Abstract
Various inflammatory dermatoses have been described in association with human immunodeficiency virus (HIV) infection. These either present in the usual way or in varied atypical presentations. This article gives a brief review about the etiopathogenesis and clinical presentation of the common inflammatory dermatoses associated with HIV such as psoriasis, reactive arthritis, seborrheic dermatitis, eosinophilic folliculitis, pruritic papular eruption, photosensitivity disorders prurigo nodularis, atopic dermatitis, and ichthyosis.
Key words: AIDS, clinical marker, human immunodeficiency virus, immunodeficiency, pruritic papular eruption
INTRODUCTION
A variety of inflammatory skin conditions have been described in human immunodeficiency virus (HIV)-infected patients since the advent of the disease. The group of inflammatory dermatoses in HIV has been enlisted in Table 1. These have a tremendous impact on the quality of life. In this article, we will be discussing only few important conditions which will bring us one step closer to early diagnosis and appropriate management of these inflammatory dermatoses in HIV patients.
Table 1.
Various inflammatory dermatoses in human immunodeficiency virus
PSORIASIS
Psoriasis is a chronic papulosquamous skin disease which can worsen or appear for the first time (often very severely) in HIV infection.
Incidence
It develops in 2%–5% of patients with HIV infection.[1] Psoriatic arthritis may develop in up to 10% of cases.[2]
Etiopathogenesis
The occurrence of psoriasis in HIV appears paradoxical as psoriasis is a Th1-mediated disorder and HIV shows Th2 immune predominance in advanced disease.[3] A strong association of two psoriasis major histocompatibility complex (MHC) variants, rs9264942 and rs3021366, and another Class III MHC variant rs9368699 is found to be associated with viral load.[4] Human retrovirus 5 has been implicated in the pathogenesis of psoriatic arthropathy, though not psoriasis. Human papillomavirus can also trigger psoriasis.[5]
Clinical features
Psoriasis in HIV-infected patients may be florid, severe, and atypical. The severity of presentation often correlates with the degree of immunosuppression. A higher frequency of guttate, inverse, rupioid, pustular, and generalized erythrodermic-type psoriasis has been reported in HIV-positive patients. Ostraceous and inverse psoriasis with psoriatic arthritis was the presenting features of advanced HIV infection in a 29-year-old male.[6] Secondary syphilis in an HIV-positive patient can mimic palmoplantar psoriasis.[7] The clinical course deteriorates as immunodeficiency advances. Erythrodermic psoriasis in HIV-infected patients may be a sign of Staphylococcus aureus septicemia, and psoriasis may improve dramatically with only intravenous antibiotics.[8] Joint involvement is severe enough to cause joint destruction and is usually associated with nail involvement and palmoplantar lesions.
Histopathology
The histology is frequently atypical. Munro's microabscesses are seen less frequently, with more irregular acanthosis and less suprapapillary thinning in HIV-associated psoriasis.
Treatment
Psoriasis in HIV-positive patients is often refractory to standard psoriasis treatments. Many effective drugs for psoriasis are immunosuppressive making the therapy more challenging. When started on highly active antiretroviral therapy (HAART), patient's psoriasis tends to subside as the immune system is reconstituted. Significant improvement of psoriasis was seen with zidovudine therapy. The response was dose related.
Topical therapy is the first-line recommended treatment for mild-to-moderate disease. Topical steroids, anthralin, ultraviolet light B (UVB) with or without tar, and psoralen plus ultraviolet A (PUVA) have been used with marginal success. PUVA reduces T-cell numbers and also eliminates circulating atypical T-lymphocytes. It is also a cutaneous carcinogen and hence Kaposi sarcoma is a complication that should be looked for, while on PUVA therapy. For moderate-to-severe disease, phototherapy is the first-line therapeutic agent. Oral retinoids (acitretin/etretinate) may be used as second-line treatment. Both skin and joint manifestations respond to acitretin therapy.[9] Acitretin has the advantage of not having any immunosuppressive properties, adverse effects being moderate and well tolerated. Etretinate followed by Re-PUVA is also effective systemic therapy, with rare adverse effects.[10] For more refractory, severe disease, cautious use of cyclosporine, methotrexate, hydroxyurea, and tumor necrosis factor-alpha inhibitors may be considered. Methotrexate should be considered a last resort owing to its immunosuppressive properties. Cyclosporine has shown moderate efficacy; however, its immunosuppressive properties limit its usage. Biologic therapy appears as a boon to patients with psoriasis and HIV as they do not have any negative effect on the CD4 count or viral load nor increase the rates of morbidity and mortality.[11] Infliximab and etanercept have shown good efficacy. Ustekinumab has been successful in treating severe psoriasis in a HIV patient.[12]
REACTIVE ARTHRITIS
Reactive arthritis (ReA) was first described in association with HIV in 1987. It is a triad of arthritis, conjunctivitis, and urethritis.
Incidence
Two small studies reported an incidence between 5% and 10% while another study reported it to be 0.3%–0.5%.[13]
Etiopathogenesis
Human leukocyte antigen B27, which is disease susceptibility factor, has been linked to the pathogenesis of ReA. Microbial peptides of certain infectious agents cause cytotoxic T-cell reaction by acting on the CD8 T-cells. HIV infection increases the relative number of CD8 T-cells, thereby increasing the pathogenesis.[14]
Clinical features
The course of ReA in HIV is more severe, progressive, and refractory to treatment than in non-HIV-positive patients. As in HIV-uninfected patients, antecedent history of genitourinary and gastrointestinal infection is common.[15]
Palmoplantar involvement is more common than truncal involvement. The skin lesions present as hyperkeratotic annular waxy papules, which usually progress to horny plaques. Mucocutaneous features are common classically keratoderma blenorrhagicum and circinate balanitis. The joint involvement is severe and disabling with multidigit dactylitis, synovitis, enthesitis, and knee involvement being more common. Involvement of the hip joint and spine and uveitis is less common.
Treatment
Nonsteroidal anti-inflammatory drugs remain the first line. Sulfasalazine 1–3 g/day forms the second line and should be given indefinitely. Sulfa allergy is common in HIV patients and may limit its usage. Acitretin is found to be very effective in these patients. Low-dose corticosteroids have not been found to be beneficial in HIV-infected individuals with ReA. Methotrexate, other immunosuppressive agents, and phototherapy should be used only with extreme caution. Antiretroviral therapy (ART) was found to effective in patients who failed to respond to conventional therapy.[16]
SEBORRHEIC DERMATITIS
Seborrheic dermatitis (SD) is one of the most common skin manifestations of HIV infection.
Incidence
It occurs in 85%–95% of patients with advanced HIV infection.[17] SD may occur at any stage of HIV infection; however, it often begins when CD4 counts are below 450–550 cells/μL range and becomes more severe at CD4 T-cell counts of 100 cells/μL.[18] The incidence of SD is increased in HIV-positive patients in whom zinc deficiency has been found.[19]
Etiopathogenesis
The etiology of SD is not entirely clear. Overgrowth of the Malassezia yeast (formerly called Pityrosporum ovale), failure of the immune system to regulate the fungus, and inflammation appear to be the chief factors that cause the dermatitis.[17]
Clinical features
SD is characterized by itchy erythematous plaques, accompanied by greasy flakes or scales [Figure 1]. It spreads beyond the areas of typical seborrheic distribution evolving usually into erythroderma.[20] Occasionally, sebopsoriasis or inverse psoriasis may be the presenting manifestation of HIV infection.[17]
Figure 1.
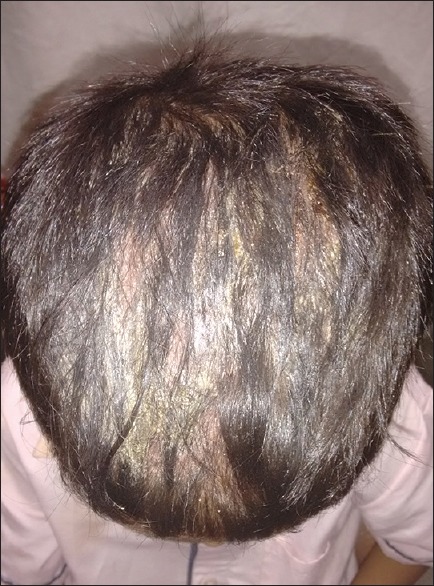
Greasy scales on the scalp in seborrheic dermatitis
Histopathology
Histologically, marked hyperkeratosis with confluent parakeratosis, follicular plugging, acanthosis with slight spongiosis, and lymphocyte and neutrophil exocytosis accompanied by keratinocyte necrosis and focal interface changes are suggestive of AIDS-related SD.[18]
Treatment
Specific treatments for SD are divided into three types: antimycotic agents remain the first choice, which include topical clotrimazole, sertaconazole, bifonazole, and selenium sulfide/sulfur preparations. The anti-inflammatory agents are second choice of treatment which can be used in combination with the first and include topical steroids, azelaic acid, and calcineurin inhibitors. Low-potency agents (e.g., hydrocortisone 1%) rather than high-potency corticosteroids (e.g., betamethasone dipropionate and triamcinolone) are recommended, especially for the face. Pimecrolimus 1% and tacrolimus cream are newer potential therapeutic options for facial SD in HIV patients.[11,21] The third group includes the keratolytic agents such as whole coal tar, crude coal tar extract, and salicylic acid either in shampoo, cream, or gel formulations. Shampoos may be used on the entire body, with avoidance of eyes and mucous membranes. Data on the use of oral antifungals are limited and the evidence is very low.
EOSINOPHILIC FOLLICULITIS OF HUMAN IMMUNODEFICIENCY VIRUS DISEASE
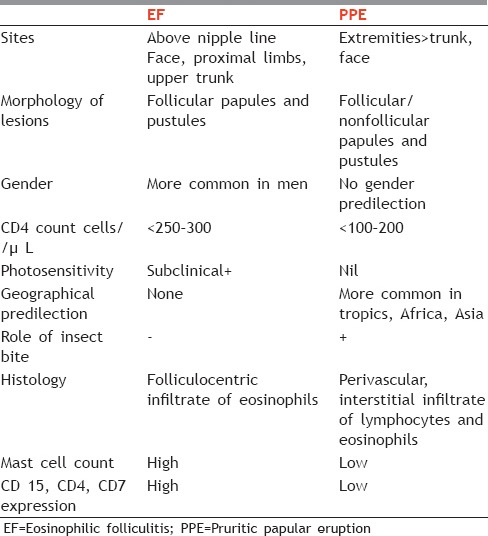
Eosinophilic folliculitis (EF) was originally described by Ofuji et al. It is a chronic, intensely pruritic condition that causes marked morbidity in affected HIV patients.
Incidence
EF occurs in patients with a CD4 count of <250 × 106/L with an incidence, in one series, of 9%.[22]
Etiopathogenesis
The pathogenesis of EF remains unclear. Fungi, yeasts, Demodex, and bacteria have been seen and implicated. EF has also been proposed to be a follicular hypersensitivity reaction or an autoimmune reaction to sebum.[23] Many patients have peripheral eosinophilia along with elevated IgE. High serum levels of CCL17, CCL26, and CCL27 may account for the development of HIV-associated EF.[24] Increased levels of interleukin (IL)-4, IL-5, RANTES, and eotaxin have been found in the lesional skin as compared to nonlesional, suggesting a Th2 pattern in HIV-associated EF.[25]
Clinical features
It is characterized by 2–3 mm erythematous papules or pustules, mostly affecting the shoulders, trunk, upper arms, neck, and forehead, where it can be disfiguring. The eruption waxes and wanes.
Histopathology
The histology of EF shows an inflammatory infiltrate, predominantly of eosinophils and lymphocytes which is folliculocentric with marked sebaceous lysis.
Treatment
Topical steroids give transient symptomatic relief of symptoms. Permethrin 5% on alternate days is beneficial by acting on the Demodex mite.[26] Ultraviolet B therapy, thrice weekly for 3–6 weeks, is an effective treatment. Relapse is frequent and weekly maintenance may be needed. Oral itraconazole (200 mg daily) is found to be effective due to its action on fungi or yeasts such as pityrosporum ovale which may be involved in the pathogenesis of EF.[27] Metronidazole 250 mg three times daily for 4 weeks was reported to be effective in clearing EF in 5 out of 5 patients in a study.[15] Oral indomethacin is efficacious, especially for classic EF.[28]
Isotretinoin at a dose of 20 mg daily for 6–8 weeks is effective and acts by decreasing the perifollicular infiltrate and sebum production.[23] Isotretinoin-loaded invasomal gel is a novel treatment which is found to be effective in EF.[29] Topical tacrolimus, dimethyl diphenyl sulfone, minocycline as well as oral prednisolone have also been successfully used in some patients.
PRURITIC PAPULAR ERUPTIONS
Pruritic papular eruption (PPE) of HIV has been used as a marker for early diagnosis of HIV infection and initiation of antiretroviral drugs in resource-poor settings.[30]
Incidence
Its prevalence is reported to be 10%–60%.[31] PPE is included in stage 2 of the WHO's clinical staging of HIV and occurs in patients with a low CD4 count (<100-200 cells/μL ).[32]
Etiopathogenesis
It is proposed to be the result of exaggerated immunological reaction to arthropod bites or stings. The total IgE levels were found to be increased in these patients.[33] In a study by Resneck et al., out of the total 108 patients, 86 patients (84%) had biopsy suggestive of arthropod bite reaction.[32]
Clinical features
PPE is characterized by chronic, intensely pruritic, follicular, or nonfollicular sterile pruritic papules and pustules on the extensor surfaces of limbs, trunk, and face with sparing of the palms and soles [Figure 2]. Secondary changes of excoriation, ulceration, pigmentation, scarring, and prurigo nodularis (PN)-like lesions can be seen. PPE closely resembles EF. The differentiating features are enumerated in Table 2. The other dermatological conditions closely simulating PPE include staphylococcal folliculitis, Demodex folliculitis, drug eruption, exuberant reactions to arthropod bites, photodermatitis, and crusted scabies.
Figure 2.
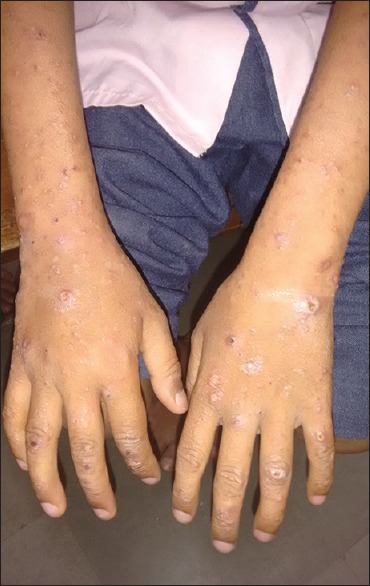
Pruritic papular eruption in a human immunodeficiency virus patient
Table 2.
Differentiating features of eosinophilic folliculitis and pruritic papular eruption
Histopathology
Histology shows a wedge-shaped perivascular and interstitial infiltrate of lymphocytes and eosinophils, similar to the microscopic findings of arthropod bites or stings.[32]
Treatment
Moderate-to-high potency topical steroids are given for symptomatic relief along with emollients and antihistamines. However, antiretroviral treatment remains the cornerstone of treatment giving dramatic results. Failure to respond to ART marks it an indicator for treatment failure and resistance. It was, therefore, suggested that PPE can be used to monitor response to ART in resource-poor areas.[34]
Narrow-band UVB (NB-UVB) phototherapy can be considered for resistant cases. Pentoxifylline by its TNF-alpha inhibitor effect, given in a dose of 400 mg three times daily for at least 8 weeks, has been effective. In a study, 10 of 11 patients showed a reduction in their pruritus, ranging from 22.6% to 87.3%.[35] Topical tacrolimus and itraconazole have also been tried but with poor outcomes. Thalidomide in a dose of 100 mg daily was found to be effective in a patient with recurrent PPE.[36]
PHOTOSENSITIVE DISORDERS
Photosensitivity in HIV-positive patients has long been a well-recognized clinical entity. It appears to be a manifestation of advanced disease. The affected individuals are sensitive to UVB, UVA, and visible light; however, sensitivity to UVB is most common.
Incidence
It is reported in approximately 5.4% of HIV-positive patients.[37] It occurs in patients with low CD4 count ≤50 cells/μL and worsens as the immunodeficiency progresses.[38]
Etiopathogenesis
It can be due to HIV infection per se or secondary to photosensitive drugs such as co-trimoxazole, nonsteroidal anti-inflammatory drugs, and HAART (protease inhibitors and nonnucleoside reverse transcriptase inhibitor classes have a porphyrinogenic risk).
Clinical features
HIV photodermatitis encompasses a variety of clinical manifestations, including polymorphic light eruption, actinic prurigo, chronic actinic dermatitis (CAD), porphyria cutanea tarda, photosensitive granuloma annulare, lichenoid photoeruption, widespread vitiligo-like depigmentation, and photodistributed hyperpigmentation. Of these, CAD and lichenoid photoeruption are the most common associations.[39] Patients with EF may also be subclinically photosensitive. Sun-exposed areas, such as the face, ears, scalp, posterior neck, upper back, “V” area of the chest, extensor aspect of arms and dorsae of hands, are involved.
Histopathology
Histology varies depending on the presentation, but features of dermatitis and eosinophilic infiltrate are common to all.
Treatment
It consists of avoidance of photoallergens, phototoxins, UV light as well as visible light. Initially, sun protection and topical steroids can be used. Administration of hydroxychloroquine at dosages of 200 mg orally once or twice daily has also been reported to be beneficial. Refractory cases have shown response to thalidomide, however, it has the potential to increase HIV viral load.[40]
PRURIGO NODULARIS (PN)
Incidence
PN was observed in 1.8% and 6.5% of HIV-positive patients in two studies.[41] It is more frequently seen in patients with CD4 cell counts below 50/μL.[42]
Etiopathogenesis
The pathogenesis of PN, particularly in HIV, is unclear and thought to be multifactorial.
Clinical features
PN is characterized by discrete, intensely pruritic, symmetric, papulonodular lesions primarily on the extensor surfaces of the extremities [Figure 3]. They may persist indefinitely and some may regress to leave scars.
Figure 3.
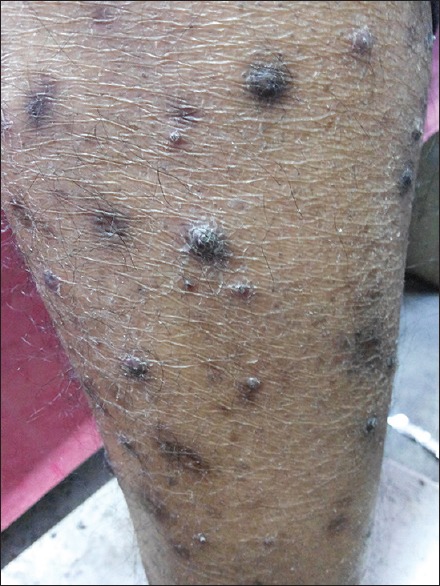
Prurigo nodularis in a human immunodeficiency virus patient
Treatment
The standard treatment includes antihistamines and topical and systemic corticosteroids. Thalidomide 100 mg/day for 5 months is well tolerated and effective. However, monitoring for the development of peripheral neuropathies should be done. Unemori et al.[43] reported dramatic improvement of recalcitrant PN in 2 HIV-infected patients within 2 weeks of adding raltegravir to ART. Other treatment options include ultraviolet phototherapy, psoralen photochemotherapy, benoxaprofen, and direct injection of the nodules with a steroid.[42]
ATOPIC DERMATITIS
Atopic-like dermatitis (AD) in HIV patients is characterized by erythematous scaly plaques with associated papules and vesicles. Both AD and HIV share a similar Th2 cytokine profile characterized by elevated IgE levels, increased eosinophils, and increased IL-4 and IL-5.[37] The stage of HIV disease does not influence the development of atopy. Patients may have disease exacerbation or recurrence or may develop allergic conjunctivitis and rhinitis. Cases of erythroderma have been reported too. On the other hand, others believe that atopic dermatitis is not affected by HIV; in fact, there is decrease in the frequency of atopic diseases and positive radioallergosorbent test in HIV-positive patients. The presence of severe pruritus and a history of atopy generally help in making a diagnosis. Histology shows superficial perivascular infiltrate of lymphocytes and eosinophils with spongiosis. Treatment includes emollients, topical steroids, oral antihistamines, and avoidance of irritants. Phototherapy has been used with success.
ICHTHYOSIS/XEROSIS
Xerosis is seen in approximately 30% of HIV-infected patients and has been attributed to impaired skin barrier function. Excessive levels of carotenoids, especially lycopene, and decrease in lipids in the dermis have been found in these patients. Poor nutritional status, chronic illness, and malabsorption are other implicated factors.[44] Acquired ichthyosis may be a marker of concomitant infection with HIV-1 and human T-cell lymphotropic virus-II in intravenous drug users.
It is more severe over lower limbs but may become generalized to a severe form of asteatotic eczema [Figure 4]. Treatment comprises generous application of emollients containing urea, lactic acid twice daily, and mid-potent steroids over eczematous areas.[44]
Figure 4.
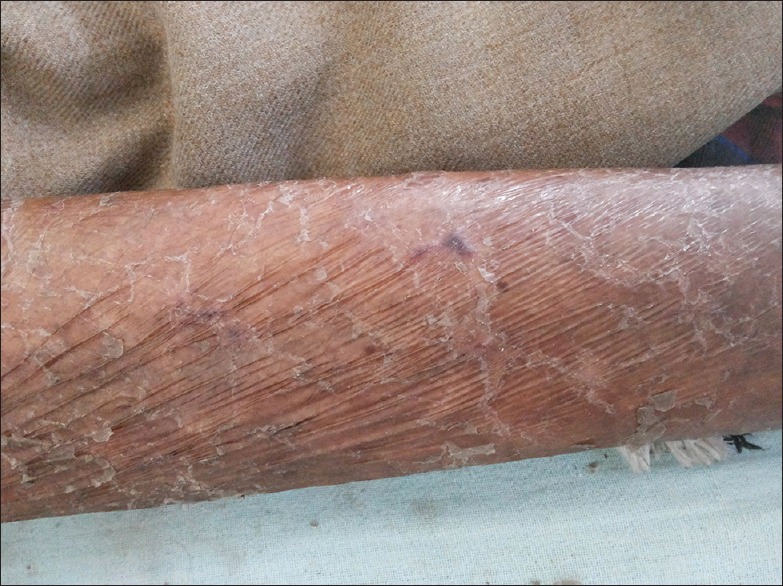
Severe xerosis of the limb leading to asteatotic eczema in a human immunodeficiency virus patient
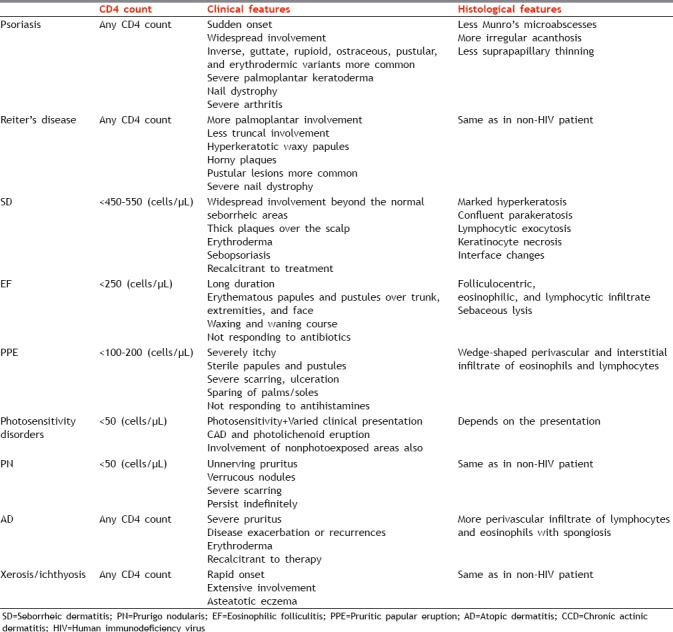
To conclude, the various inflammatory dermatoses can be present before the development of HIV infection or can exacerbate after the infection. The course is usually prolonged and recalcitrant to treatment. However, every dermatosis has certain features either clinically or histologically which are different from the features seen in non-HIV-positive patient as enumerated in Table 3. A knowledge of these features helps in early diagnosis of either the dermatoses or HIV infection and their appropriate management.
Table 3.
Features of inflammatory dermatoses in human immunodeficiency virus-positive patients
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Garman ME, Tyring SK. The cutaneous manifestations of HIV infection. Dermatol Clin. 2002;20:193–208. doi: 10.1016/s0733-8635(01)00011-0. [DOI] [PubMed] [Google Scholar]
- 2.Cockerell CJ, Friedman-Kien AE. Cutaneous manifestations of HIV infection. In: Mergan TC, Bartlett JG, Bolognesi D, editors. Textbook of AIDS Medicine. Philadelpia: Lippincott Williams and Wilkins; 1999. pp. 509–10. [Google Scholar]
- 3.Klein SA, Dobmeyer JM, Dobmeyer TS, Pape M, Ottmann OG, Helm EB, et al. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11:1111–8. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Nititham J, Gupta R, Zeng X, Hartogensis W, Nixon DF, Deeks SG, et al. Psoriasis risk SNPs and their association with HIV-1 control. Hum Immunol. 2017;78:179–84. doi: 10.1016/j.humimm.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain SP, Gulhane S, Pandey N, Bisne E. Human papilloma virus infection and psoriasis: Did human papilloma virus infection trigger psoriasis? Indian J Sex Transm Dis. 2015;36:201–3. doi: 10.4103/2589-0557.167178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castillo RL, Racaza GZ, Roa FD. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60–3. doi: 10.11622/smedj.2014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittencourt Mde J, Brito AC, Nascimento BA, Carvalho AH, Nascimento MD. A case of secondary syphilis mimicking palmoplantar psoriasis in HIV infected patient. An Bras Dermatol. 2015;90:216–9. doi: 10.1590/abd1806-4841.20153399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green MS, Prystowsky JH, Cohen SR, Cohen JI, Lebwohl MG. Infectious complications of erythrodermic psoriasis. J Am Acad Dermatol. 1996;34:911–4. doi: 10.1016/s0190-9622(96)90078-x. [DOI] [PubMed] [Google Scholar]
- 9.Jeong YS, Kim MS, Shin JH, Cho JK, Lee HI, Kim HJ, et al. A case of severe HIV-associated psoriasis successfully treated with acitretin therapy. Infect Chemother. 2014;46:115–9. doi: 10.3947/ic.2014.46.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buccheri L, Katchen BR, Karter AJ, Cohen SR. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711–5. [PubMed] [Google Scholar]
- 11.Calabrese LH, Zein N, Vassilopoulos D. Safety of antitumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: Hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis. 2004;63(Suppl 2):ii18–24. doi: 10.1136/ard.2004.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saeki H, Ito T, Hayashi M, Fukuchi O, Umezawa Y, Nobeyama Y, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653–5. doi: 10.1111/jdv.12531. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC, Fox R, Nelson KE, Saah A. HIV infection is not associated with Reiter's syndrome: Data from the Johns Hopkins Multicenter AIDS Cohort Study. AIDS. 1990;4:1149–51. [PubMed] [Google Scholar]
- 14.Altman EM, Centeno LV, Mahal M, Bielory L. AIDS-associated reiter's syndrome. Ann Allergy. 1994;72:307–16. [PubMed] [Google Scholar]
- 15.Adizie T, Moots RJ, Hodkinson B, French N, Adebajo AO. Inflammatory arthritis in HIV positive patients: A practical guide. BMC Infect Dis. 2016;16:100. doi: 10.1186/s12879-016-1389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott C, Brand A, Natha M. Reactive arthritis responding to antiretroviral therapy in an HIV-1-infected individual. Int J STD AIDS. 2012;23:373–4. doi: 10.1258/ijsa.2009.009400. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Madzia SE, Batra R. Etiology and management of seborrheic dermatitis. Dermatology. 2004;208:89–93. doi: 10.1159/000076478. [DOI] [PubMed] [Google Scholar]
- 18.Schaub NA, Drewe J, Sponagel L, Gilli L, Courvoisier S, Gyr N, et al. Is there a relation between risk groups or initial CD4 T-cell counts and prevalence of seborrheic dermatitis in HIV-infected patients? Dermatology. 1999;198:126–9. doi: 10.1159/000018087. [DOI] [PubMed] [Google Scholar]
- 19.Basset-Séguin N, Sotto A, Guillot B, Jourdan J, Guilhou JJ. Zinc status in HIV-infected patients: Relation to the presence or absence of seborrheic dermatitis. J Am Acad Dermatol. 1998;38:276–8. doi: 10.1016/s0190-9622(98)70250-6. [DOI] [PubMed] [Google Scholar]
- 20.Forrestel AK, Kovarik CL, Mosam A, Gupta D, Maurer TA, Micheletti RG, et al. Diffuse HIV-associated seborrheic dermatitis – A case series. Int J STD AIDS. 2016;27:1342–5. doi: 10.1177/0956462416641816. [DOI] [PubMed] [Google Scholar]
- 21.de Moraes AP, de Arruda EA, Vitoriano MA, de Moraes Filho MO, Bezerra FA, de Magalhães Holanda E, et al. An open-label efficacy pilot study with pimecrolimus cream 1% in adults with facial seborrhoeic dermatitis infected with HIV. J Eur Acad Dermatol Venereol. 2007;21:596–601. doi: 10.1111/j.1468-3083.2006.01923.x. [DOI] [PubMed] [Google Scholar]
- 22.Uthayakumar S, Nandwani R, Drinkwater T, Nayagam AT, Darley CR. The prevalence of skin disease in HIV infection and its relationship to the degree of immunosuppression. Br J Dermatol. 1997;137:595–8. doi: 10.1111/j.1365-2133.1997.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 23.Simpson-Dent S, Fearfield LA, Staughton RC. HIV associated eosinophilic folliculitis – differential diagnosis and management. Sex Transm Infect. 1999;75:291–3. doi: 10.1136/sti.75.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokobayashi H, Sugaya M, Miyagaki T, Kai H, Suga H, Yamada D, et al. Analysis of serum chemokine levels in patients with HIV-associated eosinophilic folliculitis. J Eur Acad Dermatol Venereol. 2013;27:e212–6. doi: 10.1111/j.1468-3083.2012.04592.x. [DOI] [PubMed] [Google Scholar]
- 25.Amerio P, Verdolini R, Proietto G, Feliciani C, Toto P, Shivji G, et al. Role of Th2 cytokines, RANTES and eotaxin in AIDS-associated eosinophilic folliculitis. Acta Derm Venereol. 2001;81:92–5. doi: 10.1080/00015550152384191. [DOI] [PubMed] [Google Scholar]
- 26.Blauvelt A, Plott RT, Spooner K, Stearn B, Davey RT, Turner ML, et al. Eosinophilic folliculitis associated with the acquired immunodeficiency syndrome responds well to permethrin. Arch Dermatol. 1995;131:360–1. [PubMed] [Google Scholar]
- 27.Berger TG, Heon V, King C, Schulze K, Conant MA. Itraconazole therapy for human immunodeficiency virus-associated eosinophilic folliculitis. Arch Dermatol. 1995;131:358–60. doi: 10.1001/archderm.131.3.358. [DOI] [PubMed] [Google Scholar]
- 28.Nomura T, Katoh M, Yamamoto Y, Miyachi Y, Kabashima K. Eosinophilic pustular folliculitis: A published work-based comprehensive analysis of therapeutic responsiveness. J Dermatol. 2016;43:919–27. doi: 10.1111/1346-8138.13287. [DOI] [PubMed] [Google Scholar]
- 29.Dwivedi M, Sharma V, Pathak K. Pilosebaceous targeting by isotretenoin-loaded invasomal gel for the treatment of eosinophilic pustular folliculitis: Optimization, efficacy and cellular analysis. Drug Dev Ind Pharm. 2017;43:293–304. doi: 10.1080/03639045.2016.1239628. [DOI] [PubMed] [Google Scholar]
- 30.Samanta M, Kundu C, Sarkar M, Bhattacharyya S, Chatterjee S. Papular pruritic eruptions: A marker of progressive HIV disease in children: Experience from Eastern India. Indian J Sex Transm Dis. 2009;30:79–83. doi: 10.4103/2589-0557.62762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naswa S, Khambhati R, Marfatia YS. Pruritic papular eruptions as presenting illness of HIV. Indian J Sex Transm Dis. 2011;32:118–20. doi: 10.4103/2589-0557.85420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resneck JS, Jr, Van Beek M, Furmanski L, Oyugi J, LeBoit PE, Katabira E, et al. Etiology of pruritic papular eruption with HIV infection in uganda. JAMA. 2004;292:2614–21. doi: 10.1001/jama.292.21.2614. [DOI] [PubMed] [Google Scholar]
- 33.Jiamton S, Kaewarpai T, Ekapo P, Kulthanan K, Hunnangkul S, Boitano JJ, et al. Total IgE, mosquito saliva specific IgE and CD4+ count in HIV-infected patients with and without pruritic papular eruptions. Asian Pac J Allergy Immunol. 2014;32:53–9. doi: 10.12932/AP0317.32.1.2014. [DOI] [PubMed] [Google Scholar]
- 34.Farsani TT, Kore S, Nadol P, Ramam M, Thierman SJ, Leslie K, et al. Etiology and risk factors associated with a pruritic papular eruption in people living with HIV in India. J Int AIDS Soc. 2013;16:17325. doi: 10.7448/IAS.16.1.17325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berman B, Flores F, Burke G., 3rd Efficacy of pentoxifylline in the treatment of pruritic papular eruption of HIV-infected persons. J Am Acad Dermatol. 1998;38:955–9. doi: 10.1016/s0190-9622(98)70159-8. [DOI] [PubMed] [Google Scholar]
- 36.Wernham AG, Vydianath B, Chua SL. Thalidomide-A novel therapeutic approach for pruritic papular eruption of HIV. JAAD Case Rep. 2015;1:109–11. doi: 10.1016/j.jdcr.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dlova NC, Mosam A. Inflammatory noninfectious dermatoses of HIV. Dermatol Clin. 2006;24:439–48, vi. doi: 10.1016/j.det.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Bilu D, Mamelak AJ, Nguyen RH, Queiroz PC, Kowalski J, Morison WL, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175–83. doi: 10.1111/j.1600-0781.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 39.Philips RC, Motaparthi K, Krishnan B, Hsu S. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6. [PubMed] [Google Scholar]
- 40.Vin-Christian K, Epstein JH, Maurer TA, McCalmont TH, Berger TG. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361–9. doi: 10.1111/j.1346-8138.2000.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 41.Matthews SN, Cockerell CJ. Prurigo nodularis in HIV-infected individuals. Int J Dermatol. 1998;37:401–9. doi: 10.1046/j.1365-4362.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- 42.Maurer TA. Dermatologic manifestations of HIV infection. Top HIV Med. 2005;13:149–54. [PubMed] [Google Scholar]
- 43.Unemori P, Leslie KS, Maurer T. Persistent prurigo nodularis responsive to initiation of combination therapy with raltegravir. Arch Dermatol. 2010;146:682–3. doi: 10.1001/archdermatol.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mischo M, von Kobyletzki LB, Bründermann E, Schmidt DA, Potthoff A, Brockmeyer NH, et al. Similar appearance, different mechanisms: Xerosis in HIV, atopic dermatitis and ageing. Exp Dermatol. 2014;23:446–8. doi: 10.1111/exd.12425. [DOI] [PubMed] [Google Scholar]


