Abstract
Background and Objectives:
Vitamin D is a key determinant of bone health and calcium homeostasis in children. Vitamin D deficiency (VDD) in early years may have an effect on total bone mass and risk of osteoporosis. Despite widespread prevalence of VDD among children, there is limited information in under-five age group. The objectives of the current study were to estimate the community-based prevalence of VDD and to identify the factors associated with children aged 1–5 years.
Materials and Methods:
A community-based cross-sectional study was conducted among 201 apparently healthy children (aged 1–5 years) in an urban slum of the selected geographical area in Mumbai. VDD was defined as serum 25-hydroxy Vitamin D (25[OH]D) levels <20 ng/ml as per the US Endocrine society classification.
Results:
The prevalence of VDD was found to be 74.6% (95% of confidence interval [68.6–80.6]). It was significantly higher (P = 0.04) among children staying indoors (44.8%). 25(OH)D was negatively correlated with parathyroid hormone (PTH) ([r = −0.199, P = 0.005]) and Alkaline phosphatase ([r = −0.140, P = 0.05]). However, the increase in PTH was observed when 25(OH)D levels were <10 ng/ml unlike anticipated increase at <20 ng/ml.
Conclusion:
The study revealed a high prevalence of VDD in 1–5 years age group. It was observed that the outdoor activities and sun exposure have a significant association with Vitamin D status. Majority of children had normal PTH levels despite VDD. The study endorses the importance of sun exposure and throws light on that fact that functional cutoffs for VDD may be lower in under-five children and also highlights the need of redefining cutoffs of Vitamin D among the Indian children.
Keywords: Hypovitaminosis D, under-five children, Vitamin D, Vitamin D deficiency
INTRODUCTION
Vitamin D is a key determinant of bone health and calcium homeostasis in children and has major implications on adult bone health. Therefore, its role in growth and development is of paramount importance.[1] However, apart from these explicit actions, Vitamin D is also believed to have effect on endocrine, immune and cardiovascular system, neuropsychological functioning.[2,3] A severe form of Vitamin D deficiency (VDD) manifests as Rickets and represents only a tip of Iceberg,[1] however, subclinical VDD is a significant risk factor for acute respiratory tract infections and tuberculosis in children.[4,5] Vitamin D supplementation in early childhood is known to be associated with a reduction in risk of Type 1 Diabetes.[6] VDD is thus likely to contribute to enormous morbidities in view of its multiple effects. Hence, maintaining optimal levels of Vitamin D is essential among under-five children.
Nearly 90% of Vitamin D requirement is met through adequate exposure of the skin to sunlight by the action of ultraviolet B radiations (between 10 am to 3 pm) and 10% is said to meet through diet.[7] Despite ample sunshine, 50%–90% Indian children have VDD.[8,9,10,11] However, there is limited information on its prevalence among under-five children as previous studies have mainly focused on breastfeeding babies and schoolgoing children.[9,10,11] Under-five children are either in preschools or indoors during 10 am to 3 pm making them more susceptible for VDD due to inadequate sun exposure. However, there is need to generate substantial evidence of the comprehensive prevalence of VDD in this age group for planning future strategies.
Three studies conducted in the age group of 9 months-5 years have reported prevalence ranging from 46% to 80%.[12,13,14,15] However, associated factors of VDD in under-five age group have not been studied so far. Most of the studies have used 25-hydroxy Vitamin D (25[OH]D) level <20 ng/ml as cutoff for defining VDD in children. However, VDD among children may be defined either on the basis of a locally developed “population-based reference value” or a “functional health-based reference value” which physiologically defines hypovitaminosis D as the concentration of 25(OH)D at which parathyroid hormone (PTH) begins to increase.[8] These deflection points may vary in children as reported in some studies.[16] However, the studies carried out so far among the Indian children have not explored Vitamin D-PTH axis in under-five age group.
The objectives of the current study were, therefore, to estimate the prevalence of VDD and its associated factors in children aged 1–5 years belonging to urban slum community in Mumbai.
MATERIALS AND METHODS
A community-based cross-sectional study was conducted among 201 apparently healthy children aged 1–5 years of both sexes in an urban slum of the conveniently selected geographical area of Mumbai. The study protocol was approved by the institutional ethics committee.
Considering anticipated prevalence of VDD among children in the community as 80%,[14] at 5% of precision, 5% level of significance and assuming 10% nonresponse rate, the required sample size was 273.
The study was planned in a community comprising of nearly 1,00,000 population having 20 anganwadies covering approximately 5000 under-five children. The study was discussed with Child Development Project Officer and Anganwadi workers. After obtaining necessary permissions, the list of children (1–5 years) was obtained from Anganwadi workers followed by household visits for screening the children in the age group of 1–5 years. A total of 330 children were screened from the slum for eligibility. The flowchart in Figure 1 represents the process of recruitment of participants.
Figure 1.
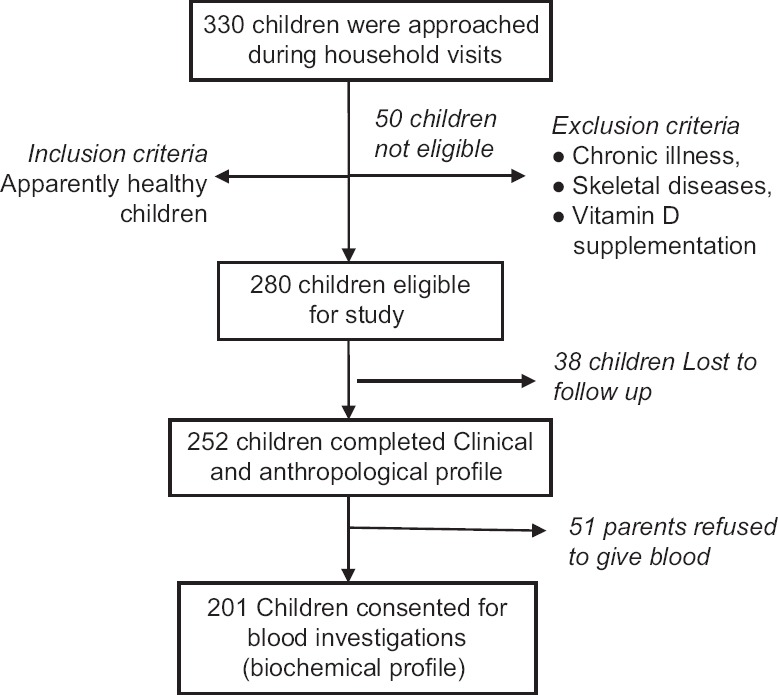
Flowchart representing the process of recruitment of participants
A total of 201 eligible apparently healthy children were included in the study from November 2015 to March 2017. The remaining children with chronic illness, skeletal diseases, and receiving Vitamin D supplementation were excluded from the study. Informed written consent was obtained from the parents or guardian. During household visits, information was gathered on sociodemographic profile,[17] physical activity profile (sun exposure, time spent outdoor). Direct sunlight exposure was assessed by documenting average duration of exposure between 10 am and 3 pm and percentage of the body surface area exposed daily using the standard method as described in the textbook of Bailey and Love's short practice of surgery. Dietary profile, i.e., 24 h recall of their food intake was collected through face-to-face interview using structured questionnaire by trained social workers. Parents with children were further invited to the clinic for clinical, anthropometric examination, and biochemical investigations. Children were evaluated for grades of malnutrition as per the WHO growth standards.[18] Clinically, Genu varum (bow legs) or Genu valgum (knock knees) was diagnosed if intercondylar and intermalleolar distances were 6 cm and 8 cm, respectively.[19] Other subjective signs, i.e., frontal bossing and epiphyseal widening were also recorded. After clinical and anthropometric evaluation, a volume of 4 ml of venous blood sample was drawn in the fasting state between for the estimation of serum calcium, phosphate, alkaline phosphatase, 25(OH)D, and PTH. Alkaline phosphatase was estimated in the serum by the pNPP (para nitrophenyl phosphate) method. 25(OH)D levels were measured by an immunochemiluminometric assay with an assay range of 3.7–150 ng/ml. The laboratory reference range for alkaline phosphatase was 145–320U/L, for serum calcium was 8.8–10.8 mg/dl, for serum phosphorus was 4.0–7.0 mg/dl, and for PTH was 14–72 pg/ml.
The US Endocrine society classification was referred for classification of VDD as 25(OH)D levels <20 ng/ml, insufficiency as 25(OH)D levels between 20 and 30 ng/ml and sufficiency as 25(OH)D >30 ng/ml.[20] However, only two groups, VDD (25[OH]D <20 ng/ml) and Vitamin D nondeficiency (25[OH]D ≥20 ng/ml) were considered as an outcome variables for analysis.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Macintosh, Version 19.0 (Armonk, NY: IBM Corp) Sociodemographic parameters such as age (12–23, 24–59 months), sex (male/female), socioeconomic status (Kuppuswami's Classification),[15,17] mother's education (literate/illiterate), physical activity profile (Sun Exposure, time spent outdoor), calcium-rich diet, growth, and clinical parameters such as age at dentition, calcium and Vitamin D supplementation during infancy, recurrent episodes of upper respiratory tract infections, z score, frontal bossing, epiphyseal widening, Genu varum, Genu valgum, and biochemical markers (calcium, phosphorus, alkaline phosphatase, and PTH) were considered as explanatory variables. Descriptive analysis using frequency percentage for categorical data and mean (±standard deviation [SD]) for continuous variables were carried out.
The association between explanatory and outcome variables was carried out using Chi-square test. The Pearson's correlation was applied to see the relationship between biochemical parameters and 25(OH)D levels. The value of P < 0.05 was considered as statistically significant.
RESULTS
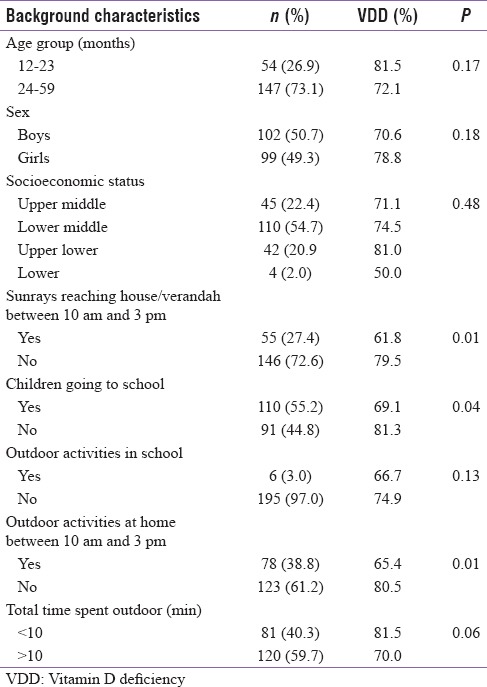
Background characteristics
Mean age of the children was 33.1 (±12.9) months. All the children had uneventful birth history, normal development and had completed universal immunization as per age. The mean age of weaning was 5.7 (±1.5) months. About 57.4% of children belonged to lower middle class, 22.4% upper middle, 20.9% upper lower and 2% lower class.
Prevalence of Vitamin D deficiency
Mean 25(OH)D level was found to be 15.37 (+7.79) ng/ml among children under study. The prevalence of VDD among children was found to be 74.6% (95% confidence interval [CI] [68.6–80.6]). Insufficiency was observed among 19.9% of children. Nearly 52.2% of children had 25(OH)D levels <15 ng/ml, 22.4% had levels <10 ng/ml and 5.5% had levels <5 ng/ml whereas only 3.5% of children had sufficient levels of Vitamin D as depicted in Figure 2. No significant difference in proportion of VDD was found between age group and sex of the children [Table 1], respectively.
Figure 2.
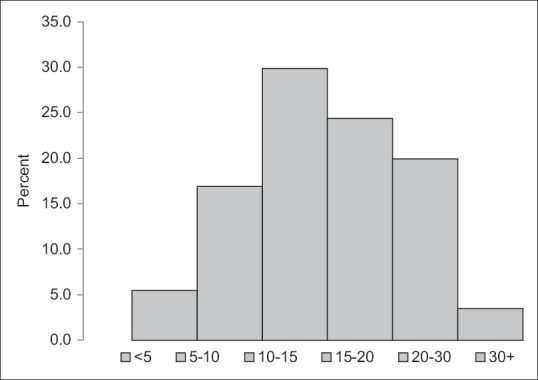
Frequency distribution of Vitamin D Level
Table 1.
Frequencies and percentage distribution of selected baseline characteristics and its association with Vitamin D deficiency
Dietary profile and Vitamin D
Assessment of dietary intake of nutrients (energy, protein, carbohydrate, total fat, diet, phytate, Ca, and P) was made using Diet-software and Nutrient guidelines and dietary recommendation for Indian by National Institute of Nutrition-Indian Council of Medical Research.[21,22]
Nearly 70%–80% of children frequently consumed street foods/packed snacks. Consumption of Vitamin D fortified foods (Dairy products, health drinks, and cereals) was nearly absent, whereas intake of oily fishes was seen among 59.2% of children and 25% consumed egg yolk. The most common calcium-rich food consumed was milk and banana (84%–93%) followed by green vegetables 66% and Ragi/Cheese-Paneer (11%–25%). Table 2 represents mean levels of calcium and phytate intakes.
Table 2.
Mean calcium and phytate intakes of children
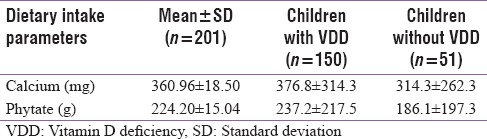
Mean calcium intake (360.96 [±18.50] mg) was less that the recommended dietary allowance (400 mg). No significant association was observed between intake of calcium and Vitamin D containing food and VDD (r = −0.062, P = 0.38). A negative correlation was observed between dietary phytate and calcium ratio (r = −0.113, P = 0.110). However, it was not significant.
Physical activity, sun exposure, and Vitamin D
As mentioned, though the children were living in overcrowded slums, in 35.3% of houses, sunrays were reaching verandas. However, in 27.4% of houses, sunrays were reaching between 10 am and 3 pm. Half of the children (55%) were going either to school or Anganwadies. Physical activity during school hours was reported only among 4.5% of children. Children playing outside houses between 10 am and 3 pm were 38.8%. Nearly 40.3% of children spent >10 min during 10 am to 3 pm outdoors for playing local games and accompanying parents while shopping. Mean body surface area exposed was 32.49% (±10.9).
On assessing the association of these factors with Vitamin D status as shown in Table 1, VDD was significantly associated with children staying in houses with no sunrays reaching between 10 am and 3 pm (P = 0.014), not playing outdoor activities between 10 am and 3 pm (P = 0.047) and not attending preschool or anganwadies (P = 0.04). However, only marginal significant association (P = 0.06) was observed between time spent outdoor (>10 min) and VDD.
Growth and clinical parameters
Nearly 74% of children had normal growth standards (z score >2SD) as per WHO growth charts without significant association with Vitamin D status. Frontal bossing (P = 0.002), Genu Varum (P = 0.004) and Epiphyseal Widening (P = 0.034) were significantly associated with VDD as shown in Table 3.
Table 3.
Association between growth and clinical parameters with Vitamin D deficiency
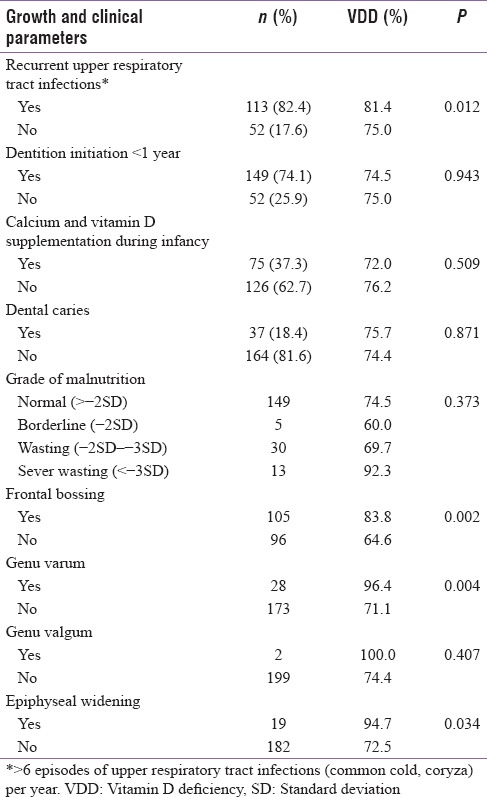
Almost 82.4% of children had >6 episodes of respiratory tract infections in a year and had a significant association with VDD (P = 0.012). Nonetheless, no significant association was observed between age of initiation of dentition (P = 0.943), dental caries (P = 0.871), and calcium and Vitamin D supplementation during infancy (P = 0.509).
Biochemical parameters
Despite having 25(OH)D levels below <20 ng/ml, normal PTH, alkaline phosphatase and calcium levels were observed among 81%, 91.2%, and 84.7% of children, respectively. However, 25(OH)D level was negatively correlated with PTH level ([r = −0.199, P = 0.005]) and alkaline phosphatase ([r = −0.140, P = 0.05]). The increase in PTH was observed at 25(OH)D concentrations <10 ng/mL as shown in Figure 3.
Figure 3.
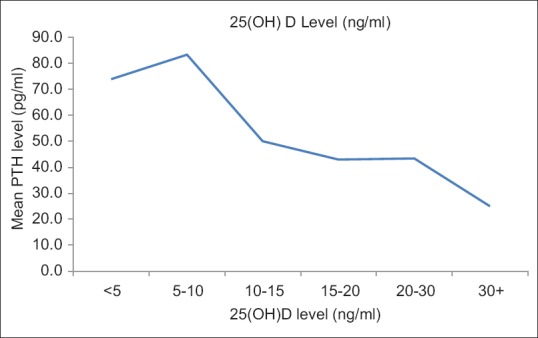
The mean parathyroid hormone level by 25-hydroxy Vitamin D levels
DISCUSSION
The study identified a high prevalence of 74.6% [95% CI (68.6–80.6)] of VDD in children of age group 1–5 years and also explored its association with various factors. The study did not find a significant difference in deficiency status of boys and girls. This is contrary to some previous studies[11,23] which have reported more deficiency in girls compared to boys. There was no significant difference in deficiency status between younger children (12–23 months) and comparatively older children (24–60 months).
As mentioned, these children belonged to overcrowded slums where factors hampering direct sunlight reaching household were also coexistent. Despite this, outdoor activities played by children and sunrays reaching houses had a significant association with normal Vitamin D status. Few studies have revealed the impact of sun exposure on Vitamin D status of children; however, the association had not been studied to a substantial extent.[12,13,14,15,24] This study endorses the need to increase sun exposure to children, especially between 10 am and 3 pm to improve Vitamin D levels and recommends the need to create community awareness on this issue among parents and school teachers.
Although diet contributes only 10% of Vitamin D requirements, certain factors such as low-dietary calcium and higher phytate are known to affect Vitamin D metabolism and significantly associated with low 25(OH)D levels. High phytate and/or low-calcium diet among rural children were associated with VDD despite plentiful sun exposure.[25] In our study, higher phytate to calcium ratio was observed only among 10.4% of children. However, a negative, but a weak correlation was observed between 25(OH)D levels and dietary phytate-calcium ratio. Inadequate calcium and Vitamin D in diet further emphasizes the importance of sun exposure as effective means of sufficient Vitamin D status in under-five children.
Only 13.9% of children showed associated signs such as Genu varum and Valgum similar to some studies.[10,23] Although the association between lower respiratory tract infection among under-five children and VDD had been studied before,[4,26] the current study reported a significant association of VDD with recurrent episodes of upper respiratory tract infections. Analysis of other factors such as the initiation of dentition, dental caries, and supplementation of calcium and Vitamin D during infancy did not seem to have any association with VDD.
The functional cutoffs for diagnosing VDD have been defined as a variable range of 25OHD level (15–30 ng/ml) when PTH levels begin to increase.[27] It is mainly based on adult studies in relation to fracture risk, intestinal calcium absorption, or bone mineral density.[28] The “optimal” 25OHD threshold which separates blood markers of abnormal bone metabolism from normal bone metabolism may differ in children as it is observed that PTH elevation levels in response to low 25(OH)D levels in the pediatric age group is not as same as that that of adults. Therefore, simply estimating deficiency on the basis of population-based reference values may not be appropriate. In children, a few studies have attempted to investigate the Vitamin D and PTH axis and have identified lower cutoff values among children on the basis of PTH response analogous with the Paediatric Endocrine Society classification. Some pediatric studies have defined VDD, based on PTH elevation, best at 25OHD level of 13.6 ng/ml.[16] The negative correlation between 25(OH)D and PTH among Indian children was first discussed by Marwah and Sripathyin the age group of 10–14 years.[10] Presumably, ours is the second and probably first study in the age group of 1–5 years wherein similar correlation was observed. Anticipated elevation of PTH in response to low 25(OH)D levels was still within the normal range. The rise in PTH was observed at 25(OH)D concentrations <10 ng/ml unlike the previous study in which it was observed at <5 ng/ml.[10] The reasons for the lack of anticipated rise in PTH above the upper limit of normal, despite low 25(OH)D levels, could be due to availability of adequate 1,25(OH) 2D levels to maintain calcium homeostasis and normal PTH values. This corresponds to functional cut off of 10 ng/ml of 25(OH)D closer to Paediatric Endocrine Society recommended cutoffs. In the present study, nearly 22.4% of children had 25(OH)D levels <10 ng/ml compared to 74.4% having 25(OH)D levels <20 ng/ml. This discrepancy suggests that optimal 25(OH)D levels required for calcium homeostasis may differ in children than those of adults thereby resulting in generally lower PTH levels in children as compared to adults.[16] This especially has clinical relevance as there are increasing reports of subclinical and overt Hypervitaminosis D, especially among children[29] which could be due to empirically treating children with massive doses of Vitamin D assuming its widespread prevalence in pediatric age group as well. The study throws light on that fact that functional or metabolic cutoffs for VDD may be lower in under-five children thereby alarming cautious and evidence-based treatment in children. It also justifies the need to relook at the functional relevance of currently defined cut offs of Vitamin D in children.[30]
Several strengths of the study include community-based approach which reflects true prevalence and information on lifestyle and dietary factors of these children along with biochemical markers of VDD. However, the potential limitations of the study were the inability to correlate and analyze seasonal variations among deficient children and to evaluate bone marrow density among these children.
CONCLUSION
This paper specifically generates information on community-based prevalence of VDD among under-five children from urban slums and also throws light on various associated factors. Healthcare practitioners need to encourage parents of younger children to adopt a healthy lifestyle, incorporating Vitamin D-containing foods in the diet and more importantly have adequate sun exposure. The study endorses the importance of sun exposure and also highlights the need of defining cutoffs of 25(OH)D for VDD among under-five children in India. High prevalence of VDD in the Indian children, however, demands further research to explore genetic and environmental factors.
Financial support and sponsorship
This was financially supported by ICMR-NIRRH, Mumbai.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and it's management: Review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Sunlight and Vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 3.Walker VP, Modlin RL. The Vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65(5 Pt 2):106R–13R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaiah J, Reddy PS, Sheshacharyulu M, Radhakrishna KV, Subrahmanyam GV. Association of Vitamin D deficiency with sever acute lower respiratory tract infection in children of less than 2 year age. Asian J Biochem Pharm Res. 2014;4:11–9. [Google Scholar]
- 5.Dhingra A, Dhanda M, Goel A, Kumar N, Mohd M, Khan MH. Hypovitaminosis and tuberculosis: A comparative study in children of Mewat. Indian J Sci Res. 2015;6:81–4. [Google Scholar]
- 6.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch Dis Child. 2008;93:512–7. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 7.Report of the Joint FAO/WHO Expert Consultation on Vitamin and Mineral Requirement in Human Nutrition: Bangkok 1998. 2nd ed. Rome: FAO; 2004. [Google Scholar]
- 8.Harinarayan CV, Joshi SR. Vitamin D status in India – Its implications and remedial measures. J Assoc Physicians India. 2009;57:40–8. [PubMed] [Google Scholar]
- 9.Bhalala U, Desai M, Parekh P, Mokal R, Chheda B. Subclinical hypovitaminosis D among exclusively breastfed young infants. Indian Pediatr. 2007;44:897–901. [PubMed] [Google Scholar]
- 10.Marwaha RK, Sripathy G. Vitamin D & bone mineral density of healthy school children in Northern India. Indian J Med Res. 2008;127:239–44. [PubMed] [Google Scholar]
- 11.Vasudevan J, Reddy MM, Jenifer A, Thayumanavan S, Devi U, Rathinasamy M. Prevalence and factors associated with Vitamin D deficiency in Indian children: A hospital based cross sectional study. Pediatr Oncall. 2014;11(3) [Doi: 10.7199/ped.oncall. 2014.47] [Google Scholar]
- 12.Basu S, Gupta R, Mitra M, Ghosh A. Prevalence of vitamin d deficiency in a pediatric hospital of Eastern India. Indian J Clin Biochem. 2015;30:167–73. doi: 10.1007/s12291-014-0428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekbote VH, Khadilkar AV, Mughal MZ, Hanumante N, Sanwalka N, Khadilkar VV, et al. Sunlight exposure and development of rickets in Indian toddlers. Indian J Pediatr. 2010;77:61–5. doi: 10.1007/s12098-009-0263-2. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM, et al. The impact of atmospheric pollution on Vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002;87:111–3. doi: 10.1136/adc.87.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari L, Puliyel JM. Vitamin D level in slum children of Delhi. Indian Pediatr. 2004;41:1076–7. [PubMed] [Google Scholar]
- 16.Atapattu N, Shaw N, Hogler W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of Vitamin D deficiency in children. Pediatr Res. 2013;74:552–6. doi: 10.1038/pr.2013.139. [DOI] [PubMed] [Google Scholar]
- 17.Oberoi SS. Updating income ranges for Kuppuswamy's socio-economic status scale for the year 2014. Indian J Public Health. 2015;59:156–7. doi: 10.4103/0019-557X.157540. [DOI] [PubMed] [Google Scholar]
- 18.WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for Height and Body Mass Index-for-Age: Methods and Development. [Last accessed on 2017 Aug 08]. Available from: http://www.who.int/childgrowth/standards/Technical_report.pdf .
- 19.Solomon L, Warwick D, Nayagam S, editors. Apley's System of Orthopedics and Fractures. 8th ed. London, United Kingdom: Arnold; 2001. pp. 449–84. [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 21.Gopalan C, Sastri BV, Balasubramanian SC, editors. Nutritive Value of Indian Foods. Hyderabad, India: National Institute of Nutrition, Indian Council of Medical Research; 2012. Food composition tables; pp. 45–95. [Google Scholar]
- 22.Software for Dietary Calculation. [Last accessed on 2017 Jul 05]. Available from: http://www.dietsoft.in/DietSoft .
- 23.Zhao X, Xiao J, Liao X, Cai L, Xu F, Chen D, et al. Vitamin D status among young children aged 1-3 years: A Cross-sectional study in Wuxi, China. PLoS One. 2015;10:e0141595. doi: 10.1371/journal.pone.0141595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puri S, Marwaha RK, Agarwal N, Tandon N, Agarwal R, Grewal K, et al. Vitamin D status of apparently healthy schoolgirls from two different socioeconomic strata in Delhi: Relation to nutrition and lifestyle. Br J Nutr. 2008;99:876–82. doi: 10.1017/S0007114507831758. [DOI] [PubMed] [Google Scholar]
- 25.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D. Vitamin D status in Andhra Pradesh: A population based study. Indian J Med Res. 2008;127:211–8. [PubMed] [Google Scholar]
- 26.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical Vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 27.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, Vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–41. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 28.Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more Vitamin D. J Clin Endocrinol Metab. 2003;88:185–91. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 29.Sharma LK, Dutta D, Sharma N, Gadpayle AK. The increasing problem of subclinical and overt hypervitaminosis D in India: An institutional experience and review. Nutrition. 2017;34:76–81. doi: 10.1016/j.nut.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Surve S, Chauhan S, Amdekar Y, Joshi B. Vitamin D deficiency in children: An update on its prevalence, therapeutics and knowledge gaps. Indian J Nutr. 2017;4:167. [Google Scholar]


