Abstract
Introduction:
Obesity is associated with several complications like metabolic syndrome. Many professional athletes adopt a sedentary lifestyle after retirement. This study was aimed at assessing the risk of developing obesity, insulin resistance (IR), and metabolic syndrome among former power-sports athletes, compared with age-matched active athletes and nonathletes.
Materials and Methods:
The study was conducted in Mashhad during 2012–2014. The individuals were recruited through announcements and were divided into three groups of active athletes (n = 34), ex-athletes (n = 30), and nonathletes (n = 30). Demographic and anthropometric data were collected and biochemical factors including low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol, triglycerides (TG), fasting plasma glucose, insulin, and high-sensitive C-reactive protein were measured.
Results:
Ex-athletes had significantly higher mean values of weight, body mass index, diastolic blood pressure, LDL-C, insulin, homeostatic model assessment (HOMA) IR, and HOMA β-cell function (HOMA-%β-cell) compared with active athletes and nonathletes (P < 0.001, P < 0.001, P < 0.001, P = 0.03, P = 0.01, P = 0.02, and P = 0.01, respectively). However, mean values of HDL-C was significantly lower in ex-athletes compared with nonathletes (P < 0.001). The prevalence of metabolic syndrome showed no significant difference among three groups, although its mean was higher among ex-athletes.
Conclusions:
The results showed that abandoning regular athletic exercise and weight cycling in power sports athletes leads to adverse outcomes such as obesity and IR. Although higher IR might not necessarily result in metabolic syndrome in short term, it could cause metabolic syndrome in the long run.
Keywords: Former athlete, insulin resistance, metabolic syndrome, obesity, risk factor
INTRODUCTION
Metabolic syndrome is a constellation of abnormalities generally considered to include abdominal obesity, high levels of fasting plasma glucose (FPG) or impaired glucose tolerance, dyslipidemia, and high blood pressure that together increase the risk of developing overt diabetes mellitus and cardiovascular diseases.[1]
According to the World Health Organization, obesity is now a global epidemic; there are estimated to be 250 million (7% of the adult population) obese people in the world. About 27% of the American adult population is obese and the prevalence of overweight and obesity for adults ranges from 15% to 60% globally.[2] Obesity is associated with several problems, including cardiovascular diseases, diabetes, high blood pressure, and other metabolic disorders.[3]
Dyslipidemia and obesity are serious public health problems and should be addressed through prevention programs and education. Treatment includes changes in lifestyle with healthy eating habits, maintenance or acquisition of adequate body mass, regular exercise, and using lipid-lowering agents.[4,5]
The importance of physical activity and its positive effects on reducing the incidence of cardiovascular diseases and type 2 diabetes mellitus is well established. Long-term exercise can reduce the risk factors of cardiovascular diseases, such as serum level of lipids, obesity, blood pressure, and glucose intolerance.[6] Regular exercise can reduce serum triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), total cholesterol, insulin resistance (IR), body mass index (BMI), and body fat to a desirable level while increasing levels of high-density lipoprotein cholesterol (HDL-C), lean body mass, and basal metabolism. Among different kinds of sports, aerobic exercise has the greatest impact on lipid profile.[7]
Many professional athletes adopt a sedentary lifestyle after their retirement from professional sports, and thus obesity could be a potential threat to their health. The purpose of this study was to assess the prevalence and risk of obesity and metabolic syndrome among former athletes and compare them to the age-matched active athletes and nonathletes.
MATERIALS AND METHODS
Ethics
The study was designed and performed in accordance with the fundamentals of Helsinki Declaration and approved by Ethics Committee of the affiliated University, Mashhad, Iran (approval code: 901121).
Before the study, all individuals were given a thorough explanation about purposes, risks, and benefits of the study. They also read and signed an informed written consent form.
Study design and population
The study was conducted during 2012–2014 in Mashhad, Iran. The study group consisted of 30 former male athletes who had participated in national and regional sports competitions before and had been retired from all sport activities 3–5 years before the study. The inclusion criteria for this group were being aged 25–40 years, being in good health condition, and history of participation in power sports (e.g., wrestling, judo, and powerlifting) competitions and events.
The first control group consisted of 34 age-matched males who were actively participating in power sport events and competitions. These individuals were randomly selected from different sport clubs and boards across Mashhad, Iran.
The second control group included 30 age-matched males with no history of participation in professional sport events and athletic trainings who were selected randomly through the announcement of a declaration in the affiliated university in Mashhad, Iran.
Exclusion criteria were being younger than 25 or older than 40 years, female gender, and having any of the following diseases: hereditary dyslipidemias, edema, cachexia, cirrhosis, ascites, and known AIDS or hepatitis-B.
Statistics
Statistical Package for Social Sciences (SPSS) version 22.0 (IBM Statistics, Chicago, IL, United States) was used to analyze data. P < 0.05 was considered as statistically significant in all the applied tests.
In order to investigate normal distribution of measured variables among the three groups, One-Sample Kolmogorov–Smirnov test was used. Descriptive statistics were used to present the data as mean and standard deviation (SD) for variables with normal distribution, as well as median and interquartile range (IQR) for variables without normal distribution.
For quantitative variables with normal distribution, parametric one-way analysis of variances (ANOVA), and Tukey honest significant difference (HSD) tests were used to compare the means between all three groups and each two of them one-by-one, respectively. For quantitative variables without normal distribution, Kruskal–Wallis and Mann–Whitney U tests were applied. For comparing qualitative variables, the Chi-squared test was performed.
Anthropometric measurements
Height was measured to the nearest 0.1 cm, using a portable stadiometer (OTM, Tehran, Iran) with the individuals removed their shoes and stretching to the maximum height and their head positioned in the Frankfort plane.[8] Weight (kg), BMI (kg/m2) and body fat percentage were determined by a TANITA body composition analyzer (type: BC–418MA; TANITA corporation, Tokyo, Japan). Waist circumference (WC) was measured to the nearest 0.1 cm at the mid-point between the lower rib and the upper margin of the iliac crest in a horizontal plane using a non-stretching tape with an insertion buckle at one end.[8] Systolic and diastolic blood pressures (SBP, DBP; mmHg) were measured while resting in a relaxed sitting position using standard auscultatory method by an ALPK2 sphygmomanometer (model: 500-v, ALPK2, Japan).
Biochemical assays
All individuals were asked to fast from 8–10 pm to 8–10 am and not to do any sports activities during the fasting period. The blood samples were taken from all individuals after the 12 h fasting during 8–10 am on the following day at a university-affiliated nutrition clinic, Mashhad, Iran. A 10 ml blood sample was collected from the cubital vein by venipuncture technique and stored in anticoagulant (heparin) included tubes for further analyses. The blood samples were then analyzed in a university-affiliated laboratory.
Lipid profile (LDL-C, HDL-C, and TG; mg/dl) and FPG (mg/dl) were measured by a Biotecnica autoanalyzer device (model: BT3500, Diamond Diagnostics, USA) using a Parsazmun test kit. High-sensitivity C-reactive protein (hs-CRP; mg/l) was measured by immunofluorescence assay technique using an I-CHROMA test kit.
Serum insulin level (μIU/ml) was measured by a DANA device (model: DA3200) using Monobind ELISA test kit. Homeostatic model assessment-IR (HOMA-IR) and HOMA-β-cell function (HOMA-%β-cell) were calculated using homeostasis model assessment.[9]
RESULTS
Overall, 94 individuals were recruited, of which 34 (36.2%) were active athletes, 30 (31.9%) were ex-athletes, and 30 (31.9%) were nonathletes. The overall mean age was 33.01 ± 4.91 years, while it was 31.62 ± 6.51, 34.07 ± 3.93, and 33.53 ± 3.16 years among active athletes, ex-athletes, and nonathletes, respectively.
Among different variables, SBP, DBP, FBS, TG, HDL-C, HOMA-IR, HOMA-%β-cell, and hs-CRP had nonnormal distribution (P < 0.05), while weight, BMI, body fat percentage, WC, height, total cholesterol, LDL-C, and insulin level were normally distributed (P > 0.05).
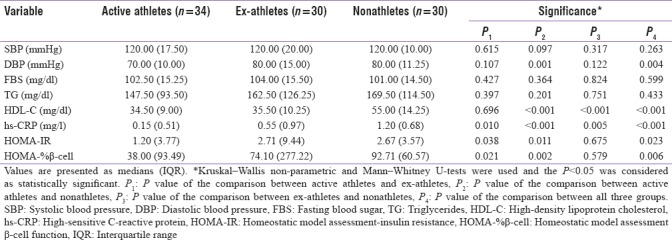
Ex-athletes had significantly higher values in weight (P = 0.004), BMI (P = 0.004), DBP (P = 0.004), LDL-C (P = 0.03), insulin (P = 0.015), HOMA-IR (P = 0.023), and HOMA-%β-cell (P = 0.006), compared with the active athletes and nonathletes.
Nonathletes had significantly higher levels of HDL-C, compared to the two other groups (P < 0.001). Besides, they showed significantly higher amounts of hs-CRP and hs-CRP concentration was significantly lower in active athletes compared with nonathletes and ex-athletes (P < 0.001). Demographic and anthropometric data of the three groups are presented and compared in Tables 1 and 2, respectively.
Table 1.
Comparison of variables with normal distribution among three groups
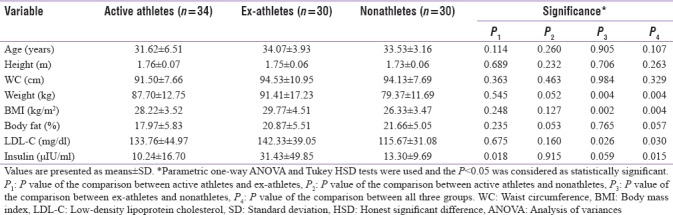
Table 2.
Comparison of variables without normal distribution among the three groups
The relative frequency of metabolic syndrome according to the National Cholesterol Education Program (NCEP) defined criteria among three groups is shown in Figure 1.[10] Pearson Chi-Square test revealed that the difference in the prevalence of metabolic syndrome between three groups was not significant (P = 0.14).
Figure 1.
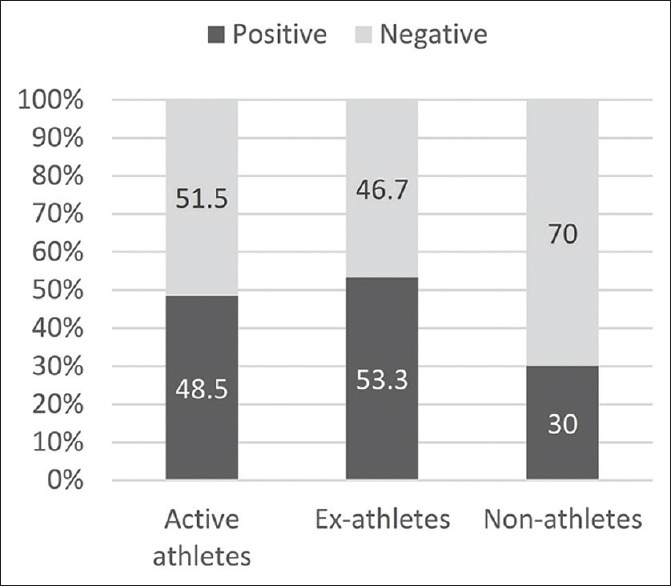
Relative frequency of metabolic syndrome in each study group
DISCUSSION
Weight gain and obesity development following the retirement from professional sports has been a major concern in professional athletes. However, the real impact of retirement and adopting sedentary lifestyles on the development of obesity and associated unhealthy conditions, which lead to metabolic syndrome, is still under controversy.
We found that average weight was 12 kg higher in ex-athletes in comparison with nonathletes. However, due to the nature of higher lean body mass in athletes, it could not be concluded that the rate of overweight and obesity was higher in ex-athletes, compared with nonathletes. The average weight of active athletes was 8 kg more than average weight of nonathletes but mean body fat percentage of active athletes was significantly lower, compared with that of nonathletes. In a study done by Hyman et al. it was shown that body fat percentage provides a more accurate evaluation of obesity than what BMI does among retired football players.[3] Moreover, the effect of genetic difference between study groups on the result of weight and body fat percentage cannot be disregarded.
No significant difference was observed in WC and height between the three groups of our study. The results of Phil and colleagues, who studied 219 male former athletes, were consistent with our findings regarding height and WC.[11]
Our results showed that DBP was significantly higher in nonathletes in comparison with active athletes and ex-athletes, but there was no significant difference between DBP of active athletes and ex-athletes. Studies have shown that higher levels of energy expenditure and physical activity are associated with lower levels of blood pressure.[12] Chang et al. have also reported the prevalence of hypertension to be lower in former football athletes, compared to general population.[13] Another study by Albuquerque et al. showed that SBP and DBP in former athletes with cardiovascular disease were higher compared with nonathletes with the same condition.[14] In order to achieve results that are more accurate, a cohort study on a group of professional athletes is recommended to measure blood pressure over a long period from their active career until their retirement.
Literature suggests that there is a relationship between exercise and lipid profile and higher levels of physical activity are associated with lower serum lipids.[12] Consistently, we observed that the serum level of TG was the least in active athletes and the most in ex-athletes, among the study groups. However, we found higher levels of LDL-C in active athletes, compared to nonathletes, though the differences were insignificant.
One possible reason for this inconsistency could be the effect of nutrition on lipid profile, which was not measured in the present study. In order to achieve a clear outcome, a cross-sectional study on the same groups to assess the current state of nutrition is suggested. It has also been reported that the effect of regular exercise on serum lipids is transient and the serum level of lipids return to preexercise values within a few days after cessation of physical activity.[12]
We observed a significantly higher level of HDL-C in nonathletes, compared with active and former athletes. This finding can be attributed to the effect of nutrition on serum lipid profile. Although it cannot be concluded that abandoning regular exercise reduces HDL-C level since there was no significant difference in HDL-C levels between ex-athletes and active athletes.
The serum insulin concentration, HOMA-IR (which is an indicator of IR) and HOMA-%β-cell (which indicates the activity of pancreatic β cells) were significantly higher in ex-athletes compared to active athletes. It is concluded that abandoning regular exercise can lead to higher levels of IR, which is a definite risk factor for diabetes mellitus.
The serum concentration of hs-CRP, which is a prominent risk factor for cardiovascular and cerebrovascular diseases, was significantly lower among active athletes, compared to both ex-and nonathletes.[15,16,17] Moreover, Voils and Cooper-DeHoff in their study confirmed a significant dose-related association between number of metabolic syndrome conditions in the participants and their likelihood of having elevated hs-CRP concentrations.[18]
In the present study, the prevalence of metabolic syndrome among three groups was determined by using NCEP-defined criteria, and although metabolic syndrome was more prevalent among ex-athletes, no significant statistical difference was seen.[10] This is in line with the findings of the study by Batista et al. in which they observed no significant difference in the likelihood of metabolic syndrome among former elite, nonelite, and nonathletes.[6]
Limitations
Although metabolic syndrome is thought to be more prevalent in adults older than 40, we assessed subjects aged 25-40 because the number of active athletes older than 40 is strictly limited.
Among the other limitations of the present study, we can mention the restriction of the study population to athletes of the male gender, measuring blood pressure only one time, not measuring the present status of physical activity, and not assessing the current nutritional status of participants. Hence, a more comprehensive cohort study with larger sample size regarding the physical activity and diet of the athletes is recommended.
CONCLUSIONS
Based on the aforementioned discussion, it can be concluded that abandoning regular athletic exercise in power sports increases BMI, body fat percentage, serum levels of LDL-C and TG, and DBP. One of the most prominent outcomes of this study was the increased level of serum insulin and IR after abandoning athletic power sports. However, no association was observed between leaving regular athletic exercising and development of metabolic syndrome in short term, but higher IR could lead to metabolic syndrome in the long run. Therefore, continuing regular physical activity is strongly recommended to maintain a healthy lifestyle after retirement in professional athletes.
Financial support and sponsorship
The study was supported by a research grant from Mashhad University of Medical Sciences, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors express their great appreciation to the nutrition department of MUMS, sports society of Mashhad, Kaveh Bahrami, Maryam Sheykhvanlu, Hamid Ghashang, and Alireza Shahidi for providing facilities to complete this study. Thanks are also due to all individuals for their participation.
REFERENCES
- 1.Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendíc S, Rydén L, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet. 2002;359:2140–4. doi: 10.1016/S0140-6736(02)09089-X. [DOI] [PubMed] [Google Scholar]
- 2.Sahin H, Ciçek B, Yılmaz M, Ongan D, Inanç N, Aykut M, et al. Obesity prevalence, waist-to-height ratio and associated factors in adult Turkish males. Obes Res Clin Pract. 2011;5:e1–78. doi: 10.1016/j.orcp.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Hyman MH, Dang DL, Liu Y. Differences in obesity measures and selected comorbidities in former national football league professional athletes. J Occup Environ Med. 2012;54:816–9. doi: 10.1097/JOM.0b013e3182572e53. [DOI] [PubMed] [Google Scholar]
- 4.Araújo F, Yamada AT, Araújo MV, Latorre M, Mansur AJ. Lipid profile of individuals without cardiopathy with overweight and obesity. Arq Bras Cardiol. 2005;84:405–9. [PubMed] [Google Scholar]
- 5.Santos RD. III Brazilian guidelines on dyslipidemia and guidelines for the prevention of atherosclerosis of the department of atherosclerosis of the Brazilian Society of Cardiology. Arq Bras Cardiol. 2001;77:1–48. [PubMed] [Google Scholar]
- 6.Batista C, Soares JM. Are former elite athletes more protected against metabolic syndrome? J Cardiol. 2013;61:440–5. doi: 10.1016/j.jjcc.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Valle VS, Mello DB, Fortes Mde S, Dantas EH, Mattos MA. Effect of diet and indoor cycling on body composition and serum lipid. Arq Bras Cardiol. 2010;95:173–8. doi: 10.1590/s0066-782x2010005000080. [DOI] [PubMed] [Google Scholar]
- 8.Nematy M, Alinezhad-Namaghi M, Rashed MM, Mozhdehifard M, Sajjadi SS, Akhlaghi S, et al. Effects of ramadan fasting on cardiovascular risk factors: A prospective observational study. Nutr J. 2012;11:69. doi: 10.1186/1475-2891-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. Third National Health and Nutrition Examination Survey (NHANES III), National Cholesterol Education Program (NCEP). et al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 11.Pihl E, Zilmer K, Kullisaar T, Kairane C, Mägi A, Zilmer M, et al. Atherogenic inflammatory and oxidative stress markers in relation to overweight values in male former athletes. Int J Obes (Lond) 2006;30:141–6. doi: 10.1038/sj.ijo.0803068. [DOI] [PubMed] [Google Scholar]
- 12.Dey SK, Ghosh C, Debray P, Chatterjee M. Coronary artery disease risk factors & their association with physical activity in older athletes. J Cardiovasc Risk. 2002;9:383–92. doi: 10.1097/01.hjr.0000049244.21319.20. [DOI] [PubMed] [Google Scholar]
- 13.Chang AY, FitzGerald SJ, Cannaday J, Zhang S, Patel A, Palmer MD, et al. Cardiovascular risk factors and coronary atherosclerosis in retired national football league players. Am J Cardiol. 2009;104:805–11. doi: 10.1016/j.amjcard.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albuquerque FN, Kuniyoshi FH, Calvin AD, Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, et al. Sleep-disordered breathing, hypertension, and obesity in retired national football league players. J Am Coll Cardiol. 2010;56:1432–3. doi: 10.1016/j.jacc.2010.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenig W, Sund M, Fröhlich M, Fischer HG, Löwel H, Döring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: Results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 16.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: The Framingham study. Stroke. 2001;32:2575–9. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 17.Shroff GR, Cen YY, Duprez DA, Bart BA. Relationship between carotid artery stiffness index, BNP and high-sensitivity CRP. J Hum Hypertens. 2009;23:783–7. doi: 10.1038/jhh.2009.17. [DOI] [PubMed] [Google Scholar]
- 18.Voils SA, Cooper-DeHoff RM. Association between high sensitivity C-reactive protein and metabolic syndrome in subjects completing the National Health and Nutrition Examination Survey (NHANES) 2009-10. Diabetes Metab Syndr. 2014;8:88–90. doi: 10.1016/j.dsx.2014.04.021. [DOI] [PubMed] [Google Scholar]


