Abstract
IL-6 is a critical cytokine in acute phase response and involved in the pathogenesis of several chronic inflammatory diseases including cancer. Studies have highlighted that levels of IL-6 and its family members can be useful for diagnosis, prognosis of relapse-free survival and recurrence. IL-6 family cytokines have been identified as cancer biomarkers through screening of inflammatory mediators in different fluids including saliva, serum, and bronchoalveolar lavage fluid (BALF). IL-6 can be modulated by chemopreventive drugs, small molecules, monoclonal antibodies and immune checkpoint inhibitors. Unveiling the different sources of IL-6, the interaction between IL-6 and its cellular targets, the IL-6-dependent tumor resistance mechanisms, and the identification of novel regulators of IL-6 are some of the highly complex topics included in this review and their understanding could aid cancer biomarkers and therapy development.
Keywords: IL-6, IL-6 family, cytokine, cancer biomarker, immunoprevention
Graphical abstract
1. Introduction
Inflammation, one of the hallmarks of cancer, is a known contributor to cancer initiation and progression [1]. The role of IL-6 in the development and progression of inflammation-associated cancers has been widely described. Several cells in the tumor microenvironment are capable of secreting IL-6, including epithelial oncogenic cells as well as stromal cells including immune cells [2]. Besides its direct effect on tumor cell proliferation, IL-6 is also a major immunomodulatory agent, playing active roles in the regulation of acute phase reactions, activation of T helper cells, inhibition of T regulatory (Tregs) cells and differentiation of B cells by orchestrating innate and adaptive immune responses [3]. Dual roles for IL-6 have been described in some specific tumors as it is the example of lung cancer in which IL-6 has a preventive role in the tumor initiation but it is also capable of enhancing cancer progression [4] [5].
IL-6 monoclonal antibody (Siltuximab) has been tested in mouse xenografts models of lung cancer and it has tumor inhibitory effect particularly potent when cancer-associated fibroblasts were coadministered, suggesting that IL-6 secreted by the stroma might be more susceptible to the antibody effect [6]. Cancer associated fibroblasts (CAF) in hepatocellular carcinoma (HCC) also secrete high levels of IL-6 which contributes to tumor progression via recruitment of immune cells with immunosuppressive phenotype. This data suggests that IL-6 blockade in addition to immune checkpoint inhibitors may potentially overcome the immune-checkpoint inhibitor resistance in some types of cancer [7]. The gp130 f/f (IL6st) knock-in mouse model exhibits hyper activation of the STAT3 arm of IL6. When this transgenic mouse is crossed with Kras(G12D) mice to study lung tumorigenesis, an increase in atypical adenomatous hyperplasia, adenocarcinoma in situ, and invasive adenocarcinoma throughout the lung are observed, suggesting that IL-6 trans-signaling can be a target for the treatment of KRAS-driven lung adenocarcinoma [8].
2. Identification of IL-6 family cytokines as cancer biomarkers
2.1. IL-6
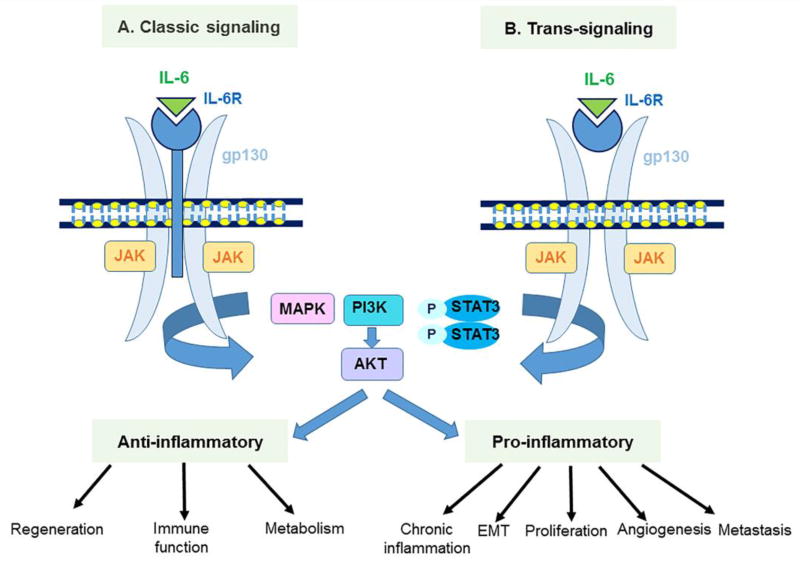
The IL-6-type family cytokines are IL-6, IL-11, IL-31, Cardiotrophin-1, Ciliary neurotrophic factor (CNTF), Cardiotrophin-like cytokine (CLC), Granulocyte-colony stimulating factor (G-CSF), Leptin, Leukemia inhibitory factor (LIF), Neuropoietin and Oncostatin M. The main cellular source of IL-6 is monocytes and T cells but it can also be produced by other cells including epithelial cells [9]. IL-6 promotes Th17 cells development when combined with TGF-β [10], inhibits TGF-β-induced Treg differentiation [11], plays a role in neutrophil and macrophage recruitment and is also associated with the pathogenesis of chronic inflammatory disease [12]. IL-6 activates STAT3 through two pathways: classical and trans-signaling. Classical signaling of IL-6 occurs in cells expressing IL-6Rα and it induces anti-inflammatory molecules [12]. On the other hand, trans-signaling is possible in all cells expressing gp130 and causes pro-inflammatory cytokines induction which drives chronic inflammation. IL-6 not only contributes to cancer related inflammation but also plays crucial roles in DNA damage repair, anti-oxidant defense system, proliferation, invasion, metastasis, angiogenesis and metabolic remodeling [12] (Figure 1).
Figure 1.
IL-6 classic- and trans-signaling pathways and their downstream targets.
Screening of cytokines can serve to identify immunological response-related soluble factors which may be increased in cancer even at early stages. A study that included 224 hepatocellular carcinoma (HCC) cases and 644 controls revealed that higher serum levels of IL-6 are predictive of increased HCC risk, independently of hepatitis virus infection, radiation exposure and lifestyle-associated factors [13]. IL-6 can also be involved in stemness and metastasis via its downstream target, Osteopontin, in HCC. Plasma levels of IL-6 and Osteopontin were found to be independent prognostic factors for HCC patients [14].
Serum levels of IL-6 have been associated with tumor progression. In bladder cancer, IL-6 serum levels were found to be remarkably higher in patients with recurrence compared to non-recurrent patients [15]. In pancreatic ductal adenocarcinoma (PDAC), serum levels of IL-6 can be useful as diagnostic biomarker and this cytokine has also been implicated in the progression of this type of tumor [16]. It has also been reported as a useful classifier for the identification of high-risk stage I lung adenocarcinoma patients [17]. In addition to serum based studies of inflammatory mediators, tissue microarray and immunohistochemistry analysis of IL-6 in human cervical cancer tissues suggest its usefulness as prognostic biomarker as well as potential therapeutic targets for treatment of cervical cancer [18]. Advanced or metastatic colorectal cancer patients with high serum IL-6 levels had poorer overall survival (OS), progression-free survival (PFS) and anti-VEGF resistance [19] than patients with lower levels. In addition to solid tumors, elevated serum levels of IL-6 can serve as a biomarker in hematological malignancies like Hodgkin’s lymphoma [20]. IL-6 and the JAK-STAT3 signaling pathway have also been found upregulated in myeloproliferative neoplasms (MPN). Interestingly, the recent JAK1/2 inhibitor trials in MPNs demonstrated that lessening inflammation can be even more helpful that targeting mutations [21].
Besides serum levels, Il-6 detection in saliva has been proposed as a useful diagnostic biomarker of cancers like oral squamous cell carcinoma (OSCC) [22]. In OSCC, IL-6 modulates resistance to radiation by inhibiting oxidative stress through the Nrf2-antioxidant pathway [23].
2.2. IL-6 as a critical inflammatory marker in murine models
IL-6 is the most highly elevated cytokine in COPD-like inflammatory mouse model and is required for lung cancer promoted by COPD-like inflammation [24]. IL-6 differently modulates both tumor initiation and progression via activating STAT3 in a mouse model of lung cancer induced by the Kras oncogene. It suppresses lung cancer initiation via sustaining lung homeostasis, modulating lung macrophages and activating cytotoxic CD8+ T cells under Kras oncogenic stress, and on the other hand it promotes lung cancer cell growth by promoting the cell proliferation regulator Cyclin D1 [4]. In a different lung cancer mouse model, CcspCre/+ KrasLSL-G12D/+ (CC-LR), IL-6 and IL-6 class cytokine LIF were highly detected in BALF from mice with tumors. Chemopreventive treatment of mice with myo-inositol diminished IL-6 and phospho-STAT3 signaling, a process likely mediated by tumor associated macrophages [25].
In the pancreas, KrasG12D activation induces premalignant lesions called pancreatic intraepithelial neoplasias (PanINs). IL-6 trans-signaling-dependent activation of Stat3/Socs3 is required to promote murine PanIN progression to PDAC [26]. In a similar fashion, IL-6 has been described as a critical tumor booster during early colitis-associated cancer (CAC). Its production by myeloid cells in the lamina propia has a protective role on normal and premalignant intestinal epithelial cells (IECs) against apoptosis [27].
IL-6 has also been found increased during the development and malignant progression of astrocytomas [28]. Although suppression of IL-6 does not influence preneoplastic astrogliosis, it prevents tumor formation in a spontaneous GFAP-v-src+/− mouse astrocytoma model. In a murine model of osteosarcoma, tumor progression and recurrence are modulated by IL-6 via promoting tumor self-seeding by CTCs [29].
In murine models of hematological malignancies such as CML, increased IL-6 levels were detected in BCR/ABL transgenic mice. IL-6 produced by myeloid CML cells inhibits lymphoid differentiation from multipotent progenitor cells [30] and shapes the CML pathogenesis.
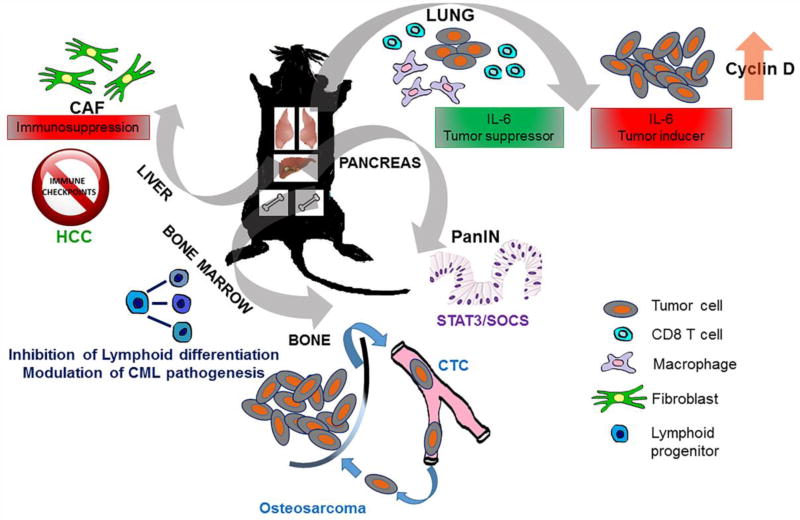
Apart from tumor cells derived IL-6 secretion, mesenchymal stem cells (OvMSC) can secrete IL-6 which contributes to tumor progression in models like ovarian cancer. Coinjection of OvMSC with ovarian cancer cells enhances ovarian tumor development in NOD-SCID mice [31]. In a murine model of hepatocellular carcinoma (HCC), IL-6 is predominantly expressed by CAFs creating an immunosuppressive environment via up-regulation of inhibitory immune checkpoints [7] (Figure 2).
Figure 2.
Graphical scheme showing the complex role of IL-6 in multiple cancer types in vivo.
A study of gastric tumorigenesis in mice challenged with N-methyl-N-nitrosourea demonstrated the importance of IL-6 in driving tumor development through STAT3 stimulation by using IL-6 knockout mice [32]. Inoculation of another chemical carcinogen, diethylnitrosamine (DEN), remarkably increased serum interleukin-6 (IL-6) concentration in males compared to females. Estrogen-mediated suppression of IL-6 production by Kupffer cells diminished liver cancer risk in females in the DEN-induced hepatocellular carcinoma mouse model [33], suggesting that suppression of IL-6 abrogates the gender differences in hepatic carcinogenesis.
2.3. IL-11
IL-11 was identified as a 19 kDa soluble factor belonging to the IL-6 cytokine family in the supernatant of bone-marrow derived stromal cell. The main cellular source of IL-11 are bone, connective tissue, and malignant cells [34]. Through transmembrane protein glycoprotein-130 beta subunit, IL-11 shows pro-tumorigenic activities such as proliferation, self-renewal, invasion and angiogenesis [34]. In a prospective cohort of 60 smokers including patients with lung cancer, COPD and both, IL-11 was found to be a specific biomarker for the diagnosis of lung adenocarcinoma in BALF specimens [35].
Although there is a limitation with cohort size and short follow-up time, IL-11 has been found to be a useful biomarker for diagnosis and prognosis in patients with pancreatic cancer [36]. Assessment of IL-11 expression by immunohistochemistry in clear-cell RCC (ccRCC) has demonstrated its association with increased risk of recurrence and poor survival for ccRCC patients with early-stage disease. As a result of immunohistochemical evaluation of tissue microarrays including paired tumor/peritumoral liver tissue from 290 patients who had undergone hepatectomy for histologically proven HCC, intra-tumoral IL-11 is significantly concordant with higher tumor node metastasis (TNM) stage and has been found to be an independent prognostic factor for progression-free survival (PFS) [37], [38].
2.4. Oncostatin M
Oncostatin M (OSM) is produced by monocytes, macrophages, T cells, neutrophils and dendritic cells. Oncostatin M plays fundamental roles in heart remodeling, inflammation, hematopoiesis, liver regeneration and cancer [39]. OSM levels are associated with inflammatory response-genes, epidermal growth factor (EGF) signaling and epithelial-to-mesenchymal transition (EMT) in human estrogen receptor (ER)-negative/human epidermal growth factor receptor 2 (HER2)-negative breast cancer [40]. High expression of OSM and OSM receptor (OSMR) mRNA have been associated with reduced ER and progesterone receptor (PR) protein levels in a cohort of 70 invasive breast cancers [41]. OSM stimulates the expression of ZEB1, Snail (SNAI1), and OSMR as well as the CSC phenotypes in pancreatic cancer, suggesting that therapeutic targeting of the OSM/OSMR axis could be useful for patients with PDAC [42]. Analysis of serum diagnostic biomarkers in PDAC showed that OSM was overexpressed in PDAC patients versus controls (AUC=0.744). OSM could also be a predictive biomarker for treatment of PDAC response to drugs like gemcitabine and erlotinib [43].
2.5. IL-31
IL-31 is mainly expressed by circulating Th2 lymphocytes and skin-homing CLA+ CD45RO+ T cells. IL-31 binds its heterodimeric receptor formed from IL-31RA and the OSMR chains and this leads to phosphorylation of Jak1/2, which in turn, triggers phosphorylation of STAT1/3/5 or PI3K/AKT. These pathways promote skin inflammation, development of T cell type-2 inflammation in asthma and allergic rhinitis as well as gut inflammation. Elevated serum levels of IL-31 contribute to the pathogenesis of different tumor types including endometrial, lung cancer, cutaneous T cell lymphoma, follicular B cell lymphoma [44] [45]. Expression of IL-31 was found to be increased in patients with mastocytosis compared with those seen in healthy control subjects (P < .0473) [46].
3. Identification of IL-6 family cytokines as potential cancer treatment target
3.1. IL-6
Activation of IL-6/STAT3 pathway has been reported in various cancer types. Blockade of IL-6/STAT3 has been targeted by potent chemopreventive drugs. As an example, disulfiram, targets cancer stem cells [47] and STAT3 signaling in triple-negative breast cancer [48]. Targeting STAT3 could cause elimination of cancer stem-like cells and contribute to blockade of recurrence in breast cancer. Recently published papers have also focused on small molecules such as Tanshinone IIA (Tan-IIA) possessing anti-cancer and anti-inflammatory activities or proteins like repebody which binds IL-6 ligand with high affinity attenuating STAT3 signaling and inhibiting human breast cancer stem cells growth and NSCLC, respectively [49] [50]. In prostate cancer, elevation of IL-6 and loss of ESE3/EHF, required for differentiation of human prostate epithelial cells, were associated with STAT3 activation. IL-6 upregulates cancer stem-like and metastatic spread-related gene expressions, indicating that identification of the novel regulator sites in IL-6 promoter could be beneficial for prostate cancer with loss of ESE3/EHF. Besides transcriptional modifiers, a long non-coding RNA identified as antisense IL6 stimulates IL-6 expression, which induces IL-6/STAT3 activation and increases invasive ability of glioblastoma cells [51].
Interleukin-6 (IL-6) is a growth factor for estrogen receptor-α (ERα)-positive breast cancer. Preclinical models have shown that breast cancer patients-derived xenografts respond to IL-6 blocking antibody [52]. Siltuximab has been well tolerated in patients with solid tumors including ovarian and KRAS-mutant cancers [53]. Siltuximab inhibits the growth of human renal cell carcinoma (RCC) in nude mice and remarkably stabilizes disease in patients with progressive metastatic RCC [54]. Since IL-6 has been involved in resistance to anti-angiogenic treatment, combinational therapy targeting angiogenic factors could be useful to prevent or minimize side effects of the monoclonal antibody.
3.2. IL-11
IL-11 is the dominant IL-6 family cytokine identified as an inducer of oncogenic STAT3 activity in the gastrointestinal (GI) epithelium during tumorigenesis, which can be targeted pharmacologically. mIL-11 Mutein treatment remarkably diminished overall tumor burden, gastric epithelial hyperplasia and reduced expression of the inflammatory mediators in the gastrointestinal tumorigesis model [55]. IL-11/IL-11Rα axis modulates human osteosarcoma through STAT3 [56–58]. Bone metastasis-targeting peptidomimetic (BMTP-11) directed against IL-11Rα retards primary tumor growth and lung metastasis in preclinical models of human osteosarcoma. BMTP-11 in combination with gemcitabine shows better outcomes than BMTP-11 alone [59]. Similarly, targeting IL-11Rα in combination with doxorubicin therapeutically improves the effect of chemotherapy in high grade Type I endometrioid cancer [60].
3.3. Oncostatin M
The role of Oncostatin M on tumor progression is controversial. A lung cancer study has shown that Oncostatin M inhibits metastasis of lung adenocarcinoma by suppressing SLUG expression via STAT1/STAT3 dependent signaling [61].
Oncostatin M (OSM) was described as a robust inducer of mesenchymal/CSC. Eradication of OSM or suppression of STAT3 or SMAD3 causes a significant reversion to a non-invasive, epithelial phenotype [62]. OSMR has been described as an inducer of mesenchymal properties in glioblastoma. Analysis of TCGA data has showed that OSMR but not IL-6R or LIFR is upregulated in GBM [63]. In contrast to osteosarcomas and chondrosarcomas, OSM induces proliferation of Ewing carcinoma cells which are rare bone malignant cells, suggesting its potential utility for therapeutic intervention [64]. In sum, OSM/OSMR could be potential cancer therapeutic targets besides their usefulness as diagnostic biomarkers [63].
4. Promising Trials targeting IL-6/ IL-6R and IL-11/IL-11Rα in cancer
Inhibition of IL-6 and IL-6R using specific monoclonal antibodies is being tested therapeutically for many different types of cancer [65]. Phase I and II studies assessed siltuximab in various solid tumors including ovarian, pancreatic, colorectal, head & neck and lung neoplasms [66]. In addition to solid tumors, siltuximab has also been evaluated for patients with multiple myeloma who are relapsed or refractory [67]. Phase I trials related to siltuximab are also undergoing in hematological tumors including non-Hodgkin’s lymphomas (NHL) and B cell Chronic Lymphocytic Leukemia [68]. In pancreatic cancer, a current phase II trial will evaluate the safety and efficacy of gemcitabine and nab-paclitaxel with or without tocilizumab (monoclonal antibody against IL-6 receptor) (Clinical trial identification: NCT02767557). IL-6R has been described as an independent prognostic factor and potential therapeutic target for ovarian cancer. Tocilizumab has been found to be well tolerated in clinical trials [69]. The on-going phase I trial will evaluate tolerability and safety of tocilizumab given with trastuzumab and pertuzumab in subject with metastatic HER2+ breast cancer (Clinical trial identification: NCT03135171).
Recombinant IL-11 (Neumega) is also under clinical development for acceleration of platelet recovery and inhibition of inflammation in cancer patients to prevent chemotherapy-induced thrombocytopenia [70]. BMTP-1 for IL-11Rα has been shown as a promising drug candidate in metastatic prostate cancer [71]. A comprehensive list with the completed and ongoing clinical trials targeting IL-6 family cytokines as well as their receptors can be found in Table 1.
Table 1.
Clinical trials registered at ClinicalTrials.gov targeting IL-6 family members or its receptors.
| Drug type | Drug target |
Single agent or combination |
Cancer type | Study Status (completed/ongoing) |
Clinical Trial Identification |
|---|---|---|---|---|---|
| Siltuximab | IL-6 | Single agent | Ovarian, pancreatic, colorectal, head and neck, lung neoplasm | Phase I and Phase II, completed | NCT00841191 |
| Multicentric Castleman's Disease | Phase II, ongoing | NCT01400503 | |||
| High-risk Smoldering Multiple Myeloma | Phase II, ongoing | NCT01484275 | |||
| Relapsed or Refractory Multiple Myeloma | Phase II, completed | NCT00402181 | |||
| Prostate cancer | Phase II, completed | NCT00433446 | |||
| in combination with Bortezomib | Multiple myeloma | Phase II, ongoing | NCT00401843 | ||
| in combination with Velcade-Melphalan-Prednisone (VMP) | Multiple myeloma | Phase II | NCT00911859 | ||
| Tocilizumab | IL-6R | in combination with standard chemotherapy Carboplatin or Doxorubicin | Recurrent ovarian cancer | Phase I, completed | NCT01637532 |
| in combination with Trastuzumab and Pertuzumab | Metastatic HER2 positive breast cancer | Phase I, ongoing | NCT03135171 | ||
| in combination with Gemcitabine/Nab-paclitaxel | Unresectable pancreatic carcinoma | Phase II, ongoing | NCT02767557 | ||
| Allogeneic or Haploidentical Stem Cell Transplant Followed By High-Dose Cyclophosphamide in Patients With Relapsed or Refractory Acute Myeloid Leukemia | Leukemia, Myeloid, Acute | Phase I, ongoing | NCT02057770 | ||
| in combination with oral chlorambucil to participants with previously untreated BCLL who have comorbidities | B-Cell Chronic Lymphocytic Leukemia | Phase I, ongoing | NCT02336048 | ||
| in combination with RO7082859 and Obinutuzumab | Non-Hodgkin's Lymphoma | Phase I, ongoing | NCT03075696 | ||
| Neumega | IL-11 | in combination with G-CSF to Mobilize Autologous Peripheral Blood Stem Cells | Breast cancer, gestational trophoblastic tumor, kidney cancer, lymphoma, neuroblastoma, ovarian cancer, sarcoma, testicular germ cell tumor | Phase II, completed | NCT00004157 |
| Single agent | Thrombocytopenia associate with imatinib or other tyrosine kinase inhibitor therapy in patients with CML | Phase II, completed | NCT00493181 | ||
| rhIL-11 | IL-11 | Single agent | Chemotherapy-induced Thrombocytopenia | Phase III, completion not been verified | NCT01663441 |
| BMTP-1 | IL-11Rα | Single agent | Patients With Castrate-Resistant Prostate Cancer With High-Volume Osseous Metastases and no Standard Treatment Options | Phase I, completed | NCT00872157 |
5. IL-6/STAT3 pathway can be targeted for cancer prevention
Chemoprevention targets many steps including tumor initiation, promotion and progression. Dietary derived products play a role in all steps of the carcinogenic process [72]. Administration of α-Tocopherol (vitamin E) in an inducible mouse model of non-Hodgkin's T-cell lymphoma diminishes IL-6 gene and protein expression [73]. In a similar manner, plant derived glycoprotein (UDN glycoprotein) inhibits the levels of IL-6 in 1,2-dimethylhydrazine-treated mice, suggesting that UDN glycoprotein may be an effective agent for colon cancer prevention [74]. Intragastric treatment with Isoliquiritigenin, originated from licorice root, downregulates IL-6 signaling in a chemically induced mouse model of colon carcinogenesis and it suppresses macrophage polarization into M2-like phenotype partially through IL-6/STAT3 pathway [75]. IL-6/STAT3 pathway can be also regulated by balsalazide (a 5-ASA prodrug) and probiotic agent VSL#3 in colorectal cancer chemoprevention [76]. The diet-derived polyphenols Apigenin and luteolin have an inhibitory effect on angiogenesis mediated by IL-6/STAT3 pathway in human endothelial cells [77]. Another example of IL-6 suppression is the mechanism of Quercetin, a compound found in many plant-based foods, which has been proposed for chemoprevention and treatment of glioblastoma [78]. Finally, the estrogen receptor modulator Evista (Raloxifene HCl), found to also inhibit IL-6/GP130 protein interaction, represents a promising chemopreventive agent for breast, colon, multiple myeloma and liver cancer [79] [80].
6. Conclusion & Perspectives
IL-6 is a multi-functional cytokine and its abnormal expression levels are associated with cancer diagnosis, prognosis and disease progression. Recently, the new area of immunoprevention has gained major attention. Direct targeting of the IL-6 family members with some of the monoclonal antibodies currently described in clinical trials for treatment of various cancers could potentially be used for cancer prevention [81] [82].
Development of monoclonal antibodies and small molecules in addition to defining de novo transcriptional regulators targeting trans-signaling of IL-6 would improve cancer therapeutic strategies. Defining the origin of IL-6 and IL-6 family cytokines in the tumor microenvironment and molecular stratification of cancer subtypes based on these key inflammatory mediators would open up novel insights for chemoresistance and immunotherapy in cancer therapy. Using in vivo models, application of combinational therapies including IL-6 blockade and conventional chemotherapeutic or immunotherapy drugs will provide basis for clinical research.
Highlights.
IL-6 and its family members can be useful for diagnosis, prognosis of relapse-free survival and recurrence.
The identification of novel regulators of IL-6 family cytokines could help cancer treatment.
Defining the origin of IL-6 family cytokines in the tumor microenvironment would open up novel insights for chemoresistance and immunotherapy.
Development of monoclonal antibodies targeting IL-6 family cytokines would improve cancer therapeutic strategies.
Acknowledgments
Dr. McAllister received support from the PanCAN/AACR Career Development Award (14-20-25 MCAL), National Pancreas Foundation and V Foundation (V Scholar). Dr McAllister is also a Paul Calabresi K12 clinical scholar (NCI grant awarded to MDACC K12CA088084-16A1).
Abbreviations
- BALF
Bronchoalveolar lavage fluid
- CAC
Colitis-associated cancer
- CAF
Cancer associated fibroblast
- ccRCC
Clear cell renal cell carcinoma
- CLC
Cardiotrophin-like cytokine
- CML
Chronic myeloid leukemia
- CNTF
Ciliary neurotrophic factor
- COPD
Chronic obstructive pulmonary disease
- CTC
Circulating tumor cells
- EGF
Epidermal Growth Factor
- EMT
Epithelial–mesenchymal transition
- GCSF
Granulocyte-colony stimulating factor
- Gp130
Glycoprotein 130
- HCC
Hepatocellular carcinoma
- IEC
Intestinal epithelial cells
- IL-11
Interleukin 11
- IL-31
Interleukin 31
- IL-6
Interleukin 6
- IL-6R
Interleukin 6 receptor
- Jak1/2
Januse kinase 1/2
- LIF
Leukemia inhibitory factor
- LIFR
Leukemia inhibitory factor receptor
- MAPK
Mitogen-activated protein kinase
- MPN
Myeloproliferative neoplasms
- Nrf-2
Nuclear factor (erythroid-derived 2)-like 2
- NHL
Non-Hodgkin’s lymphomas
- NSCLC
Non-small cell lung cancer
- OSCC
Oral squamous cell carcinoma
- PanIN
Pancreatic intraepithelial neoplasia
- PDAC
Pancreatic ductal adenocarcinoma
- PFS
Progression-free survival
- PI3K/AKT
The phosphatidylinositol-3-kinase/Protein kinase B
- RCC
Renal cell carcinoma
- STAT1
Signal transducer and activator of transcription 1
- STAT3
Signal transducer and activator of transcription 3
- TNM
Tumor node metastasis
- Treg
Regulatory T cell
Biographies

Nese Unver received her PhD in Tumor Biology and Immunology from Hacettepe University in 2012. Dr. Unver is a Postdoctoral Research Fellow in the Department of Clinical Cancer Prevention. Her research focuses on understanding of crosstalk between cancer cells and cancer-associated immune cells, especially macrophages, cytokine/chemokine mediated modulation of tumor microenvironment, uncovering the potential effects of chemopreventive drugs on lung tumor progression using in-vivo models and discovery of novel cancer biomarkers in solid tumors through utilization of translational medicine approaches.
Florencia McAllister received medical degree from National University of Rosario in 2000 and completed postdoctoral training at the University of Pittsburgh and Johns Hopkins University in Basic and Tumor Immunology, Oncology and Clinical Pharmacology. Dr. McAllister Lab at MD Anderson Cancer Center is focused on developing novel cancer immunopreventive and immunotherapy approaches for cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest statement
None.
References
- 1.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 2.Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol. 2014;26(1):38–47. doi: 10.1016/j.smim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Qu Z, Sun F, Zhou J, Li L, Shapiro SD, Xiao G. Interleukin-6 Prevents the Initiation but Enhances the Progression of Lung Cancer. Cancer Res. 2015;75(16):3209–15. doi: 10.1158/0008-5472.CAN-14-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rath T, Billmeier U, Waldner MJ, Atreya R, Neurath MF. From physiology to disease and targeted therapy: interleukin-6 in inflammation and inflammation-associated carcinogenesis. Arch Toxicol. 2015;89(4):541–54. doi: 10.1007/s00204-015-1461-5. [DOI] [PubMed] [Google Scholar]
- 6.Song L, Smith MA, Doshi P, Sasser K, Fulp W, Altiok S, Haura EB. Antitumor efficacy of the anti-interleukin-6 (IL-6) antibody siltuximab in mouse xenograft models of lung cancer. J Thorac Oncol. 2014;9(7):974–82. doi: 10.1097/JTO.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Shen J, Lu K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun. 2017;486(2):239–244. doi: 10.1016/j.bbrc.2017.02.128. [DOI] [PubMed] [Google Scholar]
- 8.Brooks GD, McLeod L, Alhayyani S, Miller A, Russell PA, Ferlin W, Rose-John S, Ruwanpura S, Jenkins BJ. IL6 Trans-signaling Promotes KRAS-Driven Lung Carcinogenesis. Cancer Res. 2016;76(4):866–76. doi: 10.1158/0008-5472.CAN-15-2388. [DOI] [PubMed] [Google Scholar]
- 9.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–5. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 11.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 12.Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37(9):11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 13.Ohishi W, Cologne JB, Fujiwara S, Suzuki G, Hayashi T, Niwa Y, Akahoshi M, Ueda K, Tsuge M, Chayama K. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer. 2014;134(1):154–63. doi: 10.1002/ijc.28337. [DOI] [PubMed] [Google Scholar]
- 14.Wang CQ, Sun HT, Gao XM, Ren N, Sheng YY, Wang Z, Zheng Y, Wei JW, Zhang KL, Yu XX, Zhu Y, Luo Q, Yang LY, Dong QZ, Qin LX. Interleukin-6 enhances cancer stemness and promotes metastasis of hepatocellular carcinoma via up-regulating osteopontin expression. Am J Cancer Res. 2016;6(9):1873–1889. [PMC free article] [PubMed] [Google Scholar]
- 15.Kumari N, Agrawal U, Mishra AK, Kumar A, Vasudeva P, Mohanty NK, Saxena S. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumour Biol. 2017;39(4) doi: 10.1177/1010428317697552. 1010428317697552. [DOI] [PubMed] [Google Scholar]
- 16.Kim HW, Lee JC, Paik KH, Kang J, Kim J, Hwang JH. Serum interleukin-6 is associated with pancreatic ductal adenocarcinoma progression pattern. Medicine (Baltimore) 2017;96(5):e5926. doi: 10.1097/MD.0000000000005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meaney CL, Zingone A, Brown D, Yu Y, Cao L, Ryan BM. Identification of serum inflammatory markers as classifiers of lung cancer mortality for stage I adenocarcinoma. Oncotarget. 2017 doi: 10.18632/oncotarget.16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Z, Lin Y, Ye X, Feng C, Lu Y, Yang G, Dong C. Expression of IL-1alpha and IL-6 is Associated with Progression and Prognosis of Human Cervical Cancer. Med Sci Monit. 2016;22:4475–4481. doi: 10.12659/MSM.898569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara M, Nagasaki T, Shiga K, Takahashi H, Takeyama H. High serum levels of interleukin-6 in patients with advanced or metastatic colorectal cancer: the effect on the outcome and the response to chemotherapy plus bevacizumab. Surg Today. 2017;47(4):483–489. doi: 10.1007/s00595-016-1404-7. [DOI] [PubMed] [Google Scholar]
- 20.Levin LI, Breen EC, Birmann BM, Batista JL, Magpantay LI, Li Y, Ambinder RF, Mueller NE, Martinez-Maza O. Elevated serum levels of sCD30 and IL-6 and detectable IL-10 precede classical Hodgkin lymphoma diagnosis. Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-16-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cokic VP, Mitrovic-Ajtic O, Beleslin-Cokic BB, Markovic D, Buac M, Diklic M, Kraguljac-Kurtovic N, Damjanovic S, Milenkovic P, Gotic M, Raj PK. Proinflammatory Cytokine IL-6 and JAK-STAT Signaling Pathway in Myeloproliferative Neoplasms. Mediators Inflamm. 2015;2015:453020. doi: 10.1155/2015/453020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahibzada HA, Khurshid Z, Khan RS, Naseem M, Siddique KM, Mali M, Zafar MS. Salivary IL-8, IL-6 and TNF-alpha as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics (Basel) 2017;7(2) doi: 10.3390/diagnostics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuoka Y, Nakayama H, Yoshida R, Hirosue A, Nagata M, Tanaka T, Kawahara K, Sakata J, Arita H, Nakashima H, Shinriki S, Fukuma D, Ogi H, Hiraki A, Shinohara M, Toya R, Murakami R. IL-6 controls resistance to radiation by suppressing oxidative stress via the Nrf2-antioxidant pathway in oral squamous cell carcinoma. Br J Cancer. 2016;115(10):1234–1244. doi: 10.1038/bjc.2016.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochoa CE, Mirabolfathinejad SG, Ruiz VA, Evans SE, Gagea M, Evans CM, Dickey BF, Moghaddam SJ. Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev Res (Phila) 2011;4(1):51–64. doi: 10.1158/1940-6207.CAPR-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unver N, Delgado O, Zeleke K, Cumpian A, Tang X, Caetano MS, Wang H, Katayama H, Yu H, Szabo E, Wistuba, Moghaddam SJ, Hanash SM, Ostrin EJ. Reduced IL-6 levels and tumor-associated phospho-STAT3 are associated with reduced tumor development in a mouse model of lung cancer chemoprevention with myo-inositol. Int J Cancer. 2018;142(7):1405–1417. doi: 10.1002/ijc.31152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algul H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, Aguzzi A, Weis J. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23(19):3308–16. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Ma Q, Liu T, Guan G, Zhang K, Chen J, Jia N, Yan S, Chen G, Liu S, Jiang K, Lu Y, Wen Y, Zhao H, Zhou Y, Fan Q, Qiu X. Interleukin-6 suppression reduces tumour self-seeding by circulating tumour cells in a human osteosarcoma nude mouse model. Oncotarget. 2016;7(1):446–58. doi: 10.18632/oncotarget.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M, Sala-Torra O, Radich JP, Passegue E. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20(5):661–73. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding DC, Liu HW, Chu TY. Interleukin-6 from Ovarian Mesenchymal Stem Cells Promotes Proliferation, Sphere and Colony Formation and Tumorigenesis of an Ovarian Cancer Cell Line SKOV3. J Cancer. 2016;7(13):1815–1823. doi: 10.7150/jca.16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinoshita H, Hirata Y, Nakagawa H, Sakamoto K, Hayakawa Y, Takahashi R, Nakata W, Sakitani K, Serizawa T, Hikiba Y, Akanuma M, Shibata W, Maeda S, Koike K. Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. PLoS One. 2013;8(4):e60914. doi: 10.1371/journal.pone.0060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone CN, Chand A, Putoczki TL, Ernst M. Emerging roles for IL-11 signaling in cancer development and progression: Focus on breast cancer. Cytokine Growth Factor Rev. 2015;26(5):489–98. doi: 10.1016/j.cytogfr.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Pastor MD, Nogal A, Molina-Pinelo S, Quintanal-Villalonga A, Melendez R, Ferrer I, Romero-Romero B, De Miguel MJ, Lopez-Campos JL, Corral J, Garcia-Carboner R, Carnero A, Paz-Ares L. IL-11 and CCL-1: Novel Protein Diagnostic Biomarkers of Lung Adenocarcinoma in Bronchoalveolar Lavage Fluid (BALF) J Thorac Oncol. 2016;11(12):2183–2192. doi: 10.1016/j.jtho.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Ren C, Chen Y, Han C, Fu D, Chen H. Plasma interleukin-11 (IL-11) levels have diagnostic and prognostic roles in patients with pancreatic cancer. Tumour Biol. 2014;35(11):11467–72. doi: 10.1007/s13277-014-2459-y. [DOI] [PubMed] [Google Scholar]
- 37.Pan D, Xu L, Liu H, Zhang W, Liu W, Liu Y, Fu Q, Xu J. High expression of interleukin-11 is an independent indicator of poor prognosis in clear-cell renal cell carcinoma. Cancer Sci. 2015;106(5):592–7. doi: 10.1111/cas.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang ZL, Zeng ZC, Fan J, Tang ZY, Zeng HY. Expression of connective tissue growth factor and interleukin-11 in intratumoral tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Mol Biol Rep. 2012;39(5):6001–6. doi: 10.1007/s11033-011-1413-y. [DOI] [PubMed] [Google Scholar]
- 39.Hermanns HM. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015;26(5):545–58. doi: 10.1016/j.cytogfr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Bottai G, Diao L, Baggerly KA, Paladini L, Gyorffy B, Raschioni C, Pusztai L, Calin GA, Santarpia L. Integrated MicroRNA-mRNA Profiling Identifies Oncostatin M as a Marker of Mesenchymal-Like ER-Negative/HER2-Negative Breast Cancer. Int J Mol Sci. 2017;18(1) doi: 10.3390/ijms18010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West NR, Murphy LC, Watson PH. Oncostatin M suppresses oestrogen receptor-alpha expression and is associated with poor outcome in human breast cancer. Endocr Relat Cancer. 2012;19(2):181–95. doi: 10.1530/ERC-11-0326. [DOI] [PubMed] [Google Scholar]
- 42.Smigiel JM, Parameswaran N, Jackson MW. Potent EMT and CSC Phenotypes Are Induced By Oncostatin-M in Pancreatic Cancer. Mol Cancer Res. 2017;15(4):478–488. doi: 10.1158/1541-7786.MCR-16-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres C, Perales S, Alejandre MJ, Iglesias J, Palomino RJ, Martin M, Caba O, Prados JC, Aranega A, Delgado JR, Irigoyen A, Ortuno FM, Rojas I, Linares A. Serum cytokine profile in patients with pancreatic cancer. Pancreas. 2014;43(7):1042–9. doi: 10.1097/MPA.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 44.Ferretti E, Corcione A, Pistoia V. The IL-31/IL-31 receptor axis: general features and role in tumor microenvironment. Journal of leukocyte biology. 2017 doi: 10.1189/jlb.3MR0117-033R. [DOI] [PubMed] [Google Scholar]
- 45.Ohmatsu H, Sugaya M, Suga H, Morimura S, Miyagaki T, Kai H, Kagami S, Fujita H, Asano Y, Tada Y, Kadono T, Sato S. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm Venereol. 2012;92(3):282–3. doi: 10.2340/00015555-1345. [DOI] [PubMed] [Google Scholar]
- 46.Hartmann K, Wagner N, Rabenhorst A, Pflanz L, Leja S, Forster A, Gehring M, Kapp A, Raap U. Serum IL-31 levels are increased in a subset of patients with mastocytosis and correlate with disease severity in adult patients. J Allergy Clin Immunol. 2013;132(1):232–5. doi: 10.1016/j.jaci.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL, Wang W. Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer. 2011;104(10):1564–74. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YJ, Kim JY, Lee N, Oh E, Sung D, Cho TM, Seo JH. Disulfiram suppresses cancer stem-like properties and STAT3 signaling in triple-negative breast cancer cells. Biochem Biophys Res Commun. 2017;486(4):1069–1076. doi: 10.1016/j.bbrc.2017.03.164. [DOI] [PubMed] [Google Scholar]
- 49.Lin C, Wang L, Wang H, Yang L, Guo H, Wang X. Tanshinone IIA inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of IL-6/STAT3/NF-kB signaling pathways. J Cell Biochem. 2013;114(9):2061–70. doi: 10.1002/jcb.24553. [DOI] [PubMed] [Google Scholar]
- 50.Lee JJ, Kim HJ, Yang CS, Kyeong HH, Choi JM, Hwang DE, Yuk JM, Park K, Kim YJ, Lee SG, Kim D, Jo EK, Cheong HK, Kim HS. A high-affinity protein binder that blocks the IL-6/STAT3 signaling pathway effectively suppresses non-small cell lung cancer. Mol Ther. 2014;22(7):1254–65. doi: 10.1038/mt.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Chen X, Tang G, Liu D, Peng G, Ma W, Liu Q, Yuan J. AS-IL6 promotes glioma cell invasion by inducing H3K27Ac enrichment at the IL6 promoter and activating IL6 transcription. FEBS Lett. 2016;590(24):4586–4593. doi: 10.1002/1873-3468.12485. [DOI] [PubMed] [Google Scholar]
- 52.Morancho B, Zacarias-Fluck M, Esgueva A, Bernado-Morales C, Di Cosimo S, Prat A, Cortes J, Arribas J, Rubio IT. Modeling anti-IL-6 therapy using breast cancer patient-derived xenografts. Oncotarget. 2016;7(42):67956–67965. doi: 10.18632/oncotarget.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angevin E, Tabernero J, Elez E, Cohen SJ, Bahleda R, van Laethem JL, Ottensmeier C, Lopez-Martin JA, Clive S, Joly F, Ray-Coquard I, Dirix L, Machiels JP, Steven N, Reddy M, Hall B, Puchalski TA, Bandekar R, van de Velde H, Tromp B, Vermeulen J, Kurzrock R. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2014;20(8):2192–204. doi: 10.1158/1078-0432.CCR-13-2200. [DOI] [PubMed] [Google Scholar]
- 54.Kaminska K, Czarnecka AM, Escudier B, Lian F, Szczylik C. Interleukin-6 as an emerging regulator of renal cell cancer. Urol Oncol. 2015;33(11):476–85. doi: 10.1016/j.urolonc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK, Nguyen PM, Preaudet A, Farid R, Edwards KM, Boglev Y, Luwor RB, Jarnicki A, Horst D, Boussioutas A, Heath JK, Sieber OM, Pleines I, Kile BT, Nash A, Greten FR, McKenzie BS, Ernst M. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24(2):257–71. doi: 10.1016/j.ccr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Lewis VO, Ozawa MG, Deavers MT, Wang G, Shintani T, Arap W, Pasqualini R. The interleukin-11 receptor alpha as a candidate ligand-directed target in osteosarcoma: consistent data from cell lines, orthotopic models, and human tumor samples. Cancer Res. 2009;69(5):1995–9. doi: 10.1158/0008-5472.CAN-08-4845. [DOI] [PubMed] [Google Scholar]
- 57.Campbell CL, Jiang Z, Savarese DM, Savarese TM. Increased expression of the interleukin-11 receptor and evidence of STAT3 activation in prostate carcinoma. Am J Pathol. 2001;158(1):25–32. doi: 10.1016/S0002-9440(10)63940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang G, Yu L, Cooper LJ, Hollomon M, Huls H, Kleinerman ES. Genetically modified T cells targeting interleukin-11 receptor alpha-chain kill human osteosarcoma cells and induce the regression of established osteosarcoma lung metastases. Cancer Res. 2012;72(1):271–81. doi: 10.1158/0008-5472.CAN-11-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis VO, Devarajan E, Cardo-Vila M, Thomas DG, Kleinerman ES, Marchio S, Sidman RL, Pasqualini R, Arap W. BMTP-11 is active in preclinical models of human osteosarcoma and a candidate targeted drug for clinical translation. Proc Natl Acad Sci U S A. 2017;114(30):8065–8070. doi: 10.1073/pnas.1704173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winship A, Van Sinderen M, Rainczuk K, Dimitriadis E. Therapeutically blocking Interleukin-11 Receptor-alpha enhances doxorubicin cytotoxicity in high grade type I endometrioid tumours. Oncotarget. 2017;8(14):22716–22729. doi: 10.18632/oncotarget.15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan CM, Wang ML, Chiou SH, Chen HY, Wu CW. Oncostatin M suppresses metastasis of lung adenocarcinoma by inhibiting SLUG expression through coordination of STATs and PIASs signalings. Oncotarget. 2016;7(37):60395–60406. doi: 10.18632/oncotarget.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Junk DJ, Bryson BL, Smigiel JM, Parameswaran N, Bartel CA, Jackson MW. Oncostatin M promotes cancer cell plasticity through cooperative STAT3-SMAD3 signaling. Oncogene. 2017;36(28):4001–4013. doi: 10.1038/onc.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Natesh K, Bhosale D, Desai A, Chandrika G, Pujari R, Jagtap J, Chugh A, Ranade D, Shastry P. Oncostatin-M differentially regulates mesenchymal and proneural signature genes in gliomas via STAT3 signaling. Neoplasia. 2015;17(2):225–37. doi: 10.1016/j.neo.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.David E, Tirode F, Baud'huin M, Guihard P, Laud K, Delattre O, Heymann MF, Heymann D, Redini F, Blanchard F. Oncostatin M is a growth factor for Ewing sarcoma. Am J Pathol. 2012;181(5):1782–95. doi: 10.1016/j.ajpath.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 65.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38(7):904–10. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Chen R, Chen B. Siltuximab (CNTO 328): a promising option for human malignancies. Drug Des Devel Ther. 2015;9:3455–8. doi: 10.2147/DDDT.S86438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chari A, Pri-Chen H, Jagannath S. Complete remission achieved with single agent CNTO 328, an anti-IL-6 monoclonal antibody, in relapsed and refractory myeloma. Clin Lymphoma Myeloma Leuk. 2013;13(3):333–7. doi: 10.1016/j.clml.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 68.Ferrario A, Merli M, Basilico C, Maffioli M, Passamonti F. Siltuximab and hematologic malignancies. A focus in non Hodgkin lymphoma. Expert Opin Investig Drugs. 2017;26(3):367–373. doi: 10.1080/13543784.2017.1288213. [DOI] [PubMed] [Google Scholar]
- 69.Isobe A, Sawada K, Kinose Y, Ohyagi-Hara C, Nakatsuka E, Makino H, Ogura T, Mizuno T, Suzuki N, Morii E, Nakamura K, Sawada I, Toda A, Hashimoto K, Mabuchi S, Ohta T, Morishige K, Kurachi H, Kimura T. Interleukin 6 receptor is an independent prognostic factor and a potential therapeutic target of ovarian cancer. PLoS One. 2015;10(2):e0118080. doi: 10.1371/journal.pone.0118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrari S, Danova M, Porta C, Brugnatelli S, Pugliese P, Bertolini A, Riccardi A. Recombinant human interleukin-11 (Neumega, rhIL-11) reduces thrombocytopenia in breast cancer patients receiving tandem autologous circulating progenitor cell transportation. Ann Hematol. 2002;81(6):354–6. doi: 10.1007/s00277-002-0459-2. [DOI] [PubMed] [Google Scholar]
- 71.Pasqualini R, Millikan RE, Christianson DR, Cardo-Vila M, Driessen WH, Giordano RJ, Hajitou A, Hoang AG, Wen S, Barnhart KF, Baze WB, Marcott VD, Hawke DH, Do KA, Navone NM, Efstathiou E, Troncoso P, Lobb RR, Logothetis CJ, Arap W. Targeting the interleukin-11 receptor alpha in metastatic prostate cancer: A first-in-man study. Cancer. 2015;121(14):2411–21. doi: 10.1002/cncr.29344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steward WP, Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013;109(1):1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma R, Vinayak M. alpha-Tocopherol attenuates NF-kappaB activation and pro-inflammatory cytokine IL-6 secretion in cancer-bearing mice. Biosci Rep. 2011;31(5):421–8. doi: 10.1042/BSR20100137. [DOI] [PubMed] [Google Scholar]
- 74.Lee SJ, Lim KT. Inhibitory effect of phytoglycoprotein on tumor necrosis factor-alpha and interleukin-6 at initiation stage of colon cancer in 1,2-dimethylhydrazine-treated ICR mice. Toxicol Appl Pharmacol. 2007;225(2):198–205. doi: 10.1016/j.taap.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 75.Zhao H, Zhang X, Chen X, Li Y, Ke Z, Tang T, Chai H, Guo AM, Chen H, Yang J. Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE2 and IL-6. Toxicol Appl Pharmacol. 2014;279(3):311–21. doi: 10.1016/j.taap.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Do EJ, Hwang SW, Kim SY, Ryu YM, Cho EA, Chung EJ, Park S, Lee HJ, Byeon JS, Ye BD, Yang DH, Park SH, Yang SK, Kim JH, Myung SJ. Suppression of colitis-associated carcinogenesis through modulation of IL-6/STAT3 pathway by balsalazide and VSL#3. J Gastroenterol Hepatol. 2016;31(8):1453–61. doi: 10.1111/jgh.13280. [DOI] [PubMed] [Google Scholar]
- 77.Lamy S, Akla N, Ouanouki A, Lord-Dufour S, Beliveau R. Diet-derived polyphenols inhibit angiogenesis by modulating the interleukin-6/STAT3 pathway. Exp Cell Res. 2012;318(13):1586–96. doi: 10.1016/j.yexcr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Michaud-Levesque J, Bousquet-Gagnon N, Beliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318(8):925–35. doi: 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 79.Shi W, Yan D, Zhao C, Xiao M, Wang Y, Ma H, Liu T, Qin H, Zhang C, Li C, Lin J, Li S, Lv J, Lin L. Inhibition of IL-6/STAT3 signaling in human cancer cells using Evista. Biochem Biophys Res Commun. 2017;491(1):159–165. doi: 10.1016/j.bbrc.2017.07.067. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Ma H, Zhao C, Liu T, Yan D, Jou D, Li H, Zhang C, Lu J, Li C, Lin J, Li S, Lin L. Growth-suppressive activity of raloxifene on liver cancer cells by targeting IL-6/GP130 signaling. Oncotarget. 2017;8(20):33683–33693. doi: 10.18632/oncotarget.16898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roeser JC, Leach SD, McAllister F. Emerging strategies for cancer immunoprevention. Oncogene. 2015;34(50):6029–39. doi: 10.1038/onc.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD, di Magliano MP. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73(20):6359–74. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]