Abstract
Mucormycosis is a devastating opportunistic fungal infection to which the immunosuppressed are particularly vulnerable. We report the case of a 60 year-old man who was found to have multifocal pulmonary mucormycosis ten weeks after concomitant heart and kidney transplantation. Despite appropriate antifungal therapy, the infection progressed rapidly and soon involved critical pulmonary vasculature. He successfully underwent staged operative resection of his pulmonary mucormycosis without recurrence of infection. Although surgical debridement of pulmonary mucormycosis is typically reserved for localized disease, this case demonstrates that surgical intervention should be considered as an adjunct to antifungal therapy in multifocal disease.
Mucormycosis is a rare, opportunistic fungal infection with frequently devastating outcomes. Transplant patients, as well as those with hematogenous malignancies or poorly controlled diabetes, are particularly vulnerable to these infections. Infections typically involve the rhino-orbital-cerebral spaces and the lungs, and are characterized by angioinvasion with subsequent infarction and necrosis of affected tissues.
Given the aggressive clinical course of mucormycosis, early diagnosis and rapid treatment are the mainstays of therapy. Surgical debridement in combination with antifungal therapy may improve outcomes for localized disease [1, 2, 5, 7, 8]. However, there are no previously reported cases of surgical resection for multi-lobar pulmonary disease. We present the case of a 60 year-old man with successful surgical treatment of multifocal pulmonary mucormycosis.
A 60 year-old man with ischemic cardiomyopathy, coronary artery disease, diabetes, and end stage renal disease on hemodialysis presented to our institution with 3 days of worsening of his chronic dyspnea. He was found to have significant multi-vessel coronary artery disease and a ventricular ejection fraction of 25%. He was evaluated for transplantation and underwent an orthotopic, bi-caval heart transplant three months later with a concomitant deceased donor kidney transplant. He had an uncomplicated postoperative course and was discharged on postoperative day fourteen. His immunosuppressive regimen included mycophenolate, prednisone, and tacrolimus with valganciclovir and trimethoprim-sulfamethoxazole antimicrobial prophylaxis.
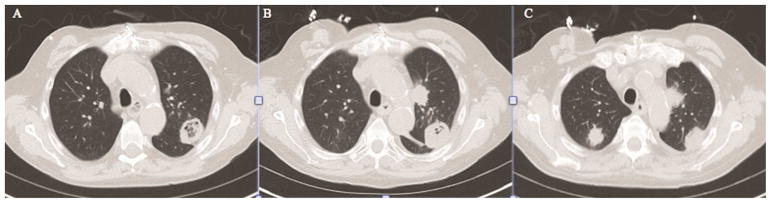
A left upper lobe mass was detected on a routine chest x-ray performed ten weeks after transplantation. Computed tomography (CT) of the chest demonstrated a 39mm well-circumscribed, cavitary mass in the posterior left upper lobe (Figure 1A) not present on CT four months prior. An additional 8mm soft tissue nodule was seen in the left upper lobe, as well as a 12mm nodule in the posterior right upper lobe. The findings were concerning for an infectious process in the setting of recent transplantation. The patient remained asymptomatic, and specifically denied cough, sputum production, chest pain, or fever.
Figure 1.
(A) Computed tomographic (CT) view showing a 39mm cavitary mass in the posterior left upper lobe. (B) Repeat CT chest again demonstrating the cavitary nodule, now with central soft tissue. (C) Additional view of the CT chest from Panel B, showing interval significant enlargement of the second left upper lobe nodule and the right upper lobe nodule.
A transthoracic fine needle aspiration was performed of the left upper lobe cavitary mass. Pathologic examination of a silver stain of the specimen revealed fungal organisms consistent with Aspergillus, and the patient was started on voriconazole. Upon further examination of the specimen several days later, broad, ribbon-like hyphal forms within necrotic tissue with possible angioinvasion raised concern for Mucor species. The patient was transitioned to amphotericin therapy, and thoracic surgery was consulted for surgical intervention. A repeat CT fourteen days after initial imaging demonstrated increased central density in the dominant left upper lobe nodule (Figure 1B). Furthermore, interval enlargement was seen of the additional left upper lobe and right upper lobe nodules to 25 mm and 22 mm, respectively (Figure 1C). Given the rapid growth of these lesions despite appropriate antifungal therapy, as well as the concern for impending invasion of the main pulmonary artery, the decision was made to proceed urgently with operative resection.
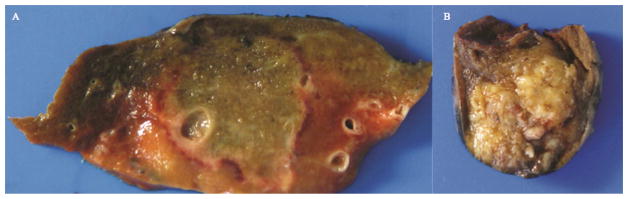
Surgical resection was performed in a staged fashion. The patient was first taken to the operating room for a left upper lobectomy via thoracotomy to allow latissimus flap buttress of the bronchial stump. Two dominant masses were identified within the left upper lobe, one of which crossed the segmental boundary with the lingula, and the second of which abutted the underside of the aortic arch. Both were carefully dissected, and the aorta was liberated from inflammatory adhesions. After vascular isolation, the left upper lobe was removed and sent for pathologic evaluation (Figure 2A). The latissimus was used as a pedicled flap to reinforce the bronchial stump. The patient tolerated the procedure well, and three days later underwent a right upper lobe wedge resection via video-assisted thorascopic surgery (VATS) for complete source control (Figure 2B).
Figure 2.
(A) Gross photograph of a section of left upper lobe with central necrosis rimmed by a hyperemic border. (B) Gross photograph of the right wedge resection, demonstrating central necrosis with organizing pneumonia.
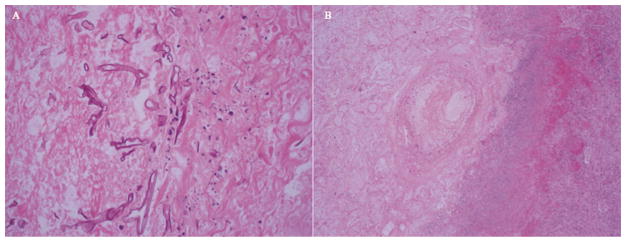
Pathology from both procedures confirmed angioinvasive mucormycosis with multiple infarcts and microabscess formation (Figure 3), and cultures were positive for Cunninghamella species as well as Nocardia Nova Complex. The patient was discharged to a subacute rehabilitation facility on isavuconazole, doripenem and trimethoprim- sulfamethoxazole. He recovered well post-operatively, and did not develop recurrent infection. He ultimately succumbed to cardiac death the following year secondary to cardiac graft rejection.
Figure 3.
(A) Photomicrograph of a section of infarcted lung with broad, ribbon-shaped hyphal structures. (B) Photomicrograph of a section of infarcted lung with angioinvasive fungal organisms.
Comment
Mucormycosis is an opportunistic infection by fungi in the order Mucorales. The most common pathogens are Mucor and Rhizopus species and, as in this patient, the less common Cunninghamella, although the latter tends to cause more severe disease [4]. The genera are characterized by broad, irregular nonseptate hyphae and have a characteristic angioinvasive quality. Although infection can have a variety of manifestations, sinopulmonary disease is the most common.
Pulmonary mucormycosis in particular is thought to be due to spore inhalation leading to necrotizing pneumonia. The classic presentation is with fever, dyspnea, cough, and often what is described as “massive” hemoptysis, all of which were notably absent in this patient. On imaging, pulmonary mucormycosis is sometimes identified by a “reverse halo sign,” a ground glass pattern surrounded by consolidation of the lung parenchyma [3]. However, the most frequent radiographic findings are nonspecific pleural effusion and pulmonary nodules, making mucormycosis difficult to diagnose noninvasively [6]. In a retrospective review of 35 patients with mucormycosis, 71% required bronchoscopy for definitive diagnosis [6]. In another series of 87 patients, 20% required open biopsy or surgical resection for diagnosis [5].
Pulmonary mucormycosis bears a grave prognosis, with in-hospital mortality reported to be as high as 65% [8]. Early diagnosis, rapid treatment, and control of underlying disease are critical for survival as antifungal therapy alone frequently fails. Current guidelines [2] recommend combined antifungal therapy and surgical debridement, with several studies demonstrating vastly improved outcomes for those patients treated with surgical resection [5, 7, 8].
A review of the literature suggests that surgical resection of pulmonary mucormycosis is reserved for localized disease [1, 8]. However, this case suggests that multilobar involvement does not necessarily preclude surgical intervention. In the case of our patient, the rapid progression of disease as well as the proximity of one of the lesions to critical pulmonary vasculature rendered surgical intervention crucial. Combining lobectomy with wedge resection achieved adequate source control while also minimizing resection of uninfected lung parenchyma. As this case illustrates, in patients with multifocal pulmonary mucormycosis, aggressive surgical debridement should be considered as an adjunct to antifungal treatment as a means to improve survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afolayan O, Copeland H, Zaheer S, Wallen JM. Pulmonary mucormycosis treated with lobectomy. Ann Thorac Surg. 2017;103(6):531–533. doi: 10.1016/j.athoracsur.2017.01.102. [DOI] [PubMed] [Google Scholar]
- 2.Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20(suppl 3):5–26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 3.Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments into a persistently devastating infection. Semin Respir Crit Care Med. 2015;36(5):692–705. doi: 10.1055/s-0035-1562896. [DOI] [PubMed] [Google Scholar]
- 4.Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693–702. doi: 10.1055/s-0031-1295717. [DOI] [PubMed] [Google Scholar]
- 5.Lee FYW, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999;159(12):1301–1309. doi: 10.1001/archinte.159.12.1301. [DOI] [PubMed] [Google Scholar]
- 6.Lin E, Moua T, Limper AH. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45(4):443–448. doi: 10.1007/s15010-017-0991-6. [DOI] [PubMed] [Google Scholar]
- 7.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 8.Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044–50. doi: 10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]