Abstract
To give an overview of the potential clinical utility of 18F-fluorocholine PET/CT (FCH PET/CT) in imaging of parathyroid adenoma. Available studies have provided preliminary results of 18F-FCH PET/CT in primary and secondary hyperparathyroidism. Results of various studies have shown that 18F-FCH is a promising upcoming tracer for the detection of parathyroid adenomas, especially when multiple, or having low size. FCH PET/CT has the potential to be a standard investigation in the detection of parathyroid lesions.
Keywords: 18F-Fluorocholine, parathyroid adenoma, PET/CT
INTRODUCTION
Primary hyperparathyroidism (PHPT) is a common endocrine disorder with prevalence estimates of one to seven cases per 1000 adults.[1] About 85% of PHPT is caused by a solitary adenoma of the parathyroid glands, less frequently (about 15%) by multiple parathyroid gland disease (MGD), and rarely (1%) by parathyroid carcinoma.
It is characterized by increased production and secretion of parathyroid hormone (PTH). The consequent biochemical changes are the result of a lack of calcium homeostasis: increased blood and urine calcium levels, decrease in blood phosphate level, and increase in urine phosphate level. Clinically, this condition causes nephrocalcinosis, urolithiasis, bone disease, neuropsychiatric disorders (from mild behavioural changes to coma), gastrointestinal disturbances (from mild abdominal pain to acute pancreatitis), and neuromuscular manifestation (weakness, cramps, and muscle pain).
Most cases of MGD are sporadic, while a small number are associated with hereditary disorders such as multiple endocrine neoplasia type 1 or type 2A, or familial hyperparathyroidism.[2]
The management of PHPT at present includes radio-guided minimally invasive surgery. The success of targeted parathyroid surgery depends not only on an experienced surgeon, but also on a sensitive and accurate imaging technique. The two main reasons for failed surgery are ectopic glands and undetected MGD.[3]
A prerequisite for minimally invasive surgery is a successful preoperative localization of the involved parathyroid tissue.
Parathyroid glands can be imaged with multiple modalities including high-resolution (7.0–10.0 MHz) ultrasonography (USG), thin-section computed tomography (CT), magnetic resonance imaging (MRI), and scintigraphy.[4]
Parathyroid scintigraphy involves a number of different radiotracers and protocols. Older tracers which are no longer in use include 75Se-selenomethionine, 57Co-vitamin B12, 131I-toluidine blue, and 123I-methylene blue. Single-photon emission computed tomography (SPECT) tracers include 201Tl, 99mTc-sestamibi, or 99mTc-tetrofosmin for parathyroid localization, 99mTcpertechnetate, and 123I for thyroid scan. 99m Tc-Sestamibi is the most common tracer used. Mechanism of action includes the abnormal accumulation in parathyroid adenoma and differential washout from thyroid and parathyroid. Various studies have reported the sensitivity to be between 77% and 89%. The addition of SPECT/CT improved diagnostic accuracy and sensitivity. However, disadvantages include low sensitivity in very small parathyroid lesions due to its poor spatial resolution, in postoperative patients with thyroid adenomas or when present in close proximity to thyroid, in MGD involvement in syndromic patients and in certain adenomas with unusual washout. Also, up to one-third of patients with adenomas are MIBI negative.[4]
PET tracers include 11C-methionine, 18F-DOPA, 18F-FET, or 18F-fluorodeoxyglucose (18F-FDG). Recently, 18F-fluorocholine (18F-FCH) is being highlighted in literature for the detection of parathyroid adenomas.
In contrast to conventional nuclear medicine imaging approaches for localization of the involved parathyroid tissue, hybrid imaging (PET/CT and SPECT/CT) offers the possibility of attenuation correction and co-registration of functional and anatomical information; an additional advantage of PET/CT over SPECT/CT is its superior spatial resolution.
Basis of imaging parathyroid adenoma with FCH PET/CT
Mechanism of uptake: Increased cell proliferation/metabolism in the adenoma or hyperplasia possibly leads to increased choline uptake which on phosphorylation by choline kinase gets trapped to form a major membrane phospholipid called phosphatidylcholine. Hence, upregulation of choline kinase activity leads to enhanced choline uptake. Based on this possible mechanism 18F-FCH, a choline analogue is used for evaluation of parathyroid adenoma.[5]
18F is positron emitter with half-life of 109.7 minutes and Emax of 1.656 MeV. 18F-FCH is cleared via the kidneys and excreted in the urine.[6]Figure 1 shows the chemical structure of 18F-FCH.
Figure 1.
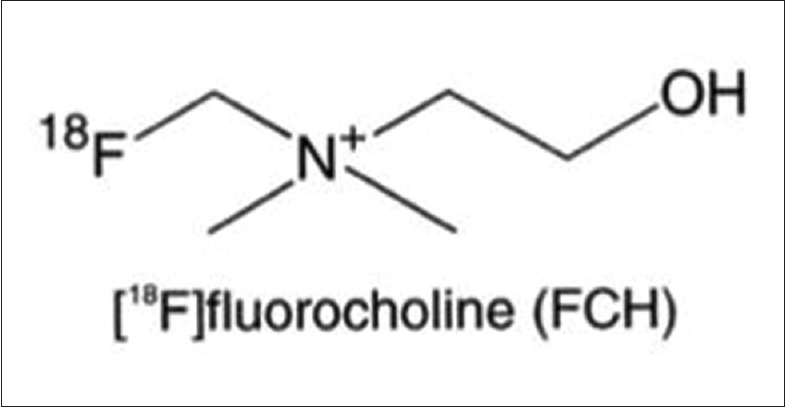
Chemical structure of 18F-FCH
PET/CT protocol: 18F-FCH doses varying from 5 to 10 mci is usually injected to the patient intravenously and scan is acquired after 45–60 minutes.
Review of literature
Studies regarding parathyroid imaging were reviewed using PUBMED dating from 1995 to January 2018. And, publications specifically pertaining to FCH PET/CT are highlighted here. Studies describing its role in primary hyperparathyroidism, those comparing FCH PET/CT with conventional imaging modailities, role in guiding minimally invasive surgery, and in secondary hyperparathyroidism are quoted below.
In the initial studies, Cazaentre et al.[7] reported incidental uptake of 18F-FCH in parathyroid hyperplasia in a case of prostate cancer.
Behera and Damle have reported the incremental role of FCH PET/CT over technetium-99m-labelled MIBI scan in hyperparathyroidism in two patients. They concluded that this investigation may not just be useful in patients with the negative 99mTc-sestamibi scan, but may even show additional lesions in patients with already positive 99mTc-sestamibi scans.[8]
Potential advantages of the use of FCH PET/CT compared to dual-Tc99m pertechnetate thyroid and/or 99mTc MIBI parathyroid scintigraphy include the better spatial resolution of PET tracers, resulting in the detection of smaller adenomas and the reduced scanning time that may be anticipated due to the rapid kinetics of choline.
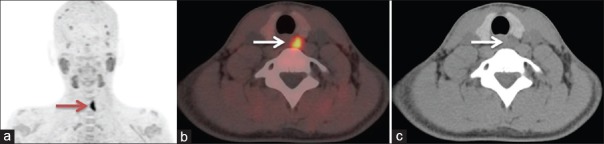
Figure 2 shows a 24-year-old male, a case of PHPT underwent FCH PET/CT for evaluation of parathyroid adenoma. (a) Maximum intensity projection (MIP) image shows focal increased tracer uptake in the left thyroid region (thick arrow). Corresponding transaxial PET/CT (b), and CT (c) images show a hypodense soft tissue lesion of about 1.2 × 1.0 cm with increased tracer uptake suggesting a parathyroid adenoma. Also, the radiation burden to the patient is lower for 18F-FCH (175 MBq; 4.3 mSv) compared to parathyroid SPECT/CT imaging (600 MBq; 5.4 mSv).[9]
Figure 2.
A 24-year-old male, a case of PHPT underwent 18F-FCH PET/CT for evaluation of parathyroid adenoma. (a) MIP image shows focal increased tracer uptake in the left thyroid region (thick arrow). Corresponding transaxial PET/CT (b), and CT (c) images show a hypodense soft tissue lesion of about 1.2 × 1.0 cm with increased tracer uptake suggesting of a parathyroid adenoma
Lezaic et al. evaluated the utility of FCH PET/CT for preoperative localization of hyperfunctioning parathyroid tissue; conducted a pilot study in 24 patients and suggested that FCH PET/CT is an accurate, efficient imaging modality for localization of hyperfunctioning parathyroid tissue, particularly in patients with multiple lesions or hyperplasia, in which the diagnostic performance of conventional scintigraphic imaging method is often unsatisfactory. FCH PET/CT was reported to have a sensitivity of 92% and specificity of 100%, in contrast to 49% and 100%, 46% and 100%, and 44% and 100% for 99mTc-sestaMIBI SPECT/CT, 99mTc-sestaMIBI/pertechnetate subtraction imaging, and 99mTc-sestaMIBI dual-phase imaging, respectively. Combined conventional scintigraphic imaging had a sensitivity and specificity of 64% and 100%, respectively. Regarding the time of acquisition, they observed that all lesions were visible at both imaging times (5–9 minutes and 60–64 minutes post-injection), with visually better lesion-to-background and lesion-to-thyroid contrast on delayed imaging.[10]
Recent study by Hocevar et al. retrospectively analyzed the results of preoperative localization with FCH PET/CT in 151 patients with primary hyperparathyroidism. Results obtained showed single adenoma in 128/151 (84.7%) patients. They concluded 18F-FCH PET/CT as a reliable preoperative localization test prior to focused parathyroidectomy, and patients need not undergo ioPTH testing. Operative success rate by this approach was reported above 95% and the risk of postoperative complications was negligible.[11]
In a study of 12 patients by Michaud et al. the authors found a detection rate of 92% for FCH PET/CT and it also solved discrepant results between MIBI scan and US. Regarding the quantification of FCH uptake, greater SUVmax was observed in adenomas than in hyperplastic glands. However, correlation between FCH uptake and PTH serum level was not significant according to this study. Also, they acquired one single PET image acquisition over a limited field of view, starting just 10 minutes after injection, permitting an effective localization of abnormal parathyroid glands with a limited occupation of the machine and mobilization of the patient.[12]
Damle et al. in one of their case reports observed that the FCH PET/CT showed a very high lesion to thyroid ratio. They postulated that with the use of 18F-FCH it may be easier to report smaller lesions which are very close to the thyroid, in cases of low gland mass and multigland involvement.[13]
Figure 3: A 24-year-old male, a case of PHPT underwent 18F-FCH PET/CT for evaluation of parathyroid adenoma. (A) MIP image shows focal increased tracer uptake in the left thyroid region (thick arrow). Corresponding transaxial PET/CT (B) and CT (C) images show a hypodense soft tissue lesion of about 1.2 × 1.0 cm with increased tracer uptake suggesting of a parathyroid adenoma.
Figure 3.
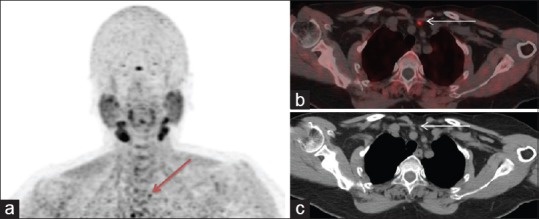
A 53-year-old female with raised PTH level was suspected to have primary hyperparathyroidism. US neck was inconclusive. Patient underwent FCH PET/CT. (a) MIP image shows focal increased tracer uptake in the suprasternal region (arrow). Corresponding transaxial PET/CT (b), and CT (c) images show a hypodense soft tissue lesion with increased tracer uptake of about 0.8 cm, just above the left brachiocephalic vein and posterior to the left sternothyroid muscle, likely a parathyroid adenoma. Subsequently 4D-CT images were performed and results were concordant with FCH PET/CT results
Few authors have reported FCH PET/CT uptake in parathyroid adenoma who are MIBI negative.[14] Kluijfhout et al.[15] reported a case where FCH PET/CT enabled a minimally invasive surgery for parathyroid adenoma in a patient with negative ultrasound and MIBI scan.
The recent prospective bicentric APACH1 study conducted by Quak et al. concluded preoperative FCH PET/CT has high sensitivity and positive predictive value for detection of parathyroid adenoma in patients with PHPT and negative or inconclusive conventional imaging results. FCH PET/CT-guided surgery in 88% of patients and avoided bilateral cervical exploration in 75% of patients.[16]
Other PET tracers such as 18FDG, 11C-methionine, 11C- choline, and 18F-FET are also being tried. Variable results have been reported with 18F-FDG in the detection of adenoma or hyperplastic parathyroid glands.[17]
In 2014, Chicklore et al.[18] compared FDG and 11C-methionine PET/CT performed in 43 patients referred for hyperparathyroidism of whom only 16 had parathyroid surgery; results favoured the amino acid 11C-methionine. Pooled patient-based sensitivity and detection rate was 81% and 70% according to a recent meta-analysis.[19]
11C-choline is also used as an alternative PET tracer for imaging of parathyroid adenoma. Orevi et al. in their prospective study of 40 patients, compared the utility of 11C-choline PET/CT imaging against 99mTc-sestamibi imaging. A majority of the patients had surgical findings and the authors concluded that 11C-choline PET/CT imaging combining both functional and anatomical modality is a promising tool for parathyroid adenoma localization, with the advantages of superior accuracy, easier acquisition, and better image quality.[20]
Parvinian et al.[21] reported high specificity of 11C-choline PET/CT in detecting parathyroid adenomas in 13 patients they studied (SUVmax, 5.6 ± 3.0).
However, logistics of 11C-labelled tracers are very demanding due to its short 20-minute half-life, requiring the presence of a cyclotron close to the PET centre. FCH would be easier to develop as a parathyroid imaging agent. Its only disadvantage, compared to 11C-choline or 11C-methionine, is its less favourable dosimetry. Krakauer et al.[22]found no significant differential uptake in parathyroid adenomas, possibly due to lack of expression of specific transmembrane transporter molecules in parathyroid tissue and hence concluded that 18F-FET is not a feasible tracer for use in preoperative localization imaging in PHP.
Another amino acid, 18F-fluorodihydroxyphenylalanine (FDOPA) has been studied in few cases, and concluded as not effective to detect hyperfunctioning parathyroid glands.[23]
Rep et al.[24] performed triple-phase PET/CT imaging at 5 minutes, 1 and 2 hours after the administration of 18F-FCH in 43 patients with PHPT and concluded that the optimal scan time of FCH PET/CT for localization of lesions representing enlarged parathyroid tissue is 1 hour after administration of the FCH.
Four-dimensional CT (4D-CT) is being used for accurate localization of parathyroid adenomas prior to surgery. Many authors describe a three- or four-phase imaging protocol consisting of precontrast, arterial (25–30 seconds after contrast injection), early-delayed (45 seconds after contrast injection), and late-delayed (80–90 seconds after contrast injection) phases. Adenomatous glands characteristically have low attenuation on the precontrast phase, maximal contrast enhancement during the arterial phase, and rapid washout during the delayed phases. After injection of contrast, normal parathyroid glands typically exhibit less contrast enhancement than the adenomatous gland during the arterial phase. Additionally, 4D-CT scanning may identify a polar feeding vessel, termed the “polar vessel sign,” in up to 63% of adenomas. The presence of this sign significantly increases the likelihood that the identified gland is an adenoma. 4D-CT scanning has reported sensitivity of 60–87% in localizing an adenoma.[25]
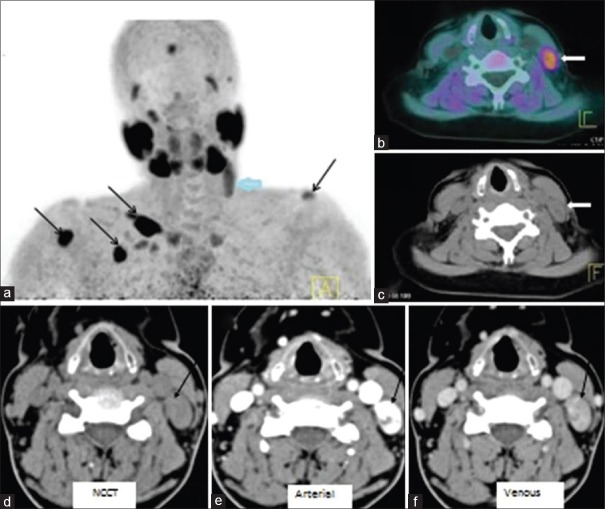
Taywadeet al. compared the diagnostic accuracy of FCH PET/CT and 4D-CT in detection and localization of eutopic and ectopic parathyroid adenoma in patients with hyperparathyroidism in five patients [Figure 4]. 18F-FCH PET/CT (a) MIP revealed focal radiotracer uptake in upper neck on the left side (thick arrow). In addition, multiple foci of increased tracer uptake noted over left shoulder, chest wall, and right arm regions (black arrows) corresponding to brown tumours. Fused PET/CT (b) and CT (c) images showed soft tissue nodule in the left lateral neck, posterior to left sternocleidomastoid muscle (thick arrow). 4D-CT (d–f) demonstrated an intensely enhancing lesion (arrow) on arterial phase with washout in venous phase posterior to the left jugular vein at thyroid cartilage level suggesting ectopically located parathyroid adenoma. Their study demonstrated 100% concordance between 4D-CT and FCH PET CT in localization of parathyroid lesions. However, the radiation dose of 4D-CT is relatively higher which is around 11–13 mSv.[5]
Figure 4.
FCH PET/CT (a) MIP shows focal uptake in left upper neck (thick arrow). Also, multiple foci of increased tracer uptake are seen over left shoulder, chest wall, and right arm regions (black arrows) – brown tumours. Fused PET/CT (b) and CT (c) images show soft tissue nodule in the left lateral neck, posterior to left sternocleidomastoid muscle (thick arrow). 4D-CT (d–f) shows an intensely enhancing lesion (arrow) on arterial phase with washout in venous phase posterior to the left jugular vein at thyroid cartilage level suggesting ectopically located parathyroid adenoma
4D-MRI is another upcoming technique. Few reports are available in literature. Merchavy et al. reported a sensitivity of 90% and after optimization 100%, positive predictive value of 91% and specificity of 100%. 4D-MRI was performed in nine patients and there was a complete match between the 4D-MRI and the US and MIBI and with the operative finding. They also compared 4D-CT and 4D-MRI. 4D-MRI has the advantages of having a higher sensitivity and specificity with no radiation exposure, but disadvantages include more artefacts related to motion and swallowing. They concluded that 4D-MRI is a reliable technique for identification of parathyroid adenomas.[26]
Nael et al. established the MR perfusion characteristics of parathyroid adenomas to differentiate from thyroid tissue and cervical lymph nodes. They conducted study on 30 patients. Parathyroid adenomas showed significantly faster time-to-peak, higher washin, and higher washout compared with cervical lymph nodes and thyroid tissue due to its hypervascular nature with diagnostic accuracies of 96%.[27]
Recently, PET/MRI is being utilized for the detection of parathyroid adenoma. 18F-FCH PET/MR might be even more suitable, owing to less radiation exposure and higher soft-tissue contrast of MR, allowing for a morphological correlation of PET findings.[28]
Kluijfhout et al. investigated the performance of 18F-FCH PET/MRI in 10 patients with hyperparathyroidism. FCH PET/MRI showed good performance for localization of 9/10 adenomas (90% sensitivity), without any false-positive results (100% positive predictive value). One patient had four-gland hyperplasia, of which three hyperplastic glands were not localized. The median SUVmax of the nine preoperatively identified adenomas was 4.9 (interquartile range, 2.45–7.35), which was significantly higher than the SUVmax 2.7 of the thyroid (interquartile range, 1.6–3.8) (P = 008).[29]
Michal Krcma described 18F-FCH PET/MRI in two cases with SPECT/CT invisible parathyroid adenoma. They reported 18F-FCH PET as a promising diagnostic option. Also, described advantages of using MRI instead of CT as less radiation load, no risk of contrast allergy, and slightly better resolution in neck region.[30]
Secondary hyperparathyroidism is a common complication in patients with chronic renal failure on maintenance dialysis and is associated with significant morbidity and mortality. In secondary hyperparathyroidism, immediate failure after surgery and delayed recurrence is common, occurring in 10–30% of patients. Preoperative imaging in secondary hyperparathyroidism has not gained wide acceptance. Sestamibi scanning is reported to have low sensitivity of about 40–50% in detecting hyperplastic glands.[2]
Role of 18F-FCH in this setting is yet to be explored. One of the previously mentioned study of Michaud et al., 5 out of the 12 patients studied were of secondary hyperparathyroidism. They concluded that FCH uptake is a general property in case of adenomatous or hyperplastic parathyroid glands, independently of the type of hyperparathyroidism, either primary or secondary.[31]
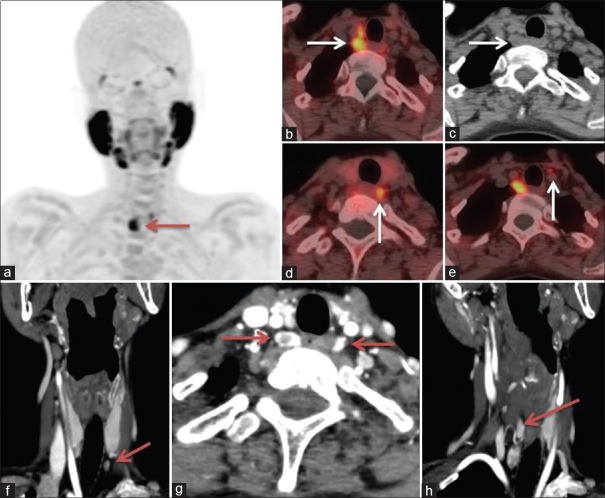
MIBI is less sensitive and specific for detecting parathyroid lesions in MGD than in single adenoma.[32]. Significance of FCH PET/CT is not clear in MGD and in syndromic patients (e.g., MEN syndrome) [Figure 5]. 18F-FCH PET/CT (a) MIP revealed focal radiotracer uptake in neck on the right side (thick arrow). Fused PET/CT (b) and CT (c) images showed two soft tissue nodules in the right tracheoesophageal groove posterior to the right lobe of thyroid (white arrow). Image (d) shows a hypodense lesion with increased tracer uptake in left tracheoesophageal groove and image (e) shows a discrete small lesion inferior to the left lobe of thyroid anterior to left common carotid artery with increased tracer uptake, suggesting of multiglandular disease in a patient with MEN 1 syndrome. Coronal and axial sections of 4D-CT images (f–h) revealed increased vascularity in the arterial phase in multiple parathyroid adenomas.
Figure 5.
FCH PET/CT (a) MIP revealed focal uptake in right neck (arrow). Fused PET/CT (b) and CT (c) images showed two soft tissue nodules in the right tracheo-esophageal groove posterior to the right lobe of thyroid (arrow). (d) Shows a hypodense lesion in left tracheoesophageal groove, (e) shows a discrete small lesion inferior to the left lobe of thyroid anterior to left common carotid artery, suggesting of multiglandular disease in a patient with MEN 1 syndrome. 4D-CT images (f–h) revealed increased vascularity in the arterial phase in multiple parathyroid adenomas
Very little is known about the utility of FCH PET/CT in parathyroid carcinoma. Hatzl et al.[33] have reported increased uptake of 18F-FCH in recurrent parathyroid carcinoma in a 71-year-old male.
CONCLUSION
To conclude, FCH PET/CT is a novel promising tool to localize parathyroid adenoma/hyperplasia. Many of the references quoted above have shown superiority of 18F-FCH over MIBI scan. However, larger prospective validation studies are required to evaluate the usefulness of F18-choline PET/CT. Future studies, are needed to compare FCH PET/CT with other upcoming modalities like 4D-CT. Also, th e impact of this new modality on the patient management is currently being explored.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yeh MW, Ituarte PHG, Zhou HC, Nishimoto S, Liu ILA, Harari A. Incidence and Prevalence of Primary Hyperparathyroidism in a Racially Mixed Population. J Clin Endocrinol Metab. 2013;98:1122–9. doi: 10.1210/jc.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hindié E, Ugur O, Fuster D, O'Doherty M, Grassetto G, Ureña P, et al. EANM parathyroid guidelines. Eur J Nucl Med Mol Imaging. 2009;36:1201–16. doi: 10.1007/s00259-009-1131-z. [DOI] [PubMed] [Google Scholar]
- 3.Shafiei B, Hoseinzadeh S, Fotouhi F, Malek H, Azizi F, Jahed A, et al. Preoperative 99mTc-sestamibi scintigraphy in patients with primary hyperparathyroidism and concomitant nodular goiter: Comparison of SPECT-CT, SPECT, and planar imaging. Nucl Med Commun. 2012;33:1070–6. doi: 10.1097/MNM.0b013e32835710b6. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins RC, Reading CC. Thyroid and parathyroid imaging. Semin Ultrasound CT. 1995;16:279–95. doi: 10.1016/0887-2171(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 5.Taywade SK, Damle NA, Behera A, Devasenathipathy K, Bal C, Tripathi M, et al. Comparison of 18F-Fluorocholine positron emission tomography/computed tomography and four-dimensional computed tomography in the preoperative localization of parathyroid adenomas-initial results. Indian J Endocr Metab. 2017;21:399–403. doi: 10.4103/ijem.IJEM_536_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeGrado TR, Baldwin SW, Wang S, Orr MD, Liao RP, Friedman HS, et al. Synthesis and evaluation of (18F)F-labeled choline analogs as oncologic PET tracers. J Nucl Med. 2001;42:1805–14. [PubMed] [Google Scholar]
- 7.Cazaentre T, Clivaz F, Triponez F. False-positive result in 18F- fluorocholine PET/CT due to incidental and ectopic parathyroid hyperplasia. Clin Nucl Med. 2014;39:e328–30. doi: 10.1097/RLU.0b013e3182a77b62. [DOI] [PubMed] [Google Scholar]
- 8.Behera A, Damle NA. Incremental role of 18F-fluorocholine PET/CT over technetium-99m-labeled MIBI scan in hyperparathyroidism. Indian J Endocr Metab. 2016;20:888–90. doi: 10.4103/2230-8210.192897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raalte DHV, Vlot MC, Zwijnenburg A, Kate RW. F18-Choline PET/CT: A novel tool to localize parathyroid adenoma. Letters to Editor. Clin Endocrinol. 2015;82:910–14. doi: 10.1111/cen.12681. [DOI] [PubMed] [Google Scholar]
- 10.Lezaic L, Rep S, Sever MJ, Kocjan T, Hocevar M, Fettich J. 18F-Fluorocholine PET/CT for localization of hyperfunctioning parathyroid tissue in primary hyperparathyroidism: A pilot study. Eur J Nucl Med Mol Imaging. 2014;41:2083–9. doi: 10.1007/s00259-014-2837-0. [DOI] [PubMed] [Google Scholar]
- 11.Hocevar M, Lezaic L, Rep S, Zaletel K, Kocjan T, Sever MJ, et al. Focused parathyroidectomy without intraoperative parathormone testing is safe after pre-operative localization with 18F-Fluorocholine PET/CT. Eur J Surg Oncol. 2017;43:133–7. doi: 10.1016/j.ejso.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Michaud L, Burgess A, Huchet V, Lefèvre M, Tassart M, Ohnona J, et al. Is 18F-fluorocholine-positron emission tomography/computerized tomography a new imaging tool for detecting hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism? J Clin Endocrinol Metab. 2014;99:4531–6. doi: 10.1210/jc.2014-2821. [DOI] [PubMed] [Google Scholar]
- 13.Damle NA, Tripathi M, Behera A, Aggarwal S, Bal C, Aggarwal S, et al. Utility of 18F-choline photon emission tomography/computed tomography in the diagnosis of parathyroid adenoma. Indian J Nucl Med. 2016;31:207–9. doi: 10.4103/0972-3919.181857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nocuń A, Kędra M, Stefaniak B, Chrapko B. Parathyroid hyperplasia detected by F-18-Choline PET/CT, negative on Tc-99m-MIBI SPECT/CT and F-18-Deoxyglucose PET/CT scans. Nucl Med Rev. 2017;20:60–1. doi: 10.5603/NMR.2017.0005. [DOI] [PubMed] [Google Scholar]
- 15.Kluijfhout WP, Vriens MR, Valk GD, Barth RE, Borel RIH, de Keizer B. (18)F- Fluorocholine PET-CT enables minimal invasive parathyroidectomy in patients with negative sestamibi SPECT-CT and ultrasound: A case report. Int J Surg Case Rep. 2015;13:73–5. doi: 10.1016/j.ijscr.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quak E, Blanchard D, Houdu B, Roux YL, Ciappuccini R, Lireux B, et al. F18-choline PET/CT guided surgery in primary hyperparathyroidism when ultrasound and MIBI SPECT/CT are negative or inconclusive: The APACH1 study. Eur J Nucl Med Mol Imaging. 2018;45:658–66. doi: 10.1007/s00259-017-3911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melon P, Luxen A, Hamoir E, Meurisse M. Fluorine-18- fluorodeoxyglucose positron emission tomography for preoperative parathyroid imaging in primary hyperparathyroidism. Eur J Nucl Med. 1995;22:556–8. doi: 10.1007/BF00817282. [DOI] [PubMed] [Google Scholar]
- 18.Chicklore S, Schulte KM, Talat N, Hubbard JG, O'Doherty M, Cook GJ. 18F-FDG PET rarely provides additional information to 11C-methionine PET imaging in hyperparathyroidism. Clin Nucl Med. 2014;39:237–42. doi: 10.1097/RLU.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 19.Caldarella C, Treglia G, Isgrò MA, Giordano A. Diagnostic performance of positron emission tomography using 11C-methionine in patients with suspected parathyroid adenoma: A meta-analysis. Endocrine. 2013;43:78–83. doi: 10.1007/s12020-012-9746-4. [DOI] [PubMed] [Google Scholar]
- 20.Orevi M, Freedman N, Mishani E, Bocher M, Jacobson O, Krausz Y. Localization of parathyroid adenoma by 11C-choline PET/CT: Preliminary results. Clin Nucl Med. 2014;39:1033–8. doi: 10.1097/RLU.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 21.Parvinian A, Martin-Macintosh EL, Goenka AH, Durski JM, Mullan BP, Kemp BJ, et al. 11C-Choline PET/CT for Detection and Localization of Parathyroid Adenomas. AJR Am J Roentgenol. 2018;210:418–22. doi: 10.2214/AJR.17.18312. [DOI] [PubMed] [Google Scholar]
- 22.Krakauer M, Kjaer A, Bennedbæk FN. 18F-FET-PET in Primary Hyperparathyroidism: A Pilot Study. Diagnostics (Basel) 2016;17:6. doi: 10.3390/diagnostics6030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange-Nolde A, Zajic T, Slawik M, Brink I, Reincke M, Moser E, et al. PET with 18FDOPA in the imaging of parathyroid adenoma in patients with primary hyperparathyroidism: A pilot study. Nuklearmedizin. 2006;45:193–6. [PubMed] [Google Scholar]
- 24.Rep S, Lezaic L, Kocjan T, Pfeifer M, Sever MJ, Simoncic U, et al. Optimal scan time for evaluation of parathyroid adenoma with [18F]-fluorocholine PET/CT. Radiol Oncol. 2015;49:327–33. doi: 10.1515/raon-2015-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bann DV, Zacharia T, Goldenberg D, Goyal N. Parathyroid localization using 4D-computed tomography. Ear Nose Throat J. 2015;94:E55–7. doi: 10.1177/014556131509404-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merchavy S, Luckman J, Guindy M, Segev Y, Khafif A. 4D MRI for the Localization of Parathyroid Adenoma: A Novel Method in Evolution. Otolaryngol Head Neck Surg. 2016;154:446–8. doi: 10.1177/0194599815618199. [DOI] [PubMed] [Google Scholar]
- 27.Nael K, Hur J, Bauer A, Khan R, Sepahdari A, Inampudi R, et al. Dynamic 4D MRI for Characterization of Parathyroid Adenomas: Multiparametric Analysis. AJNR Am J Neuroradiol. 2015;36:2147–52. doi: 10.3174/ajnr.A4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huellner MW, Aberle S, Sah BR, Veit-Haibach P, Bonani M, Schmid C, et al. Visualization of parathyroid hyperplasia using 18F-fluorocholine PET/MR in a patient with secondary hyperparathyroidism. Clin Nucl Med. 2016;41:e159–61. doi: 10.1097/RLU.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 29.Kluijfhout WP, Pasternak JD, Gosnell JE, Shen WT, Duh QY, Vriens MR, et al. 18F Fluorocholine PET/MR imaging in patients with Primary Hyperparathyroidism and in inconclusive conventional imaging: A Prospective Pilot Study. Radiology. 2017;284:460–7. doi: 10.1148/radiol.2016160768. [DOI] [PubMed] [Google Scholar]
- 30.Krcma M. 18F-choline PET/MRI in patients with primary hyperparathyroidism and negative sestamibi SPECT/CT - report of two cases. Endocrine Abstracts 2017. 49 EP293. DOI: 10.1530/endoabs.49.EP293. [Google Scholar]
- 31.Michaud L, Balogova S, Burgess A, Ohnona J, Huchet V, Kerrou K, et al. A pilot comparison of 18F-fluorocholine PET/CT, Ultrasonography and 123I/99mTc-sestaMIBI dual-phase dual-isotope scintigraphy in the preoperative localization of hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism. Influence of thyroid anomalies. Medicine (Baltimore) 2015;94:e1701. doi: 10.1097/MD.0000000000001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols KJ, Tomas MB, Tronco GG, Palestro CJ. Sestamibi parathyroid scintigraphy in multigland disease. Nucl Med Commun. 2012;33:43–50. doi: 10.1097/MNM.0b013e32834bfeb1. [DOI] [PubMed] [Google Scholar]
- 33.Hatzl M, Röper-Kelmayr JC, Fellner FA, Gabriel M. 18F-Fluorocholine, 18F-FDG, and 18F-Fluoroethyl Tyrosine PET/CT in Parathyroid Cancer. Clin Nucl Med. 2017;42:448–50. doi: 10.1097/RLU.0000000000001652. [DOI] [PubMed] [Google Scholar]