Abstract
The purpose of this study was to investigate the hypothesis that individuals with dyslexia and individuals with childhood apraxia of speech share an underlying persisting deficit in processing sequential information. Levels of impairment (sensory encoding, memory, retrieval, motor planning/programming) were also investigated. Participants were 22 adults with dyslexia, 10 adults with a probable history of childhood apraxia of speech (phCAS), and 22 typical controls. All participants completed nonword repetition, multisyllabic real word repetition, and nonword decoding tasks. Using phonological process analysis, errors were classified as sequence or substitution errors. Adults with dyslexia and adults with phCAS showed evidence of persisting nonword repetition deficits. In all three tasks, the adults in the two disorder groups produced more errors of both classes than the controls, but disproportionally more sequencing than substitution errors during the nonword repetition task. During the real word repetition task, the phCAS produced the most sequencing errors, whereas during the nonword decoding task, the dyslexia group produced the most sequencing errors. Performance during multisyllabic motor speech tasks, relative to monosyllabic conditions, was correlated with the sequencing error component during nonword repetition. Results provide evidence for a shared persisting sequential processing deficit in the dyslexia and phCAS groups during linguistic and motor speech tasks. Evidence for impairments in sensory encoding, short-term memory, and motor planning/programming was found in both disorder groups. Future studies should investigate clinical applications regarding preventative and targeted interventions towards crossmodal treatment effects.
Keywords: Adults, Reading, Speech motor control, Phonology, Short-term memory
INTRODUCTION
According to the International Dyslexia Association, dyslexia is a disability affecting the acquisition of written language at the word level, characterized by deficits in accurate and/or fluent word recognition, decoding, and spelling in the presence of generally intact spoken language skills (Lyon, Shaywitz, & Shaywitz, 2003; Shaywitz & Shaywitz, 2003). These difficulties are unrelated to cognitive ability or quality of reading instruction; rather, they are thought to be neurobiological in origin. A deficit in phonological processing ability is widely thought to underlie dyslexia (Bus & van IJzendoorn, 1999; Ehri et al., 2001; Troia, 1999). Despite intense treatment, some aspects of dyslexia persist into adulthood, including poor spelling and writing abilities (Berninger, Abbott, Thomson, & Raskind, 2001; Shaywitz, Shaywitz, Fletcher, & Escobar, 1990).
Several other developmental disorders are comorbid with dyslexia, including speech sound disorder (SSD), a disorder that interferes with a child’s ability to produce clearly intelligible speech. One SSD subtype is childhood apraxia of speech (CAS), described by the American Speech- Language-Hearing Association as a “[n]eurological childhood (pediatric) speech sound disorder in which the precision and consistency of movements underlying speech are impaired in the absence of neuromuscular deficits.” The definition further describes the core deficit as interfering with planning and/or programming spatiotemporal parameters of movement sequences, which results in errors in speech sound production and prosody (http://www.asha.org/docs/html/PS2007-00277.html). It is thought that SSD does not pose a risk for dyslexia unless the SSD is severe and persistent, which includes CAS (Bishop & Edmundson, 1987; Lewis, Freebairn, Hansen, Iyengar, & Taylor, 2004; Stothard, Snowling, Bishop, Chipchase, & Kaplan, 1998).
The purpose of this study was to investigate the hypothesis that individuals with dyslexia and individuals with CAS share deficits in information processing, specifically regarding complex sequential information. It is well established that children with dyslexia and children with CAS struggle with nonword repetitions (Catts, 1986; Shriberg, Lohmeier, Strand, & Jakielski, 2012) and rapid syllable repetitions (Malek, Amiri, Hekmati, Pirzadeh, & Gholizadeh, 2013; Thoonen, Maassen, Gabreels, & Schreuder, 1999). Both of these task types require sequential processing, the former with respect to encoding, storing, retrieving, and motor execution of variegated phoneme sequences (e.g., /lɪ́səʃral/) and the latter, regarding mostly motor planning and motor execution of syllable sequences (e.g., /papapapa …/; /patapatapata …/). Whether or not deficits in sequential processing persist into adulthood is one question addressed in this study. Ultimately, the hypothesis of a deficit in sequential processing shared by individuals with dyslexia and individuals with CAS implies a shared genetic etiology expressed in the brain. The present study does not address this biological aspect; rather, it provides motivation to address it in the future.
In the remainder of this section, a theoretical framework for sequential processing in speech and reading is provided, followed by further evidence for sequential processing deficits in dyslexia and CAS. We show how phonological process analysis has been adapted as a methodological framework for estimating the locus of impairment in the information processing cascade and for quantifying sequential processing errors. The research questions motivating this study are formulated in the context of our hypotheses regarding dyslexia and CAS.
Linguistic sequences as inputs for cognitive and motor processing
Sequential processing involves converting complex information into serial structures and vice versa, or converting serial structures from one format into another. Two fundamental and mutually orthogonal aspects are relevant for investigating sequential processing: the nature of the sequence to be processed and the cascade of cognitive and motor processes acting on the information in the sequence. Regarding the first, sequential structures, regardless of their modality, can be characterized in three dimensions: (1) serial order of elements in sequences, as illustrated by multi-colored beads on a string, where the number of possible element types and the length of the sequence contribute to its complexity; (2) temporal durations of elements forming rhythm patterns, as illustrated by Morse code messages; and (3) abstract dimensions where serial order is recoded, so that ABCBAC has the same abstract pattern as 123213 (Dominey, 1995, 2013; Dominey, Hoen, Blanc, & Lelekov-Boissard, 2003). Consequently, spoken and written language can be parameterized as (1) serially ordered sequences of sounds, words, phrases, and/or symbols with (2) temporal characteristics (e.g., stressed and unstressed syllables) and (3) abstract patterns such as spoken phonotactics and orthographic rules.
The second fundamental aspect in sequential processing is information processing, which, like information structures, can be modeled irrespective of modality. Regarding speech and language, the information processing machinery involves a cascading flow of information through stages of sensory encoding, storing (sensory, short-term/working, and long-term memory), retrieval, phonological assembly, motor programming, motor planning, and motor execution (Levelt, 1999; Levelt, Roelofs, & Meyer, 1999; Stackhouse & Wells, 1997). Because the terms “motor programming” and “motor planning” are used inconsistently in the linguistics and psychology literature, Shriberg and colleagues propose the term “transcoding” to refer to the translation of an intended phoneme sequence into a set of motor commands (Shriberg et al., 2012). Here, we use van der Merwe’s terminology where “motor planning” refers to creating the motor activation at the level of the articulators, and “motor programming” refers the fine-tuning at the level of the individual motor units (van der Merwe, 2009). Some models posit a phonological output buffer necessary for motor planning and programming, where sequences of phonemes are briefly stored in short-term memory prior to motor execution (Levelt, 1999). In adults with acquired forms of apraxia of speech, the phonological output buffer may have reduced storage capacity (Rogers & Storkel, 1999) and the time required for planning and programming prior to motor execution appears to be longer (Maas et al., 2008), compared to control adults.
Different linguistic tasks require different pathways through the information processing cascade (Table 1). During nonword repetition, the phoneme sequence is presented once, in real time, in the auditory modality. The sequence must be perceptually encoded into short-term memory, retrieved from there, passed through a phonological assembly module, stored in a phonological output buffer, and translated into a set of complex motor planning/programming commands controlling the entire speech production system before the final step of motor execution, which involves contraction of precisely specified muscle groups to generate the acoustic output. Imitating real words involves a different path through the information processing cascade, as hearing the word triggers word recognition and access to the word form in long-term memory, from where it is retrieved and passed through the phonological assembly, phonological buffer, motor planning/programming, and execution stages. During nonword decoding, the target is presented in a more stable form in the visible modality. It must be perceptually encoded and passed through the sequential grapheme-phoneme conversion (commonly referred to as decoding), phonological assembly, buffer, motor planning/programming, and motor execution stages. Task-specific sequencing errors, hence, may offer clues regarding the locus of impairment in the information processing cascade. Table 1 summarizes the levels of processing for the three task types of nonword repetition, real word repetition, and nonword decoding.
Table 1.
Conceptualization of information processing requirements in nonword repetition (input and output units = phonemes), real word repetition (input and output units = phonemes), and nonword decoding (input units = graphemes, output units = phonemes)
| Information Processing | Repeating Nonwords |
Repeating Real Words |
Decoding Nonwords |
|---|---|---|---|
| Auditory encoding into short-term memory |
• | ||
| Auditory encoding and word recognition in long-term memory |
• | ||
| Visual encoding into grapheme- phoneme conversion |
• | ||
| Retrieval from short- or long-term memory |
• | • | |
| Phonological assembly | • | • | • |
| Storage in phonological output buffer |
• | • | • |
| Motor planning/programming | • | • | • |
| Motor execution | • | • | • |
Known sequential processing deficits in dyslexia
According to the cerebellar hypothesis of dyslexia (Frank & Levinson, 1973; Levinson, 1988; Nicolson, Fawcett, & Dean, 1995), affected individuals struggle with tasks across many domains that are influenced by cerebellar functions, e.g., peg moving, rapid pointing, and control of eye movement, but note that not all results have been replicated and that cerebellar signs are not found in all individuals with dyslexia (Ramus, Pidgeon, & Frith, 2003; Stoodley & Stein, 2013). In recent years, the role of the cerebellum in processing nonmotor sequential information including linguistic information has been established (Marien & Beaton, 2014). For instance, the cerebellum plays a crucial role in infants during speech perception (Deniz Can, Richards, & Kuhl, 2013), in children and adults during tasks involving syntactic rules (Koziol et al., 2014; Pliatsikas, Johnstone, & Marinis, 2014), and in children during reading tasks (Feng et al., 2016).
Recent studies have investigated serial order learning in children and adults with typical and disordered reading ability. This work was motivated by prior findings in children, for instance correlations between performance on verbal immediate serial recall tasks and nonword repetition (Gupta, 2003) and vocabulary (Service, 1992), respectively. Similarly, immediate recall of serial order was found to be associated with early oral language learning (Leclercq & Majerus, 2010). A longitudinal study of children with and without dyslexia risk showed that capacity for verbal and nonverbal serial order learning was associated with reading ability and phonological awareness (Bogaerts, Szmalec, De Maeyer, Page, & Duyck, 2016). A deficit in maintaining serial order in short-term memory across several modalities was confirmed in a sample of children with dyslexia with and without concomitant language impairment (Cowan et al., 2017). Several studies showed that serial-order learning, both in the verbal and nonverbal modality, was found to be deficient in adults with dyslexia as well (Bogaerts, Szmalec, Hachmann, Page, & Duyck, 2015; Hachmann et al., 2014; Szmalec, Loncke, Page, & Duyck, 2011). Similarly, in a study investigating how type of presentation (i.e., simultaneous versus serial) affected recall of visual symbols in a sequence, Romani, Tsouknida, and Olson (2015) reported that the adults with dyslexia did not differ from the typical controls in the simultaneous condition but performed significantly worse in the sequential condition. A recent review provides further detail regarding the role of serial order in short-term memory in children and adults with dyslexia (Majerus & Cowan, 2016).
Together, these findings are consistent with the possibility that domain-general difficulty with encoding and transferring sequential information into long-term memory may be an underlying deficit in dyslexia, that this deficit persists into adulthood, and that it is evident at the level of the graphemes and the corresponding phonemes in words, leading to the disordered processing of written language characteristic of dyslexia. Evidence for such a persisting sequential processing deficit at the level of graphemes and phonemes in dyslexia is provided by several studies. For instance, children with dyslexia were less accurate than controls in judging the order of phonemes in consonant clusters (Rey, De Martino, Espesser, & Habib, 2002), and college students with a history of dyslexia imitated complex words and phrases at a slower rate and with more errors than typical peers (Catts, 1989).
Known sequential processing deficits in CAS
A recent series of studies has shown that a domain-general deficit in rapid sequential processing may underlie CAS. In multigenerational families with familial CAS, motor speech tasks (diadochokinetics; DDK) involving rapid alternating-sequential movements (repetitions of /pata/, /taka/, and /pataka/) and hand motor tasks involving rapid alternating key tapping were associated with presence or history of CAS, whereas repetitive motor speech tasks (repetitions of /pa/, /ta/, and /ka/) and repetitive keyboard tapping were associated to a much lesser extent (Peter & Raskind, 2011). In one family with familial CAS, the motor sequencing deficit was used as a binary input trait in a genome-wide genetic linkage analysis, which identified four new genomic regions of interest (Peter, Matsushita, & Raskind, 2012) including one on chromosome 6 that was also implicated in a dyslexia study (Konig et al., 2011), suggesting a genetic influence on motor sequencing. A study of a large multigenerational family with familial CAS led to the insight that sequential processing deficits can be observed across a wide range of task types, not only in oral and hand motor tasks but also during nonword decoding, spelling, and nonword repetition (Peter, Button, Stoel-Gammon, Chapman, & Raskind, 2013). In this family, CDH18 and eight other genes were identified as genes of interest (Peter et al., 2016), further supporting the view that sequential processing deficits have a genetic etiology. Interestingly, most of the identified genes in this family and the only candidate gene in another family with familial CAS (Peter et al., 2016) are highly expressed in the cerebellum.
Results from a study of adults from five other families, two of them with familial CAS, replicated the findings regarding sequential processing and showed correlations among scores from a variety of tasks requiring rapid sequential processing including motor tasks (multisyllabic DDK, alternating key tapping), and tasks related to written language (spelling, rapidly reading words and nonwords) (Button, Peter, Stoel-Gammon, & Raskind, 2013).
Together, these deficits are consistent with a domain-general deficit in sequential processing. We hypothesize that difficulties with the sequential order of phonemes and graphemes are an instantiation of this deficit at the level of spoken and written language and that it is shared by individuals with CAS and dyslexia.
Phonological processes as a cognitive framework for word-based sequential processing errors
Phonological process analysis is a systematic approach to speech sound errors, traditionally applied to child speech (Edwards, 1983; Grunwell, 1981; McLeod et al., 1990; Stoel-Gammon & Dunn, 1985; Vihman & Greenlee, 1987). Errors are parameterized as violations of specific rules acting not on individual phonemes but phoneme classes. Common examples are reductive processes such as cluster reductions ([tap] for /stap/) and final consonant deletions ([bʊ] for /bʊk/), positional changes such as migrations ([los] for /slo/) and metatheses ([æmɪnəl] for /ænɪməl/), additive processes such as reduplications ([bʊbʊk] for /bʊk/) and insertions ([bəlu] for /blu/), assimilatory processes, i.e., anticipatory assimilations ([lɛlo] for /jɛlo/) and perseveratory assimilations ([pap] for /pat/), and substitution processes related to articulatory place ([ti] for /ki/), manner ([ti] for /si/), and voicing ([baɪ] for /paɪ/).
A subset of phonological processes has been used to allocate putative cognitive levels of impairment in the information processing cascade in CAS. In a group of 40 children and young adults with CAS, accuracy during a nonword repetition task was significantly lower than that in controls (Shriberg et al., 2012). Based on an analysis of error types, the authors conclude that the observed errors in the CAS group are consistent with deficits on three levels of information processing: encoding (manifested as articulatory place substitutions, e.g., [m]/n), memory (manifested as more errors during longer nonwords), and transcoding (manifested as inserted phonemes). Note that this model does not parameterize the serial order of elements and the cascade of information processing as orthogonal aspects; rather, it convolves them.
In studies of families with CAS of likely genetic origin, we have shown that affected adults and children had lower standard scores during nonword repetition tasks than their unaffected relatives (Button et al., 2013; Peter et al., 2013). Toward a qualitative error type analysis, errors were classified as errors of phoneme sequence (assimilation, migration, metathesis, omission, and insertion) or errors of identity (substitution). As predicted by their lower standard scores, the affected family members made many more errors than their unaffected relatives in general, but especially errors affecting the phoneme sequence. The most common type of sequencing error was assimilation, similar to a study of error types in children with CAS (Thoonen, Maassen, Gabreels, & Schreuder, 1994). The direction of the assimilations (anticipatory or perseverative) was not further analyzed in any of these studies. Note that in the cited studies of sequential processing abilities (Button et al., 2013; Peter et al., 2013), only the serial order of elements in the sequences was considered; aspects of information processing (e.g., sensory encoding, storage, retrieval; see Table 1) were not addressed.
In the present study, we leverage phonological process analysis not only in the context of nonword repetitions but also real word repetitions and nonword decoding. These are three tasks during which individuals with dyslexia and individuals with CAS have documented deficits (Button et al., 2013; Facoetti et al., 2006; Peter et al., 2013). These tasks provide an opportunity to consider not only the serial order of elements but also aspects of information processing (Table 1) as two orthogonal aspects of sequential information processing.
Purpose
Previous studies have reported on nonword and real word repetition in children with suspected CAS, adults who likely had CAS in childhood (Button et al., 2013; Peter et al., 2013), and children with dyslexia (Melby-Lervåg & Lervåg, 2012). However, few studies to date have examined performance on these tasks in adults with dyslexia, and no studies have compared adults with dyslexia to adults with a probable childhood history of CAS (phCAS). Focusing on adults provides the opportunity to evaluate deficits at a point in time when developmental fluctuations in performance are minimal and when the tasks can be of higher complexity than those used in child studies, yielding a greater spectrum of errors.
The purpose of this project was to assess sequential processing ability in adults with dyslexia, adults with phCAS, and typical controls. The qualitative error analysis was based on phonological process analysis using two classes of errors, sequencing errors and identity (i.e., substitution) errors (Button et al., 2013; Peter et al., 2013). Research questions, hypotheses, and rationales were as follows:
Do adults with phCAS and adults with dyslexia show deficits in nonword repetitions, similar to those previously described in children with these disorders? Hypothesis: Adults with dyslexia and adults with phCAS will obtain lower standard scores during nonword repetition than typical controls. Rationale: This core deficit does not diminish over time.
Do adults with phCAS and adults with dyslexia show relatively more sequential than substitution errors in a nonword repetition task, and, if so, is this increase different from the error patterns seen in typical controls? Hypothesis: In adults with dyslexia and adults with phCAS, disproportionally more sequencing than substitution errors will be observed. Rationale: Adults in the two disorder groups may have difficulty with processing sequential information at any or all levels of the task (sensory encoding, storage in short-term memory, retrieval, phonological assembly, phonological output buffer, motor planning/programming), whereas such a deficit is not expected in typical controls.
Do adults with phCAS and adults with dyslexia show different assimilation direction patterns during nonword repetition, compared to typical adults? Hypothesis: Adults in the two disorder groups will produce more errors of both assimilation types, compared to controls, but especially regarding perseverative assimilations. Rationale: Typical children and adults produce relatively few assimilation errors, and of those, an estimated 75% of total assimilation errors are anticipatory (Schwartz, Saffran, Bloch, & Dell, 1994). This bias is explained in models where all segments and motor plans/programs are stored in the phonological output buffer, which is part of short-term memory, at the onset of word production. These plans are deactivated as they are deployed, so that word-final segments present more triggers than word-initial ones (Dell, Burger, & Svec, 1997). Adults with dyslexia and adults with phCAS will produce relatively more anticipatory errors than typical controls if a motor plan is present but the representation of the phoneme sequence in the phonological output buffer is weak. They will produce more perseverative errors, compared to typical controls, if, in addition to weak representation of the phoneme sequence in the buffer, the motor plan is impaired.
Do adults with phCAS and adults with dyslexia produce relatively more sequencing than substitution errors during real word repetitions, and, if so, is this pattern different from that in controls? Hypothesis: Adults in both disorder groups will produce more sequencing than substitution errors during this task, whereas controls will make few errors of either type. Rationale: During real word repetitions, the words are retrieved from long-term memory rather than from short-term memory, then passed through the phonological component assembly, buffer, motor planning and programming, and motor execution stages. Adults with phCAS have more difficulty with the sequential assembly of phonological units into the phonological output buffer and the planning and programming of the motoric components than typical adults and will show more sequencing errors in real word repetitions. Adults with dyslexia likely retain residual motor planning/programming deficits similar to those observed in children with dyslexia during DDK tasks and therefore will produce elevated levels of sequencing errors during real word repetition, compared to typical adults.
Do adults with phCAS and adults with dyslexia produce relatively more sequencing than substitution errors during nonword decoding, and is this pattern different from that in typical adults? Hypothesis: Adults in both disorder groups will produce higher numbers of sequencing than substitution errors, whereas controls will make few errors of either type. Rationale: Nonword decoding forces sequential conversions of graphemes to phonemes during visual encoding into short-term memory prior to retrieval, phonological assembly, buffering, and motor planning/programming. Difficulty with sequential processing will lead to high numbers of sequencing errors in this task in the two disorder groups.
Are sequencing errors in a primarily linguistic task (nonword repetition) correlated with measures of sequencing during a primarily motor task (DDK) ? Hypothesis: The sequential aspect during nonword repetition will be correlated with the sequential aspect during DDK testing. Rationale: If sequential processing ability is modality-general, it is expressed during linguistic as well as motor tasks.
METHOD
Participants
This study was completed with the approval of the University of Washington Institutional Review Board acting on behalf of the Institutional Review Board at Arizona State University. All participants gave written consent.
Three groups of adults participated, a control group (CTR; n = 22), a phCAS group (n = 10), and a dyslexia group (DYX; n = 22). All participants were required to be free of any neurological, psychiatric, or sensory impairment that could confound the results of the study. Inclusion in the CTR group required absence of any diagnoses of speech or reading disorder and performance on all five standardized reading and spelling measures, further described below, above −1 z. Approximately half of the CTR participants were undergraduate university students. For inclusion in the phCAS group, participants had to self-report a history of severe childhood speech difficulties and obtain a score below −1 z score on at least one of three multisyllabic DDK tasks (/pata/, /taka/, /pataka/), a task type that frequently reveals residual deficits in adults with a CAS history (Button et al., 2013; Peter et al., 2013; Peter et al., 2012; Peter & Raskind, 2011). The average number of scores below −1 z = 1.9, SD = 0.6, range [1, 2]. One fifth of the members of this group were university students. Further, all adults in the phCAS group had at least two biological relatives with a current CAS diagnosis and a family history of CAS. In two of these families, candidate genes have already been identified and the family members who participated in this study were carriers of the genetic variants, a CDH18 mutation on chromosome 5 in one family (Peter et al., 2016) and a heterozygously deleted IGF2R–AIRN-SLC22A2–SLC22A3 gene cluster on chromosome 6 in another family (Peter et al., 2017). In an additional family, a different candidate region on chromosome 6 was identified (Peter et al., 2012). These biological associations provide further support for the apraxic type of the childhood speech disorder in the adults in this group, whose conversational speech had normalized. For inclusion in the DYX group, a professional dyslexia diagnosis and scores below −1 z score on at least one out of five standardized reading and/or spelling measures were required (average number of scores below −1 z = 1.9, SD = 1.2, range [1, 5]). All participants in the dyslexia group had a low TOWRE PDE score, consistent with low automatized decoding skills in the entire group, whereas low spelling scores were only seen in some participants and only in the presence of one or more low reading scores, consistent with persisting word reading deficits but not primary spelling deficits. In addition, a positive family history of dyslexia was required, with at least two biologically related family members also affected. Approximately one third of the DYX participants were undergraduate university students.
Table 2 shows per-group means and standard deviations for age and test scores. Magnitude of group difference is expressed as effect size. Group differences for percent male are not shown in this table. Rather, numbers of males and females in the DYX and phCAS groups were compared to those in the CTR group with chi square tests. Group differences were not statistically significant (overall ; CTR versus DYX: ; CTR versus phCAS: ). The phCAS group was slightly older than the CTR and DYX groups.
Table 2.
Descriptive measures (mean (standard deviation) [min, max]) for the three participant groups (CTR, phCAS, DYX) and effect sizes for the DYX and phCAS groups with respect to the CTR group
| CTR (n = 22) | phCAS (n = 10) |
DYX (n = 22) | phCAS – CTR d |
DYX – CTR d |
|
|---|---|---|---|---|---|
| Age (Years) | 31.09 (15.39) [18, 71] |
43.30 (19.02) [19, 69] |
36.27 (15.43) [18, 70] |
0.74 | 0.34 |
| Percent Male/Female |
45.5%/54.5% | 50.0%/50.0% | 40.9%/49.1% | n/a | n/a |
| RIAS VIXa | 115.14 (10.56) [94, 134] |
101.70 (11.37) [82, 119] |
112.81 (8.76) [91, 126] |
1.24 | 0.24 |
| RIAS NIXa | 113.85 (7.39) [97, 130] |
107.5 (10.10) [90, 122] |
113.05 (10.35) [88, 129] |
0.77 | 0.09 |
| DDK Monosyll.b | 0.97 (0.54) [−0.07, 1.69] |
−0.15 (0.70) [−1.16, 0.67] |
0.28 (0.88) [−1.81, 1.57] |
1.89 | 0.95 |
| DDK Multisyllb | 0.65 (0.52) [−0.29, 1.59] |
−1.65 (0.89) [−3.97, −0.88] |
−0.06 (0.89) [−1.52, 1.89] |
3.52 | 0.97 |
| WIDa | 106.05 (6.98) [95, 122] |
91.69 (7.44) [80, 102] |
94.00 (7.35) [74, 106] |
2.03 | 1.68 |
| WATTa | 104.86 (9.59) [88, 123] |
97.60 (9.85) [83, 109] |
94.73 (7.84) [79, 113] |
0.85 | 1.16 |
| TOWRE - SWEa | 106.86 (8.55) [87, 113] |
92.89 (10.73) [79, 113] |
89.18 (13.37) [57, 113] |
1.51 | 1.58 |
| TOWRE - PDEa | 97.45 (8.92) [86, 120] |
84.33 (10.01) [71, 103] |
78.14 (7.13) [62, 85] |
1.42 | 2.39 |
| WIAT II Spellinga |
111.82 (10.13) [87, 128] |
93.90 (17.50) [63, 117] |
91.41 (14.72) [57, 111] |
1.40 | 1.62 |
Notes. Mean = 100, standard deviation = 15
Mean = 0, standard deviation = 1
RIAS VIX = Reynolds Intellectual Assessment Scales Verbal index; RIAS NIX = Reynolds Intellectual Assessment Scales Nonverbal Index; DDK = diadochokinetics; WID = Word Identification; WATT = Word Attack; TOWRE - SWE = Test of Word Reading Efficiency: Sight Word Efficiency; TOWRE - PDE = Test of Word Reading Efficiency: Phonemic Decoding Efficiency; WIAT II = Wechsler Individual Achievement Test - II.
As expected, all three groups showed typical performance during nonverbal and verbal processing tasks. Lower scores in measures of reading and spelling in the DYX group confirm the inclusionary criteria for that group, whereas in the phCAS group, lower reading and spelling scores are consistent with previous observations (Button et al., 2013; Peter et al., 2013). Conversely, lower DDK scores in the phCAS group, especially in the multisyllabic condition, confirm inclusionary criteria for this group. In the DYX group, lower DDK scores are consistent with previous observations (Malek et al., 2013; Thoonen et al., 1999).
To investigate the effects of memory impairment on word-level imitation and reading tasks, an additional participant with short-term memory impairment but intact cognitive, sensory, and linguistic abilities was recruited. This case study is reported in a companion study (Peter, in press).
Experimental measures and data reduction
Nonword repetition
The Nonword Repetition (NWR) subtest from the Comprehensive Test of Phonological Processing (CTOPP) (Wagner, Torgesen, & Rashotte, 1999) was administered using the sound files provided by the manufacturer. This subtest contains 18 items of increasing complexity. The qualitative error analysis was based on all 18 nonword repetitions, regardless of ceiling rules. The average number of phonemes in the set of nonwords was 8.2 (SD = 4.0, range [3, 15]), with a total sum of 147 phonemes. Phonological complexity in terms of phonological mean length of utterance (pMLU; per-word sum of two points per consonant plus one point per vowel) (Ingram, 2002) was 12.9 (SD = 6.2, range [5, 24]).
Real word repetition
To elicit real word repetitions, a list of multisyllabic words (MSWs) (Catts, 1986) was administered using a sound file of a male adult speaker. A subset of these words was selected for further analysis, consisting of 20 multisyllabic words with complex phoneme sequences such as consonant clusters at the onset and/or coda of syllables. The average number of phonemes in these words was 8.9 (SD = 1.7, range [6, 13]), with a total sum of 183 phonemes, and the average pMLU was 14.5 (SD = 2.7, range [11, 21]).
Nonword decoding
To evaluate the ability to decode words, the Word Attack (WATT) subtest of the Woodcock Reading Mastery Tests – Revised (WRMT-R) (Woodcock, McGrew, & Mather, 2001) was administered. The nonwords in this subtest follow standard English orthography and must be sounded out sequentially. To quantify errors in nonword decoding, we selected all multisyllabic words and one monosyllabic word with a complex grapheme sequence (vowel digraph plus three consonants), a total of 11 items. The average number of graphemes in these nonwords was 7.5 (SD = 2.8, range [4, 12]) with a total sum of 83 graphemes, and the average pMLU of the spoken targets was 11.4 (SD = 10.2, range [7, 18]).
Thus, the stimuli for the nonword and real word repetition tasks were roughly equivalent in terms of numbers of items, length, and phonological complexity. For the nonword decoding task, there were fewer items and these had slightly shorter sequences and lower phonological complexity, compared to the stimuli in the two repetition tasks.
Diagnostic measures
Reading
In addition to measuring nonword decoding ability with the WATT, the Word Identification (WID) subtest of the Woodcock Reading Mastery Tests – Revised (Woodcock et al., 2001) was administered to measure sight word reading ability. The sight words in this subtest do not necessarily follow the rules of standard orthography (e.g., “ache,” “some,” “brought”) and the task, hence, requires whole-chunk word recognition. Word reading abilities under timed conditions were also assessed using the Sight Word Efficiency (SWE) subtest and the Phonemic Decoding Efficiency subtest (PDE) of the Test of Word Reading Efficiency (Torgesen, Wagner, & Rashotte, 2012). In both subtests, the participant is given 45 seconds to read as many of the stimuli as possible.
Spelling
Participants were asked to complete the Spelling (SP) subtest from the Wechsler Individual Achievement Test – II (WIAT-II) (Wechsler, 2005). This subtest contains words that do not follow standard English orthography (e.g., “eight,” “knight”) and their letter sequences must be stored in long-term memory, retrieved from there, and motorically converted to written letter sequences upon dictation.
Motor speech
Participants completed DDK testing, rapidly repeating the monosyllables (/pa/, /ta/, /ka/) and multisyllables (/pata/, /taka/, /pataka/), following published procedures (Fletcher, 1972). Using the acoustic analysis package Praat (Boersma & Weenink, 2014), version 5.1.25, average syllable durations were calculated for each syllable type and participant. First and last syllables in each breath group and inhalations were excluded from the analysis. For statistical processing, raw syllable durations were converted to z scores based on published norms (Fletcher, 1972) for the oldest available age, 13 years. Z scores were used because of the inherent durational differences between the DDK tasks reflected in the norms, which makes averaging raw durations across syllable types problematic.
Verbal processing
Verbal processing ability was measured with two subtests from the Reynolds Intellectual Assessment Scales (RIAS) (Reynolds & Kamphaus, 2003). Participants are asked to name terms that fit a given definition (Guess What, GWH), a task that relies on conceptual verbal knowledge, and to complete verbal analogies (Verbal Reasoning, VRZ). The Verbal Intelligence Index (VIX) is calculated based on the combined GWH and VRZ scores.
Nonverbal processing
The two nonverbal subtests from the RIAS (Reynolds & Kamphaus, 2003) were administered. The Odd Item Out (OIO) subtest requires comparing items in an array, then identifying the item that does not share a similarity with all others. The What’s Missing (WHM) subtest requires forming a mental construct based on a complex image, then determining which element is missing. The Nonverbal Intelligence Index (NIX) is a normed composite from the sum of the OIO and WHM standard scores.
Error codes, transcription, and reliability
As in two preceding studies of sequencing and substitution errors (Button et al., 2013; Peter et al., 2013) and similar to phonological assessments of child speech productions (Edwards, 1983; Grunwell, 1981; Roberts, Burchinal, & Footo, 1990; Stoel-Gammon & Dunn, 1985; Stoel-Gammon & Stone, 1991; Vihman & Greenlee, 1987), incorrect real word or nonword repetitions were transcribed into International Phonetic Alphabet (IPA) and the errors were classified with respect to the sequence and identity of the phonemes (Table 3). Regarding the nonword decoding task, all incorrect productions were transcribed into IPA and orthographically re-transcribed. By comparing the orthographical re-transcription of the production with the grapheme sequence in the target, error codes analogous to the ones used for the repetition tasks were applied. For all three tasks, both consonants and vowels were included in the analysis. In all cases, the first complete production of the word was selected for analysis, even if the participant added a revised production.
Table 3.
Definitions and examples of error classes and types
| Error Class |
Error Type | Definition | Example | |
|---|---|---|---|---|
| Target | Response | |||
| Sequence | Assimilation (Ant.) |
A phoneme occurring later in the word replaces a phoneme occurring earlier in the word |
/baɪlidóʤ/ | [bolidóʤ] |
| Assimilation (Pers.) |
A phoneme occurring earlier in the word replaces a phoneme occurring later in the word |
/baɪlidóʤ/ | [baɪlidáɪʤ] | |
| Migration | A phoneme is moved to an incorrect position |
/lɪ́səʃral/ | [lɪ́srəʃal] | |
| Metathesis | Two phonemes switch places |
/baɪlidóʤ/ | [daɪlibóʤ] | |
| Omission | A phoneme is removed | /lɪ́səʃral/ | [lɪ́səʃal] | |
| Insertion | A phoneme is added | /vozətúv/ | [vozəvtúv] | |
| Identity | Substitution | A phoneme is substituted for a target phoneme |
/vozətúv/ | [vozətúb] |
The class of sequencing errors consisted of total assimilations based on whole phonemes with at least one intervening phoneme (not phonetic assimilations), migrations, metatheses, omissions, and insertions. Assimilation errors were further coded for direction (anticipatory or perseverative). In cases where an assimilation direction was ambiguous, the error was scored as 0.5 point for both directions. An example of ambiguous assimilation direction is [ʃʌbəraɪˡhʌʃɔɪmʌʃI for /ʃʌbəraɪˡhʌvɔɪmʌʃ/, where the [ʃ/v] error could represent either an anticipatory or perseveratory assimiliation because /ʃ/ occurs both at the beginning and the end of the item. Identity errors occurred when an incorrect phoneme was substituted for a correct one. The rationale for these two classes of errors is as follows: As in our previous work (Button et al., 2013; Peter et al., 2013), the phonemes and graphemes in a word are parameterized here as a sequence of ordered elements. Using the 4-element sequence of 1 2 3 4 as a model, an assimilation alters the sequence via a copy-and-paste mechanism where the copied element is superimposed on another (1 2 1 4 for a perseveratory assimilation and 3 2 3 4 for an anticipatory one). A migration is a cut-and-paste mechanism where an element moves to an incorrect position in the sequence (2 3 1 4). In a metathesis, two elements in the sequence switch places (3 2 1 4). In an omission, one element drops out of the sequence without replacement, shortening it by one count (1 3 4). In an insertion, the length of the sequence increases by one count as an element, usually but not necessarily from the sequence, intrudes into the sequence, lengthening the sequence by one count (1 2 1 3 4). In a substitution, by contrast, an element is replaced by an element from outside the sequence (1 2 X 4). Thus, sequencing errors are those where the elements in a sequence are serially re-arranged, either with or without altering the length of the sequence. Substitution errors do not alter the serial arrangement of the elements or the length of the sequence; rather, the identity of an element is lost as it is replaced by a foreign one. This taxonomy, especially regarding errors of serial order, is consistent with previously published work, not only in studies of speech production (Thoonen et al., 1994) but also in other fields, for instance psychology and cognitive neuroscience where errors of serial order have traditionally been described as errors of insertion, omission, and transposition (Elvevag, Fisher, & Goldberg, 2003; McCormack, Brown, Vousden, & Henson, 2000; McNeilage, 1964). In some studies, however, omissions and insertions were captured under a separate category outside of serial order errors (Snyder & Logan, 2014). Table 3 provides examples for each error type.
All transcriptions were completed by teams of two to four undergraduate and graduate research assistants trained in phonetics who were blind to group status. Video recordings as well as acoustic analysis of sound recordings were used to resolve discrepancies in the transcriptions. For instance, acoustically similar phonemes such as [m] versus [n] were disambiguated using the video recording, whereas acoustic analysis was used to disambiguate visually indistinct phonemes such as voiced versus unvoiced consonants. The first, second, and third authors reviewed transcription and coding for all stimuli. Approximately 5% of the codings were modified by consensus.
Statistical analysis
For the standardized tests, standard and z scores were calculated individually for each participant, following the manufacturers’ instructions. Also for each participant, error counts were summed by type and class. To answer Research Question 1, NWR standard scores were tested for group differences using a one-way ANOVA test with posthoc between-group testing. For Research Question 2, the proportion of sequential errors out of total errors (# Sequencing Errors / (# Sequencing Errors + # Substitution Errors) was evaluated for group level differences using Kruskal-Wallis rank sum analysis of variance, followed by Dunn testing as a posthoc investigation of pairwise group differences. Nonparametric tests were used for this and all other question involving sequencing and substitution errors because these measures do not represent a ratio scale due to the unknown weights of the individual errors and error types whose counts were summed. A second method for determining if the DYX and phCAS groups differed from the typical pattern was to compare the groups on the total number of sequence errors and the total number of substitution errors. To address Research Question 3, group-level differences for the proportion of perseverative assimilation errors, as well as anticipatory and perseverative errors individually, were evaluated using the Kruskal-Wallis rank sum analysis of variance, followed by Dunn testing. For Research Questions 4 and 5, the same analysis path was used as for Research Question 2 (see above) for errors on MSW and WATT, respectively, except that a proportion of sequencing errors could not be computed due to a high occurrence of zero errors (see Results). To evaluate the association between sequencing ability during linguistic and motor tasks (Research Question 6), a Spearman correlation coefficient was computed between two measures designed to capture the sequencing component in the nonword repetition task (# sequencing errors - # substitution errors) and the DDK tasks (averaged monosyllabic DDK z score - averaged multisyllabic DDK z score). The nonword task was selected over the nonword decoding and real word repetition tasks because it has the potential to capture the greatest number of sequencing errors as it involves the largest number of processing steps (Table 1). The difference in sequencing vs. substitution errors captures the relative weakness in maintaining the serial order of the phonemes in the NWR task. The difference between the averaged mono- and multisyllabic DDK z scores should be near zero under random conditions but large where motor planning/programming deficits affect performance during multisyllabic DDK more than monosyllabic DDK.
Nominal statistical significance was determined at = 0.05. Omnibus group differences were evaluated with one parametric ANOVA and 13 planned (only 11 actual) nonparametric ANOVAs, resulting in a Bonferroni-corrected experimentwise = 0.0038 (0.0045 for the 11 actual tests). Note, however, that multiple tests were conducted for the same task, so that these tests of group differences were not mutually independent and the Bonferroni correction was, hence, overly conservative.
RESULTS
Research Question 1: Nonword repetition performance in adults
The NWR test is normed on a population mean of 10 and a standard deviation of 3, so that typical values will fall between standard scores of 7 and 13. The average NWR standard scores (standard deviations) [ranges] and z scores per group were as follows: CTR 8.86 (1.73) [6, 12], z = −0.38, phCAS 6.20 (1.48) [4, 9], z = −1.27, and DYX 7.36 (1.71) [5, 11], z = −0.88. In the CTR group, 1 of 22 participants (4%) obtained a score lower than 1 SD below the population mean. In the phCAS group, such a low performance was obtained by 5 of 10 participants (50%), and in the DYX group, by 7 of 22 participants (32%). The ANOVA model was experimentwise significant (F (2) = 9.72, p = 0.0003). Posthoc testing showed differences between the CTR and phCAS groups (p < 0.0001) and the CTR and DYX groups (p = 0.0140), but not between the two disorder groups (p = 0.2240).
Research Question 2: Sequential and substitution errors during nonword repetition
In the NWR task, omnibus group differences for proportions of sequence errors out of total errors were nominally statistically significant (Table 5). Posthoc Dunn testing showed a large group difference between the CTR and phCAS groups but not between the CTR and DYX groups and between the two disorder groups. The phCAS group produced the highest proportion of sequencing errors (median = 0.69), followed by the DYX (0.60) and CTR (0.55) groups.
Table 5.
Summary of nonparametric statistical tests of group difference
| Research Question |
Measure | Variable of Interest | χ2 (2) | p | Posthoc C-A p |
Posthoc C-D p |
Posthoc A-D p |
|---|---|---|---|---|---|---|---|
| 2 | NWR | Prop. Sequ./Subst. Err. | 8.34 | 0.0155 | 0.0023 | 0.0516 | 0.0611 |
| Sequ. Err. | 21.22 | <0.0001 | <0.0001 | 0.0005 | 0.0456 | ||
| Subst. Err. | 7.54 | 0.0230 | 0.0092 | 0.0131 | 0.274 | ||
| 3 | NWR | Prop. Persev./Ant. Err. | 2.07 | 0.3556 | |||
| Perseveratory Err. | 11.93 | 0.0026 | 0.0037 | 0.0011 | 0.3999 | ||
| Anticipatory Err. | 4.07 | 0.0874 | |||||
| 4 | MSW | Prop. Sequ./Subst. Err. | N/A | N/A | N/A | N/A | N/A |
| Sequ. Err. | 17.96 | <0.0001 | <0.0001 | 0.0272 | 0.0034 | ||
| Subst. Err. | 16.81 | 0.0002 | <0.0001 | 0.0716 | 0.0016 | ||
| 5 | WATT | Prop. Sequ./Subst. Err. | N/A | N/A | N/A | N/A | N/A |
| Sequ. Err. | 17.84 | 0.0001 | 0.0022 | <0.0001 | 0.3827 | ||
| Subst. Err. | 13.2 | 0.0014 | 0.0249 | 0.0002 | 0.1914 |
Omnibus group differences for sequencing errors were experimentwise significant (Table 5). Pairwise between-group differences were more significant between the CTR and phCAS groups and the CTR and DYX groups than between the two disorder groups. The phCAS group produced the most sequencing errors, followed by the DYX and CTR groups (Table 4).
Table 4.
Errors by participant group, task, class, and type (mean (standard deviation) median [range])
| Sequencing | Identity | |||||||
|---|---|---|---|---|---|---|---|---|
|
Type |
Assimilation (Ant.) |
Assimilation (Pers.) |
Migration |
Metathesis |
Omission |
Insertion |
Sum Sequencing |
Substitution |
| Nonword Repetition (NWR) | ||||||||
| CTR | 1.43 (1.00) 1 [0, 3] |
1.75 (1.60) 2 [0, 7] |
0.32 (0.65) 0 [0, 2] |
0.64 (0.95) 0 [0, 3] |
0.86 (0.89) 1 [0, 2] |
0.86 (0.94) 1 [0, 3] |
5.86 (3.94) 6 [2, 20] |
5.32 (2.81) 5 [0, 11] |
| phCAS | 2.50 (1.53) 2.5 [0, 4.5] |
5.20 (5.08) 3.5, [0, 18] |
3.60 (2.99) 2.5 [0, 10] |
1.40 (1.07) 1 [0, 3] |
4.20 (2.53) 4 [2, 10] |
4.30 (4.79) 2.5 [0, 14] |
21.20 (12.04) 19.5 [7, 47] |
9.90 (6.08) 8 [3, 22] |
| DYX | 2.43 (1.90) 2 [0, 7.5] |
4.16 (2.83) 4, [0, 9.5] |
1.64 (1.97) 1 [0, 8] |
1.09 (0.87) 1 [0, 3] |
1.55 (1.82) 1 [0, 7] |
2.32 (2.42) 2 [0, 9] |
13.18 (7.64) 12.5 [2, 28] |
8.32 (4.37) 8 [2, 18] |
|
Multisyllabic Word (MSW) Repetition | ||||||||
| CTR | 0.09 (0.29) 0 [0, 1] |
0.05 (0.21) 0 [0, 1] |
0 | 0 | 0.18 (0.50) 0 [0, 2] |
0 | 0.32 (0.78) 0 [0, 3] |
0 |
| phCAS | 0.70 (1.06) 0 [0, 3] |
0.40 (0.52) 0 [0, 1] |
0.50 (1.08) 0 [0, 3] |
0 |
2.50 (2.37) 1.5 [0, 8] |
1.40 (1.51) 1 [0, 4] |
5.50 (4.43) 5 [0, 13] |
2.10 (2.42) 1 [0, 6] |
| DYX | 0.11 (0.38) 0 [0, 1.5] |
0.16 (0.36) 0 [0, 1] |
0.32 (0.78) 0 [0, 3] |
0.05 (0.21) 0 [0, 1] |
0.95 (1.73) 0 [0, 7] |
0.27 (0.70) 0 [0, 3] |
1.86 (2.83) 0 [0, 9] |
0.36 (0.84) 0 [0, 3] |
|
Nonword Decoding (Word Attack; WATT) | ||||||||
| CTR | 0.05 (0.21) 0 [0, 1] |
0.18 (0.50) 0 [0, 2] |
0.18 (0.39) 0 [0, 1] |
0.32 (0.72) 0 [0, 3] |
0.82 (1.62) 0 [0, 7] |
0.18 (0.39) 0 [0, 1] |
1.72 (2.23) 1 [0, 8] |
0.59 (1.01) 0 [0, 3] |
| phCAS | 0.60 (0.66) 0.5 [0, 2] |
0.30 (0.79) 0 [0, 2.5] |
0.50 (0.53) 0.5 [0, 1] |
0.70 (0.82) 0.5 [0, 2] |
2.00 (2.31) 1 [0, 6] |
1.40 (1.65) 1 [0, 5] |
5.50 (3.50) 6 [0, 11] |
1.70 (1.57) 2 [0, 5] |
| DYX | 0.18 (0.50) 0 [0, 2] |
0.50 (0.60) 0 [0, 2] |
0.86 (1.36) 0 [0, 5] |
0.77 (0.75) 0 [0, 2] |
2.32 (2.90) 1.5 [0, 12] |
1.68 (1.64) 1 [0, 7] |
6.32 (4.83) 6 [1, 18] |
2.27 (1.78) 2 [0, 7] |
Notes. NWR = Comprehensive Test of Phonological Processing - Nonword Repetition subtest, MSW = Catts’ (1986) Multisyllabic Word repetition task, WATT = Woodcock Johnson Tests of Achievement - Word Attack subtest. CTR n = 22; DYX n = 22; phCAS n = 10. Mean (standard deviation), median, [min, max].
The omnibus group differences for substitution errors were only nominally statistically significant. Posthoc testing showed a pairwise group difference between the CTR and phCAS groups and between the CTR and DYX groups but not between the two disorder groups, consistent with the highest number of substitution errors seen in the phCAS group, followed by the DYX and CTR groups (Table 4).
Research Question 3: Assimilation direction in the NWR task
In all three groups, the most prevalent sequencing error type was assimilation, especially perseveratory assimilation (Table 4). The proportion of perseveratory assimilations did not differ among the groups (Table 5), but the three groups differed significantly with respect to number of perseveratory assimilation errors. Posthoc testing showed group differences between the CTR and phCAS groups and the CTR and DYX groups but not between the two disorder groups. The phCAS group showed the highest number of perseverative assimilations, followed by the DYX and CTR groups (Table 4). The three groups did not differ regarding anticipatory assimilation errors (Table 5).
Research Question 4: Sequential and substitution errors during multisyllabic real word repetition
Due to the high frequency of zero sequencing (57.41%) and substitution (81.48%) errors, it was not feasible to calculate the proportions of sequence errors out of total errors for each participant, as was done for the NWR task. The per-group medians for sequencing errors were much larger, compared to the substitution errors, in the two disorder groups (Table 4). The omnibus group differences for sequence errors were experimentwise significant (Table 5). Pairwise comparisons showed greater differences between the CTR and phCAS groups and the two disorder groups than the CTR and DYX groups. The phCAS group produced by far the most errors, followed by the DYX and CTR groups (Table 4). The group differences for substitution errors were experimentwise significant as well (Table 5). Group differences between the CTR and phCAS groups and the two disorder groups were more significant than between the DYX and CTR groups. Although individual sequencing error types were not analyzed for group differences, Table 4 shows highest error counts for omissions and insertions, especially in the phCAS group.
Research Question 5: Sequencing and substitution errors during nonword decoding
Similar to the MSW task, there was a high frequency of zero sequencing (18.52%) and substitution (40.74%) errors; therefore the proportion of sequence errors was not calculated for individual participants. The medians for the sequencing errors were greater than those for substitution errors for all groups (Table 4). The omnibus group differences for sequence errors were experimentwise significant (Table 5). Posthoc testing showed group differences between the CTR and phCAS groups and the CTR and DYX groups but not between the two disorder groups. Omnibus group differences for substitution errors were experimentwise significant as well. Posthoc testing showed group differences between the CTR and DYX groups and, to a lesser extent, between the CTR and phCAS groups but not between the two disorder groups. Again, individual sequencing error types were not analyzed for group differences, but Table 4 shows highest counts of omissions and insertions, especially in the DYX group.
Research Question 6: Association between sequencing deficits in nonword repetition and motor speech
The nonparametric correlation between the measure of sequential deficit in the nonword repetition task (difference between sequencing and substitution errors) and the measure of motor sequencing during the DDK task (difference between the average monosyllabic and multisyllabic z scores) was statistically significant (rho = 0.43, p = 0.0011). Figure 1 shows the corresponding scatterplot. By contrast, the correlation between the sequential deficit during the nonword repetition task and the average z score for monosyllabic DDK was not significant (rho = −0.16, p = 0.2448).
Figure 1.
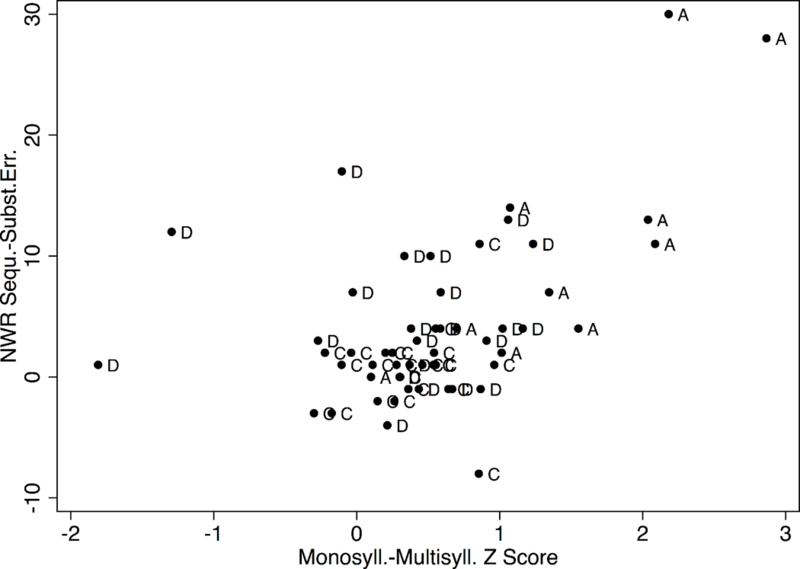
Difference between sequencing and substitution errors during nonword repetition as a function of the difference between monosyllabic and multisyllabic DDK z scores. C = control group, A = probable history of CAS, D = dyslexia.
Table 4 provides an overview of means, standard deviations, medians, and ranges for error classes and types by group and task in units of raw sums. Figure 2 shows boxplots of the error classes by task and group in units of percent of sequence errors and substitution errors. Separately for each task, the percent scores are based on sum of errors divided by the total number of elements for the task, multiplied by 100. For instance, an error count of 5 during the NWR task is equivalent to 3.4 % given the sum of 147 phonemes in this task. Table 5 summarizes the results from the statistical tests of group difference for Research Questions 2, 3, 4, and 5.
Figure 2.
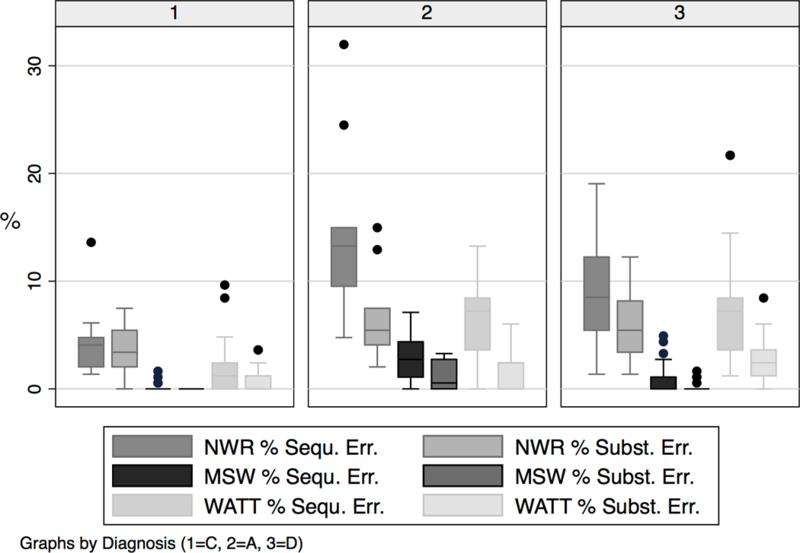
Percent of elements (phonemes for NWR and MSW, graphemes for WATT) in error by group, task, and error class
DISCUSSION
The purpose of this study was to investigate the hypothesis that adults with dyslexia and adults who likely had a childhood history of CAS share a general sequential processing deficit that may represent a biomarker shared by these two disorders. Overall, results of this study are consistent with this hypothesis. In what follows, the results specific to each research question are interpreted individually and then integrated into a systematic framework of sequential information in the cascade of processing levels in CAS and dyslexia. The final section describes limitations of this study and points to future directions, including investigating clinical applications.
Findings regarding individual research questions
Persistent deficit in nonword repetition (Research Question 1)
The DYX and phCAS groups demonstrated nonword repetition abilities far below normal limits, whereas the CTR group did not. These results are consistent with the hypothesis that deficits in this task persist past childhood despite years of treatment in the areas of reading and speech production, respectively, raising the possibility that nonword repetition, although not a functional communication behavior, taps into a persisting core deficit underlying both dyslexia and CAS. Low nonword repetition scores in the DYX group are consistent with the frequently cited deficit in phonemic awareness (Bus & van IJzendoorn, 1999; Ehri et al., 2001; Lyon, 1995; Lyon et al., 2003; Troia, 1999) and, in the phCAS group, with previous observations (Button et al., 2013; Peter et al., 2013).
Sequencing errors during nonword repetition (Research Question 2)
Adults with phCAS and dyslexia demonstrated the expected pattern of producing not only more errors in both error classes than the CTR group but also disproportionally more sequencing errors than substitution errors. This was evident in the nominally statistical group differences regarding the proportions of sequencing errors that were driven primarily by very large differences in sequencing errors between the CTR and each of the disorder groups. A high number of sequencing errors is consistent with preserving the identity of the phonemes in the sequence but not their serial order, whereas a high number of substitution errors is consistent with preserving the word shape in terms of number of phonemes but not the identity of the phonemes. High numbers of either error type in a nonword repetition task do not allocate the locus of impairment unambiguously to specific levels in the information processing cascade, as these errors can plausibly occur at any of these levels (Table 1). Note, however, that the relatively high number of insertion errors in the disorder groups would be consistent with motor programming and planning deficits (“transcoding errors”) within the framework described by Shriberg et al. (2012). To obtain insights into the locus of impairment, error profiles in two other types of tasks, multisyllabic real word repetition and nonword decoding, are discussed below, although it should be noted that results from the three tasks can only be compared indirectly due to the fact that the stimuli were not completely equivalent in terms of number, length, and complexity.
Direction of assimilation errors (Research Question 3)
The most prevalent type of sequencing error in all three groups in the nonword repetition task was assimilation, accounting for roughly half of the sequencing errors in the CTR and DYX groups and over one third in the phCAS group. Highest numbers were observed in the phCAS group, followed by the DYX and CTR groups (Table 4). This finding matches and validates our previous findings in adults with phCAS during the same task (Button et al., 2013; Peter et al., 2013). A high number of assimilation errors was also seen in a sample of children with CAS (Thoonen et al., 1994), suggesting that this error type is present in childhood; our results suggest that it persists into adulthood.
In the previous studies, the direction of the assimilations, perseveratory versus anticipatory, was not analyzed. In the present study, both disorder groups produced more perseverative errors than the control group, whereas the three groups did not differ with respect to anticipatory errors. In typical adults, assimilatory errors tend to be anticipatory (Schwartz, Saffran, Bloch, & Dell, 1994), likely because the motor plan for the entire word is thought to be cued up at the onset of the word (Dell et al., 1997; Levelt, 1999), triggering errors in the anticipatory direction. The presence of anticipatory errors implies an active motor plan, but its deployment on an additional segment earlier in the sequence may result from a weak representation of the phoneme sequence in short-term memory. The high prevalence of perseverative assimilations, rarely discussed in the child and adult phonology literature, may be caused by an underspecified motor plan for the target in addition to weak representation of the sequence in short-term memory, so that an existing motor plan is deployed in the correct position and a second time later in the sequence, replacing the target element in that position. Alternatively, participants in the DYX and phCAS groups may struggle with producing phoneme sequences with variable content, as opposed to repetitive content. Support for this view is found in the observation that, during DDK, the performance of both groups was far slower during the alternating conditions than during the repetitive conditions (Table 2). Perseverating on a phoneme may be a motorically and/or cognitively less taxing way to reduce phonological complexity, compared to anticipatory processes. Note that in assimilations produced by children, the direction is influenced by the place of articulation of the trigger and target phonemes such as velars in final position triggering anticipatory assimilations replacing labials (Stoel-Gammon, 1996). In the present study, however, the place of articulation was not further analyzed.
The CTR group produced very few assimilation errors, with slightly more perseveratory than anticipatory errors. This differs from the expected 75% of anticipatory errors, which may be due not only to the low error counts in general but also to the fact that the stimuli included tokens with possible triggers in either direction.
Sequencing errors during multisyllabic real word repetition (Research Question 4)
Although the proportions of sequencing errors could not be evaluated for group differences due to high counts of zero errors, adults with phCAS produced significantly more sequencing and substitution errors than the control group, with the highest error counts of both classes and especially omissions observed in the phCAS group. High numbers of sequencing errors in this task are consistent with a sequential deficit at the level of phonological assembly and/or downstream from there (Table 1), given the assumption that real words are retrieved from long-term memory, not short-term memory, before being passed through the preparatory steps towards motor execution. This assumption was confirmed in the error-free performance of real word repetitions but highly degraded nonword repetitions by a participant with short-term memory impairment (Peter, in press). Deficits in the motor planning and programming stages are well documented in children with CAS, and slowed speeds during DDK in children with dyslexia are consistent with a similar deficit in that group as well. The present findings are consistent with a persisting deficit in motor programming/planning. This deficit was additionally documented with slow speeds in the DDK task and especially the multisyllabic condition (Table 2). It was greatest in adults with phCAS but was also seen in adults with dyslexia.
An alternate interpretation is the proposition that adults with phCAS and adults with dyslexia have an incorrectly stored phonemic representation of multisyllabic real words in their long-term memory, or have difficulty accessing this stored information. Regarding individuals with dyslexia, studies have shown that they make more errors on phonologically complex stimuli than typical controls (Apthorp, 1995; Blalock, 1982; Catts, 1986; Snowling, 1981), including the study whose real word stimuli were used in the present study (Catts, 1986). Evidence that the locus of impairment lies more in the area of motor planning/programming than in the area of phonological memory comes from a study investigating accuracy and speed during production of phonologically complex words and phrases. College students with dyslexia were able to produce complex familiar phrases in isolation, consistent with intact phonological memory, but struggled with rapid repetition of the same phrases, consistent with difficulties during motor planning/programming (Catts, 1989). Further evidence for motor planning/programming deficits in dyslexia was provided by a study showing an association between production of complex stimuli and reading ability even after controlling for memory factors (Apthorp, 1995).
Regarding individuals with CAS, studies in children with CAS have provided evidence that deficits are not restricted to motor planning/programming errors but may also include errors during encoding and memory storage and retrieval. A review of this literature (Velleman, 2011) mentions reduced perception and production of vowels, syllables, and phoneme sequences and difficulties with spelling, suggesting impoverished phonemic representations. In our studies, adults with phCAS obtained lower reading and spelling scores than their unaffected relatives (Button et al., 2013; Peter et al., 2013), consistent with the possibility that cognitive and linguistic sequencing deficits, upstream from the motor planning/programming stages, persist into adulthood in individuals with CAS, along with the known motor planning/programming deficits.
Sequencing errors during nonword decoding (Research Question 5)
Adults with dyslexia have a known deficit in word and nonword decoding. Therefore, a high error count during WATT is expected in this group, and indeed, of the three groups, the DYX group produced the highest number of sequencing and substitution errors, closely followed, however, by the phCAS group. Although group differences could not be calculated regarding the proportion of sequencing errors, the two disorder groups differed from the CTR group more significantly regarding sequential than substitution errors. Examples of omissions, the most prevalent sequential error type, were producing the target “sprawn’t” (/sprɔnt/) as “spawn’t” ([spɔnt]) and the target “byrcal” (/bɝkəl/ or /bərkəl/ in some transcription conventions) as “bycal” ([baɪkəl]).
Interestingly, the phoneme change from /ɝ/ or /ər/ to [aɪ] in “byrcal” is evidence that this error occurred at the level of visual encoding. Producing “byrcal” as if it were spelled “bycal” is evidence of skipping the letter “r.” The [aɪ] sound is not part of the target phoneme sequence and the most parsimonious explanation is that the “r” in the grapheme sequence was skipped. Similarly, a frequently seen example of a grapheme metathesis in both disorder groups was [braɪkəl] for the target /bɝkəl/ or /bərkəl/, where the grapheme sequence “byrcal” was rendered as if it were spelled “brycal.” Other examples pointing to visual encoding as the locus of impairment are [ralut] for “wrault” as if it were spelled “wralut” and [trob] for “throbe” as if it were spelled “trobe.” Whether or not other omission errors also resulted from faulty encoding (e.g., by visually skipping over letters) is less clear. Evidence in favor of faulty visual encoding in individuals with dyslexia is found in studies reporting transient visual processing deficits. The transient visual system is sensitive to global visual features and guides eye movement. Deficits in this system have been described not only in verbal but also nonverbal visual tasks (Livingstone, Rosen, Drislane, & Galaburda, 1991; Stuart & Lovegrove, 1992). According to one study, this deficit affects 29% of individuals with dyslexia (Ramus, 2003). The adults with dyslexia produced the highest number of omissions and also total sequencing errors during this task, an indication that if visual encoding is a relevant locus of impairment, it affects this group more than the phCAS group. Whether or not visual recognition and visual motor control are impaired in individuals with CAS is unknown.
The error profile in the phCAS group resembled that in the DYX group but was expressed to a lesser extent, as evident in slightly lower error counts in each error class and type. The sequential processing deficit at the level of motor planning/programming has been well established, not only in the motor speech domain but also in the hand motor domain (Button et al., 2013; Peter et al., 2013; Peter et al., 2012; Peter & Raskind, 2011). Here, we show evidence that sequencing errors also occur during the encoding stage of visual information, manifested as phonemes not present in the target word due to visual grapheme rearrangements. This finding is consistent with the encoding errors described in children and young adults with CAS during a nonword repetition task, where the modality was auditory (Shriberg et al., 2012).
Cross-modality of the sequential processing deficit (Research Question 6)
Evidence that the sequential processing deficit observed in nonword and real word imitations as well as nonword decoding is not specific to linguistic tasks comes from the statistically significant correlation between a measure of sequential processing deficit during the nonword repetition task (difference between sequencing and substitution errors) and a measure of motor sequencing (z score difference between monosyllabic and multisyllabic DDK scores). A large positive difference between the two DDK conditions indicates disproportional difficulty during the multisyllabic tasks, consistent with difficulty integrating sequential sets of motor commands efficiently. This specific difficulty was found to be highly associated with the difficulty of maintaining the serial order of phonemes in the nonword repetition task, whereas no such association was observed for the monosyllabic condition.
Two participants with dyslexia did not fit the general pattern of association. Their multisyllabic DDK speeds were faster than their monosyllabic DDK speeds, whereas their nonword repetition errors were similar to other participants with dyslexia. One of these participants had experience with DDK in her role as a special education professional and produced multisyllables at extremely rapid speeds; the other had z scores for /pata/ and /pataka/ within normal limits but extremely slow rates for the other DDK tasks for unknown reasons.
Integrating inferences from error analysis into a systematic view of sequential processing
Results from this study are consistent with the hypothesis that adults with dyslexia and adults with phCAS share a fundamental deficit regarding sequential information that is distinct from weak phonemic awareness. The phenotypic similarities between individuals with dyslexia and an apraxia history were supported, not only in the results from error analyses and especially in the nonword repetition task (Table 4 and Figure 2), but also in standardized scores of tasks with a high sequential processing load including reading, spelling, and multisyllabic DDK tests (Table 2). The fact that some of these similarities have previously been described in children with these disorders and demonstrated here in adults is consistent with the hypothesis that the sequential processing deficit persists into adulthood. The fact that substitution errors occurred at greater numbers in these two groups, compared to the typical controls, is evidence that maintaining the serial order of elements in sequences was not their only difficulty; they also struggled with maintaining the identity of individual elements.
Shared underlying substrates likely include deficits in sequential processing at multiple levels of the information processing cascade. A comparison of performance during the different tasks based on percent errors (Figure 2) offers some clues regarding the loci of impairment in this cascade. Adults in both disorder groups produced nonword decoding errors that clearly resulted from faulty encoding as the switched order of graphemes resulted in phonemes not present in the target word. A locus of impairment at the motor planning/programming level was implicated during real word repetition and further supported by discrepant performance during monosyllabic and multisyllabic DDK. The task with the greatest demand on short-term memory is nonword repetition. The additional participant with short-term memory impairment (Peter, in press) produced many sequencing as well as substitution errors during this task while resembling the control group during real word repetition, consistent with the loss of sequential as well as segmental information at the level of short-term memory. Nonword repetition also heavily taxes levels of sensory encoding, similar to nonword decoding, and speech production, common to all three tasks (Table 1). It is possible that deficits at multiple levels of processing including encoding, short-term memory, retrieval, phonologic assembly, and motor planning/programming produce a compound effect on NWR task performance in both of the disorder groups. Regarding loci of impairment in the information processing cascade, segmental errors during encoding, storage in short-term memory, and motor planning/programming have been described in children and young adults with CAS (Shriberg et al., 2012). The present results extend these findings from segments to complex serial order, from children with CAS to adults with phCAS, and from CAS to dyslexia.
It is possible that the two disorder groups are affected by the shared underlying deficits in slightly different ways. Consistent with the known deficits in visual encoding (Ramus, 2003) and forming grapheme-phoneme associations in dyslexia, adults with dyslexia may struggle to encode sequential information visually, leading to high error counts during nonword decoding, whereas adults with a CAS history may be affected by this deficit to a slightly lesser extent (Tables 2 and 4). Similarly, individuals with CAS may have greater deficits in motor planning/programming than individuals with dyslexia, affecting their performance to a greater extent than individuals with dyslexia during tasks with complex motor planning/programming demands such as the MSW task and the multisyllabic DDK tasks in the present study (Tables 2 and 4). It is possible that, similar to adults with acquired apraxia of speech, their phonological buffer capacity is limited (Rogers & Storkel, 1999) and/or their motor planning/programming time are relatively long (Maas et al., 2008).
One possible explanation for the shared phenotypes is a shared deficit in cerebellar function, caused by genetic variations. As mentioned, the cerebellum plays a crucial role not only in motor coordination but also in linguistic and cognitive functions, including reading. Our recent discoveries of CAS candidate genes that are highly expressed in the cerebellum (Peter et al., 2016) are consistent with the hypothesis that variations in different or multiple genes converge on the cerebellum, causing downstream difficulty with processing complex sequential information across domains. If so, a sequential processing deficit represents a shared biomarker of genetic etiology for dyslexia and CAS.
LIMITATIONS AND FUTURE DIRECTIONS
This study has several limitations. First, whereas the adults in the DYX group showed current evidence of dyslexia, the adults in the phCAS group had normalized conversational speech and the inclusionary criteria for them were based more on circumstantial than overt evidence. Note, however, that poor performance during multisyllabic DDK, a known residual weakness in adults with a probable history of CAS, was used to help determine inclusion, and the recent genetic findings strengthen the construct of inherited forms of CAS in some of these participants. Ultimately, their performance across tasks confirmed the hypotheses guiding the present study, supporting the validity of the group assignment. Second, inferences regarding deficits that persist into adulthood are based on cross-sectional samples of adults and published findings in samples of children; longitudinal data from childhood into adulthood were not available. Third, the error types in the sequence error class are qualitatively different from each other, but were collapsed together for quantitative analysis. This lack of homogeneity was accounted for in the selection of nonparametric statistical procedures. Fourth, the stimuli in the three tasks were selected from standardized tests. They were not custom-designed, nor were they completely equivalent in terms of number and complexity. Therefore, comparisons across tasks and inferences regarding levels of processing are indirect, but note that Figure 2 shows boxplots of errors scaled to percent errors out of total elements for each task, facilitating comparisons across tasks. In addition, errors of omission were not independent of prosodic information. Where syllables were omitted, this involved not only omitting the vocalic syllable core but also consonants in the onset and/or coda. A close analysis of the assimilation errors in the nonword repetition task was completed because assimilation errors were the most frequently occurring errors in all tasks. However, group differences regarding error types within the sequence error class could not be explored due to lack of statistical power. To complete such fine-grained analyses, a larger sample size, especially including more adults with phCAS, and a greater volume of data would be required. Adding an analysis of the real word reading task for purposes of comparing error types during real word reading against nonword decoding and real word repetition was considered but not done because of overall low error counts in that task.
The present study offers strong support for a biomarker centered on a sequential processing deficit shared by individuals with dyslexia and CAS. Future studies should expand on the nature of sequencing errors beyond speech and reading disorders to also include language disorders. Future studies should also investigate shared genetic etiologies linked to component phenotypes most closely associated with sequential processing deficits. Candidate genes already identified in CAS include several genes that are strongly expressed in the cerebellum or associated with cerebellar anomalies, for instance FOXP2 (Belton, Salmond, Watkins, Vargha-Khadem, & Gadian, 2003; Liegeois, Morgan, Connelly, & Vargha-Khadem, 2011), CDH18, MYO10, and C4orf21 (ZGRF1) (Peter et al., 2016). It is possible that variations in these and other genes influence cerebellar function with converging effects on sequential processing during speech and reading tasks. Brain imaging studies should be conducted to provide insights into the downstream effects of genetic variations on brain structures and functions, core deficits, and behaviorally observable communication deficits. Inconsistency of word and phrase productions was recently shown to differentiate between children with CAS and children with other types of speech delay (Iuzzini-Seigel, Hogan, & Green, 2017). Future investigations should explore the relationship between inconsistency of performance and loss of serial order. Similarly, whether the deficits in motor planning that are posited here for individuals with dyslexia are based on neural substrates should be investigated.
Finally, future studies should investigate the clinical relevance of sequential processing deficits in individuals with speech and reading disorders. For clinicians serving children with these disorders, potential topics include (1) providing early preventative support in preschoolers with a CAS diagnosis toward stronger reading skills later on, (2) targeting underlying core deficits in the area of sequential processing in addition to treating the overt symptoms in reading and speech production, and (3) implementing interprofessional approaches where core deficits manifest in additional areas such as fine and gross motor development. Regarding (1), a global deficit in sequential processing shared by at least a subset of individuals with CAS and dyslexia provides additional motivation beyond the observed comorbidities for initiating support in the area of preliteracy skills in children with a preschool diagnosis of CAS. This may head off difficulties with reading and spelling that are anticipated to surface later on during the school years. The proposed sequential processing deficit helps explain not only the presence of the comorbidity but also the shared substrate. Regarding (2), if sequential processing deficits underlie both CAS and dyslexia, then the question arises whether treating this core deficit directly may have beneficial effects on speech, reading, and spelling. For instance, one could envision training programs addressing sequential processing ability linguistically by teaching concepts like “first,” “last,” “second,” “then,” “before,” and “after,” or visually by creating patterns with beads or blocks, or motorically through music and dance, then observing whether these activities are associated with improvements in speech and reading. Regarding (3), speech-language pathologists, occupational therapists, and physical therapists frequently serve children with CAS and dyslexia who have concomitant fine motor and gross motor concerns (Iversen, Berg, Ellertsen, & Tonnessen, 2005; Teverovsky, Bickel, & Feldman, 2009). The underlying deficits being addressed by each of these disciplines may in fact be the same, particularly in the case of the child’s overall coordination and sequencing abilities. Interprofessional approaches to addressing weak sequencing skills might lead to increased generalization of abilities across the various domains. Clinical trials would be necessary, however, to explore whether these kinds of clinical translations have practical merit.
Acknowledgments
The authors wish to thank all participants for their time and efforts. The following funding sources are gratefully acknowledged: American Speech-Language-Hearing Foundation New Century Scholars Research Grant (B. Peter), NIDCD T32DC00033 (B. Peter), NIDCD R03DC010886 (B. Peter), University of Washington Royalty Research Fund (B. Peter), and Arizona State University New Faculty Startup Fund (B. Peter). Special thanks to the following undergraduate and graduate students for their assistance with data collection and analysis: Linda Eng, Natalie Heldenbrand, Hannah Holtz, Andrea Kretchmer, Jennifer Philp, Whitney Stine, Mckayla Tully, and Christen Webb.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- Apthorp HS (1995). Phonetic coding and reading in college students with and without learning disabilities. Journal of Learning Disabilities, 28(6), 342–352. [DOI] [PubMed] [Google Scholar]
- Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, & Gadian DG (2003). Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Human Brain Mapping, 18(3), 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger VW, Abbott R, Thomson JB, & Raskind W (2001). Phenotype for reading and writing disability: A lifespan approach. Scientific Studies of Reading, 5, 59–105. [Google Scholar]
- Bishop DV, & Edmundson A (1987). Language-impaired 4-year-olds: distinguishing transient from persistent impairment. Journal of Speech and Hearing Disorders, 52(2), 156–173. [DOI] [PubMed] [Google Scholar]
- Blalock JW (1982). Persistent auditory language deficits in adults with learning disabilities. Journal of Learning Disabilities, 15(10), 604–609. [DOI] [PubMed] [Google Scholar]
- Boersma P, & Weenink D (2014). Praat: Doing phonetics by computer. 5.4 Retrieved from http://www.praat.org/
- Bogaerts L, Szmalec A, De Maeyer M, Page MP, & Duyck W (2016). The involvement of long-term serial-order memory in reading development: A longitudinal study. Journal of Experimental Child Psychology, 145, 139–156. [DOI] [PubMed] [Google Scholar]
- Bogaerts L, Szmalec A, Hachmann WM, Page MP, & Duyck W (2015). Linking memory and language: Evidence for a serial-order learning impairment in dyslexia. Research in Developmental Disabilities, 43-44, 106–122. [DOI] [PubMed] [Google Scholar]
- Bus AG, & van IJzendoorn MH (1999). Phonological awareness and early reading: A meta-analysis of experimental training studies. Journal of Educational Psychology, 91(3), 403–414. [Google Scholar]
- Button L, Peter B, Stoel-Gammon C, & Raskind WH (2013). Associations among measures of sequential processing in motor and linguistics tasks in adults with and without a family history of childhood apraxia of speech: a replication study. Clinical Linguistics and Phonetics, 27(3), 192–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts HW (1986). Speech production/phonological deficits in reading-disordered children. Journal of Learning Disabilities, 19(8), 504–508. [DOI] [PubMed] [Google Scholar]
- Catts HW (1989). Speech production deficits in developmental dyslexia. Journal of Speech and Hearing Disorders, 54(3), 422–428. [DOI] [PubMed] [Google Scholar]
- Cowan N, Hogan TP, Alt M, Green S, Cabbage KL, Brinkley S, & Gray S (2017). Short-term memory in childhood dyslexia: Deficient serial order in multiple modalities. Dyslexia, 23(3), 209–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Burger LK, & Svec WR (1997). Language production and serial order: a functional analysis and a model. Psychological Review, 104(1), 123–147. [DOI] [PubMed] [Google Scholar]
- Deniz Can D, Richards T, & Kuhl PK (2013). Early gray-matter and white-matter concentration in infancy predict later language skills: a whole brain voxel-based morphometry study. Brain and Language, 124(1), 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominey PF (1995). Complex sensory-motor sequence learning based on recurrent state representation and reinforcement learning. Biological Cybernetics, 73(3), 265–274. [PubMed] [Google Scholar]
- Dominey PF (2013). Recurrent temporal networks and language acquisition-from corticostriatal neurophysiology to reservoir computing. Frontiers in Psychology, 4, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominey PF, Hoen M, Blanc JM, & Lelekov-Boissard T (2003). Neurological basis of language and sequential cognition: evidence from simulation, aphasia, and ERP studies. Brain and Language, 86(2), 207–225. [DOI] [PubMed] [Google Scholar]
- Edwards ML (1983). Phonological processes documented in the speech of children with normal and disordered phonological development. Seminars in Speech and Language, 4, 351–374. [Google Scholar]
- Ehri LC, Nunes SR, Willows DM, Schuster BV, Yaghoub-Zadeh Z, & Shanahan T (2001). Phonemic awareness instruction helps children learn to read: Evidence from the National Reading Panel’s meta-analysis. Reading Research Quarterly, 36(3), 250–287. [Google Scholar]
- Elvevag B, Fisher JE, & Goldberg TE (2003). Probed recall for serial order deficits in short-term memory in schizophrenic patients. Schizophrenia Resesarch, 59(2–3), 127–135. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Zorzi M, Cestnick L, Lorusso ML, Molteni M, Paganoni P, Umilta C, & Mascetti GG (2006). The relationship between visuo-spatial attention and nonword reading in developmental dyslexia. Cognitive Neuropsychology, 23(6), 841–855. [DOI] [PubMed] [Google Scholar]
- Feng X, Li L, Zhang M, Yang X, Tian M, Xie W, Lu Y, Liu L, Belanger NN, Meng X, & Ding G (2016). Dyslexic children show atypical cerebellar activation and cerebro-cerebellar functional connectivity in orthographic and phonological processing. Cerebellum. [DOI] [PubMed] [Google Scholar]
- Fletcher SG (1972). Time-by-count measurement of diadochokinetic syllable rate. J Speech Hear Res, 15(4), 763–770. [DOI] [PubMed] [Google Scholar]
- Frank J, & Levinson H (1973). Dysmetric dyslexia and dyspraxia. Hypothesis and study. Journal of the American Academy of Child and Adolescent Psychiatry, 12(4), 690–701. [DOI] [PubMed] [Google Scholar]
- Grunwell P (1981). The development of phonology. First Language, 2, 161–191. [Google Scholar]
- Gupta P (2003). Examining the relationship between word learning, nonword repetition, and immediate serial recall in adults. Quarterly Journal of Experimental Psychology (A), 56(7), 1213–1236. [DOI] [PubMed] [Google Scholar]
- Hachmann WM, Bogaerts L, Szmalec A, Woumans E, Duyck W, & Job R (2014). Short-term memory for order but not for item information is impaired in developmental dyslexia. Ann Dyslexia, 64(2), 121–136. [DOI] [PubMed] [Google Scholar]
- Ingram D (2002). The measurement of whole-word productions. Journal of Child Language, 29(4), 713–733. [DOI] [PubMed] [Google Scholar]
- Iuzzini-Seigel J, Hogan TP, & Green JR (2017). Speech inconsistency in children with childhood apraxia of speech, language impairment, and speech delay: Depends on the stimuli. J Speech Lang Hear Res, 60(5), 1194–1210. [DOI] [PubMed] [Google Scholar]
- Iversen S, Berg K, Ellertsen B, & Tonnessen FE (2005). Motor coordination difficulties in a municipality group and in a clinical sample of poor readers. Dyslexia, 11(3), 217–231. [DOI] [PubMed] [Google Scholar]
- Konig IR, Schumacher J, Hoffmann P, Kleensang A, Ludwig KU, Grimm T, Neuhoff N, Preis M, Roeske D, Warnke A, Propping P, Remschmidt H, Nothen MM, Ziegler A, Muller-Myhsok B, & Schulte-Korne G (2011). Mapping for dyslexia and related cognitive trait loci provides strong evidence for further risk genes on chromosome 6p21. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 156B(1), 36–43. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, Pezzulo G, Ramnani N, Riva D, Schmahmann J, Vandervert L, & Yamazaki T (2014). Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum, 13(1), 151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq AL, & Majerus S (2010). Serial-order short-term memory predicts vocabulary development: evidence from a longitudinal study. Developmental Psychology, 46(2), 417–427. [DOI] [PubMed] [Google Scholar]
- Levelt WJ (1999). Models of word production. Trends in Cognitive Sciences, 3(6), 223–232. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22(1), 1–38; discussion 38–75. [DOI] [PubMed] [Google Scholar]
- Levinson HN (1988). The cerebellar-vestibular basis of learning disabilities in children, adolescents and adults: hypothesis and study. Perceptual and Motor Skills, 67(3), 983–1006. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Freebairn LA, Hansen AJ, Iyengar SK, & Taylor HG (2004). School-age follow-up of children with childhood apraxia of speech. Language, Speech, and Hearing Services in Schools, 35(2), 122–140. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Morgan AT, Connelly A, & Vargha-Khadem F (2011). Endophenotypes of FOXP2: dysfunction within the human articulatory network. European Journal of Paediatric Neurology, 15(4), 283–288. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, & Galaburda AM (1991). Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proceedings of the National Academies of Science of the United States of America, 88(18), 7943–7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GR (1995). Toward a definition of dyslexia. Annals of Dyslexia, 45, 3–27. [DOI] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, & Shaywitz BA (2003). A definition of dyslexia. Annals of Dyslexia, 53, 1–14. [Google Scholar]
- Maas E, Robin DA, Austermann Hula SN, Freedman SE, Wulf G, Ballard KJ, & Schmidt RA (2008). Principles of motor learning in treatment of motor speech disorders. Am J Speech Lang Pathol, 17(3), 277–298. [DOI] [PubMed] [Google Scholar]
- Majerus S, & Cowan N (2016). The Nature of Verbal Short-Term Impairment in Dyslexia: The Importance of Serial Order. Frontiers in Psychology, 7, 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek A, Amiri S, Hekmati I, Pirzadeh J, & Gholizadeh H (2013). A comparative study on diadochokinetic skill of dyslexic, stuttering, and normal children. ISRN Pediatrics, 2013, 165193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P, & Beaton A (2014). The enigmatic linguistic cerebellum: clinical relevance and unanswered questions on nonmotor speech and language deficits in cerebellar disorders. Cerebellum Ataxias, 1, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack T, Brown GD, Vousden JI, & Henson RN (2000). Children’s serial recall errors: implications for theories of short-term memory development. Jornal of Experimental Child Psychology, 76(3), 222–252. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Fowlow SB, Robertson A, Samcoe D, Burgess I, & Hoo JJ (1990). Chromosome 6q deletions: a report of two additional cases and a review of the literature. American Journal of Medical Genetics, 35(1), 79–84. [DOI] [PubMed] [Google Scholar]
- McNeilage PF (1964). Typing errors as clues to serial ordering mechanisms in language behaviour. Language and Speech, 7(3), 144–159. [Google Scholar]
- Melby-Lervåg M, & Lervåg A (2012). Oral language skills moderate nonword repetition skills in children with dyslexia: A meta-analysis of the role of nonword repetition skills in dyslexia. Scientific Studies of Reading, 16(1), 1–34. [Google Scholar]
- Nicolson RI, Fawcett AJ, & Dean P (1995). Time estimation deficits in developmental dyslexia: evidence of cerebellar involvement. Proceedings of the Royal Society B: Biological Sciences, 259(1354), 43–47. [DOI] [PubMed] [Google Scholar]
- Peter B (in press). The role of memory impairment in nonword repetition: A case study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Button L, Stoel-Gammon C, Chapman K, & Raskind WH (2013). Deficits in sequential processing manifest in motor and linguistic tasks in a multigenerational family with childhood apraxia of speech. Clinical Linguistics and Phonetics, 27(3), 163–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Lancaster H, Vose C, Fares A, Schrauwen I, & Huentelman M (2017). Two unrelated children with overlapping 6q25.3 deletions, motor speech disorders, and language delays. American Journal of Medical Genetics Part A. [DOI] [PubMed] [Google Scholar]
- Peter B, Matsushita M, & Raskind WH (2012). Motor sequencing deficit as an endophenotype of speech sound disorder: a genome-wide linkage analysis in a multigenerational family. Psychiatric Genetics, 22(5), 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, & Raskind WH (2011). A multigenerational family study of oral and hand motor sequencing ability provides evidence for a familial speech sound disorder subtype. Topics in Language Disorders, 31(2), 145–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Wijsman EM, Nato AQ Jr., Genomics U o. W. C. f. M., Matsushita, M. M., Chapman, K. L., Stanaway, I. B., Wolff, J., Oda, K., Gabo, V. B., & Raskind, W. H. (2016). Genetic candidate variants in two multigenerational families with childhood apraxia of speech. PLoS One, 11(4), e0153864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliatsikas C, Johnstone T, & Marinis T (2014). Grey matter volume in the cerebellum is related to the processing of grammatical rules in a second language: a structural voxel-based morphometry study. Cerebellum, 13(1), 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F (2003). Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Current Opinions in Neurobiology, 13(2), 212–218. [DOI] [PubMed] [Google Scholar]
- Ramus F, Pidgeon E, & Frith U (2003). The relationship between motor control and phonology in dyslexic children. Journal of Child Psychology and Psychiatry, 44(5), 712–722. [DOI] [PubMed] [Google Scholar]
- Rey V, De Martino S, Espesser R, & Habib M (2002). Temporal processing and phonological impairment in dyslexia: effect of phoneme lengthening on order judgment of two consonants. Brain and Language, 80(3), 576–591. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, & Kamphaus RW (2003). RIAS, Reynolds Intellectual Assessment Scales. Lutz, FL: Psychological Assessment Resources [Google Scholar]
- Roberts JE, Burchinal M, & Footo MM (1990). Phonological process decline from 2 1/2 to 8 years. Journal of Communication Disorders, 23(3), 205–217. [DOI] [PubMed] [Google Scholar]
- Rogers MA, & Storkel HL (1999). Planning one syllable at a time: the reduced buffer hypothesis in apraxia of speech. Aphasiology, 13, 793–805. [Google Scholar]
- Romani C, Tsouknida E, & Olson A (2015). Encoding order and developmental dyslexia: a family of skills predicting different orthographic components. Quarterly Journal of Experimental Psychology (Hove), 68(1), 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Saffran EM, Bloch DE, & Dell GS (1994). Disordered speech production in aphasic and normal speakers. Brain and Language, 47(1), 52–88. [DOI] [PubMed] [Google Scholar]
- Service E (1992). Phonology, working memory, and foreign-language learning. Quarterly Journal of Experimental Psychology A, 45(1), 21–50. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, & Shaywitz BA (2003). The science of reading and dyslexia. Journal of Aapos, 7(3), 158–166. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fletcher JM, & Escobar MD (1990). Prevalence of reading-disability in boys and girls - Results of the Connecticut Longitudinal-Study. Jama-Journal of the American Medical Association, 264(8), 998–1002. [PubMed] [Google Scholar]
- Shriberg LD, Lohmeier HL, Strand EA, & Jakielski KJ (2012). Encoding, memory, and transcoding deficits in Childhood Apraxia of Speech. Clinical Linguistics and Phonetics, 26(5), 445–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling MJ (1981). Phonemic deficits in developmental dyslexia. Psychological Research, 43(2), 219–234. [DOI] [PubMed] [Google Scholar]
- Snyder KM, & Logan GD (2014). The problem of serial order in skilled typing. Journal of Experimental Psychology: Human Perception and Performance, 40(4), 1697–1717. [DOI] [PubMed] [Google Scholar]
- Stackhouse J, & Wells B (1997). Children’s speech and literacy difficulties: Book 1. A psycholinguistic framework. Chichester: Wiley. [Google Scholar]
- Stoel-Gammon C (1996). On the acquisition of velars in English. In B. Bernhardt, J. Gilbert, & D. Ingram (Eds.), Proceedings of the UBC international conference on phonological acquisition (pp. 201–214). Somerville: Cascadilla Press. [Google Scholar]
- Stoel-Gammon C, & Dunn C (1985). Normal and disordered phonology in children. Baltimore: University Park Press. [Google Scholar]
- Stoel-Gammon C, & Stone JR (1991). Assessing phonology in young children. Clinical Communication Disorders, 1(2), 25–39. [PubMed] [Google Scholar]
- Stoodley CJ, & Stein JF (2013). Cerebellar function in developmental dyslexia. Cerebellum, 12(2), 267–276. [DOI] [PubMed] [Google Scholar]
- Stothard SE, Snowling MJ, Bishop DV, Chipchase BB, & Kaplan CA (1998). Language-impaired preschoolers: a follow-up into adolescence. Journal of Speech, Language, and Hearing Research, 41(2), 407–418. [DOI] [PubMed] [Google Scholar]
- Stuart GW, & Lovegrove WJ (1992). Visual processing deficits in dyslexia: receptors or neural mechanisms? Perceptual and Motor Skills, 74(1), 187–192. [DOI] [PubMed] [Google Scholar]
- Szmalec A, Loncke M, Page MP, & Duyck W (2011). Order or disorder? Impaired Hebb learning in dyslexia. Journal of Experimental Psychology: Learning, Memory, and Cognition, 37(5), 1270–1279. [DOI] [PubMed] [Google Scholar]
- Teverovsky EG, Bickel JO, & Feldman HM (2009). Functional characteristics of children diagnosed with Childhood Apraxia of Speech. Disability and Rehabilitation, 31(2), 94–102. [DOI] [PubMed] [Google Scholar]
- Thoonen G, Maassen B, Gabreels F, & Schreuder R (1994). Feature analysis of singleton consonant errors in developmental verbal dyspraxia (DVD). Journal of Speech and Hearing Research, 37(6), 1424–1440. [DOI] [PubMed] [Google Scholar]
- Thoonen G, Maassen B, Gabreels F, & Schreuder R (1999). Validity of maximum performance tasks to diagnose motor speech disorders in children. Clinical Linguistics and Phonetics, 13(1), 1–23. [Google Scholar]
- Torgesen JK, Wagner RK, & Rashotte CA (2012). Test of Word Reading Efficiency - Second Edition. Austin: Pro-Ed. [Google Scholar]
- Troia GA (1999). Phonological awareness intervention research: A critical review of the experimental methodology. Reading Research Quarterly, 34(1), 28–52. [Google Scholar]
- van der Merwe A (2009). A theoretical framework for the characterization of pathological speech sensorimotor control. In McNeil MR (Ed.), Clinical management of sensorimotor speech disorders (2nd edition) (pp. 3–18). New York: Thieme Medical Publishers. [Google Scholar]
- Velleman SL (2011). Lexical and phonological development in children with childhood apraxia of speech--a commentary on Stoel-Gammon’s ‘Relationships between lexical and phonological development in young children’. Journal of Child Language, 38(1), 82–86. [DOI] [PubMed] [Google Scholar]
- Vihman MM, & Greenlee M (1987). Individual differences in phonological development: ages one and three years. Journal of Speech and Hearing Research, 30(4), 503–521. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, & Rashotte CA (1999). CTOPP, Comprehensive Test of Phonological Processing Austin, Tex.: PRO-ED. [Google Scholar]
- Wechsler D (2005). Wechsler Individual Achievement Test, Second Edition London: The Psychological Corporation. [Google Scholar]
- Woodcock R, McGrew K, & Mather N (2001). Woodcock-Johnson Tests of Achievement. Itasca: Riverside Publishing. [Google Scholar]


