Abstract
Objectives:
The objective of the study was to understand the role of self-monitoring of blood glucose (SMBG) for better management of glycemic fluctuations, reducing the risk of complications, and the associated cost benefits for diabetes patients in India.
Materials and Methods:
An Excel-based Cost Impact Model was developed to analyze the impact of SMBG by calculating the savings over a 10-year time period. A literature review was undertaken to model the impact of SMBG on the risk of complications and cardiovascular morbidities. The model was developed based on inputs from previous studies.
Results:
In the base case, SMBG cohort was associated with a 10-year discounted cost of INR 718,340, resulting in an estimated saving of INR 120,173 compared to no SMBG cohort. Implementation of a once-daily SMBG protocol, for a decade, can reduce the complication-related costs. More frequent SMBG and tri-monthly hemoglobin A1c tests along with lifestyle changes can significantly reduce the financial burden on the patient over the lifespan.
Conclusion:
Our study has shown that proactive management of diabetes with SMBG can improve treatment outcomes and reduce morbidity and mortality associated with this disease. Near-normal blood glucose levels can bring in cost savings in the form of reduced long-term complications and avoidance of repeated hospitalization for the management of such complications, along with an improved quality of life.
Keywords: Control and complications, current status of diabetes care, DiabCare India, diabetes mellitus, self-Monitoring of blood glucose
INTRODUCTION
Diabetes mellitus is a systemic and progressive disease involving multiple organ systems with a profound impact on the quality of life (QOL). Persistent hyperglycemia results in microvascular and macrovascular complications affecting renal, ophthalmic, cardiac, neurological, and dermatological systems.
Postprandial hyperglycemia (PPHG) is an independent and a well-recognized risk factor for cardiovascular disease and mortality in newly diagnosed diabetes patients as well as in patients with established disease.[1,2]
Maintaining a tight glycemic control is, therefore, critical for optimal and cost-effective disease management; yet, on the contrary, ~70% of the diagnosed diabetes population has “uncontrolled/suboptimally controlled” diabetes (hemoglobin A1c [HbA1c] levels >7%).[3,4] A recent study conducted in Bangladesh demonstrated that the annual median cost of managing diabetes for patients with good glycemic control was lower when compared to patients with poor glycemic control, which was statistically significant (P = 0.006). Cost-effectiveness improves when there is good glycemic control with practices such as self-monitoring of blood glucose (SMBG).[5]
Comparative studies in patients with type 2 diabetes on insulin[1,6,7] across cohorts of regular SMBG users versus SMBG nonusers have demonstrated that HbA1c levels in regular SMBG users were lower by 0.7% to 1.1%.
Research shows that every 1% decrease in the HbA1c level in a diabetes patient can remarkably lower the risk of complications.[8,9,10] With each 1 unit reduction in HbA1c subsequent to SMBG, the risk of cardiovascular, pedal, ocular, and renal complications reduces by 14%, 43%, 19%, and 37%, respectively. However, HbA1c does not depict short-term glycemic variability and is of little value in short-term decision-making.[11,12,13]
Mean amplitude of glycemic excursion (MAGE) is also an important marker in assessing the damaging effect of fluctuating hyperglycemia in diabetes patients.[14]
The one-time cost of the equipment for SMBG and the recurrent cost of disposables are considered a barrier for compliance. However, several studies have revealed that SMBG can significantly lower the overall financial implications on the patient.[15,16]
The International Diabetes Federation (IDF) and American Diabetes Association (ADA) recommend SMBG as an integral component of effective diabetes management. Despite substantial evidence of the benefits of SMBG, compliance to self-monitoring is reported “low” globally,[17] and especially in India, where patients usually seek treatment after complications have set in. This may be attributed to various factors such as lack of awareness, literacy levels, and the perception that SMBG is painful and costly.[18]
There is a general lack of information on the economic analysis of the cost benefit of regular SMBG versus no SMBG over an extended period of time in the Indian context. This study aims to understand the role of SMBG for better management of glycemic fluctuations, reducing the risk of complications, and the associated cost benefits for diabetes patients in India. The study, therefore, highlights the need for a more structured intervention at an early stage of the disease through improved awareness of the benefits of good glycemic control with SMBG.[4,10,19]
MATERIALS AND METHODS
An Excel-based economic model with a 10-year time horizon was developed to estimate the impact of once daily SMBG testing on the risk of diabetes-related complications and the cost of managing such complications. The model is based on three key associations:
Regular SMBG testing results in a reduction in HbA1c levels
Lower HbA1c levels maintained over the long term are associated with lower risks of diabetes-related complications
A lower risk of complications results in a lower financial risk to patients.
The model compared the outcomes of two cohorts: type 2 diabetes patients not conducting SMBG and patients conducting SMBG at least once daily.
Risk of complications
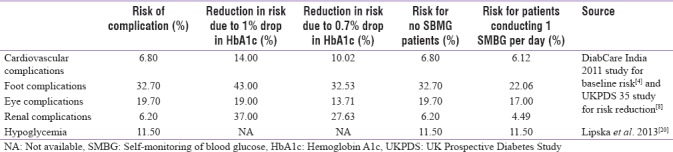
Literature searching was conducted to identify relevant inputs for the model. According to the DiabCare India 2011 Study,[4] of 6168 Indian participants with predominantly type 2 diabetes, the mean HbA1c level was 8.9 ± 2.1%, which we used as the base HbA1c level in our model.[4] Separately, a large health-care database study from the USA reported that daily SMBG testing was associated with an average reduction in HbA1c levels of 0.7% (i.e., to 8.2%).[1]
The prevalence rates of diabetes-related complications were taken from the DiabCare India 2011 Study [Table 1].[4] Risk of complications is directly related to HbA1c level in type 2 diabetes mellitus patients, i.e., the risk of complications decreases with a decrease in HbA1c level. Due to the absence of suitable studies from India on the reduction in risk of complications due to lower HbA1c levels, we used the risk reduction from a seminal study conducted on diabetes patients in the UK, which presented the risk reduction due to a 1% decrease in HbA1c levels[8] [Table 1]. Given that our model assumed a 0.7% drop in HbA1c with the use of SMBG once daily, the reduction in risk of complications was calculated for such a decrease based on the 1% drop in HbA1c level from the UK study [Table 1]. The risk of hypoglycemic events was taken from another large, database study from the USA;[20] although no difference was reported for patients with HbA1c levels of 8.9% and 8.2% [Table 1]. The risks of complications for the two cohorts are summarized in the final two columns of Table 1.
Table 1.
Risk of Complications
Costs
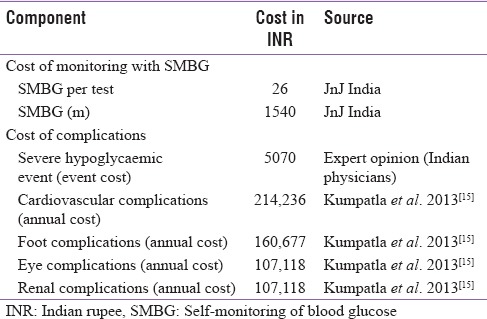
The cost inputs for managing complications were obtained from a study conducted in an Indian health-care institution[15] and from a survey of practicing physicians. The expected cost of monitoring diabetes and treating complications associated with diabetes is shown in Table 2. An annual medical inflation rate of 13% (from the Insurance and Regulatory Departmental Report – Medical Inflation in India) was applied to derive costs for 2015. When projecting over a 10-year time horizon, we used the standard discounting rate of 3% (WHO guidelines on cost-effectiveness) so as to estimate the present value of projected costs. As evident from Table 2, the cost of managing diabetes-related complications represents a significant burden of the disease.
Table 2.
Costs of monitoring type 2 diabetes mellitus and disease-related complications
Model outcomes
This model enables the comparison of the cost for patients who measure their blood glucose by self-monitoring once a day to those patients who do not measure their blood glucose levels regularly.
The model calculated the risk-adjusted costs over 10 years for each cohort by multiplying the risk of each complication by the cost of managing that complication. Costs for future years were discounted to obtain the present-day equivalent cost. The costs were then summed to obtain a total potential financial risk for patients over 10 years.
RESULTS
With a baseline HbA1c of 8.9% and no SMBG monitoring, a type 2 diabetes patient is at high risk of developing complications, thereby exposing him to a financial risk of INR 838,513 over a 10-year time horizon. In comparison, a patient performing SMBG once a day, with an additional cost of ~INR 26 every day over 10 years, would have a reduced risk of complications and a lower financial risk of INR 718,340 [Table 3].
Table 3.
Cost burden reduction with single self-monitoring of blood glucose over 10 years
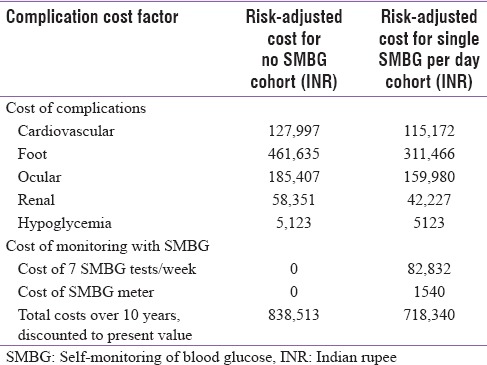
Comparing the two cohorts, patients conducting daily SMBG testing would have a reduction in financial risk over 10 years of approximately INR 120,173 compared to those who do not conduct SMBG testing regularly. This cost saving is due to the reduced cost of managing diabetes-related complications in patients conducting SMBG testing [Table 3].
Implementation of a once-daily SMBG protocol, for a decade, can substantially reduce the potential complication-related costs [Table 3]. More frequent SMBG and trimonthly HbA1c tests along with lifestyle changes can substantially reduce the financial burden on the patient over their lifespan.
It should be noted that diabetes patients require adequate training in SMBG to ensure optimal adherence to recommended protocols. Affordability of equipment and easy availability of test strips are vital factors for compliance.
DISCUSSION
Comparative studies have already demonstrated that across cohorts of regular SMBG users versus no SMBG users among type 2 diabetes patients on insulin, HbA1c levels in regular SMBG users were lower in comparison to the SMBG nonusers.[1,6,7] Studies have also demonstrated that, with every 1% reduction in HbA1c, there is a considerable reduction in the risk of complications.[4,8] When modeled for ascertaining a consequent reduction in economic burden, the cost-benefit model demonstrates that at a cost of INR 26 spent per day over 10 years, the SMBG cohort was associated with a 10-year discounted, risk-adjusted cost of INR 718,340, resulting in an estimated financial risk reduction worth INR 120,173 compared to the no SMBG cohort. This indicates that the use of SMBG is a cost-beneficial intervention that not only helps reduce the financial risk but also allows better disease management by improving the patient's risk profile and disease prognosis.
Landmark trials such as diabetes control and complications trial and UK Prospective Diabetes Study[21] have also highlighted the importance of blood glucose control in reducing the risk of complications in diabetes. SMBG provides evidence-based blood glucose targets that may be included in the routine management of diabetes. It also provides a stimulus to the patients for a better understanding of their disease, improving medication compliance and lifestyle changes, eventually resulting in an improved glycemic control.[22] IDF and ADA also recommend regular SMBG to ensure the success of the diabetes management plan. Patient record of SMBG values, food intake, medication, and duration of exercise would help to interpret the SMBG results. This would provide health-care professionals' invaluable information on hypoglycemic events, to recommend lifestyle changes and targeted therapy for the management of diabetes.
Despite evidence of the role of SMBG in reducing the complications of diabetes and the recommendations of IDF and ADA regarding SMBG, its uptake continues to remain low. This is probably because an average diabetes patient in India has low awareness of target HbA1c levels, role of SMBG, risk of long-term complications, and financial risk thereof, if appropriate diet, lifestyle and medications are not adhered to. The perceived high cost and lack of access to regular monitoring such as glucometer and test strips are another impediment in the overall disease management. Lack of a fail-safe method of documentation and analysis of blood glucose levels further complicates disease management among diabetes patients. Poverty, illiteracy, low disease awareness, and hectic everyday schedule encumber the diabetes patients to monitor and document their blood glucose levels on a daily basis.
Education on the importance of pre- and post-prandial blood glucose levels, HbA1c, SMBG, and MAGE in portending the impending complications could improve compliance, QOL, and patient outcomes.
Diabetes is a chronic progressive disease affecting major systems in the body, resulting in serious complications that lay a huge economic burden on the patient. This economic burden increases multifold in case it afflicts an earning member of the family due to loss of income, at times forcing the family into a catastrophic financial situation. This assumes more significance in a largely out-of-pocket market such as India, where >70% of health-care expenditure is out of pocket.
With the public health-care system mostly focused on addressing the burden of communicable diseases, there is little room for resources and infrastructure to be targeted to address the burden of noncommunicable diseases such as diabetes.
A targeted two-pronged strategy involving primary preventive measures of improved health education and lifestyle changes as well as secondary prevention through prompt diagnosis, regular monitoring, and timely intervention can arrest the impending disease burden.
Access to less expensive glucometers and test strips augmented with better awareness of benefits of regular glucose monitoring, enhanced focus on teaching self-management skills, and motivating patients to make lifestyle changes in response to blood glucose readings would help improve disease outcomes.
A key consideration is that performing SMBG alone does not lower blood glucose levels. To be useful, the information must be integrated into clinical and self-management plans.[23,24]
This analysis is associated with a number of limitations. While the prevalence of diabetes complications was based on a large national survey from India, the reduction in complications due to a decrease in HbA1c levels was based on a study of diabetes patients in the UK, of whom only 10% were of Indian ethnicity.[4,8] Furthermore, the data on the reduction of HbA1c levels due to regular SMBG testing were derived from a US study, due to the lack of similar data from India.[1] However, Indian clinicians reported that the results from these studies were applicable to an Indian population. The costs of each complication were taken from an existing study at one Indian diabetes care center.[15] However, the costing methodology of the study relied on patient recall and the complications measured may be more severe than those experienced by all patients in our model. As such, future evaluations should adopt a more robust approach in costing the complications to improve accuracy. Overall, this model serves as an estimate of the potential financial risk associated with diabetes complications, rather than providing a definitive cost.
CONCLUSION
Diabetes patients need to be cognizant of the impending long-term disease complications, the poor QOL, and associated financial burden if blood glucose levels are not managed optimally. Knowledge of real-time blood glucose levels on a daily basis can serve as a stimulus for improved medication compliance and lifestyle measures. Daily SMBG and HbA1c every 3 months provide a MAGE construct and PPHG to guide treating physicians to adapt treatment regimens best suited to individual patient needs. Proactive management of diabetes with SMBG can improve treatment outcomes and reduce morbidity and mortality associated with this disease. Near-normal blood glucose levels can bring in cost savings in the form of reduced long-term complications and avoidance of repeated hospitalization for the management of such complications, along with an improved QOL.
Financial support and sponsorship
This study was financially supported by Johnson and Johnson Pvt. Ltd., SmartAnalyst India Pvt. Ltd., and Costello Medical Singapore Pte Ltd.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was supported by Johnson and Johnson Pvt. Ltd. The authors would also like to thank SmartAnalyst India Pvt., Ltd. and Costello Medical Singapore Pte Ltd., for statistical analysis, data management, and project management services.
REFERENCES
- 1.Karter AJ, Ackerson LM, Darbinian JA, D'Agostino RB, Jr, Ferrara A, Liu J, et al. Self-monitoring of blood glucose levels and glycemic control: The Northern California Kaiser Permanente Diabetes Registry. Am J Med. 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 2.Unnikrishnan R, Mohan V. Pancreatic diseases and diabetes. In: Holt RI, Cockram CS, Flyvbjerg A, Goldstein BJ, editors. Textbook of Diabetes. 4th ed. Oxford: Wiley-Blackwell; 2010. pp. 298–309. [Google Scholar]
- 3.Wambui Charity K, Kumar AMV, Hinderaker SG, Chinnakali P, Pastakia SD, Kamano J, et al. Do diabetes mellitus patients adhere to self-monitoring of blood glucose (SMBG) and is this associated with glycemic control? Experiences from a SMBG program in Western Kenya. Diabetes Res Clin Pract. 2016;112:37–43. doi: 10.1016/j.diabres.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Mohan V, Shah SN, Joshi SR, Seshiah V, Sahay BK, Banerjee S, et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: Results from the DiabCare India 2011 study. Indian J Endocrinol Metab. 2014;18:370–8. doi: 10.4103/2230-8210.129715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afroz A, Chowdhury HA, Shahjahan M, Hafez MA, Hassan MN, Ali L, et al. Association of good glycemic control and cost of diabetes care: Experience from a tertiary care hospital in Bangladesh. Diabetes Res Clin Pract. 2016;120:142–8. doi: 10.1016/j.diabres.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Mast O, Tan A, Punjabi K. Usage of Self-monitoring of Blood Glucose (SMBG) by Diabetes Therapy Type in India, ISPOR. 17th Annual European Congress 2014; Amsterdam, The Netherlands, Roche. 2014. p. PDB174. [DOI] [PubMed] [Google Scholar]
- 7.Kibriya MG, Ali L, Banik NG, Khan AK. Home monitoring of blood glucose (HMBG) in type-2 diabetes mellitus in a developing country. Diabetes Res Clin Pract. 1999;46:253–7. doi: 10.1016/s0168-8227(99)00093-5. [DOI] [PubMed] [Google Scholar]
- 8.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerich J, Raskin P, Jean-Louis L, Purkayastha D, Baron MA. PRESERVE-beta: Two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care. 2005;28:2093–9. doi: 10.2337/diacare.28.9.2093. [DOI] [PubMed] [Google Scholar]
- 10.Rajasekharan D, Kulkarni V, Unnikrishnan B, Kumar N, Holla R, Thapar R, et al. Self-care activities among patients with diabetes attending a tertiary care hospital in Mangalore Karnataka, India. Ann Med Health Sci Res. 2015;5:59–64. doi: 10.4103/2141-9248.149791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrivastava SR, Shrivastava PS, Ramasamy J. Health-care of elderly: Determinants, needs and services. Int J Prev Med. 2013;4:1224–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Khadilkar KS, Bandgar T, Shivane V, Lila A, Shah N. Current concepts in blood glucose monitoring. Indian J Endocrinol Metab. 2013;17:S643–9. doi: 10.4103/2230-8210.123556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain JJ, Rhinehart AS, Shaefer CF, Jr, Neuman A. Diagnosis and Management of Diabetes: Synopsis of the American Diabetes Association Standards of Medical Care in Diabetes. 2016 doi: 10.7326/M15-3016. [DOI] [PubMed] [Google Scholar]
- 14.Hurel SJ, Mohan V. Clinical decision making: Managing postprandial hyperglycemia. J Assoc Physicians India. 2006;54:871–6. [PubMed] [Google Scholar]
- 15.Kumpatla S, Kothandan H, Tharkar S, Viswanathan V. The costs of treating long-term diabetic complications in a developing country: A study from India. J Assoc Physicians India. 2013;61:102–9. [PubMed] [Google Scholar]
- 16.Leventhal H, Phillips LA, Burns E. The common-sense model of self-regulation (CSM): A dynamic framework for understanding illness self-management. J Behav Med. 2016;39:935–46. doi: 10.1007/s10865-016-9782-2. [DOI] [PubMed] [Google Scholar]
- 17.Karter AJ, Ferrara A, Darbinian JA, Ackerson LM, Selby JV. Self-monitoring of blood glucose: Language and financial barriers in a managed care population with diabetes. Diabetes Care. 2000;23:477–83. doi: 10.2337/diacare.23.4.477. [DOI] [PubMed] [Google Scholar]
- 18.Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord. 2013;12:14. doi: 10.1186/2251-6581-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirk JK, Stegner J. Self-monitoring of blood glucose: Practical aspects. J Diabetes Sci Technol. 2010;4:435–9. doi: 10.1177/193229681000400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipska KJ, Warton EM, Huang ES, Moffet HH, Inzucchi SE, Krumholz HM, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes: The diabetes and aging study. Diabetes Care. 2013;36:3535–42. doi: 10.2337/dc13-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill J, Hicks D, James J, Agbasi N, Cook J, Diggle J, et al. Blood Glucose Monitoring Guidelines, Consensus Document [Version 1.0] 2014 [Google Scholar]
- 22.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: Results from the structured testing program study. Diabetes Care. 2011;34:262–7. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkin CG, Davidson JA. Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol. 2009;3:500–8. doi: 10.1177/193229680900300314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aschner P, Adler A, Bailey C, Chan JCN, Colagiuri S, Day C, et al. IDF Clinical Practice Recommendations for Managing Type 2 Diabetes in Primary Care: International Diabetes Federation. 2017 doi: 10.1016/j.diabres.2017.09.002. [DOI] [PubMed] [Google Scholar]


