Abstract
Background:
On diagnosis of human immunodeficiency virus (HIV) infection, a person may have been infected already for many years. This study aimed to estimate the duration of HIV infection at the time of diagnosis.
Methods:
Newly diagnosed HIV cases in Dehong, China, from 2008 to 2015 were studied. Duration of infection at the time of diagnosis was calculated using the first CD4 cell count result after diagnosis and a CD4 depletion model of disease progression. Multiple linear regression analysis was used to investigate the associated risk factors.
Results:
A total of 5867 new HIV cases were enrolled. Overall, mean duration of infection was 6.3 years (95% confidence interval [CI]: 6.2, 6.5). After adjusting for confounding, significantly shorter durations of infection were observed among participants who were female (beta: −0.37, 95% CI: −0.64, −0.09), Dai ethnicity (beta: −0.28, 95% CI: −0.57, 0.01), and infected through injecting drug use (beta: −1.82, 95% CI: −2.25, −1.39). Compared to the hospital setting, durations were shorter for those diagnosed in any other settings, and compared to 2008, durations were shorter for those diagnosed all years after 2010.
Results:
A total of 5867 new HIV cases were enrolled. Overall, mean duration of infection was 6.3 years (95% confidence interval [CI]: 6.2, 6.5). After adjusting for confounding, significantly shorter durations of infection were observed among participants who were female (beta: −0.37, 95% CI: −0.64, −0.09), Dai ethnicity (beta: −0.28, 95% CI: −0.57, 0.01), and infected through injecting drug use (beta: −1.82, 95% CI: −2.25, −1.39). Compared to the hospital setting, durations were shorter for those diagnosed in any other settings, and compared to 2008, durations were shorter for those diagnosed all years after 2010.
Conclusion:
Although the reduction in duration of infection at the time of diagnosis observed in Dehong was significant, it may not have had a meaningful impact.
Keywords: China, Delayed Diagnosis, Human Immunodeficiency Virus Infection
摘要
目的:
HIV感染者在被诊断发现时可能已经感染多年了。本研究的目的是估计HIV感染者在被诊断发现时的感染时间。
方法:
研究对象为中国德宏州2008至2015年新诊断报告的HIV病例。诊断发现时的感染时间是依据CD4数值与感染时间的衰减 模型,利用感染者首次CD4检测值反推计算获得的。使用多重线性回归模型分析影响诊断时感染时间的危险因素。
结果:
研究共纳入5867例新诊断发现的HIV病例。研究对象诊断时的感染时间均值为6.3年(95%可信区间:6.2~6.5)。多重 线性回归模型结果显示,在被诊断发现时,女性的感染时间显著短于男性(回归系数: -0.37;95%可信区间: -0.64~-0.09),傣 族的感染时间显著短于汉族、景颇族和其它民族(回归系数: -0.28;95%可信区间:-0.57~0.01),经注射吸毒感染的人群其 感染时间显著短于性传播感染人群(回归系数: -1.82;95%可信区间:-2.25~-1.39)。相较其他来源的感染者,医院来源的感 染者其感染时间更久。相较2008年新诊断发现的感染者,自2010年后新诊断发现的感染者其感染时间更短。
结论:
德宏州HIV感染者在被诊断发现时的感染时间呈明显缩短趋势,但其影响不大。
INTRODUCTION
The individual and public health benefits of “early” antiretroviral therapy (ART), meaning ART initiation when CD4 counts are still high, have been clearly demonstrated by both clinical trials and observational studies.[1,2,3,4,5,6,7] Thus, early ART is now recommended for all people living with human immunodeficiency virus (PLWH).[8] However, late presentation, meaning CD4 counts are very low or clinical manifestations of acquired immune deficiency syndrome (AIDS) are present, is a persistent and prevalent problem in many settings, including resource-rich and resource-limited countries alike, and is an important driver of elevated morbidity and mortality, onward transmission, and health system burden.[9,10,11,12,13] Unfortunately, late presentation, or delayed diagnosis, also puts the added benefits of “early” ART out of reach. Thus, focus is turning toward “immediate” ART, or ART initiation as soon as possible after diagnosis, regardless of CD4 level.[14,15,16,17,18]
Despite dramatic scale-up of a range of testing services, including voluntary counseling and testing (VCT, an opt-in approach), provider-initiated testing and counseling (PITC, an opt-out approach), and mandatory HIV testing (MHT) in detoxification settings (e.g., detention centers and reeducation-through-labor camps),[19,20] China continues to struggle with the challenge of late presentation to human immunodeficiency virus (HIV) care.[21,22,23] However, the length of delays in diagnosis has not yet been thoroughly studied in the China setting. Therefore, the primary aim of this study was to test the hypothesis that the duration of infection at the time of diagnosis has decreased over time from 2008 to 2015 in Dehong Prefecture, Yunnan Province, China. Secondarily, we sought to assess the factors associated with differences in duration of infection.
METHODS
Ethical consideration
The study protocol was reviewed and approved by the Institutional Review Board of National Center for AIDS/STD Control and Prevention (NCAIDS), Chinese Center for Disease Control and Prevention (China CDC), with approval code number KX180208497. All individuals signed written informed consent on entry into the Comprehensive Response Information Management System (CRIMS) that allows the future use of their records in epidemiological studies. Therefore, no further consent was required. All records were de-identified before analysis.
Study design and setting
A serial cross-sectional study design was used to examine the differences in durations of HIV infection at the time of diagnosis among new cases in Dehong Prefecture, Southern Yunnan Province, China, from January 1, 2008 to December 31, 2015.
Data source
Data were extracted from China's web-based national database for real-time collection and maintenance of information related to the HIV epidemic. This system, called the HIV/AIDS CRIMS, has been described elsewhere.[24] All confirmed cases of HIV infection require timely reporting to CRIMS, which is managed by the NCAIDS, China CDC. All CRIMS records contain demographic information, self-reported transmission routes, dates of HIV testing, and dates and results of CD4 testing, which are collected during the normal course of routine care in China.
Data collection and inclusion criteria
All records of persons with confirmed diagnoses of HIV infection between January 1, 2008 and December 31, 2015, were extracted from CRIMS and then further screened for study eligibility criteria, which were (1) being ≥13 years of age at the time of diagnosis and (2) having acquired HIV through heterosexual contact, male-to-male sexual contact, or injecting drug use. During the study period, these transmission routes accounted for over 90% of newly diagnosed HIV infections in China.[20] All eligible individuals were included in the analysis.
Estimation of the duration of infection at diagnosis
The regular progression of HIV disease, including the decline in numbers of CD4+ T-lymphocytes in general circulation in the absence of ART, has been well documented,[25] and the mathematical modeling of CD4 depletion has commonly been used to estimate the time delay between HIV infection and diagnosis.[26,27,28,29] The model we used employed the equation given below:
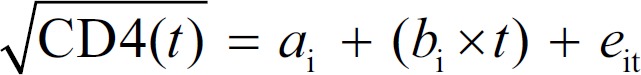
Where t is the duration of infection at the date of diagnosis, and the values for intercepts ai and slopes bi were obtained from a recently published study.[26] Ti was the study's main outcome measure – the duration from estimated date of infection to the date of diagnosis. Ti for each participant was calculated by:
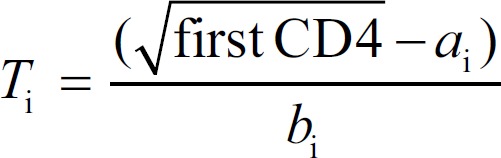
Statistical analysis
Numbers of newly reported cases overall and by year were tabulated and stratified by baseline participant characteristics. Categorical variables are presented as number and percent, and comparisons were made using Chi-square analysis, generating P values. The duration of HIV infection at the time of diagnosis (Ti) was calculated and expressed in years. Since this outcome was continuous, means and 95% confidence intervals (CIs) are reported. To examine the distribution of durations of infection at the time of diagnosis by diagnosis setting, we constructed histograms with best-fit Gaussian curves. Multiple linear regression analyses were performed to examine the factors associated with longer or shorter durations of HIV infection at diagnosis (Ti). Both univariate and multivariate regressions were performed, and beta values, 95% CIs, and P values are also reported. All P values presented are two-sided, and P < 0.05 was considered statistically significant. All analyses were conducted using the Statistical Analysis System (SAS) software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
A total of 6032 individual records with a date of confirmed HIV diagnosis between January 1, 2008 and December 31, 2015, were screened for study eligibility. However, 132 potential participants were <13 years of age, and a further 33 had reported infection routes other than sexual contact or injecting drug use. These individuals were excluded. Thus, a total of 5867 (of 6032, 97.3%) participants were included in the analysis.
Characteristics of the participants
Most participants were male (63.7%), aged 26–45 years (64.7%), Han Chinese (42.2%), and married or cohabiting (57.2%). A majority reported their route of HIV infection as sexual contact (81.0%). The most common diagnosis settings were VCT (27.8%) and hospitals (20.3%; Table 1).
Table 1.
Characteristics of participants
| Baseline characteristics | Newly reported HIV cases, n (%) | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||
| Overall | 5867 (100.0) | 1217 (100.0) | 1074 (100.0) | 796 (100.0) | 809 (100.0) | 646 (100.0) | 521 (100.0) | 443 (100.0) | 361 (100.0) | |
| Gender | ||||||||||
| Male | 3738 (63.7) | 764 (62.8) | 675 (62.9) | 490 (61.6) | 528 (65.3) | 421 (65.2) | 350 (67.2) | 288 (65.0) | 222 (61.5) | 0.210 |
| Female | 2129 (36.3) | 453 (37.2) | 399 (37.2) | 306 (38.4) | 281 (34.7) | 225 (34.8) | 171 (32.8) | 155 (35.0) | 139 (38.5) | |
| Age | ||||||||||
| ≤25 years | 914 (15.6) | 251 (20.6) | 175 (16.3) | 116 (14.6) | 99 (12.2) | 92 (14.2) | 72 (13.8) | 70 (15.8) | 39 (10.8) | <0.001 |
| 26–35 years | 2149 (36.6) | 507 (41.7) | 444 (41.3) | 292 (36.7) | 274 (33.9) | 205 (31.7) | 186 (35.7) | 123 (27.8) | 118 (32.7) | |
| 36–45 years | 1648 (28.1) | 313 (25.7) | 289 (26.9) | 235 (29.5) | 254 (31.4) | 173 (26.8) | 149 (28.6) | 136 (30.7) | 99 (27.4) | |
| >45 years | 1156 (19.7) | 146 (12.0) | 166 (15.5) | 153 (19.2) | 182 (22.5) | 176 (27.2) | 114 (21.9) | 114 (25.7) | 105 (29.1) | |
| Ethnicity | ||||||||||
| Han | 2474 (42.2) | 507 (41.7) | 416 (38.7) | 354 (44.5) | 322 (39.8) | 276 (42.7) | 211 (40.5) | 209 (47.2) | 179 (49.6) | 0.013 |
| Dai | 1808 (30.88) | 350 (28.7) | 365 (34.0) | 249 (31.3) | 259 (32.0) | 217 (33.6) | 158 (30.3) | 130 (29.4) | 80 (22.2) | |
| Jingpo | 1265 (21.6) | 295 (24.2) | 245 (22.8) | 150 (18.8) | 173 (21.4) | 115 (17.8) | 129 (24.8) | 81 (18.3) | 77 (21.3) | |
| Other | 320 (5.5) | 65 (5.3) | 48 (4.5) | 43 (5.4) | 55 (6.8) | 38 (5.9) | 23 (4.4) | 23 (5.2) | 25 (6.9) | |
| Marital status* | ||||||||||
| Married | 3354 (57.2) | 698 (57.4) | 669 (62.3) | 461 (57.9) | 486 (60.1) | 362 (56.0) | 285 (54.7) | 225 (50.8) | 168 (46.5) | <0.001 |
| Divorced/windowed | 1241 (21.2) | 246 (20.2) | 210 (19.6) | 158 (19.9) | 163 (20.2) | 140 (21.7) | 119 (22.8) | 103 (23.3) | 102 (28.3) | |
| Unmarried | 1272 (21.7) | 273 (22.4) | 195 (18.2) | 177 (22.2) | 160 (19.8) | 144 (22.3) | 117 (22.5) | 115 (26.0) | 91 (25.2) | |
| Transmission route | ||||||||||
| Sexual contact | 4754 (81.0) | 903 (74.2) | 873 (81.3) | 655 (82.3) | 687 (84.9) | 537 (83.1) | 426 (81.8) | 362 (81.7) | 311 (86.2) | <0.001 |
| Injecting drug use | 1113 (19.0) | 314 (25.8) | 201 (18.7) | 141 (17.7) | 122 (15.1) | 109 (16.9) | 95 (18.2) | 81 (18.3) | 50 (13.9) | |
| Diagnosis setting | ||||||||||
| Hospital | 1191 (20.3) | 294 (24.2) | 173 (16.1) | 111 (13.9) | 120 (14.8) | 142 (22.0) | 111 (21.3) | 122 (27.5) | 118 (32.7) | <0.001 |
| VCT | 1632 (27.8) | 210 (17.3) | 303 (28.2) | 276 (34.7) | 234 (28.9) | 192 (29.7) | 183 (35.1) | 133 (30.0) | 101 (28.0) | |
| Detoxification† | 751 (12.8) | 212 (17.4) | 124 (11.6) | 97 (12.2) | 85 (10.5) | 79 (12.2) | 62 (11.9) | 62 (14.0) | 30 (8.3) | |
| Premarital checkup | 462 (7.9) | 108 (8.9) | 101 (9.4) | 72 (9.1) | 74 (9.2) | 30 (4.6) | 29 (5.6) | 28 (6.3) | 20 (5.5) | |
| Partner testing | 446 (7.6) | 170 (14.0) | 85 (7.9) | 28 (3.5) | 42 (5.2) | 26 (4.0) | 38 (7.3) | 27 (6.1) | 30 (8.3) | |
| Other | 1385 (23.6) | 223 (18.3) | 288 (26.8) | 212 (26.6) | 254 (31.4) | 177 (27.4) | 98 (18.8) | 71 (16.0) | 62 (17.2) | |
*For the marital status variable, those who were cohabitating were categorized as married; †The detoxification category of the diagnosis setting variable includes detoxification centers and reeducation-through-labor camps but does not include methadone maintenance treatment clinics. VCT: Voluntary counseling and testing; HIV: Human immunodeficiency virus. Characteristics of newly reported HIV cases in Dehong Prefecture, Yunnan Province, China, overall and annually from 2008 to 2015.
Over the 8-year study period, the annual total number of newly diagnosed cases decreased substantially from 1217 in 2008 to 361 in 2015. In more recent years, greater proportions of participants were older (P < 0.001), were Han Chinese (P = 0.013), were unmarried or divorced/widowed (P < 0.001), and reported sexual contact as their route of infection (P < 0.001; Table 1).
Duration from human immunodef iciency virus infection to diagnosis
Overall, the mean duration of infection at diagnosis was 6.3 years (95% CI: 6.2, 6.5). The longest durations were observed for participants who were >45 years of age (6.8 years, 95% CI: 6.5, 7.1), were Han ethnicity (6.8 years, 95% CI: 6.6, 7.0), were reported sexual contact as their route of infection (6.8 years, 95% CI: 6.6, 6.9), and were diagnosed in hospital (8.6 years, 95% CI: 8.3, 8.9) or VCT settings (7.2 years, 95% CI: 7.0, 7.5). The shortest durations were experienced by participants who reported their route of HIV infection as injecting drug use (4.6 years, 95% CI: 4.3, 4.8) and were diagnosed in detoxification settings, which included detention centers and reeducation-through-labor camps but did not include methadone maintenance treatment (MMT) clinics (4.3 years, 95% CI: 4.0, 4.5; Table 2).
Table 2.
Mean duration between HIV infection and diagnosis
| Baseline characteristics | Duration between HIV infection and diagnosis (years), mean (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 5867) | 2008 (n = 1217) | 2009 (n = 1074) | 2010 (n = 796) | 2011 (n = 809) | 2012 (n = 646) | 2013 (n = 521) | 2014 (n = 443) | 2015 (n = 361) | |
| Overall | 6.3 (6.2, 6.5) | 6.7 (6.4, 7.0) | 6.5 (6.2, 6.8) | 6.6 (6.2, 7.0) | 6.2 (5.9, 6.5) | 6.4 (6.0, 6.7) | 5.6 (5.2, 6.0) | 5.8 (5.4, 6.3) | 6.1 (5.5, 6.6) |
| Gender | |||||||||
| Male | 6.4 (6.2, 6.5) | 6.8 (6.4, 7.2) | 6.4 (6.0, 6.8) | 6.8 (6.3, 7.2) | 6.2 (5.8, 6.6) | 6.4 (5.9, 6.8) | 5.6 (5.1, 6.1) | 5.8 (5.3, 6.4) | 6.0 (5.3, 6.7) |
| Female | 6.3 (6.1, 6.5) | 6.5 (6.0, 7.0) | 6.7 (6.2, 7.2) | 6.3 (5.7, 6.9) | 6.3 (5.7, 6.8) | 6.3 (5.7, 7.0) | 5.7 (4.9, 6.4) | 5.8 (5.0, 6.6) | 6.1 (5.3, 6.9) |
| Age | |||||||||
| ≤25 years | 5.9 (5.6, 6.3) | 6.1 (5.5, 6.8) | 5.9 (5.1, 6.7) | 6.3 (5.3, 7.3) | 5.5 (4.5, 6.6) | 6.0 (4.9, 7.1) | 4.4 (3.3, 5.5) | 5.8 (4.7, 7.0) | 7.1 (5.5, 8.8) |
| 26–35 years | 6.3 (6.1, 6.5) | 6.6 (6.2, 7.1) | 6.3 (5.8, 6.8) | 6.0 (5.4, 6.6) | 6.2 (5.6, 6.8) | 6.7 (6.0, 7.3) | 5.3 (4.6, 6.0) | 4.8 (4.0, 5.6) | 4.7 (3.9, 5.5) |
| 35–45 years | 6.7 (6.5, 6.9) | 7.0 (6.4, 7.6) | 6.8 (6.3, 7.4) | 7.1 (6.4, 7.7) | 6.3 (5.7, 6.9) | 5.9 (5.2, 6.7) | 6.1 (5.4, 6.9) | 6.1 (5.2, 7.0) | 6.7 (5.7, 7.7) |
| >45 years | 6.8 (6.5, 7.1) | 7.2 (6.4, 8.0) | 7.2 (6.5, 8.0) | 7.3 (6.5, 8.1) | 6.5 (5.8, 7.2) | 6.6 (5.9, 7.3) | 6.3 (5.4, 7.2) | 6.5 (5.7, 7.4) | 6.6 (5.7, 7.6) |
| Ethnicity | |||||||||
| Han | 6.8 (6.6, 7.0) | 7.0 (6.6, 7.5) | 7.2 (6.7, 7.7) | 6.9 (6.4, 7.4) | 6.6 (6.0, 7.2) | 6.9 (6.3, 7.5) | 5.9 (5.2, 6.6) | 6.2 (5.5, 6.9) | 6.5 (5.8, 7.3) |
| Dai | 6.0 (5.8, 6.3) | 6.9 (6.4, 7.5) | 6.0 (5.5, 6.5) | 6.2 (5.6, 6.9) | 6.1 (5.5, 6.7) | 5.7 (5.0, 6.3) | 5.3 (4.6, 6.0) | 5.4 (4.6, 6.2) | 5.3 (4.2, 6.3) |
| Jingpo | 6.0 (5.8, 6.3) | 5.9 (5.4, 6.5) | 6.3 (5.7, 7.0) | 6.4 (5.6, 7.3) | 5.6 (4.9, 6.3) | 6.9 (6.0, 7.8) | 5.7 (4.9, 6.5) | 5.1 (4.1, 6.2) | 5.8 (4.7, 6.9) |
| Other | 6.0 (5.5, 6.5) | 6.1 (4.8, 7.3) | 5.8 (4.1, 7.4) | 6.7 (5.2, 8.2) | 6.3 (5.0, 7.5) | 4.8 (3.4, 6.2) | 4.9 (2.6, 7.2) | 7.2 (5.4, 9.1) | 6.1 (4.0, 8.2) |
| Marital status* | |||||||||
| Married | 6.3 (6.2, 6.5) | 6.9 (6.5, 7.3) | 6.7 (6.3, 7.1) | 6.4 (5.9, 6.8) | 5.9 (5.5, 6.3) | 6.4 (5.9, 6.9) | 5.4 (4.9, 5.9) | 5.6 (5.0, 6.2) | 6.0 (5.3, 6.8) |
| Divorced/windowed | 6.5 (6.3, 6.8) | 6.6 (5.9, 7.2) | 6.2 (5.6, 6.9) | 7.0 (6.2, 7.9) | 6.7 (5.9, 7.4) | 6.3 (5.5, 7.1) | 6.9 (5.9, 7.8) | 6.5 (5.5, 7.5) | 6.0 (5.1, 6.9) |
| Unmarried | 6.2 (5.9, 6.5) | 6.3 (5.7, 6.9) | 6.3 (5.6, 7.1) | 6.8 (6.0, 7.5) | 6.7 (5.8, 7.5) | 6.4 (5.6, 7.2) | 4.9 (4.1, 5.8) | 5.6 (4.7, 6.5) | 6.2 (5.1, 7.2) |
| Transmission route | |||||||||
| Sexual contact | 6.8 (6.6, 6.9) | 7.4 (7.1, 7.7) | 7.0 (6.7, 7.4) | 7.0 (6.6, 7.4) | 6.4 (6.1, 6.8) | 6.7 (6.3, 7.1) | 6.0 (5.5, 6.5) | 6.2 (5.7, 6.7) | 6.3 (5.7, 6.8) |
| Injecting drug use | 4.6 (4.3, 4.8) | 4.7 (4.2, 5.1) | 4.4 (3.9, 5.0) | 4.7 (4.0, 5.4) | 4.9 (4.3, 5.6) | 4.7 (4.1, 5.4) | 3.9 (3.2, 4.6) | 4.2 (3.4, 5.0) | 4.8 (3.7, 5.9) |
| Diagnosis setting | |||||||||
| Hospital | 8.6 (8.3, 8.9) | 9.4 (8.8, 10.0) | 9.8 (9.1, 10.5) | 8.8 (7.9, 9.7) | 8.7 (7.8, 9.7) | 7.8 (6.9, 8.7) | 6.9 (6.0, 7.8) | 7.7 (6.7, 8.6) | 7.8 (6.8, 8.7) |
| VCT | 7.2 (7.0, 7.5) | 7.6 (6.8, 8.3) | 7.3 (6.7, 7.9) | 8.2 (7.6, 8.8) | 7.6 (7.0, 8.3) | 7.3 (6.6, 8.0) | 6.2 (5.5, 7.0) | 6.2 (5.4, 7.1) | 6.0 (5.0, 6.9) |
| Detoxification† | 4.3 (4.0, 4.5) | 4.6 (4.0, 5.1) | 4.2 (3.5, 4.9) | 4.7 (3.9, 5.4) | 4.4 (3.7, 5.1) | 4.1 (3.4, 4.8) | 3.6 (2.7, 4.4) | 4.2 (3.4, 5.1) | 3.4 (2.0, 4.7) |
| Premarital checkup | 5.4 (5.0, 5.8) | 5.4 (4.6, 6.3) | 5.9 (4.9, 6.9) | 4.7 (3.6, 5.8) | 5.1 (4.1, 6.2) | 6.5 (4.4, 8.5) | 6.1 (4.7, 7.5) | 5.7 (3.9, 7.5) | 3.0 (1.4, 4.6) |
| Partner testing | 5.1 (4.6, 5.5) | 5.3 (4.6, 5.9) | 5.3 (4.3, 6.4) | 3.8 (2.0, 5.5) | 5.6 (4.1, 7.0) | 5.1 (3.5, 6.8) | 4.5 (2.8, 6.2) | 3.5 (2.2, 4.8) | 5.7 (4.1, 7.3) |
| Other | 5.2 (5.0, 5.5) | 5.7 (5.2, 6.2) | 5.4 (4.9, 5.9) | 5.1 (4.5, 5.7) | 4.9 (4.3, 5.4) | 5.3 (4.7, 6.0) | 4.6 (3.8, 5.4) | 4.0 (3.2, 4.8) | 5.5 (4.6, 6.4) |
*For the marital status variable, those who were cohabitating were categorized as married; †The detoxification category of the diagnosis setting variable includes detoxification centers and reeducation through labor camps but does not include methadone maintenance treatment clinics. CI: Confidence interval; VCT: Voluntary counseling and testing; HIV: Human immunodeficiency virus.
Factors associated with duration of infection
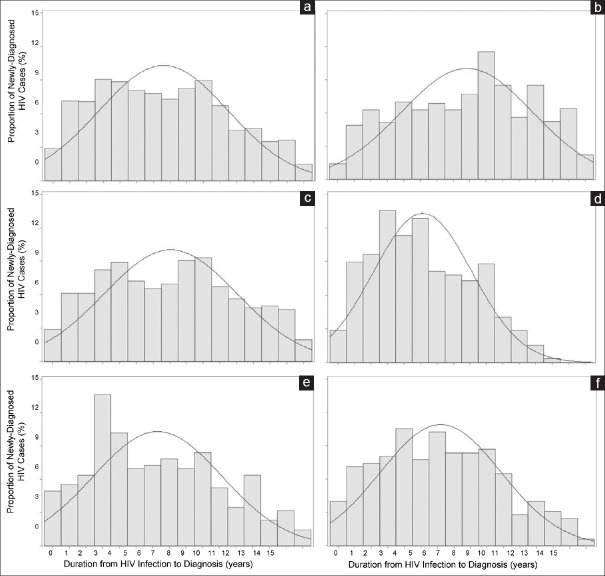
Histograms and best-fit Gaussian curves of the distribution of durations of infection at time of diagnosis by diagnosis setting show that durations were longest among those diagnosed in hospital settings (curve shifted right) and shortest among those diagnosed in detoxification settings [curve shifted left; Figure 1].
Figure 1.
Distributions of delays in diagnosis by diagnosis setting. Histograms (gray bars) and best-f it Gaussian curves (blue lines) showing the distributions of durations from HIV infection to HIV diagnosis (in years) among newly diagnosed cases in Dehong Prefecture, Yunnan Province, China: (a) overall, (b) in hospitals, (c) at VCT sites, (d) within detoxif ication settings, (e) during premarital checkups, and (f) as a result of partner testing. These findings suggest that durations from HIV infection to diagnosis were longer among those diagnosed in hospital settings (shifted right; b) and shorter among those diagnosed in detoxif ication settings (shifted left; d). VCT: Voluntary counseling and testing; HIV: Human immunodef iciency virus.
After controlling for confounding, factors found to be associated with significantly shorter durations of infection at the time of diagnosis were female sex (adjusted beta: −0.37, 95% CI: −0.64, −0.09), injecting drug use infection route (adjusted beta: −1.82, 95% CI: −2.25, −1.39), and any diagnosis setting compared to hospital setting (VCT adjusted beta: −1.31, detoxification adjusted beta: −3.34, premarital checkup adjusted beta: −3.34, partner testing adjusted beta: −3.59, and other adjusted beta: −3.19; Table 3).
Table 3.
Determinants of long-time interval between infection and diagnosis
| Characteristic | Unadjusted Beta (95% CI)* | P* | Adjusted Beta (95% CI)* | P* |
|---|---|---|---|---|
| Gender | ||||
| Male | 0.00 | 0.00 | ||
| Female | −0.03 (−0.30, 0.24) | 0.820 | −0.37 (−0.64, −0.09) | 0.010 |
| Age | ||||
| ≤25 years | −0.89 (−1.32, −0.45) | <0.001 | −0.26 (−0.72, 0.21) | 0.280 |
| 26–35 years | −0.71 (−1.07, −0.35) | <0.001 | −0.01 (−0.38, 0.36) | 0.960 |
| 36–45 years | −0.22 (−0.60, 0.16) | 0.260 | 0.28 (−0.08, 0.65) | 0.130 |
| >45 years | 0.00 | 0.00 | ||
| Ethnicity | ||||
| Han | 0.00 | 0.00 | ||
| Dai | −0.72 (−1.02, −0.42) | <0.001 | −0.28 (−0.57, 0.01) | 0.060 |
| Jingpo | −0.73 (−1.07, −0.39) | <0.001 | 0.03 (−0.30, 0.37) | 0.840 |
| Other | −0.77 (−1.35, −0.19) | 0.010 | −0.33 (−0.89, 0.23) | 0.250 |
| Marital status† | ||||
| Married | 0.00 | 0.00 | ||
| Divorced/windowed | 0.21 (−0.12, 0.53) | 0.220 | 0.22 (−0.10, 0.55) | 0.180 |
| Unmarried | −0.11 (−0.43, 0.22) | 0.510 | 0.77 (0.42, 1.13) | <0.001 |
| Infection route | ||||
| Sexual contact | 0.00 | 0.00 | ||
| Injecting drug use | −2.20 (−2.52, −1.88) | <0.001 | −1.82 (−2.25, −1.39) | <0.001 |
| Diagnosis setting | ||||
| Hospital | 0.00 | 0.00 | ||
| VCT | −1.34 (−1.70, −0.98) | <0.001 | −1.31 (−1.67, −0.95) | <0.001 |
| Detoxification‡ | −4.28 (−4.72, −3.84) | <0.001 | −3.34 (−3.89, −2.79) | <0.001 |
| Premarital checkup | −3.17 (−3.69, −2.65) | <0.001 | −3.18 (−3.71, −2.65) | <0.001 |
| Partner testing | −3.50 (−4.02, −2.98) | <0.001 | −3.59 (−4.12, −3.06) | <0.001 |
| Other | −3.32 (−3.70, −2.95) | <0.001 | −3.19 (−3.57, −2.81) | <0.001 |
| Diagnosis year | ||||
| 2008 | 0.00 | 0.00 | ||
| 2009 | −0.17 (−0.58, 0.25) | 0.430 | −0.25 (−0.64, 0.15) | 0.220 |
| 2010 | −0.10 (−0.55, 0.35) | 0.660 | −0.31 (−0.74, 0.12) | 0.160 |
| 2011 | −0.49 (−0.94, −0.04) | 0.032 | −0.66 (−1.10, −0.23) | 0.003 |
| 2012 | −0.34 (−0.82, 0.14) | 0.170 | −0.72 (−1.19, −0.26) | 0.002 |
| 2013 | −1.06 (−1.58, −0.55) | <0.001 | −1.52 (−2.02, −1.03) | <0.001 |
| 2014 | −0.88 (−1.42, −0.33) | 0.002 | −1.46 (−1.98, −0.94) | <0.001 |
| 2015 | −0.64 (−1.23, −0.05) | 0.034 | −1.44 (−2.01, −0.88) | <0.001 |
*Univariate and multivariate regression modeling resulted in beta, 95% CIs, and P values; †For the marital status variable, those who were cohabitating were categorized as married; ‡The detoxification category of the diagnosis setting variable includes detoxification centers and reeducation-through-labor camps but does not include methadone maintenance treatment clinics. CI: Confidence interval; VCT: Voluntary counseling and testing.
Furthermore, compared to diagnosis in 2008, significantly shorter durations of infection at the time of diagnosis were observed for 2011 and every year afterward (2011 adjusted beta: −0.66, 2012 adjusted beta: −0.72, 2013 adjusted beta: −1.52, 2014 adjusted beta: −1.46, and 2015 adjusted beta: −1.44; Table 3). The adjusted beta values and 95% CIs for each study year from 2008 to 2015 were plotted to show the change in duration of infection at the time of diagnosis among participants. The graph shows a clear step-down from 2008 to 2012 and then a second step-down of 1.52 years for 2013 compared to 2008 [Figure 2].
Figure 2.
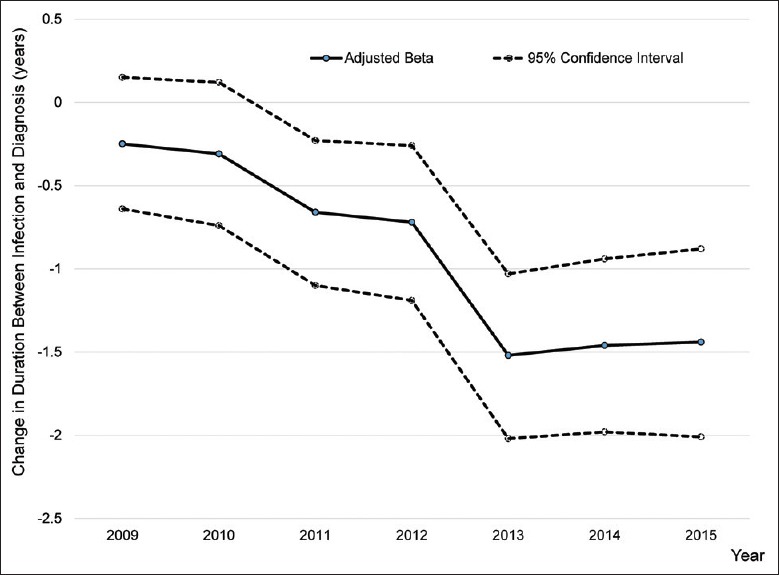
Change in overall duration between HIV Infection and diagnosis overtime. Using 2008 as a reference, annual adjusted beta values (blue markers) and 95% conf idence intervals (gray lines) show a decreasing trend in the overall duration between HIV infection and diagnosis (in years) among newly diagnosed cases in Dehong Prefecture, Yunnan Province, China. HIV: Human immunodeficiency virus.
DISCUSSION
The main finding of our study was a modestly decreasing trend in the duration of infection at the time of diagnosis among new HIV cases in Dehong over our 8-year study period from 2008 to 2015. Factors associated with shorter durations of infection included being female, reporting injecting drug use as the route of HIV infection, being diagnosed in any setting other than hospital, and being diagnosed after 2010.
We found that the mean duration of infection was 6.3 years. After reviewing both domestic and international literature, we have found no other report of this figure in China. However, we have found a study in the United States that reported duration of infection at diagnosis of 7.0 years in 2003 and 5.6 years in 2011,[30] which was similar to our study results.
China has shown great determination in its endeavors to bring Dehong's HIV epidemic under control, providing strong political commitment and consistent financial support from central and local governments. Dehong was given a special epidemic designation in 2004 and thereafter has been directly supported by the China CDC using a special central government AIDS surplus fund of 11 million Renminbi (approximately 1.8 million United States Dollars) per year. Although these special funds were used to fully implement HIV testing and treatment programs, coverage, service quality, and other factors were not consistent across our whole study period. For example, at the provincial level, the Yunnan Government engaged in a series of campaigns against HIV: the first was 2004–2008, then 2008–2010, 2011–2015, and 2016–2020 currently. At the end of each, the effectiveness of the HIV response was reevaluated for the purpose of iteratively improving it. As another example, Dehong was selected as a study site for the National Science and Technology Major Project on Prevention and Treatment of Major Infectious Diseases including AIDS and Viral Hepatitis. As a result, from 2008 to 2015, focus on universal testing and treatment was very strong, and many observational studies and interventional trials conducted in this setting strongly influenced HIV testing and treatment services. Taken together, it is possible that these types of changes to HIV services in Dehong over the study period may have been the driver for the moderate decline in the duration of infection at the time of diagnosis observed in our study.
The World Health Organization began recommending PITC in 2007 as a strategy to expand access to HIV-related services.[31] China quickly adopted these recommendations, first introducing HIV PITC the same year, and subsequently scaling it up dramatically.[19] However, our findings show that cases identified in hospital settings were diagnosed significantly later than in any other settings examined, suggesting that perhaps, PITC in China is not working effectively. This observation is consistent with a study by Cheng et al.[21] among newly identified cases in Guangzhou from 2008 to 2013. In this study, individuals diagnosed through PITC had greater odds of late presentation and greater odds of presentation with advanced HIV disease compared to individuals diagnosed through VCT and MHT. Furthermore, in a recently published study by Xie et al.,[23] among patients attending a tertiary hospital in Beijing between 1997 and 2012, not only was a consistent majority of patients diagnosed very late but also there was evidence of immunocompromised well before HIV testing was finally performed, demonstrating the failure of the PITC strategy to identify cases in a timely manner. These results suggest that PITC in Chinese hospital settings is not targeted for maximally efficient identification of new infections. Failure of PITC to identify new HIV infections in a timely fashion is not unique to the China setting, and some researchers have called on policymakers to shift the way PITC is targeted, from a symptom-oriented approach to risk group-oriented approach.[32]
Interestingly, we found significantly shorter durations of infection among those who reported injecting drug use as their route of infection and among those diagnosed in detoxification settings (i.e., detention centers and reeducation-through-labor camps), where MHT has been enforced since the early stages of China's epidemic. Many of those who were diagnosed outside detoxification settings yet reported injecting drug use as their route of HIV infection may have been diagnosed in the context of MMT. The rapid expansion of China's National MMT Program has clearly had a positive impact on China's dual epidemics of opioid use and HIV infection, and MHT every 6 months for all MMT clients has been important for early detection of new HIV infections in this key population.[20,33]
Development and implementation of improvements to conventional HIV testing strategies (i.e., VCT and PICT) and promotion of new testing strategies (i.e., HIV self-testing) must be a top priority for China's policymakers and public health institutions. However, our results suggest that perhaps expansion of MHT to other settings may be worth considering also. For example, perhaps, MHT for all attendees of STI clinics and hospital departments may be an effective and efficient way to identify new HIV infections earlier. For several years already, China's HIV epidemic has been driven predominantly by sexual transmission.[20,34] China must take urgent action to help PLWH who are yet unaware of their status – their diagnosis and treatment are of critical importance to individual and community outcomes, as well as national and international epidemic response goals.
This study had several limitations. First, the cross-sectional design precluded us from examining causality. Second, since dates of infection were not available, we had to estimate the durations of infection at the time of diagnosis using a CD4 depletion model, and because of some restrictive assumptions (e.g., the mean square root of CD4 count is linearly related to the time elapsed since infection), durations may have been underestimated. As a result, CIs may not cover the true value with the desired confidence level. Nevertheless, this model has been validated and accepted as a means of generating reasonably accurate estimates of duration of infection at the time of diagnosis.[26,28] Third, factors affecting delayed diagnosis are complex. Important determinants may have been missing from our dataset and therefore not included in the model. Fourth, the study did not consider HIV subtype because there was no HIV subtype information available for enrolled participants. However, although this factor may have caused some confounding, this effect would have been minor since our population-based study was took into account different stratified populations. Finally, categorization of participants for the infection route variable relied on self-reporting at the time of diagnosis, a mean of 6 years after the infection event. Thus, this variable was likely affected by recall bias, and possibly by social desirability bias, perhaps resulting in under- or over-estimation of the importance of this factor.
Although the duration of infection at the time of diagnosis observed in Dehong did significantly decline over the 8-year study period from 2008 to 2015, the decline was modest and may not have had a meaningful impact on individual, public health, and epidemic level outcomes. As of the end of 2015, an estimated 276,000 PLWH in China were unaware of their status,[35] and China is offtrack for meeting the UNAIDS 90-90-90 Targets.[36,37] China must do more to encourage early and frequent HIV testing uptake, and PITC testing strategies in hospital settings need to be urgently reevaluated and improved.
Financial support and sponsorship
This study was supported by grants from the National Science and Technology Major Project on Prevention and Treatment of Major Infectious Diseases including AIDS and Viral Hepatitis, with funding through National Health and Family Planning Commission of the People's Republic of China (No. 2012ZX10001-007).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Rou-Tao Wang for his invaluable comments on the early manuscript.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Lima VD, Reuter A, Harrigan PR, Lourenço L, Chau W, Hull M, et al. Initiation of antiretroviral therapy at high CD4+ cell counts is associated with positive treatment outcomes. AIDS. 2015;29:1871–82. doi: 10.1097/QAD.0000000000000790. doi: 10.1097/QAD.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borges ÁH, Neuhaus J, Babiker AG, Henry K, Jain MK, Palfreeman A, et al. Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clin Infect Dis. 2016;63:1668–76. doi: 10.1093/cid/ciw621. doi: 10.1093/cid/ciw621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: Results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–90. doi: 10.1016/S1473-3099(13)70692-3. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman SM, Vaidya NK, Zou X. Impact of early treatment programs on HIV epidemics: An immunity-based mathematical model. Math Biosci. 2016;280:38–49. doi: 10.1016/j.mbs.2016.07.009. doi: 10.1016/j.mbs.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin NK, Devine A, Eaton JW, Miners A, Hallett TB, Foster GR, et al. Modeling the impact of early antiretroviral therapy for adults coinfected with HIV and hepatitis B or C in South Africa. AIDS. 2014;28(Suppl 1):S35–46. doi: 10.1097/QAD.0000000000000084. doi: 10.1097/QAD.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Geneva: World Health Organization; 2016. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. [PubMed] [Google Scholar]
- 9.Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J, et al. Late presentation of HIV infection: A consensus definition. HIV Med. 2011;12:61–4. doi: 10.1111/j.1468-1293.2010.00857.x. doi: 10.1111/j.1468-1293.2010.00857.x. [DOI] [PubMed] [Google Scholar]
- 10.Darling KE, Hachfeld A, Cavassini M, Kirk O, Furrer H, Wandeler G, et al. Late presentation to HIV care despite good access to health services: Current epidemiological trends and how to do better. Swiss Med Wkly. 2016;146:w14348. doi: 10.4414/smw.2016.14348. doi: 10.4414/smw.2016.14348. [DOI] [PubMed] [Google Scholar]
- 11.Ford N, Mills EJ, Egger M. Editorial commentary: Immunodeficiency at start of antiretroviral therapy: The persistent problem of late presentation to care. Clin Infect Dis. 2015;60:1128–30. doi: 10.1093/cid/ciu1138. doi: 10.1093/cid/ciu1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC, et al. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002-2013: A meta-analysis. Clin Infect Dis. 2015;60:1120–7. doi: 10.1093/cid/ciu1137. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mocroft A, Lundgren JD, Sabin ML, Monforte A, Brockmeyer N, Casabona J, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: Results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE) PLoS Med. 2013;10:e1001510. doi: 10.1371/journal.pmed.1001510. doi: 10.1371/journal.pmed.1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Wu Z, McGoogan JM, Shi CX, Li A, Dou Z, et al. Immediate antiretroviral therapy decreases mortality among patients with high CD4 counts in China: A nationwide, retrospective cohort study. Clin Infect Dis. 2018;66:727–34. doi: 10.1093/cid/cix878. doi: 10.1093/cid/cix878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen S, Maskew M, Fox MP, Nyoni C, Mongwenyana C, Malete G, et al. Initiating antiretroviral therapy for HIV at a patient's first clinic visit: The rapIT randomized controlled trial. PLoS Med. 2016;13:e1002015. doi: 10.1371/journal.pmed.1002015. doi: 10.1371/journal.pmed.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown LB, Havlir DV, Ayieko J, Mwangwa F, Owaraganise A, Kwarisiima D, et al. High levels of retention in care with streamlined care and universal test and treat in East Africa. AIDS. 2016;30:2855–64. doi: 10.1097/QAD.0000000000001250. doi: 10.1097/QAD.0000000000001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodi S, Phillips A, Logan R, Olson A, Costagliola D, Abgrall S, et al. Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: Observational cohort study. Lancet HIV. 2015;2:e335–43. doi: 10.1016/S2352-3018(15)00108-3. doi: 10.1016/S2352-3018(15)00108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoenigl M, Chaillon A, Moore DJ, Morris SR, Mehta SR, Gianella S, et al. Rapid HIV viral load suppression in those initiating antiretroviral therapy at first visit after HIV diagnosis. Sci Rep. 2016;6:32947. doi: 10.1038/srep32947. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China's response to HIV/AIDS. Lancet. 2007;369:679–90. doi: 10.1016/S0140-6736(07)60315-8. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z. Beijing: People's Medical Publishing House; 2016. HIV/AIDS in China Beyond the Numbers; p. 4. [Google Scholar]
- 21.Cheng W, Tang W, Han Z, Tangthanasup TM, Zhong F, Qin F, et al. Late presentation of HIV infection: Prevalence, trends, and the role of HIV testing strategies in Guangzhou, China, 2008-2013. Biomed Res Int. 2016;2016:1631878. doi: 10.1155/2016/1631878. doi: 10.1155/2016/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin KY, Cheng CY, Li CW, Yang CJ, Tsai MS, Liu CE, et al. Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among HIV-positive taiwanese patients in the era of treatment scale-up. PLoS One. 2017;12:e0179870. doi: 10.1371/journal.pone.0179870. doi: 10.1371/journal.pone.0179870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Hsieh E, Sun MQ, Wang HL, Lv W, Fan HW, et al. Delays in HIV diagnosis and associated factors among patients presenting with advanced disease at a tertiary care hospital in Beijing, China. PLoS One. 2017;12:e0182335. doi: 10.1371/journal.pone.0182335. doi: 10.1371/journal.pone.0182335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao Y, Wu Z, Poundstone K, Wang C, Qin Q, Ma Y, et al. Development of a unified web-based national HIV/AIDS information system in China. Int J Epidemiol. 2010;39(Suppl 2):ii79–89. doi: 10.1093/ije/dyq213. doi: 10.1093/ije/dyq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–63. doi: 10.7326/0003-4819-124-7-199604010-00006. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiébaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 cells/mm 3: Assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011;53:817–25. doi: 10.1093/cid/cir494. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 27.Touloumi G, Pantazis N, Pillay D, Paraskevis D, Chaix ML, Bucher HC, et al. Impact of HIV-1 subtype on CD4 count at HIV seroconversion, rate of decline, and viral load set point in European seroconverter cohorts. Clin Infect Dis. 2013;56:888–97. doi: 10.1093/cid/cis1000. doi: 10.1093/cid/cis1000. [DOI] [PubMed] [Google Scholar]
- 28.Song R, Hall HI, Green TA, Szwarcwald CL, Pantazis N. Using CD4 data to estimate HIV incidence, prevalence, and percent of undiagnosed infections in the United States. J Acquir Immune Defic Syndr. 2017;74:3–9. doi: 10.1097/QAI.0000000000001151. doi: 10.1097/QAI.0000000000001151. [DOI] [PubMed] [Google Scholar]
- 29.Dailey AF, Hoots BE, Hall HI, Song R, Hayes D, Fulton P., Jr Vital signs: Human immunodeficiency virus testing and diagnosis delays – United States. MMWR Morb Mortal Wkly Rep. 2017;66:1300–6. doi: 10.15585/mmwr.mm6647e1. doi: 10.15585/mmwr.mm6647e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall HI, Song R, Szwarcwald CL, Green T. Brief report: Time from infection with the human immunodeficiency virus to diagnosis, United States. J Acquir Immune Defic Syndr. 2015;69:248–51. doi: 10.1097/QAI.0000000000000589. doi: 10.1097/QAI.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Geneva: World Health Organization; 2007. Guidance on Provider-Initiated HIV Testing and Counseling in Health Facilities. [Google Scholar]
- 32.Topp SM, Li MS, Chipukuma JM, Chiko MM, Matongo E, Bolton-Moore C, et al. Does provider-initiated counselling and testing (PITC) strengthen early diagnosis and treatment initiation? Results from an analysis of an urban cohort of HIV-positive patients in Lusaka, Zambia. J Int AIDS Soc. 2012;15:17352. doi: 10.7448/IAS.15.2.17352. doi: 10.7448/IAS.15.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin W, Hao Y, Sun X, Gong X, Li F, Li J, et al. Scaling up the national methadone maintenance treatment program in China: Achievements and challenges. Int J Epidemiol. 2010;39(Suppl 2):ii29–37. doi: 10.1093/ije/dyq210. doi: 10.1093/ije/dyq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z. Characteristics of HIV sexually transmission and challenges for controlling the epidemic in China(in Chinese) Chin J Epidemiol. 2018;39:707–9. doi: 10.3760/cma.j.issn.0254-6450.2018.06.002. doi: 10.3760/cma.j.issn.0254-6450.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y, Dou Z, Guo W, Mao Y, Zhang F, McGoogan JM, et al. The human immunodeficiency virus care continuum in China: 1985-2015. Clin Infect Dis. 2018;66:833–9. doi: 10.1093/cid/cix911. doi: 10.1093/cid/cix911. [DOI] [PubMed] [Google Scholar]
- 36.Geneva: UNAIDS; 2014. UNAIDS.90.90.90: An Ambitious Target to Help end the AIDS Epidimic. [Google Scholar]
- 37.Geneva: UNAIDS; 2017. UNAIDS. Ending AIDS-Progress Towards the 90-90-90 Targets. [Google Scholar]