Med J. 2014;127:1868–73. doi: 10.3760/cma.j.issn.0366-6999.20132528. [PubMed] [Google Scholar]
- 4.Wilson MS, Wynn TA. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Semin Respir Crit Care Med. 2010;2:103–21. doi: 10.1038/mi.2008.85. doi: 10.1038/mi.200885.Pulmonary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selman M, King TE, Pardo A. American Thoracic Society, European Respiratory Society, American College of Chest Physicians, et al. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–51. doi: 10.7326/0003-4819-134-2-200101160-00015. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 6.Noble PW, Homer RJ. Idiopathic pulmonary fibrosis: New insights into pathogenesis. Clin Chest Med. 2004;25:749–58. doi: 10.1016/j.ccm.2004.04.003. doi: 10.1016/j.ccm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Geng J, Huang X, Li Y, Xu X, Li S, Jiang D, et al. Down-regulation of USP13 mediates phenotype transformation of fibroblasts in idiopathic pulmonary fibrosis. Respir Res. 2015;16:124. doi: 10.1186/s12931-015-0286-3. doi: 10.1186/s12931-015-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014;9:157–79. doi: 10.1146/annurev-pathol-012513-104706. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YJ, Azuma A, Usuki J, Abe S, Matsuda K, Sunazuka T, et al. EM703 improves bleomycin-induced pulmonary fibrosis in mice by the inhibition of TGF-beta signaling in lung fibroblasts. Respir Res. 2006;7:16. doi: 10.1186/1465-9921-7-16. doi: 10.1186/1465-9921-7- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–66. doi: 10.1136/thx.48.10.959. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Velden JL, Ye Y, Nolin JD, Hoffman SM, Chapman DG, Lahue KG, et al. JNK inhibition reduces lung remodeling and pulmonary fibrotic systemic markers. Clin Transl Med. 2016;5:36. doi: 10.1186/s40169-016-0117-2. doi: 10.1186/s40169-016-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi-Wen X, Rodríguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, et al. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: Evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol. 2006;26:5518–27. doi: 10.1128/MCB.00625-06. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galuppo M, Esposito E, Mazzon E, Di Paola R, Paterniti I, Impellizzeri D, et al. MEK inhibition suppresses the development of lung fibrosis in the bleomycin model. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:21–37. doi: 10.1007/s00210-011-0637-7. doi: 10.1007/s00210-011-0637-7. [DOI] [PubMed] [Google Scholar]
- 14.Khalil N, Xu YD, O’Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem. 2005;280:43000–9. doi: 10.1074/jbc.M510441200. doi: 10.1074/jbcM510441200. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka H, Arai T, Mori M, Goya S, Kida H, Morishita H, et al. Ap38 MAPK inhibitor, FR-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;283:L103–12. doi: 10.1152/ajplung.00187.2001. doi: 10.1152/ajplung.00187.2001. [DOI] [PubMed] [Google Scholar]
- 16.Underwood DC, Osborn RR, Bochnowicz S, Webb EF, Rieman DJ, Lee JC, et al. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L895–902. doi: 10.1152/ajplung.2000.279.5.L895. doi: 10.1152/ajplung.2000.279.5.L895. [DOI] [PubMed] [Google Scholar]
- 17.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–9. doi: 10.1111/j.1432-1033.1993.tb18212.x. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 18.Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y, et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med. 2015;208:1459–71. doi: 10.1084/jem.20121878. doi: 10.1084/jem.20121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Y, Zhang S, Li X, Jiang F, Ye Q, Ning W, et al. Follistatin like-1 aggravates silica-induced mouse lung injury. Sci Rep. 2017;7:399. doi: 10.1038/s41598-017-00478-0. doi: 10.1038/s41598-017-00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108:7058–63. doi: 10.1073/pnas.1007293108. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning W, Song R, Li C, Park E, Mohsenin A, Choi AM, et al. TGF-beta1 stimulates HO-1 via the p38 mitogen-activated protein kinase in A549 pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1094–102. doi: 10.1152/ajplung.00151.2002. doi: 10.1152/ajplung.00151.2002. [DOI] [PubMed] [Google Scholar]
- 22.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–9. doi: 10.1172/JCI16861. doi: 10.1172/JCI200416861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2004;101:14895–900. doi: 10.1073/pnas.0401168101. doi: 10.1073/pnas.0401168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–82. doi: 10.1016/j.biocel.2007.08.011. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu MW, Liu R, Wu HY, Li YY, Su MX, Dong MN, et al. Radix puerariae extracts ameliorate paraquat-induced pulmonary fibrosis by attenuating follistatin-like 1 and nuclear factor erythroid 2p45-related factor-2 signalling pathways through downregulation of miRNA-21 expression. BMC Complement Altern Med. 2016;16:11. doi: 10.1186/s12906-016-0991-6. doi: 10.1186/s12906-016-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo J, Liang W, Li J, Long J. Knockdown of FSTL1 inhibits oxLDL-induced inflammation responses through the TLR4/MyD88/NF-κB and MAPK pathway. Biochem Biophys Res Commun. 2016;478:1528–33. doi: 10.1016/j.bbrc.2016.08.138. doi: 10.1016/j.bbrc.2016.08.138. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida K, Kuwano K, Hagimoto N, Watanabe K, Matsuba T, Fujita M, et al. MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J Pathol. 2002;198:388–96. doi: 10.1002/path.1208. doi: 10.1002/path.1208. [DOI] [PubMed] [Google Scholar]
- 28.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: A disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. doi: 10.1183/09031936.00077309. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 29.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–6. doi: 10.1513/pats.200601-001TK. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxena N, Lahiri SS, Hambarde S, Tripathi RP. RAS: Target for cancer therapy. Cancer Invest. 2008;26:948–55. doi: 10.1080/07357900802087275. doi: 10.1080/07357900802087275. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]