Abstract
Under the U.S. Lung Allocation Score (LAS) system, older and sicker patients are prioritized for lung transplantation (LT). The impact of these changes on health-related quality of life (HRQL) after transplant has not been determined. In a single-center prospective cohort study, from 2010–2016 we assessed HRQL before and repeatedly after LT for up to 3 years using the SF12-Physical and Mental Health, the respiratory-specific Airway Questionnaire 20-Revised, and the Euroqol 5D/Visual Analog Scale utility measures by multivariate linear mixed models jointly modeled with death. We also tested changes in LT-Valued Life Activities disability, BMI, allograft function, and 6-minute walk test exercise capacity as predictors of HRQL change. Amongst 211 initial participants (92% of those eligible), LT improved HRQL by all five measures (p<0.05) and all but SF12-Mental Health improved by three-fold or greater than the minimally clinically important difference. Compared to younger participants, those aged ≥65 improved less in SF12-Physical and Mental Health (p<0.01). Improvements in disability accounted for much of the HRQL improvement. In the LAS era, LT affords meaningful and durable HRQL improvements, mediated by amelioration of disability. Identifying factors limiting HRQL improvement in selected subgroups, especially those aged ≥65, are needed to maximize the net-benefits of LT.
INTRODUCTION
A primary aim of lung transplantation is to improve health-related quality of life (HRQL) for persons suffering from advanced lung disease. This is especially true in situations in which the decision to transplant is not clear, for example in older candidates (aged 65 or more) whose median survival after transplant is only 3.5 years or for even younger persons with conditions such as COPD for which an absolute survival benefit from transplant is uncertain.(1, 2) Accurate assessment of the effect of lung transplantation on HRQL is fundamental to making informed clinical decisions and gauging more comprehensively the personalized “net-benefit” of transplant.
In 2005, organ allocation policy in the U.S. was overhauled to one driven by urgency of need. The resultant Lung Allocation Score (LAS) sought to maximize “transplant benefit” by reducing wait-list mortality without negatively impacting one-year post-operative survival.(3) The LAS policy – along with advances in bridging the critically-ill to transplant (4, 5) – shifted lung allocation to older, sicker patients. Compared to 4% in 2002, patients aged ≥65, for example, account now for 30% of new U.S. transplant recipients.(6) Morbidity and mortality after the first post-operative year have also increased in this period.(7, 8)
Much of the contemporary literature relevant to this topic has focused on important individual domains of health that are influential determinants of HRQL, such as depression, anxiety, cognitive function, and functional status.(9–22) Nonetheless, relatively few studies focused on HRQL itself as a primary outcome in lung transplantation have been published since the LAS overhaul.(23) Thus, given the clinical primacy of HRQL, there is a need for contemporary information on the effect of lung transplantation on general and respiratory-specific HRQL
We aimed to comprehensively evaluate the effect of lung transplantation on HRQL in an adult U.S. cohort undergoing transplant under the LAS allocation system. Beginning before lung transplantation, we followed subjects for up to 3 years after surgery. We evaluated HRQL over a spectrum of ages and disease groups using multiple measures including generic HRQL, respiratory-specific HRQL, and health utility instruments. We also evaluated the extent to which changes in body mass index (BMI), allograft function, and exercise capacity affect HRQL over time. Finally, we examined the extent to which changes in HRQL are accounted for by improvements in physical disability. Preliminary results have been reported in abstracts and in a manuscript based on our initial recruitment experience.(24–30)
METHODS
Study design, participants, and setting
We performed this study among participants in the University of California San Francisco “Breathe Again” study, an ongoing prospective cohort study of English-speaking adults (age ≥ 18) able to read, write, and complete structured survey batteries undergoing first-time lung transplantation. Initiated in February 2010, Breathe Again aims to study the effect of lung transplantation on patient-centered outcomes, including HRQL, and to identify pulmonary and extra-pulmonary determinants of these outcomes.
Adults with advanced lung disease are enrolled at the time of placement on the wait-list for lung transplantation. At enrollment, we perform a structured research visit that includes a structured survey battery to ascertain HRQL by multiple measures. Participant self-assessed disability is determined at the same time. Quantitative functional assessments include the six-minute walk distance (6MWD) as well as selected other measures of physical performance. Research visits are conducted in hospital if study participants are inpatients at the time of listing. While wait-listed, assessments are repeated quarterly; the data most proximal to the date of transplant are treated as the pre-transplant baseline values for analytic purposes. The same survey and physical assessments are administered at three and six months after transplantation and semi-annually thereafter for up to 3 years. To minimize selection bias, home or in-hospital visits are conducted within one month of the planned study visit if subjects cannot attend the outpatient study facility or miss a study visit due to illness or other reasons. Follow-up continued through May 12, 2016. The University of California, San Francisco Committee on Human Research approved the study and written informed consent was obtained from all study participants.
Clinical eligibility for lung transplantation continues to evolve at UCSF. During the study period (and at the time of this report), our program considers adults up to the age of 75 years for lung transplantation. Given the heightened risk of mortality after transplant for older candidates, use of extra-corporeal membrane oxygenation (ECMO) is currently restricted to candidates aged 65 or younger. In general, immunosuppressive and infection prophylaxis strategies after transplant are applied uniformly across age and diagnostic groups.
More detailed descriptions of the multiple HRQL instruments’ properties, a conceptual model of disablement and analytic approaches may be found in the online supplemental materials.
Outcome Variables: HRQL
By definition, HRQL is multidimensional.(31) To evaluate generic HRQL we utilized the Medical Outcomes Survey Short Form-12 version 2 (SF12) Physical and Mental Component Summary scores (SF12-PCS and -MCS, respectively; a change of 5 points is generally considered to meet criteria as the minimally clinically important difference [MCID] in HRQL).(32–34) To evaluate respiratory-specific HRQL, we utilized the Airways Questionnaire 20-Revised (AQ20-R; MCID: 1.75)).(35) To measure health utility, we administered the Euroqol 5D 3L (EQ5D; MCID: 0.06) and Visual Analog Scale (EQVAS; MCID: 10).(36–38) Higher scores indicate better health status.
Confounding and Predictor Variables
We were interested in evaluating the relative association of changes in commonly evaluated clinical measures of transplant efficacy with HRQL. A conceptual model of disablement first proposed by Nagi and later adapted by the Institute of Medicine informed our variable selection (Figure 1; Supplemental Methods).(39, 40) In this model, pathology leads to functional limitations, which are easily quantifiable actions (e.g., distance walked in six minutes). Functional limitations lead to disability, defined as performing activities in daily life (e.g., walking to the grocery store). In this model, disability is an upstream precursor to and determinant of both HRQL and mortality.
Figure 1. Conceptual Model of Disablement.
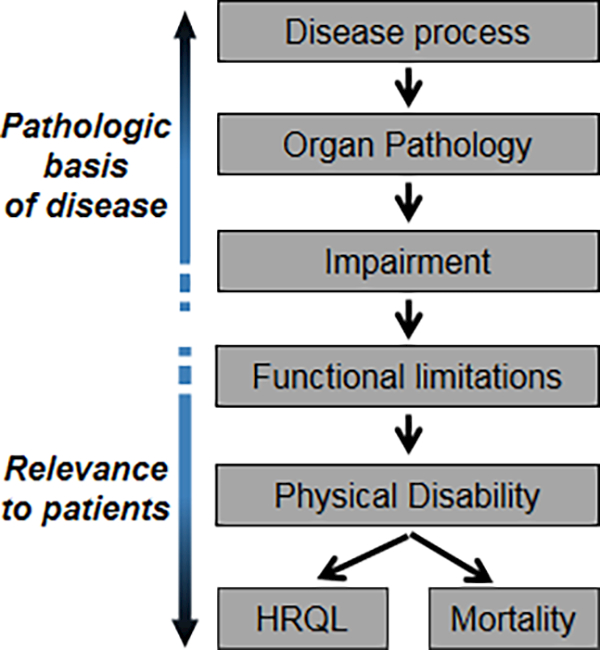
Proposed by Nagi(43), the pathway begins with a disease process that causes organ pathology. As this pathology becomes clinically relevant, organ dysfunction emerges, termed impairment. Impairment, in turn, leads to reductions in actions, termed functional limitations. Functional limitations may then lead to disability, defined as difficulty performing activities in daily life. Disability is an upstream precursor to and determinant of both health-related quality of life (HRQL) and mortality.
Within the context of this model, clinical measures included Forced Expiratory Volume in one second (FEV1, liters), 6MWD (meters), and BMI (kg/m2). We also included age, sex, diagnostic indication for transplant (categorized by the groupings used in the LAS(3)), and LAS for inclusion in our analyses as potential confounders. Baseline demographic and clinical variables were abstracted from medical records. After transplantation, FEV1 and BMI were abstracted from clinical visit records occurring in the same week as research visits. Since 6MWD is not routinely performed for clinical purposes, we performed this test at research study visits in the UCSF Clinical and Translational Sciences Institute Exercise and Body Composition Laboratory after transplantation.
Disability
HRQL in lung transplantation is a multidimensional patient-centered outcome that has many contributors. Among these, physical functioning is a particularly important.(12, 41, 42) Indeed, patients often cite impairment in physical functioning as a primary motivation for considering lung transplantation. Physical disability can impact activities extending far beyond activities of daily living. These activities can include, for example, working or attending school as well as activities that many believe make life meaningful such as spending time with friends and family, other forms of social engagement, and traveling.(43) Although whether lung transplantation improves disability is, in and of itself, an important outcome, we hypothesized that some of the changes in HRQL lung transplantation affords may be accounted for by improved physical disability. To quantify disability across this broad range of activities, we used the Lung Transplant Valued Life Activities scale (LT-VLA).(28) The LT-VLA is a 15-item validated measure of disability across the full spectrum of functioning in lung transplantation.
Analytic Approach
Exploratory plots of HRQL over time identified the need for a piecewise longitudinal modeling approach. Based on visual inspection of the plots for each HRQL measure, “early” effects were defined as changes in HRQL from pre-transplant baseline up to 3 months after transplantation; “delayed” effects were defined as changes from beyond 3 months after transplantation to completion of 3-year follow-up, death, dropout, or the end of the study period, whichever came first.
We estimated the effect of lung transplantation on HRQL using piecewise linear mixed models with random intercepts. To account for survivorship, HRQL was jointly modeled with death, which allows the HRQL component of the model to focus on subjects still living. By jointly modeling HRQL and death, the model is able to distinguish between observable missing HRQL (a subject who is alive but did not complete survey) and unobservable missing HRQL (a subject who has died). In Model 1, we included piecewise terms to allow for different coefficients for time (early versus delayed), pre-transplant age, sex, diagnostic group, LAS, BMI, FEV1, and 6MWD as fixed effects. Since the effect of lung transplant on HRQL might vary based on age or disease indication, we performed secondary analyses stratified by age groups defined a priori (18–49, 50–64, 65+) with ordinal tests of trend and by diagnostic groups (COPD, pulmonary arterial hypertension [PAH], cystic fibrosis [CF], and pulmonary fibrosis [PF]) with tests of differences. Model testing for each HRQL instrument demonstrated stability in the key parameter estimates of study interest whether including age and diagnostic group together or separately, thus supporting that there were not substantive issues with collinearity between these two variables. To determine the extent to which change in HRQL was mediated by improvements in disability, Model 2 added LT-VLA as a random effect to Model 1 and the difference in estimates was compared. We also supplementally checked our mediation modeling using a validated four-step method.(44)
To evaluate the effect of changes in BMI, FEV1, and 6MWD on changes in HRQL over the same time period, we utilized linear mixed models jointly modeled with death. We evaluated the univariate effect of each variable and then combined them in multivariate models. Changes in each variable were scaled to its clinically meaningful difference: 2.2 units for BMI (0.5 its standard deviation), 200 milliliters in FEV1, and 30 meters in 6MWD.
Finally, given the importance of one-year survival after lung transplantation, we determined the proportion of subjects who were alive but did not derive a HRQL benefit at one-year from the date of transplant. We defined HRQL benefit as an improvement in magnitude at least twice its MCID, an approach previously taken in other major thoracic surgery.(45) We tested differences in baseline characteristics between those who improved and those who did not by Fisher’s exact or Wilcoxon rank-sum tests. As a secondary analysis, we defined HRQL as an improvement in magnitude of one time its MCID and also determined the proportion of subjects whose HRQL worsened at one-year compared to before transplant.
Not all subjects completed all study visits. While mixed models can handle occasional missing data, this advantage applies only to data missing at random. We deemed survey data to be missing at random if subjects did not complete surveys for reasons other than their health and, on review of their concurrent clinical status, were not hospitalized, had stable allograft function, and were not dealing with acute medical issues (e.g., missing data because the clinic appointment ran late and the subject needed to leave promptly to avoid traffic or if a coordinator was not available). Surveys were deemed missing not at random if subjects were too ill to complete the survey. As a sensitivity analysis, we assigned subjects with non-random missing data (including those who dropped out) the median of the lowest quartile of HRQL scores for all other participants at that time point.
We addressed survivorship through joint modeling rather than by other techniques such as assigning those who died the worst HRQL possible or imputation. This approach is more relevant to data intended for clinical counseling because it provides more accurate estimates of the effect of lung transplantation on HRQL for patients still living. It is less useful for estimating of the effect of transplant on population-level HRQL.
Uniquely, the EQ5D allows for explicit accounting for death by assigning those who died a score of 0, but allowing the living to self-rate their utility state even lower than that. As EQ5D-specific sensitivity analysis, we reanalyzed the effect of lung transplant on EQ5D by linear mixed models, assigning subjects a score of 0 for all time points following their date of death. It should be noted that this particular analytic approach provides population-level estimates of EQ5D HRQL than estimates relevant for patient-specific clinical counseling.
Analyses were conducted using SAS 9.4.
RESULTS
Of the 228 patients eligible for this study, 17 declined to participate. Among the 211 subjects enrolled during the study period (93% consent rate), 46% were female; the median age, 58 years (interquartile range [IQR]: 48–64); and the median LAS, 45.2 (IQR: 37.8–64.7) (Figure 2; Table 1). Of the cohort, 24% were aged 65 or older; 33% were already hospitalized at the time of transplantation (11% of the overall cohort were intubated and 9% received extra-corporeal membrane oxygenation support). At baseline, older subjects reported better SF12-MCS and AQ-20R HRQL compared to younger subjects (p-values 0.01 and 0.005, respectively) but similar SF12-PCS, EQ5D and EQ VAS based HRQL. Over the study period, six subjects dropped out (3%), 31 died (14%), and 40 developed chronic lung allograft dysfunction (19%; [CLAD]) defined by a validated spirometry-based approach that considers both FEV1 as well as FVC.(46, 47) The overall survey completion rate was 86%; rates ranged from 71% to 92% across time points (Table S1).
Figure 2.
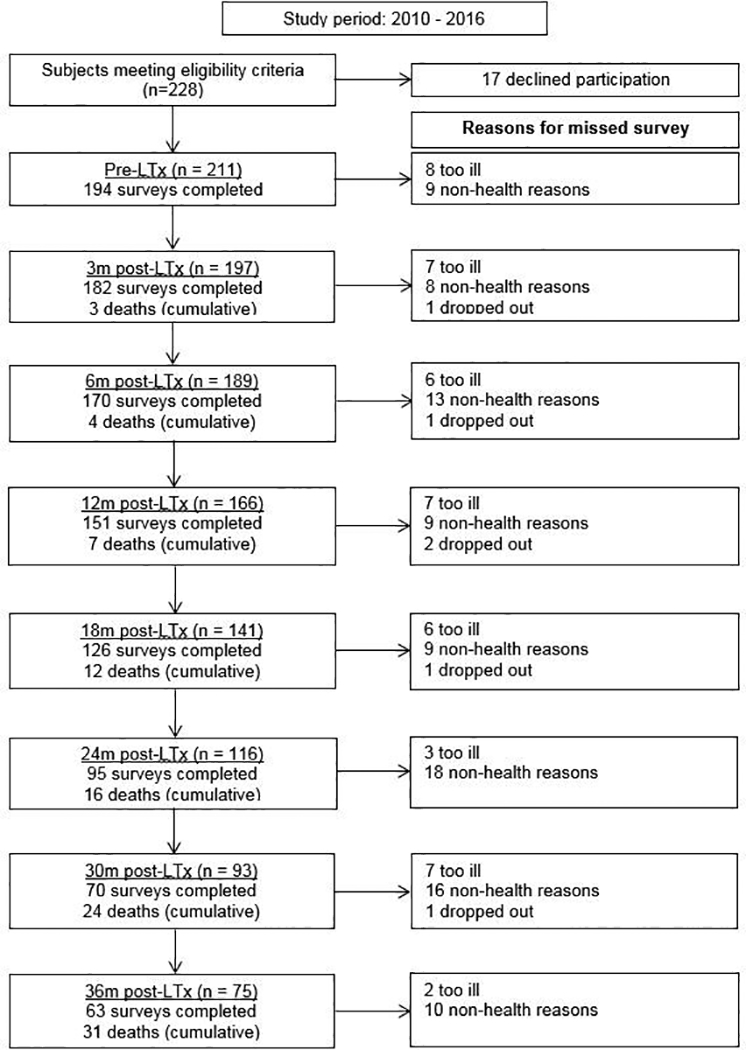
Flow chart of transplanted study participants over the duration of the study. LTx = lung transplantation. Study number in the left column represents the number of subjects providing data for analysis at each time point (deaths accounted for in analytic approach). Study number in the right column explains reasons for missed surveys at each time point.
Table 1.
Demographics and baseline characteristics among 211 transplanted study participants
| Overall | COPD | PAH | CF | PF | |
|---|---|---|---|---|---|
| n=211 | n=36 | n=8 | n=19 | n=148 | |
| Age (years) | 58 (48, 64) | 63 (56, 65) | 47 (38, 52) | 28 (24, 40) | 60 (52, 65) |
| Age group | |||||
| 18–49 | 56 (27%) | 3 (8%) | 6 (75%) | 19 (100%) | 28 (19%) |
| 50–64 | 103 (49%) | 20 (56%) | 2 (25%) | 0 (0%) | 81 (55%) |
| 65+ | 52 (25%) | 13 (36%) | 0 (0%) | 0 (0%) | 39 (26%) |
| Female | 97 (46%) | 20 (56%) | 5 (63%) | 11 (58%) | 61 (41%) |
| BMI (kg/m2) | 25.5 (22.1, 28.5) | 24.1 (21.2, 27.6) | 25.2 (20.8, 27.7) | 19.1 (18.3, 21.1) | 26.5 (23.4, 29.4) |
| Hospitalized at transplant** | 68 (33%) | 7 (19%) | 1 (13%) | 7 (39%) | 53 (36%) |
| Intubated at transplant | 22 (11%) | 2 (6%) | 0 (0%) | 4 (22%) | 16 (11%) |
| ECMO at transplant | 19 (9%) | 1 (3%) | 1 (13%) | 2 (11%) | 15 (10%) |
| FEV1 | 1.31 (0.86, 1.86) | 0.61 (0.42, 1.05) | 1.87 (1.05, 2.49) | 0.78 (0.54, 0.95) | 1.61 (1.11, 2.03) |
| FEV1 % Predicted | 45 (28, 61) | 24 (15.5, 42) | 54 (39, 66) | 23 (16, 28) | 51 (39, 64) |
| 6MWD (m) | 254 (151, 365) | 247 (139, 320) | 308 (173, 529) | 348 (171, 472) | 247 (153, 366) |
| LAS | 45.2 (38.1, 64.4) | 35.9 (33.0, 39.8) | 38.6 (34.7, 49.6) | 40.3 (38.5, 48.6) | 52.4 (40.8, 70.4) |
| SF12 PCS | 22.9 (16.5, 26.8) | 22.0 (18.4, 28.3) | 25.3 (22.8, 31.1) | 24.1 (14.8, 25.9) | 22.9 (16.1, 25.7) |
| SF12 MCS | 50.6 (40.6, 56.7) | 52.6 (47.3, 58.5) | 48.8 (28.9, 62.3) | 44.1 (37.8, 52.6) | 50.9 (40.6, 57.1) |
| AQ20-R | 6 (4, 9) | 6 (3, 11) | 7 (2, 12) | 5 (4, 7) | 7 (4, 9) |
| EQ5D | 0.69 (0.57, 0.78) | 0.69 (0.59, 0.79) | 0.79 (0.60, 0.82) | 0.60 (0.44, 0.78) | 0.69 (0.55, 0.78) |
| VAS | 40 (25, 60) | 50 (30, 65) | 45 (40, 70) | 29 (20, 30) | 40 (25, 60) |
Data presented as n (%) or median (IQR).
Diagnosis categories used for calculation of the Lung Allocation score.
Subject was hospitalized at the time of receiving a donor offer for transplant. COPD = Chronic Obstructive Pulmonary Disease; PAH = Pulmonary Arterial Hypertension; CF = Cystic Fibrosis; PF = Pulmonary Fibrosis; BMI = Body Mass Index; 6MWD = six-minute walk distance; ECMO = extra-corporeal membrane oxygenationFEV1 = forced expiratory capacity in 1 second; LAS = Lung Allocation Score; SF12 PCS = Medical Outcomes Study Short Form 12 Physical Component Summary scale; SF12 MCS = Medical Outcomes Study Short Form 12 Mental Component Summary scale; AQ20-R = Airway Questionairre 20 - revised; EQ5D = Euroqol 5D; VAS = Euroqol Visual Analog Scale
Generally, lung transplantation was associated with improvements in HRQL that achieved three to four times each instrument’s MCID; the SF12-MCS was the exception for which improvements minimally exceeded its MCID (Table 2). While improvements were generally observed by 3 months, improvements in SF12-PCS continued through 6 months (Figure 3). After early improvements, HRQL was stable overall or declined by clinically negligible amounts in survivors for the remainder of the follow-up period. For example, on average, subjects experienced a 15-point improvement in SF12-PCS within the first 3 months after transplantation (95%CI, 13.4–16.7; MCID = 5). The decline thereafter was −0.2 points (95%CI, −0.4 – 0.05). Sensitivity analysis with imputed scores for missing surveys did not appreciably change these effect estimates (data not shown). Effect estimates for EQ5D were attenuated in analyses assigning subjects who died a score of 0, especially in older subjects (Table 3; Tables S2, S3).
Table 2.
The effect of lung transplantation on HRQL and Valued Life Activities (LT-VLA) disability as a mediator of this effect
| SF12-PCS (MCID = 5) |
SF12-MCS (MCID = 5) |
AQ20-R (MCID = 1.75) |
EQ5D (MCID = 0.06) |
EQVAS (MCID =10) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early | Delayed | Early | Delayed | Early | Delayed | Early | Delayed | Early | Delayed | |
| Model 1 | 15.0 (13.4, 16.7) |
−0.2 (−0.4, 0.05) |
4.9 (3.5, 6.4) |
−0.4 (−0.6, −0.2) |
7.9 (7.4, 8.4) |
−0.03 (−0.10, 0.04) |
0.17 (0.14, 0.20) |
−0.004 (−0.007, −0.0003) |
30.4 (27.5, 33.3) |
−0.4 (−0.8, −0.1) |
| Model 2 | 4.9 (3.2, 6.6) |
−0.1 (−0.4, 0.05) |
−1.0 (−2.5, 0.6) |
−0.4 (−0.5, −0.2) |
5.4 (4.8, 6.0) |
−0.01 (−0.08, 0.05) |
0.007 (−0.018, 0.032) |
−0.003 (−0.006, −0.0004) |
18.3 (15.2, 21.3) |
−0.4 (−0.7, −0.1) |
Early: From before to 3 months after transplant Delayed: 3 months after transplant to censoring which is completion of 3-year follow-up, death, dropout, or the end of the study period, whichever came first. Effect estimates reflect average change in HRQL over the early or late time period.
Model 1: Adjusted for baseline age, sex, diagnosis, baseline body mass index, forced expiratory volume in 1 second, six minute walk distance, and Lung Allocation Score; Model 2: Model 1 + change in Lung Transplant Valued Life Activities (LT-VLA) disability over time. Model 2 reflects the average change in HRQL that is not accounted for by change in LT-VLA disability.
SF12-PCS = Medical Outcomes Study Short Form 12 Physical Component Summary scale ; SF12-MCS = Medical Outcomes Study Short Form 12 Mental Component Summary scale; AQ20-R = Airway Questionnaire 20 - Revised; score reversed for ease of interpretation); EQ5D = Euroqol 5D; EQVAS = Euroqol Visual Analog Scale; MCID = Minimally Clinically Important Difference
Figure 3.
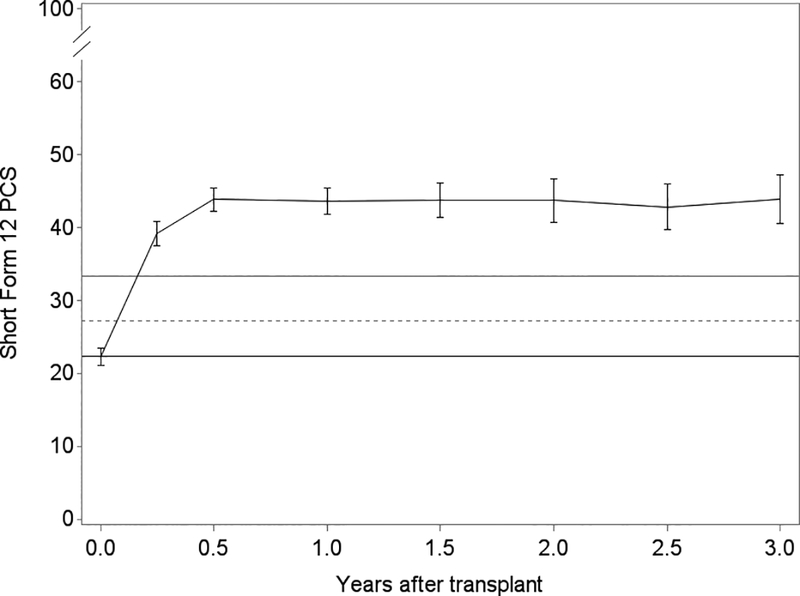
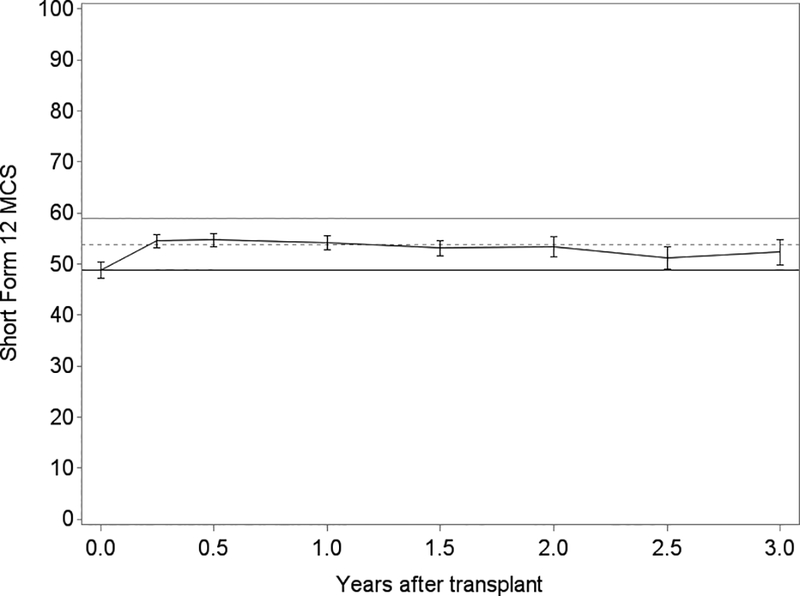
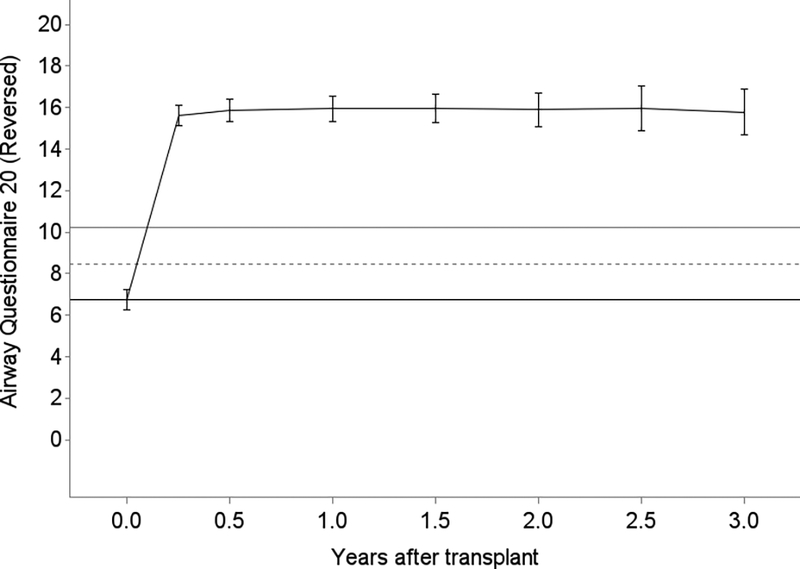
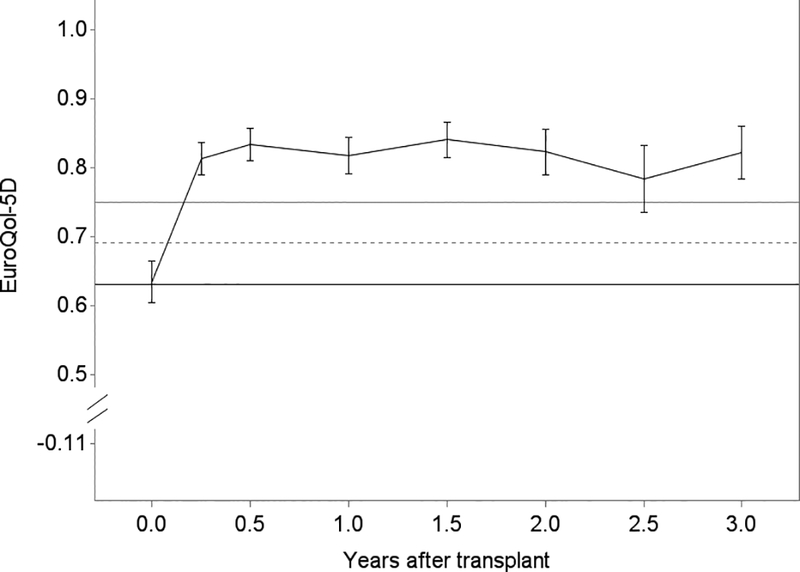
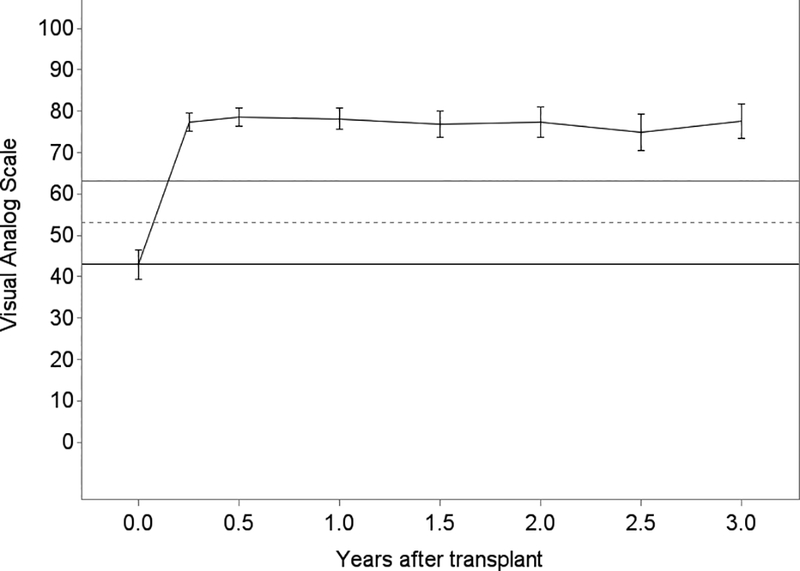
Unadjusted plots of average health-related quality of life (HRQL) from before transplant to up to 3 years after lung transplantation for Panel A. SF12 Physical Component Summary (PCS); Panel B. SF12 Mental Component Summary (MCS); Panel C. Airway Questionnaire 20-revised; Panel D. Euroqol 5D; and Panel E. Euroqol Visual Analog Scale. The plotted line reflects the mean score and whisker bars reflect the bounds of the 95% confidence intervals at each time point. On the Y-axis, the first solid horizontal line marks the baseline mean; the dashed horizontal line reflects the minimally clinically important difference (MCID) and the solid line reflects twice the MCID. The number of subjects who contributed HRQL data, missed survey responses, and died at each time point is shown in Table S1.
Table 3.
Effect of lung transplantation on EQ5D by age group, assigning a score of 0 to those who died
| Early | Test of trend for early change |
Delayed | ||
|---|---|---|---|---|
| EQ5D (MCID = 0.06) |
18–49 | 0.19 (0.12, 0.26) | p = 0.035 | −0.02 (−0.03, −0.01) |
| 50–64 | 0.16 (0.10, 0.22) | −0.03 (−0.03, −0.02) | ||
| 65+ | 0.08 (0.02, 0.15) | −0.02 (−0.03, −0.01) |
Adjusted for sex, diagnosis, baseline body mass index, forced expiratory volume in 1 second, six minute walk distance and Lung Allocation Score
Early: From before to 3 months after transplant Delayed: 3 months after transplant to censoring which is completion of 3-year follow-up, death, dropout, or the end of the study period, whichever came first. Effect estimates reflect average change in HRQL over the early or late time period.
COPD = Chronic Obstructive Pulmonary Disease; PAH = Pulmonary Arterial Hypertension; CF = Cystic Fibrosis; PF = Pulmonary Fibrosis; BMI = Body Mass Index; 6MWD = six-minute walk distance; FEV1 = forced expiratory capacity in 1 second; LAS = Lung Allocation Score; EQ5D = Euroqol 5D
A substantial proportion of the effect of lung transplantation on HRQL was accounted for by improvements in disability. After accounting for improvements in LT-VLA disability, lung transplantation was associated with improvements in SF12-PCS, AQ20-R, and EQVAS HRQL that reached only one to two times the MCID and the changes in SF12-MCS and EQ5D were no longer significant (Table 2).
Notable differences emerged in the age-stratified analyses. After controlling for diagnosis and other factors, older subjects (age ≥ 65) had substantially smaller improvements in generic HRQL compared to younger subjects (test of trend p-value= 0.05) (Table 4). For the SF12-MCS, older subjects experienced no improvement, whereas those in the in the two younger strata improved by 1.5 times the MCID (test of differences p-value = 0.002). Although older subjects experienced smaller improvements in respiratory-specific HRQL and health utility than younger subjects, these differences were not statistically significant.
Table 4.
The effect of lung transplantation on HRQL by age group
| Early change | Test of trend | ||
|---|---|---|---|
| SF12-PCS (MCID = 5) |
18–49 | 17.7 (14.8, 20.6) | P=0.05 |
| 50–64 | 14.3 (11.8, 16.7) | ||
| 65+ | 13.4 (10.0, 16.7) | ||
| SF12-MCS (MCID = 5) |
18–49 | 6.4 (3.9, 8.8) | P=0.002 |
| 50–64 | 6.5 (4.3, 8.6) | ||
| 65+ | 0.2 (−2.8, 3.3) | ||
| AQ20-R (MCID = 1.75) |
18–49 | 8.3 (7.3, 9.2) | P=0.122 |
| 50–64 | 8.2 (7.4, 9.0) | ||
| 65+ | 7.1 (6.1, 8.1) | ||
| EQ5D (MCID = 0.06) |
18–49 | 0.20 (0.15, 0.25) | P=0.124 |
| 50–64 | 0.17 (0.13, 0.21) | ||
| 65+ | 0.14 (0.09, 0.19) | ||
| EQVAS (MCID = 10) |
18–49 | 33.2 (27.6, 38.9) | P=0.174 |
| 50–64 | 30.8 (26.7, 34.9) | ||
| 65+ | 27.2 (21.5, 33.0) | ||
For age group 18 – 49, n = 56; 50–64, n = 103; n = 52. Adjusted for sex, diagnosis, baseline body mass index, forced expiratory volume in 1 second, six minute walk distance and Lung Allocation Score
Early: Average change from before to 3 months after transplant. Effect estimates reflect average change in HRQL over the early or late time period.
COPD = Chronic Obstructive Pulmonary Disease; PAH = Pulmonary Arterial Hypertension; CF = Cystic Fibrosis; PF = Pulmonary Fibrosis; BMI = Body Mass Index; 6MWD = six-minute walk distance; FEV1 = forced expiratory capacity in 1 second; LAS = Lung Allocation Score; SF12-PCS = Medical Outcomes Study Short Form 12 Physical Component Summary scale; SF12-MCS = Medical Outcomes Study Short Form 12 Mental Component Summary scale; AQ20-R = Airway Questionnaire 20- Revised; score reversed for ease of interpretation); EQ5D = Euroqol 5D; EQVAS = Euroqol Visual Analog Scale
Changes in HRQL differed significantly by condition in the diagnosis-stratified analyses (Table 5). After controlling for age and other cofactors, participants with cystic fibrosis experienced the largest improvements in HRQL across all measures (p ≤ 0.021). Improvements were generally similar in those with pulmonary fibrosis or COPD and were the smallest in pulmonary hypertension.
Table 5.
Effect of lung transplantation on HRQL by disease category
| Early change | Test of difference |
||
|---|---|---|---|
| SF12-PCS (MCID = 5) |
Group A (COPD) | 15.9 (11.5, 20.3) | P<0.001 |
| Group B (PAH) | 7.9 (1.0, 14.7) | ||
| Group C (CF) | 23.8 (19.5, 28.1) | ||
| Group D (PF) | 13.8 (11.9, 15.8) | ||
| SF12-MCS (MCID = 5) |
Group A (COPD) | 2.7 (−0.9, 6.4) | P=0.020 |
| Group B (PAH) | 0.1 (−5.6, 5.7) | ||
| Group C (CF) | 10.3 (6.4, 14.1) | ||
| Group D (PF) | 4.8 (3.1, 6.6) | ||
| AQ20-R (MCID = 1.75) |
Group A (COPD) | 7.7 (6.4, 9.1) | P=0.021 |
| Group B (PAH) | 4.5 (2.1, 6.9) | ||
| Group C (CF) | 9.4 (8.2, 10.6) | ||
| Group D (PF) | 7.9 (7.3, 8.6) | ||
| EQ5D (MCID = 0.06) |
Group A (COPD) | 0.15 (0.08, 0.21) | P=0.003 |
| Group B (PAH) | 0.07 (−0.05, 0.19) | ||
| Group C (CF) | 0.30 (0.22, 0.39) | ||
| Group D (PF) | 0.16 (0.13, 0.19) | ||
| EQVAS (MCID = 10) |
Group A (COPD) | 23.3 (16.2, 30.5) | P=0.003 |
| Group B (PAH) | 18.4 (2.6, 34.2) | ||
| Group C (CF) | 43.0 (36.8, 49.3) | ||
| Group D (PF) | 30.8 (27.4, 34.3) | ||
For Group A, n = 36, Group B, n = 8, Group C, n = 19, Group D, n = 148) Adjusted for sex, age group, baseline body mass index, forced expiratory volume in 1 second, six minute walk distance and Lung Allocation Score
Early: Average change from before to 3 months after transplant. Effect estimates reflect average change in HRQL over the early or late time period. COPD = Chronic Obstructive Pulmonary Disease; PAH = Pulmonary Arterial Hypertension; CF = Cystic Fibrosis; PF = Pulmonary Fibrosis; BMI = Body Mass Index; 6MWD = six-minute walk distance; FEV1 = forced expiratory capacity in 1 second; LAS = Lung Allocation Score; SF12-PCS = Medical Outcomes Study Short Form 12 Physical Component Summary scale; SF12-MCS = Medical Outcomes Study Short Form 12 Mental Component Summary scale; AQ20-R = Airway Questionnaire 20- Revised; score reversed for ease of interpretation); EQ5D = Euroqol 5D; EQVAS = Euroqol Visual Analog Scale
Next, we examined the effect of potential determinants of HRQL. Although statistically significant, changes in BMI, FEV1, and 6MWD did not independently explain a clinically relevant amount of the changes in HRQL (Table S5). Although the absolute differences were small, improvement in FEV1 was associated with larger point estimates of improvement in AQ20-R relative to BMI and 6MWD, whereas improvement in 6MWD was associated with larger improvements in EQ5D.
Finally, a substantial subset of subjects did not achieve improved HRQL one-year after lung transplantation (Table 6). Depending on the metric, the proportion failing to improve by twice the MCID ranged from 11% to 60%. Across measures, there were inconsistent differences in baseline factors between those who improved and those who did not (Table 7). In EQ5D, for example, those who improved had lower BMI, higher LAS scores, shorter 6MWD, and were hospitalized at the time of transplantation, whereas in AQ20-R those who did not improve had better pre-transplant lung function. A relatively small proportion of subjects reported worse HRQL at one-year after lung transplantation compared to before, most notably in SF12-MCS and EQ5D HRQL measures (Table S5)
Table 6.
Proportion of subjects without improved HRQL at 1-year after lung transplantation defined by either one or two times the minimally clinically important difference
| Not improved by at least the MCID |
Not improved by two times or greater the MCID |
|
|---|---|---|
| SF12-PCS | 11 (8%) | 30 (21%) |
| SF12-MCS | 65 (46%) | 84 (60%) |
| AQ20-R | 8 (6%) | 16 (11%) |
| EQ5D | 50 (35%) | 60 (43%) |
| EQVAS | 12 (11%) | 27 (25%) |
MCID = Minimally Clinically Important Difference; SF12-PCS = Short Form 12 Physical Component Summary scale, MCID=5; SF12-MCS = Short Form 12 Mental Component Summary scale, MCID=5; AQ20-R = Airway Questionnaire 20 – revised, MCID=1.75; EQ5D = Euroqol 5D, MCID=0.06; EQVAS = Euroqol Visual Analog Scale, MCID=10
Table 7.
Differences in baseline characteristics in subjects with improved HRQL 1-year after lung transplantation compared to those who did not improve
| SF12-PCS | SF12-MCS | AQ20-R | EQ5D | EQVAS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Not improved |
Improved | Not Improved |
Improved | Not Improved |
Improved | Not Improved |
Improved | Not improved |
Improved | ||
| N (%) | 30 (21%) | 110 (79%) | 93 (66%) | 47 (34%) | 16 (11%) | 125 (89%) | 60 (43%) | 81 (57%) | 27 (25%) | 82 (75%) | |
| Diagnosis | * | * | |||||||||
| Group A (COPD) | 7 (23%) | 16 (15%) | 21 (23%) | 2 (4%) | 1 (6%) | 22 (18%) | 14 (23%) | 9 (11%) | 6 (22%) | 11 (13%) | |
| Group B (PAH) | 2 (7%) | 3 (3%) | 4 (4%) | 1 (2%) | 3 (19%) | 2 (2%) | 5 (8%) | 0 (0%) | 2 (7%) | 2 (2%) | |
| Group C (CF) | 3 (10%) | 11 (10%) | 8 (9%) | 6 (13%) | 0 (0%) | 14 (11%) | 3 (5%) | 11 (14%) | 0 (0%) | 11 (13%) | |
| Group D (PF) | 18 (60%) | 80 (73%) | 60 (65%) | 38 (81%) | 12 (75%) | 87 (70%) | 38 (63%) | 61 (75%) | 19 (70%) | 58 (71%) | |
| BMI | 25.4 (23.9, 29.5) |
25.7 (21.4, 28.3) |
26.2 (22.8, 28.7) |
24.4 (22.1, 27.5) |
28.0 (23.3, 29.8) |
25.5 (22.2, 28.2) |
26.7 (23.1, 30.3)* |
24.4 (21.1, 27.5)* |
26.2 (24.2, 30.4) |
25.1 (21.4, 28.1) |
|
| LAS | 40.5 (35.4, 62.4) |
46.3 (39.4, 63.6) |
44.1 (36.4, 55.8)* |
56.9 (42.8, 79.0)* |
39.0 (35.0, 55.9) |
46.5 (39.6, 65.4) |
40.5 (35.4, 52.7)* |
52.4 (40.4, 73.7)* |
42.9 (37.3, 56.1) |
48.4 (40.0, 67.0) |
|
| FEV1 | 1.33 (0.91, 2.30) |
1.30 (0.89, 1.73) |
1.22 (0.89, 1.73) |
1.46 (0.93, 1.80) |
2.17 (1.15, 2.48)* |
1.29 (0.85, 1.72)* |
1.33 (0.95, 2.13) |
1.30 (0.87, 1.69) |
1.47 (0.91, 2.00) |
1.31 (0.85, 1.74) |
|
| 6MWD | 261 (149, 363) |
281 (150, 377) |
305 (191, 375)* |
215 (95, 366)* |
325 (223, 365) |
278 (146, 376) |
320 (247, 376)* |
219 (112, 370)* |
280 (222, 366) |
289 (155, 379) |
|
| Hospitalized at transplant** |
8 (27%) | 33 (30%) | 20 (21%)* | 21 (45%)* | 2 (13%) | 39 (31%) | 7 (12%)* | 34 (42%)* | 6 (22%) | 28 (34%) | |
|
Intubated at transplant |
2 (7%) | 12 (11%) | 7 (8%) | 7 (15%) | 0 (0%) | 14 (11%) | 2 (3%)* | 12 (15%)* | 2 (3%) | 12 (15%) | |
Data presented as median (IQR)
Improvement defined as increase by twice the minimally clinically important difference for each HRQL instrument
Differences that were statistically significant noted by bold and asterisk.
Subject was already hospitalized at the time of transplant (e.g., not called in from home for transplant surgery)
COPD = Chronic Obstructive Pulmonary Disease; PAH = Pulmonary Arterial Hypertension; CF = Cystic Fibrosis; PF = Pulmonary Fibrosis; BMI = Body Mass Index; 6MWD = six-minute walk distance; FEV1 = forced expiratory capacity in 1 second; LAS = Lung Allocation Score; SF12-PCS = Medical Outcomes Study Short Form 12 Physical Component Summary scale (MCID = 5) ; SF12-MCS = Medical Outcomes Study Short Form 12 Mental Component Summary scale (MCID = 5) ; AQ20-R = Airway Questionnaire 20 - revised (MCID = 1.75); EQ5D = Euroqol 5D (MCID = 0.06) ; EQVAS = Euroqol Visual Analog Scale (MCID =0.06); deaths were assigned a score of 0.
DISCUSSION
In this study of the effect of lung transplantation in the era of the LAS, we found that lung transplantation delivers large and clinically meaningful improvements in HRQL to most adults with advanced lung disease. The improvements were generally 3–4 times the magnitude what are considered to meet minimally clinically important differences. Further, improvements were achieved within the first six-months after surgery and were durable - among survivors - for up to three-years. Amelioration in disability appeared to account for a substantial proportion of this improvement. Although all age groups and disease types experienced substantial HRQL gains, some (most notably, older persons) did not benefit to the same degree. Moreover, a subset of patients failed to improve to a clinically meaningful degree at the one-year post-transplant mark and those who did not survive experienced a sharp fall-off in HRQL prior to death.
The durability of the improvements in generic and disease-specific HRQL in survivors was encouraging and somewhat surprising. Other studies (from the pre-LAS era) have identified a slow, relentless HRQL decline after the first post-operative year, although rarely declining to the low levels observed before lung transplantation.(33, 48, 49) Further, we found that improvements were achieved within the first 3 post-operative months, which contrasts to prior studies that found improvements continuing through the first year.(49, 50)
Our findings have immediate clinical implications. These data provide information desired by patients considering lung transplantation today to make more fully informed decisions. Equally relevant, these data provide clinicians with information to guide counseling tailored to specific age and diagnostic groups. Also, given the increasingly common scenario of transplanting acutely ill patients, it is reassuring that patients hospitalized in the run-up to transplant have the same likelihood of improved HRQL as those who are called in from home.
Our study identifies other important issues. In our cohort, patients age 65 or older derived substantially less HRQL benefit than younger patients. It is known that patients older than 65 have a median survival time after lung transplantation of only 3.5 years- fully 3 years less than for patients younger than 50.(51) We also found that routinely collected clinical measures, including BMI, lung function, and exercise capacity, did not explain a substantial degree of changes in HRQL. Higher baseline HRQL scores among older compared to younger subjects, which only differed statistically for two of the five measures, are unlikely to account for the smaller degree of improvement observed for that stratum across all the HRQL measures. Taken together, these findings identify the need for novel measures and approaches to deliver both improved HRQL and survival, especially to those of older age. Emerging work suggests that constructs relevant in older populations such as frailty, depression, and cognitive impairment may be important.(10, 11, 19, 20)
Importantly, our data should not be used to exclude older patients from lung transplantation for lack of benefit on an individual level. While they derived less benefit relative to younger patients, the benefits across measures were, nevertheless, two to three times what is considered to be minimally important. The benefit of lung transplantation in terms of HRQL also differed by diagnostic group, even after accounting for age. Reasons for this differential benefit are not clear. Efforts to understand why some groups, such as cystic fibrosis, derive larger benefits may inform interventions to improve HRQL in others.(45, 48, 52)
Our LT-VLA data and recent investigations focused on other individual health domains important in lung transplantation (e.g., depression and anxiety(9, 10, 14, 19, 21), physical functioning/disability(12, 17, 28), symptom burden(16, 18), and cognitive impairment(13, 15, 22)) suggest that increased attention to individual health domains may ultimately yield more informative data that can be used to design interventions to improve HRQL.
Our study also has policy implications. U.S. organ allocation is based on the LAS, a system that defines “transplant benefit” by one-year survival alone. This definition, however, falls far short of reflecting the full range of clinical benefits lung transplantation strives to deliver. How patient-centered outcomes can (or should) best be incorporated to more comprehensively quantify transplant benefit remains a topic of ongoing debate.(53, 54) Depending on the metric applied, the substantial variation in the proportion of subjects failing to achieve a HRQL benefit emphasizes the importance of choosing the right instrument to quantify HRQL. As we show in this manuscript, the influence of mortality on HRQL in instruments such as the EQ5D varies depending on the scoring approach employed. Lastly, instrument selection can be challenging since brevity may come at the expense of content validity and missed conceptual health domains of importance to lung transplant recipients. Ongoing efforts to meet this challenge may help to more fully realize the true clinical aims of lung transplantation.(42, 48, 52)
Comparing our findings to a recent large study of HRQL in patients undergoing lung transplant in Canada – a country with an allocation system different from the LAS – raises additional questions.(23) While subjects in both that cohort and ours experienced similar improvements in SF12-PCS defined HRQL, we observed substantially smaller improvements in EQ5D and EQVAS in absolute terms. Further, we observed that older patients had significantly less improvement in SF12 whereas the effect of increasing age on HRQL in the Canada cohort was negligible. Reasons for these differences are not clear but may be important. If they reflect center-specific findings, this suggests that HRQL could be improved by adopting practices from high performing centers. Alternatively, our cohort participants were older, more likely to have pulmonary fibrosis, and included a greater proportion hospitalized at the time of transplantation. It is possible that these differences reflect trends in the U.S. following the adoption of the LAS.(7, 8) Indeed, in the U.S., the transplant surgery hospitalization now exceeds one month for 25% of recipients and over 50% are discharged to places other than home without skilled support (e.g., skilled nursing facilities).(7) This morbidity could plausibly effect HRQL measures that emphasize pain, functional status, mental health, or one’s overall state of well-being. If this is indeed the case, our findings further underscore how important assessing metrics of transplant efficacy other than one-year survival are to fully maximizing the individual and societal benefit of lung transplantation.
The limitations of our findings should be kept in view. Our data were based on a relatively modest sized cohort from a single center with follow-up only up to three years. While our study is one of the largest to focus on HRQL, its limitations in size and duration nonetheless are relevant. Indeed, only 20% of our cohort developed CLAD during the study period, which is associated with poorer HRQL. Further, the instruments used to measure HRQL in our study were not developed specifically for use in lung transplantation. While some have been validated in this population, all fail to some extent to measure certain health domains important in lung transplant recipients.(18, 42, 48, 55, 56) Although we repeatedly measured HRQL, the state or trajectory of HRQL in between sampling periods is unknown and estimates might have been different with different sampling timeframes. Also, although we considered disability as an upstream predictor of HRQL, the SF12-PCS, AQ20-R, and EQ5D all include items that do query physical functioning. Thus, the constructs of impairment, disability and HRQL are difficult to completely disentangle. Notably, changes in SF12-MCS were small relative to the other HRQL measures. Although our findings are consistent with prior literature(54), it is not clear whether this is attributable to high baseline scores in our cohort at or near the population norm, insensitivity of the instrument as a mental health measure, or emergence of incident problems such as depression and anxiety after transplant. Finally, our modeling approach to address survivorship bias is most relevant for patient-specific clinical decision making and counseling (e.g., “If you survive lung transplantation, you might reasonably expect your HRQL to be X at 1-year, Y at 2-years, and Z at 3-years”). This approach, however, limits our ability to provide estimates on the effect of lung transplantation on population-level HRQL.
Counterbalancing these limitations are the notable strengths of our approach. By quantifying HRQL before and repeatedly after lung transplantation for up to three years of follow-up, performing multivariate adjustments, evaluating HRQL across several conceptual health domains, explicitly attempting to account for selection and survivorship bias, and limiting dropout, we were able to address important limitations in the existing literature.(8, 42) We also provided effect estimates of the effect of lung transplantation across clinically relevant age and diagnostic groups, novel insights into determinants of change in HRQL after transplantation, and findings on differences in HRQL benefit depending on the instrument employed. Most saliently, our study population reflects the types of patients undergoing lung transplantation today.
In summary, our study provides contemporary evidence that lung transplantation in the LAS era affords adults with advanced lung disease large and durable improvements in HRQL across age groups and diagnoses. These improvements are largely mediated by amelioration of disabilities affecting valued life activities. Despite these gains, a notable subset of patients appears to be left behind in improved HRQL. Multipronged and multi-center efforts to identify valid and patient-centered metrics of assessing transplant efficacy will help the lung transplant community maintain its focus on the outcomes that matter most to patients and society.
Supplementary Material
Acknowledgments
The authors greatly appreciate the patients who have and continue to participate the UCSF Breathe Again Study.
National Heart, Lung, and Blood Institute (Grant K23 HL111115, JPS). A portion of this study was supported by the National Center for Advancing Translational Sciences at the National Institutes of Health (UCSF-CTSI UL1 RR024131).
Abbreviations
- 6MWD
Six minute walk distance
- AQ20-R
Airway Questionnaire 20 – Revised
- BMI
Body Mass Index
- CF
Cystic Fibrosis
- COPD
Chronic Obstructive Pulmonary Disease
- ECMO
Extra-corporeal membrane oxygenation
- EQ5D
Euroqol 5D
- EQVAS
Euroqol Visual Analog Scale
- FEV1
Forced expiratory volume in one second
- HRQL
Health-related quality of life
- LAS
Lung allocation score
- LT
Lung transplantation
- LT-VLA
Lung Transplant Valued Life Activities scale
- MCID
Minimally Clinically Important Difference
- PAH
Pulmonary arterial hypertension
- PF
Pulmonary fibrosis
- SF12 PCS
Medical Outcomes Survey Short Form-12 Physical Component Summary
- SF12 MCS
Medical Outcomes Survey Short Form-12 Mental Component Summary
Footnotes
AUTHOR CONTRIBUTIONS
- JPS, PPK, and PDB made substantial contributions to the conception and design of the work.
- JPS wrote the first draft of the manuscript.
- JPS and DH performed the statistical analyses.
- JPS, PPK, AS, PS, DH, JH, SH, MM, JRG, JG, JK, RJS, PDB made substantial contributions to the acquisition, analysis, or interpretation of data for the work.
- All authors approved the manuscript.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Table S1: Survey completion rates, deaths, and dropouts over the study period (2010 – 2016)
Table S2: The effect of lung transplantation on change in EQ5D and disability as a mediator of this effect, assigning a score of 0 to those who died
Table S3: Effect of lung transplantation on EQ5D by disease category, assigning a score of 0 to those who died
Table S4: Effect of changes in body composition, allograft function, and exercise capacity on HRQL
Table S5: Proportion of subjects with worse HRQL at 1-year after lung transplantation defined by either decrease of one or two times the minimally clinically important difference
REFERENCES
- 1.Thabut G, Ravaud P, Christie JD, Castier Y, Fournier M, Mal H, et al. Determinants of the Survival Benefit of Lung Transplantation in Patients with Chronic Obstructive Pulmonary Disease. Am J Resp Crit Care Med. 2008;177(10):1156–1163. doi: 10.1164/rccm.200708-1283OC. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 4.George TJ, Beaty CA, Kilic A, Shah PD, Merlo CA, Shah AS. Outcomes and temporal trends among high-risk patients after lung transplantation in the United States. J Heart Lung Transplant. 2012;31(11):1182–1191. doi: 10.1016/j.healun.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg. 2013;145(3):862–867. doi: 10.1016/j.jtcvs.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 6.UNOS Data Report. [Website] 2016 [cited 2016 5/14/2016]; Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#.
- 7.Maxwell BG, Mooney JJ, Lee PH, Levitt JE, Chhatwani L, Nicolls MR, et al. Increased resource use in lung transplant admissions in the lung allocation score era. Am J Respir Crit Care Med. 2015;191(3):302–308. doi: 10.1164/rccm.201408-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxwell BG, Levitt JE, Goldstein BA, Mooney JJ, Nicolls MR, Zamora M, et al. Impact of the lung allocation score on survival beyond 1 year. Am J Transplant. 2014;14(10):2288–2294. doi: 10.1111/ajt.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dew MA, DiMartini AF. Psychological disorders and distress after adult cardiothoracic transplantation. J Cardiovasc Nurs. 2005;20(5 Suppl):S51–S66. doi: 10.1097/00005082-200509001-00007. [DOI] [PubMed] [Google Scholar]
- 10.Dew MA, DiMartini AF, DeVito Dabbs AJ, Fox KR, Myaskovsky L, Posluszny DM, et al. Onset and risk factors for anxiety and depression during the first 2 years after lung transplantation. Gen Hosp Psychiatry. 2012;34(2):127–138. doi: 10.1016/j.genhosppsych.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dew MA, Rosenberger EM, Myaskovsky L, DiMartini AF, DeVito Dabbs AJ, Posluszny DM, et al. Depression and Anxiety as Risk Factors for Morbidity and Mortality After Organ Transplantation: A Systematic Review and Meta-Analysis. Transplantation. 2015;100(5):988–1003. doi: 10.1097/TP.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suhling H, Knuth C, Haverich A, Lingner H, Welte T, Gottlieb J. Employment after lung transplantation--a single-center cross-sectional study. Dtsch Arztebl Int. 2015;112(13):213–219. doi: 10.3238/arztebl.2015.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen DG, Christie JD, Anderson BJ, Diamond JM, Judy RP, Shah RJ, et al. Cognitive function, mental health, and health-related quality of life after lung transplantation. AnnalsATS. 2014;11(4):522–530. doi: 10.1513/AnnalsATS.201311-388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courtwright AM, Salomon S, Lehmann LS, Wolfe DJ, Goldberg HJ. The Effect of Pretransplant Depression and Anxiety on Survival Following Lung Transplant: A Meta-analysis. Psychosomatics. 2016;57(3):238–245. doi: 10.1016/j.psym.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman BM, Blumenthal JA, Carney RC, O’Hayer CV, Freedland K, Smith PJ, et al. Changes in neurocognitive functioning following lung transplantation. Am J Transplant. 2012;12(9):2519–2525. doi: 10.1111/j.1600-6143.2012.04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kugler C, Fischer S, Gottlieb J, Tegtbur U, Welte T, Goerler H, et al. Symptom experience after lung transplantation: impact on quality of life and adherence. Clin Transplant. 2007;21(5):590–596. doi: 10.1111/j.1399-0012.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 17.Langer D, Burtin C, Schepers L, Ivanova A, Verleden G, Decramer M, et al. Exercise training after lung transplantation improves participation in daily activity: a randomized controlled trial. Am J Transplant. 2012;12(6):1584–1592. doi: 10.1111/j.1600-6143.2012.04000.x. [DOI] [PubMed] [Google Scholar]
- 18.Lanuza DM, Lefaiver CA, Brown R, Muehrer R, Murray M, Yelle M, et al. A longitudinal study of patients’ symptoms before and during the first year after lung transplantation. Clin Transplant. 2012;26(6):E576–E589. doi: 10.1111/ctr.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberger EM, DiMartini AF, DeVito Dabbs AJ, Bermudez CA, Pilewski JM, Toyoda Y, et al. Psychiatric Predictors of Long-term Transplant-Related Outcomes in Lung Transplant Recipients. Transplantation. 2016;100(1):239–247. doi: 10.1097/TP.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PJ, Blumenthal JA, Carney RM, Freedland KE, O’Hayer CV, Trulock EP, et al. Neurobehavioral functioning and survival following lung transplantation. Chest. 2014;145(3):604–611. doi: 10.1378/chest.12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith PJ, Blumenthal JA, Trulock EP, Freedland KE, Carney RM, Davis RD, et al. Psychosocial Predictors of Mortality Following Lung Transplantation. Am J Transplant. 2016;16(1):271–277. doi: 10.1111/ajt.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith PJ, Rivelli S, Waters A, Reynolds J, Hoyle A, Flowers M, et al. Neurocognitive changes after lung transplantation. AnnalsATS. 2014;11(10):1520–1527. doi: 10.1513/AnnalsATS.201406-232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer LG, Chowdhury NA, Faughnan ME, Granton J, Keshavjee S, Marras TK, et al. Effects of Recipient Age and Diagnosis on Health-related Quality-of-Life Benefit of Lung Transplantation. Am J Respir Crit Care Med. 2015;192(8):965–973. doi: 10.1164/rccm.201501-0126OC. [DOI] [PubMed] [Google Scholar]
- 24.Singer JP, Katz PP, Chen H, Phelan T, Golden T, Leard LE, et al. Lung transplantation improves health-related quality of life, particularly in those with the poorest status before transplant. Chest. 2011;140:1023A. (abstract) [Google Scholar]
- 25.Singer JP, Katz PP, Chen J, Golden T, Leard LE, Hays SR, et al. Change in Disability is a Determinant of Quality of Life in Persons Undergoing Lung Transplant. J Heart Lung Transplant. 2012;31(S):504. (abstract) [Google Scholar]
- 26.Singer JP, Chen J, Dean YM, Su B, Katz PP, Leard L, et al. Lung Transplant Improves Health Related Quality of Life for Many, But Not All, Patients With Advanced Lung Disease. Am J Respir Crit Care Med. 2013:A3775. (abstract) [Google Scholar]
- 27.Singer JP, Katz P, Dean YM, Chen J, Su B, Kern R, et al. Frailty is Common in Lung Transplant Candidates and Associated with Poorer Health-Related Quality of Life. J Heart Lung Transplant. 2013;32:S892. (abstract) [Google Scholar]
- 28.Singer JP, Blanc PD, Dean YM, Hays S, Leard L, Kukreja J, et al. Development and validation of a lung transplant-specific disability questionnaire. Thorax. 2014;69(5):445–450. doi: 10.1136/thoraxjnl-2013-204557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer JP, Soong A, Shrestha P, Huang D, Mindo M, Katz PP, et al. Lung Transplantation Improves Health-Related Quality of Life for Most, but Not All, with Advanced Lung Disease. Am J Respir Crit Care Med. 2016:A4670. (abstract) [Google Scholar]
- 30.Shah RJ, Collard HR, Soong A, Shrestha P, Hays S, Leard L, et al. Changes in Quality of Life After Lung Transplantation in Recipients with Connective Tissue Related ILD (CTD-ILD) Am J Respir Crit Care Med. 2016:A4666. (abstract) [Google Scholar]
- 31.McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. 3rd. New York: Oxford University Press; 2006. [Google Scholar]
- 32.Stewart AL, Ware JE., Jr . Measuring Functioning and Well-being: The Medical Outcomes Study Approach. Duke University Press; 1992. [Google Scholar]
- 33.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH. Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL) Arthritis Care Res. 2011;63(Suppl 11):S383–S412. doi: 10.1002/acr.20541. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Eisner MD, Katz PP, Yelin EH, Blanc PD. Measuring disease-specific quality of life in obstructive airway disease - Validation of a modified version of the airways questionnaire 20. Chest. 2006;129(6):1644–1652. doi: 10.1378/chest.129.6.1644. [DOI] [PubMed] [Google Scholar]
- 36.Euroqol G The EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 37.Marra CA, Woolcott JC, Kopec JA, Shojania K, Offer R, Brazier JE, et al. A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med. 2005;60(7):1571–1582. doi: 10.1016/j.socscimed.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 38.Coteur G, Feagan B, Keininger DL, Kosinski M. Evaluation of the meaningfulness of health-related quality of life improvements as assessed by the SF-36 and the EQ-5D VAS in patients with active Crohn’s disease. Aliment Pharmacol Ther. 2009;29(9):1032–1041. doi: 10.1111/j.1365-2036.2009.03966.x. [DOI] [PubMed] [Google Scholar]
- 39.Enabling America: Assessing the Role of Rehabilitation Science and Engineering. Washington (DC): 1997. [PubMed] [Google Scholar]
- 40.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54(4):439–467. [PubMed] [Google Scholar]
- 41.Abecassis M, Bridges ND, Clancy CJ, Dew MA, Eldadah B, Englesbe MJ, et al. Solid-organ transplantation in older adults: current status and future research. Am J Transplant. 2012;12(10):2608–2622. doi: 10.1111/j.1600-6143.2012.04245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer JP, Chen J, Katz PP, Blanc PD, Kagawa-Singer M, Stewart AL. Defining novel health-related quality of life domains in lung transplantation: a qualitative analysis. Qual Life Res. 2014 doi: 10.1007/s11136-014-0875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagi SZ. Disability concepts revisited: implications for prevention. Washington, DC: National Academy Press; 1991. [Google Scholar]
- 44.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 45.Benzo R, Farrell MH, Chang CC, Martinez FJ, Kaplan R, Reilly J, et al. Integrating health status and survival data: the palliative effect of lung volume reduction surgery. Am J Respir Crit Care Med. 2009;180(3):239–246. doi: 10.1164/rccm.200809-1383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DerHovanessian A, Todd JL, Zhang A, Li N, Mayalall A, Finlen Copeland CA, et al. Validation and Refinement of Chronic Lung Allograft Dysfunction Phenotypes in Bilateral and Single Lung Recipients. AnnalsATS. 2016;13(5):627–635. doi: 10.1513/AnnalsATS.201510-719OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Todd JL, Jain R, Pavlisko EN, Finlen Copeland CA, Reynolds JM, Snyder LD, et al. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2014;189(2):159–166. doi: 10.1164/rccm.201306-1155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman BM, Stonerock GL, Smith PJ, O’Hayer CV, Palmer S, Davis RD, et al. Development and psychometric properties of the Pulmonary-specific Quality-of-Life Scale in lung transplant patients. J Heart Lung Transplant. 2015;34(8):1058–1065. doi: 10.1016/j.healun.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myaskovsky L, Dew MA, McNulty ML, Switzer GE, DiMartini AF, Kormos RL, et al. Trajectories of Change in Quality of Life in 12-Month Survivors of Lung or Heart Transplant. Am J Transplant. 2006;6(8):1939–1947. doi: 10.1111/j.1600-6143.2006.01395.x. [DOI] [PubMed] [Google Scholar]
- 50.Kugler C, Strueber M, Tegtbur U, Niedermeyer J, Haverich A. Quality of life 1 year after lung transplantation. Prog Transplant. 2004;14(4):331–336. doi: 10.1177/152692480401400408. [DOI] [PubMed] [Google Scholar]
- 51.Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb SB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant. 2015;34(10):1264–1277. doi: 10.1016/j.healun.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Yusen RD. Technology and outcomes assessment in lung transplantation. Proc Am Thorac Soc. 2009;6(1):128–136. doi: 10.1513/pats.200809-102GO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yusen RD. Lung transplantation outcomes: the importance and inadequacies of assessing survival. Am J Transplant. 2009;9(7):1493–1494. doi: 10.1111/j.1600-6143.2009.02698.x. [DOI] [PubMed] [Google Scholar]
- 54.Singer JP, Chen J, Blanc PD, Leard LE, Kukreja J, Chen H. A thematic analysis of quality of life in lung transplant: the existing evidence and implications for future directions. Am J Transplant. 2013;13(4):839–850. doi: 10.1111/ajt.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song MK, Devito Dabbs AJ, Studer SM, Arnold RM, Pilewski JM. Exploring the meaning of chronic rejection after lung transplantation and its impact on clinical management and caregiving. J Pain Symptom Manage. 2010;40(2):246–255. doi: 10.1016/j.jpainsymman.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 56.De Vito Dabbs A, Dew MA, Stilley CS, Manzetti J, Zullo T, McCurry KR, et al. Psychosocial vulnerability, physical symptoms and physical impairment after lung and heart-lung transplantation. J Heart Lung Transplant. 2003;22(11):1268–1275. doi: 10.1016/s1053-2498(02)01227-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


