Abstract
Introduction:
A kaleidoscope of coagulation disorders has been reported in patients with thyroid dysfunctions. Globally, these disorders involve both primary and secondary hemostasis and range from subclinical laboratory abnormalities to, more rarely, life-threatening hemorrhages or thrombotic events. While overt hypothyroidism appears to be associated with a bleeding tendency, hyperthyroidism emerged to have an increased risk of thrombotic events. As a controversy, subclinical hypothyroidism and mild hypothyroidism have been reported as prothrombotic state. The mechanisms involved in these observations are also not conformed.
Objective:
To study the levels of prothrombotic coagulation factor VIII and fibrinogen in patients with thyroid disorder at baseline and to correlate the change in these factors after attaining euthyroid state by treatment.
Study Design:
This was a longitudinal interventional study.
Subjects and Methods:
Forty patients were recruited based on the inclusion and exclusion criteria, and their coagulation profile (prothrombin time, aPTT, Factor VIII, and fibrinogen levels), routine hematological, and biochemical profile was done at baseline and 6 weeks after attaining euthyroid state.
Results and Conclusion:
Hyperthyroidism and mild hypothyroidism were found to be hypercoagulable states and moderate-to-severe hypothyroidism as hypocoagulable states. Nevertheless, further observational and intervention studies are needed to provide more definitive information on the clinical relevance of this association, along with the potential implication for prevention and treatment of coagulation/fibrinolytic abnormalities in patients with thyroid dysfunction.
Keywords: Factor VIII, fibrinogen, hyperthyroidism, hypothyroidism
INTRODUCTION
Several disorders of coagulation and fibrinolysis have been widely reported in patients with thyroid dysfunctions. From a clinical standpoint, it is important to note that these coagulation-fibrinolytic disorders usually range from mild to moderate and rarely to severe laboratory abnormalities. Moreover, because they are rapidly reversible after pharmacologic treatment of the hormonal dysfunction, they would appear to be usually of limited importance in clinical practice, provided the underlying disorder is recognized quickly and treated appropriately.[1,2,3,4,5]
The first time a relation between thyroid disorder and hemostatic system was described in 1913 by Kaliebe. He described a patient with Graves' disease and cerebral venous thrombosis and proposed a relation between thyroid hormone and venous thrombosis which was subsequently published by Squizzato et al.[6] Subsequent studies focused on alterations in levels of coagulation factors in patients with thyroid disease and mostly confirmed that hyperthyroidism was associated with prothrombotic changes.[7,8,9,10,11] Even though the exact mechanism is still unproven, the most suggested one is by increasing Von Willebrand factor (vWF) and coagulation factor VIII levels.[7,9] Bleeding tendencies also reported in hyperthyroidism with probable mechanism of platelet dysfunction or development of autoimmune thrombocytopenia.[12,13]
In individuals with decreased levels of thyroxine, an increased bleeding time was seen together with a prolonged activated partial thromboplastin time and prothrombin time (PT) and decreased levels of factor VIII, von Willebrand factor, and fibrinogen. However, the reports from previous literature are still controversial in hypothyroidism. Some of the literature data have suggested hypothyroidism produces a hypocoagulable state. However, more recent data evidenced the contradictory fact.[14,15,16,17,18]
The aims of this study were to evaluate the potential association between thyroid disorders and disturbances in the coagulation system and to determine whether concentrations of serum lipids, renal functions, other hematological and biochemical indices, and markers of coagulation (PT, aPTT, factor VIII, and fibrinogen) are affected in patients with different thyroid-stimulating hormone (TSH) levels. The effects of levothyroxine replacement/antithyroid medications on these parameters were also evaluated.
SUBJECTS AND METHODS
Patients
The study was conducted in Department of Medicine, Maulana Azad Medical College and associated Lok Nayak Hospital, New Delhi, after clearance from Institutional Ethics Committee. The duration of study was from October 2015 to April 2017. It was a longitudinal interventional study. Forty patients with thyroid disorders were recruited from medical outpatient department (OPD), endocrinology OPD, and medical wards. All diagnosed cases of either hypothyroidism or hyperthyroidism (treatment naïve or uncontrolled with medication), more than 12 years of age, of both female and male sex willing to give consent and assent were included in the study. Patients with any other diseases which can alter factor VIII levels, fibrinogen levels, or coagulability of blood such as hemophilia, immune disorders as with systemic lupus erythematosus, rheumatoid arthritis, and Sjogren's syndrome, various malignancies, pregnant patients, with clinically known hypercoagulable state, and patients on any type of anticoagulant therapy were excluded from the study.
Methods
An informed written consent was taken from all patients before the inclusion to the study. A detailed history of all individuals was taken by using a predesigned pro forma. Questions with regard to risk factors associated with the development of changes in coagulability of blood were inquired such as family history of thrombophilia/bleeding disorders, past history of any bleeding or thrombosis, personal history of smoking/alcoholism, concurrent infection or malignancy, and type of thyroid disorder as patient-related factors. Patients were also inquired about the treatment received, duration of treatment, and intake of any other drugs affecting the coagulation as treatment-related factors. Out of 40 patients, 10 were hyperthyroid with TSH <0.05 mIU/L, 10 were mild hypothyroid with TSH 5–10 mIU/L, 10 were moderate hypothyroid with TSH 10–50 mIU/L, and 10 were severely hypothyroid with TSH >50 mIU/L. TSH levels were measured by commercially available automated chemiluminescence system kits. 10 ml of venous sample was drawn using venepuncture. Following investigations were done in every case at the baseline, complete blood count including platelet counts, liver function tests (LFTs), kidney function tests (KFTs), serum cholesterol and triglycerides, random blood sugar, serum TSH level, coagulation profile (PT/aPTT/INR, factor VIII, and fibrinogen level). Whole blood analyses were done using an autoanalyzer. Factor VIII activity, PT, aPTT and fibrinogen level were measured by ACL ADVANCE (automated analyser) and kits used were HemosIL kits by Instrumentation Laboratory, Monza 338–20128, Milano (Italy).
Patents were started on treatment according to their thyroid state either by levothyroxine or by antithyroid medications. Patients were followed up until they attained euthyroid state. Six weeks after attaining euthyroid state, all above-mentioned investigations were repeated. The association between each index at the baseline was evaluated and the alteration in each index after attaining euthyroid state was noted. Correlations between TSH and prothrombotic factors (Factor VIII and fibrinogen) were calculated at baseline.
Statistical analysis
Following statistics was used to analyze the data. Continuous variables were presented as mean and were compared by paired test. P < 0.05 was considered to be significant. Appropriate correlation using Pearson's correlation coefficient was performed for finding the association between the prothrombotic factor levels and TSH values. All data gathered were processed by SPSS software version 22 (Armonk, NY: IBM Corp).
RESULTS
Demographic profile
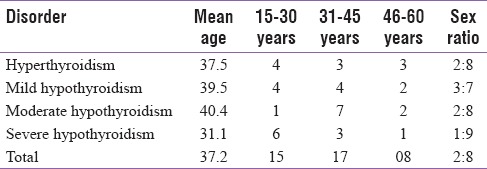
The mean age of the patients in this study group was 37.17 years with a range of 15–60. Out of 40 patients included in the study, 32 (80%) were females and the remaining 8 (20%) were males. The detailed distribution is shown in Table 1.
Table 1.
Mean age and age-wise distribution of the patients
Baseline and follow-up hematological and biochemical parameters in the study groups
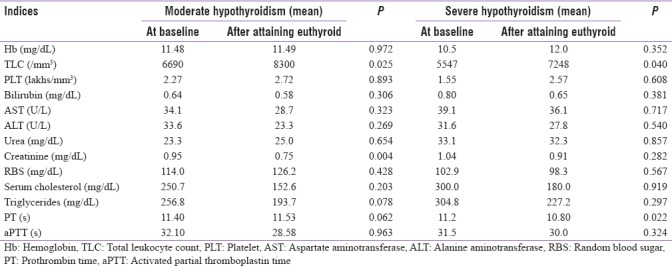
In hyperthyroid patients, no significant variations were noted in hematological and biochemical parameters while comparing at the baseline and after attaining euthyroid state. In mild hypothyroidism, statistically significant increase has occurred in hemoglobin concentration (P = 0.002), and significant decrease has occurred in PT values (P = 0.0006) comparing at baseline and after attaining euthyroid state. In moderate hypothyroidism, statistically significant increase has occurred in thin layer chromatography (TLC) (P = 0.025) and significant decrease has occurred in creatinine concentration (P = 0.004) comparing at baseline and after attaining euthyroid state. Statistically significant increase has occurred in TLC with P = 0.040 and significant decrease has occurred PT with P = 0.022 in severe hypothyroid patients comparing at baseline and after attaining euthyroid state. The variations in other indices were not statistically significant in all these subgroups of hypothyroidism. These data are tabulated in Tables 2 and 3.
Table 2.
Baseline and follow-up hematological and biochemical parameters in hyperthyroidism and mild hypothyroidism
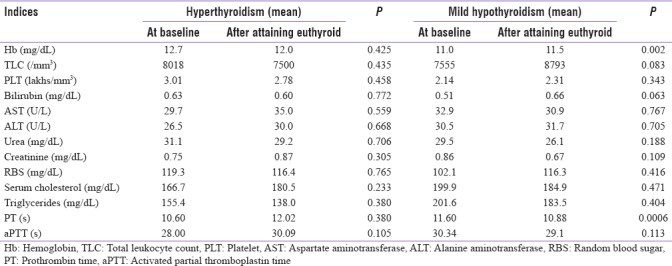
Table 3.
Baseline and follow-up hematological and biochemical parameters in moderate hypothyroidism and severe hypothyroidism
Baseline and follow-up coagulation parameters in the study groups
The mean value of PT in hyperthyroidism at baseline was 10.6 ± 0.59 s, and after attaining euthyroid status, it was 12.02 ± 0.45 s, and in hypothyroidism, it was 11.4 ± 0.76 s and 11.08 ± 0.87 s, respectively. The mean value of activated partial thromboplastin time (aPPT) in hyperthyroidism at baseline was 28 ± 3.83 s, and after attaining euthyroid status, it was 30.09 ± 3.20 s, and in hypothyroidism, it was 31.31 ± 3.49 s and 29.19 ± 3.99 s, respectively. The mean value of factor VIII levels in hyperthyroidism at baseline was 193.92 ± 49.77%, and after attaining euthyroid status, it was detected as decreased to 148.35 ± 37.28%. This is shown in Table 4. At baseline, 7 out of 10 patients (70%) were having factor VIII levels of >150%. In mild hypothyroidism, the mean value of factor VIII levels at baseline was 188.89 ± 58.13%, and after attaining euthyroid status, it was detected as decreased to 137.83 ± 30.55%. In this group also, at baseline, 7 out of 10 patients (70%), were having factor VIII levels of >150%. In moderate hypothyroidism, the mean value of factor VIII levels at baseline was 105.89 ± 16.23%, and after attaining euthyroid status, it was detected as increased to 143.92 ± 46.85%. In severe hypothyroidism, the mean value of factor VIII levels at baseline was 89.85 ± 48.26%, and after attaining euthyroid status, it was increased to 146.6 ± 34.26%. At baseline, 5 out of 10 patients (50%) were having factor VIII levels >50%. The mean value of fibrinogen level in hyperthyroidism at baseline was 254.45 ± 51.26 mg/dL, and after attaining euthyroid status, it was detected as increased to 289.62 ± 43.59 mg/dL, and in hypothyroidism, it was 237.30 ± 49.09 mg/dL and 266.52 ± 50.76 mg/dL, respectively.
Table 4.
Variations in prothrombotic factors (factor VIII and fibrinogen levels) and thyroid-stimulating hormone level at baseline and after attaining euthyroid state in thyroid patients
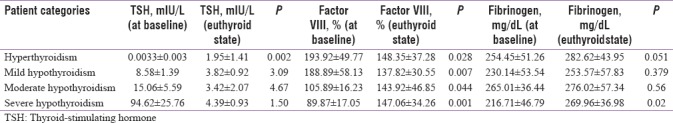
Variations in prothrombotic factors (factor VIII and fibrinogen levels) and thyroid-stimulating hormone level at baseline and after treatment
In hyperthyroid patients, the mean TSH value at baseline was 0.0033 ± 0.003 mIU/L. After attaining euthyroid, it increased to 1.95 ± 1.41 mIU/L. This increase in TSH is statistically significant with P = 0.002. At the same time, the mean value for factor VIII level at baseline was 193.92 ± 49.77%, and after attaining euthyroid state, it decreased to 148.35 ± 37.28%. This decrease in factor VIII levels is also statistically significant with P = 0.028. The mean value for fibrinogen level at baseline was 254.45 ± 51.26 mg/dL, and after attaining euthyroid state, it increased to 282.62 ± 43.95 mg/dL. This decrease in fibrinogen levels is statistically insignificant with P = 0.051.
In mild hypothyroid patients, the mean TSH value at baseline was 8.58 ± 1.39 mIU/L. After attaining euthyroid, it decreased to 3.82 ± 0.92 mIU/L. This decrease in TSH is statistically insignificant with P = 3.09. At the same time, the mean value for factor VIII level at baseline was 188.89 ± 58.13%, and after attaining euthyroid state, it decreased to 137.82 ± 30.55%. This decrease in factor VIII levels was statistically significant with P = 0.007. The mean value for fibrinogen level at baseline was 230.14 ± 53.54 mg/dL, and after attaining euthyroid state, it decreased to 253.57 ± 57.83 mg/dL. This increase in factor VIII levels was statistically insignificant with P = 0.379.
In moderate hypothyroid patients, the mean TSH value at baseline was 15.06 ± 5.59 mIU/L. After attaining euthyroid, it decreased to 3.42 ± 2.07 mIU/L. This decrease in TSH is statistically insignificant with P = 4.67. At the same time, the mean value for factor VIII level at baseline was 105.89 ± 16.23%, and after attaining euthyroid state, it increased to 143.92 ± 46.85%. This increase in factor VIII levels was statistically significant with P = 0.044. The mean value for fibrinogen level at baseline was 265.01 ± 36.44 mg/dL, and after attaining euthyroid state, it increased to 276.02 ± 57.34 mg/dL. This increase in fibrinogen was statistically not significant with P = 0.56.
In severe hypothyroid patients, the mean TSH value at baseline was 94.62 ± 25.76 mIU/L. After attaining euthyroid, it decreased to 4.39 ± 0.93 mIU/L. This decrease in TSH is statistically insignificant with P = 1.50. At the same time, the mean value for factor VIII level at baseline was 89.87 ± 17.05%, and after attaining euthyroid state, it increased to 147.06 ± 34.26%. This increase in factor VIII levels was statistically significant with P = 0.001. The mean value for fibrinogen level at baseline was 216.71 ± 46.79 mg/dL, and after attaining euthyroid state, it increased to 269.96 ± 36.98 mg/dL. This increase in factor VIII levels was statistically significant with P = 0.02. This is depicted in Table 4.
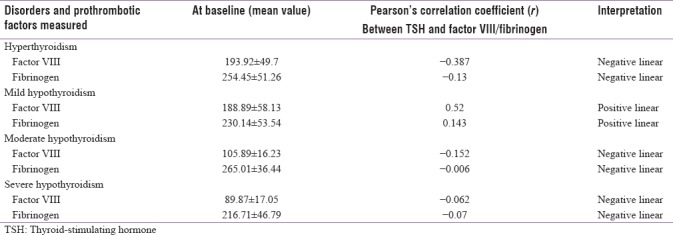
Correlation between prothrombotic factors (factor VIII and fibrinogen levels) and thyroid-stimulating hormone level at baseline in thyroid patients
The correlation between factor VIII and TSH levels at the baseline was found to have a linear negative relation except in mild hypothyroidism which shows a linear positive relation. The correlation between fibrinogen and TSH levels at the baseline was found to have a linear negative relation except in mild hypothyroidism which shows a linear positive relation. This is shown in Table 5.
Table 5.
Correlation between prothrombotic factors (factor VIII and fibrinogen levels) and thyroid-stimulating hormone level at baseline in thyroid patients
DISCUSSION
The link between thyroid disorders and hemostatic system is well known and well established. However, the type of thyroid disorder leading to which type of coagulation disorder and mechanism behind these links are confusing and controversial. The mean age for hyperthyroidism is found to have 37.5 years which is in accordance with previous studies,[19] and the mean age for hypothyroidism was 37 years which was a slightly lower than the mean age for hypothyroidism in various other Indian studies (46 years,[20] 42.5 years[21]). This could be due to regional bias. 42.5 years.[21] Anemia is seen in hypothyroid patients in our study (mean hemoglobin-10.99 g/dL) with gradual decrease in hemoglobin with severity of hypothyroidism as observed by Horton et al.[22] There was a gradual decline in both TLC and platelet count in hypothyroidism according to severity. This decrease was corrected after attaining euthyroid state. In hyperthyroidism, no significant changes were seen in these indices in our study. No significant variation in KFTs or LFT was observed in both hyperthyroidism and hypothyroidism. Lipid profile test in hyperthyroid patients has shown low level of total cholesterol and triglycerides in our study comparable to study conducted by Jalal et al.[23] In our study, both cholesterol and triglycerides level increased with the increase in the severity of the hypothyroidism as reported in previous studies.[24,25] The mean value for cholesterol and triglycerides in mild hypothyroidism was 199.0 and 201.6 mg/dL, respectively, and in severe hypothyroidism, these were 300.2 and 304.8 mg/dL, respectively. This impairment in lipid profile improved on supplementation with thyroid hormones.
Mohammed Ali et al. observed a significant decrease in PT in hyperthyroid patients compared to the control group. Activated thromboplastin time was also significantly decreased in hyperthyroid patients, compared to the control group in the same study;[26] our study also revealed the same. In our study, PT showed a mild increase at baseline in mild and severe hypothyroidism, and there was statistically significant decrease in PT after attaining euthyroid state on treatment in these patients. In aPTT analysis, it was shown a mild increase at the baseline and decline after attaining euthyroid state, but this variation was not statistically significant.
In our study, it was observed that factor VIII levels were significantly high in hyperthyroidism and mild hypothyroidism with mean values at baseline 193.92% and 188.89%, respectively, and after attaining euthyroid state decreased to 148.35% and 137.82%, respectively. These variations were statistically significant with P = 0.028 and 0.007, respectively. Our results are consistent with previous studies conducted by Roger et al.[9] and Mouton et al.[27] In mild/subclinical hypothyroidism, Muller et al. reported a hypercoagulable state, and they found increase in factor VIII and vWF activities.[28] Chadarevian et al. and Canturk et al. also explained mild/subclinical hypothyroidism as a hypofibrinolytic hypercoagulable state.[29,30] Egeberg and Simone also reported the same results.[3,31] It has been found that the thyroxin increases the factor VIII levels by a heightened direct genetic transcription of coagulation factor VIII or decreased clearance of factor VIII by increased release of vWF.[32] In our study, 8 out of 10 cases of mild hypothyroidism were those who were taking thyroxin for more than 3 months but their TSH level was not in the normal range at the time of recruitment. This could be the reason for the high factor VIII levels in mild hypothyroidism in our study. Significant elevations of factor VIII have been reported to occur in conditions such as strenuous exercise, epinephrine infusions, fever induction, pregnancy, and intravascular hemolysis, treatment with progestational agents, renal failure, hyperglobulinemia, and treatment with prednisone.[3] However, all these confounding factors were excluded in our study.
In moderate and severe hypothyroidism, we found that factor VIII levels were notably low at the baseline with mean values 105.89% and 89.87%, respectively. The factor VIII levels decreased with increase in the severity of hypothyroidism suggesting hypothyroidism to be a hypocoagulable state. After supplementation with thyroid hormones, these patients attained euthyroid state, and factor VIII levels increased to 143.92% and 147.06%, respectively. This increase was statistically significant with P = 0.044 and 0.001, respectively. These findings were consistent with observations of Chadarevian et al.[29] and Cantürk et al.[30]
As far as fibrinogen levels were concerned, we found that there was no increase in levels of fibrinogen in hyperthyroidism (mean value = 254.45 mg/dL). However, the levels increased to some extent after attaining euthyroid state (mean = 282.62 mg/dL) even though this increase was statistically insignificant (0.051). This result is contradictory to previous studies conducted by Erem et al.[33] and Lippi et al.[8] where they found that fibrinogen levels are increased in hyperthyroidism. This can be explained by small number of patients included in our study, genetic, environmental variation in Indian patients, and variations in the methods used for measurements. In hypothyroidism, no definitive pattern of alteration in fibrinogen levels was detected in mild or moderate hypothyroidism. However, in severe hypothyroidism, the fibrinogen level was 216.71 mg/dL at baseline and it increased to 269.96 mg/dL after attaining euthyroid state. This increase was statistically significant with P = 0.02. Chadarevian et al.,[29] Cantürk et al.[30] and Shih et al.[31] reported decreased levels of fibrinogen in hypothyroidism. However, other studies Dörr et al.[34] and Li et al.[35] suggested no significant decrease in fibrinogen levels in hypothyroidism.
The correlation between factor VIII and TSH levels at the baseline was found to have a linear negative relation except in mild hypothyroidism which shows a linear positive relation. The correlation between fibrinogen and TSH levels at the baseline was found to have a linear negative relation except in mild hypothyroidism which shows a linear positive relation.
From our study, we conclude that hyperthyroidism and mild hypothyroidism can be considered as hypercoagulable state and moderate-to-severe hypothyroidism as hypocoagulable state. Our study forms the basis for future studies for the comprehensive assessment of coagulation abnormalities in thyroid disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Squizzato A, Romualdi E, Büller HR, Gerdes VE. Clinical review: Thyroid dysfunction and effects on coagulation and fibrinolysis: A systematic review. J Clin Endocrinol Metab. 2007;92:2415–20. doi: 10.1210/jc.2007-0199. [DOI] [PubMed] [Google Scholar]
- 2.Franchini M, Montagnana M, Manzato F, Vescovi PP. Thyroid dysfunction and hemostasis: An issue still unresolved. Semin Thromb Hemost. 2009;35:288–94. doi: 10.1055/s-0029-1222607. [DOI] [PubMed] [Google Scholar]
- 3.Simone JV, Abildgaard CF, Schulman I. Blood coagulation in thyroid dysfunction. N Engl J Med. 1965;273:1057–61. doi: 10.1056/NEJM196511112732001. [DOI] [PubMed] [Google Scholar]
- 4.Myrup B, Bregengård C, Faber J. Primary haemostasis in thyroid disease. J Intern Med. 1995;238:59–63. doi: 10.1111/j.1365-2796.1995.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 5.Hofbauer LC, Heufelder AE. Coagulation disorders in thyroid diseases. Eur J Endocrinol. 1997;136:1–7. doi: 10.1530/eje.0.1360001. [DOI] [PubMed] [Google Scholar]
- 6.Squizzato A, Gerdes VE, Brandjes DP, Büller HR, Stam J. Thyroid diseases and cerebrovascular disease. Stroke. 2005;36:2302–10. doi: 10.1161/01.STR.0000181772.78492.07. [DOI] [PubMed] [Google Scholar]
- 7.Homoncik M, Gessl A, Ferlitsch A, Jilma B, Vierhapper H. Altered platelet plug formation in hyperthyroidism and hypothyroidism. J Clin Endocrinol Metab. 2007;92:3006–12. doi: 10.1210/jc.2006-2644. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G, Franchini M, Targher G, Montagnana M, Salvagno GL, Guidi GC, et al. Hyperthyroidism is associated with shortened APTT and increased fibrinogen values in a general population of unselected outpatients. J Thromb Thrombolysis. 2009;28:362–5. doi: 10.1007/s11239-008-0269-z. [DOI] [PubMed] [Google Scholar]
- 9.Rogers JS, 2nd, Shane SR, Jencks FS. Factor VIII activity and thyroid function. Ann Intern Med. 1982;97:713–6. doi: 10.7326/0003-4819-97-5-713. [DOI] [PubMed] [Google Scholar]
- 10.Erem C. Blood coagulation, fibrinolytic activity and lipid profile in subclinical thyroid disease: Subclinical hyperthyroidism increases plasma factor X activity. Clin Endocrinol (Oxf) 2006;64:323–9. doi: 10.1111/j.1365-2265.2006.02464.x. [DOI] [PubMed] [Google Scholar]
- 11.Loeliger EA, Van Der Esch B, Mattern MJ, Hemker HC. The biological disappearance rate of prothrombin, factors VII, IX and X from plasma in hypothyroidism, hyperthyroidism, and during fever. Thromb Diath Haemorrh. 1964;10:267–77. [PubMed] [Google Scholar]
- 12.Kurata Y, Nishioeda Y, Tsubakio T, Kitani T. Thrombocytopenia in Graves' disease: Effect of T3 on platelet kinetics. Acta Haematol. 1980;63:185–90. doi: 10.1159/000207396. [DOI] [PubMed] [Google Scholar]
- 13.Cordiano I, Betterly C, Spadaccino CA, Soini B, Fabris F. Autoimmune thrombocyte purpura and thyroid autoimmune disease: Overlapping syndrome? Clin Exp Immunol. 1998;113:373–8. doi: 10.1046/j.1365-2249.1998.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palareti G, Biagi G, Legnani C, Bianchi D, Serra D, Savini R, et al. Association of reduced factor VIII with impaired platelet reactivity to adrenalin and collagen after total thyroidectomy. Thromb Haemost. 1989;62:1053–6. [PubMed] [Google Scholar]
- 15.Levesque H, Borg JY, Cailleux N, Vasse M, Daliphard S, Gancel A, et al. Acquired von willebrand's syndrome associated with decrease of plasminogen activator and its inhibitor during hypothyroidism. Eur J Med. 1993;2:287–8. [PubMed] [Google Scholar]
- 16.Erem C, Kavgaci H, Ersöz HO, Hacihasanoglu A, Ukinç K, Karti SS, et al. Blood coagulation and fibrinolytic activity in hypothyroidism. Int J Clin Pract. 2003;57:78–81. [PubMed] [Google Scholar]
- 17.Chaudhary A, Jha K, Chaudhary TS. Study of effect of hypothyroidism on platelet aggregability. Res J Biol. 2012;2:182–5. [Google Scholar]
- 18.Cantürk Z, Cetinarslan B, Tarkun I, Cantürk NZ, Ozden M, Duman C, et al. Hemostatic system as a risk factor for cardiovascular disease in women with subclinical hypothyroidism. Thyroid. 2003;13:971–7. doi: 10.1089/105072503322511382. [DOI] [PubMed] [Google Scholar]
- 19.Devereaux D, Tewelde SZ. Hyperthyroidism and thyrotoxicosis. Emerg Med Clin North Am. 2014;32:277–92. doi: 10.1016/j.emc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N, et al. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2013;17:647–52. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhanwal DK, Bajaj S, Rajput R, Subramaniam KA, Chowdhury S, Bhandari R, et al. Prevalence of hypothyroidism in pregnancy: An epidemiological study from 11 cities in 9 states of India. Indian J Endocrinol Metab. 2016;20:387–90. doi: 10.4103/2230-8210.179992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horton L, Coburn RJ, England JM, Himsworth RL. The haematology of hypothyroidism. Q J Med. 1976;45:101–23. [PubMed] [Google Scholar]
- 23.Jalal NA, Al-Samarrai AH, Al-Tikriti KA. Biochemical changes in patients with hyperthyroidism. Tikrit J Pure Sci. 2010;15:204–8. [Google Scholar]
- 24.Lithell H, Boberg J, Hellsing K, Ljunghall S, Lundqvist G, Vessby B, et al. Serum lipoprotein and apolipoprotein concentrations and tissue lipoprotein-lipase activity in overt and subclinical hypothyroidism: The effect of substitution therapy. Eur J Clin Invest. 1981;11:3–10. doi: 10.1111/j.1365-2362.1981.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 25.Nikkila E, Kekki M. Plasma triglyceride metabolism in thyroid disease. J Clin Invest. 1973;51:203. doi: 10.1172/JCI107017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed-Ali MS, Ahmed RO. Coagulation profiles in hypothyroid and hyperthyroid female patients in Sudan. Saudi Med J. 2008;29:1289–93. [PubMed] [Google Scholar]
- 27.Mouton S, Nighoghossian N, Berruyer M, Derex L, Philippeau F, Cakmak S, et al. Hyperthyroidism and cerebral venous thrombosis. Eur Neurol. 2005;54:78–80. doi: 10.1159/000087717. [DOI] [PubMed] [Google Scholar]
- 28.Müller B, Tsakiris DA, Roth CB, Guglielmetti M, Staub JJ, Marbet GA, et al. Haemostatic profile in hypothyroidism as potential risk factor for vascular or thrombotic disease. Eur J Clin Invest. 2001;31:131–7. doi: 10.1046/j.1365-2362.2001.00777.x. [DOI] [PubMed] [Google Scholar]
- 29.Chadarevian R, Bruckert E, Giral P, Turpin G. Relationship between thyroid hormones and fibrinogen levels. Blood Coagul Fibrinolysis. 1999;10:481–6. doi: 10.1097/00001721-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Cantürk Z, Cetinarslan B, Tarkun I, Cantürk NZ, Ozden M, Duman C, et al. Hemostatic system as a risk factor for cardiovascular disease in women with subclinical hypothyroidism. Thyroid. 2003;13:971–7. doi: 10.1089/105072503322511382. [DOI] [PubMed] [Google Scholar]
- 31.Egeberg BO. Influence of thyroid function on the blood clotting system. Scand J Clin Lab Invest. 1963;15:1–7. [Google Scholar]
- 32.Shih CH, Chen SL, Yen CC, Huang YH, Chen CD, Lee YS, et al. Thyroid hormone receptor-dependent transcriptional regulation of fibrinogen and coagulation proteins. Endocrinology. 2004;145:2804–14. doi: 10.1210/en.2003-1372. [DOI] [PubMed] [Google Scholar]
- 33.Erem C, Ersoz HO, Karti SS, Ukinç K, Hacihasanoglu A, Deǧer O, et al. Blood coagulation and fibrinolysis in patients with hyperthyroidism. J Endocrinol Invest. 2002;25:345–50. doi: 10.1007/BF03344016. [DOI] [PubMed] [Google Scholar]
- 34.Dörr M, Robinson DM, Wallaschofski H, Schwahn C, John U, Felix SB, et al. Low serum thyrotropin is associated with high plasma fibrinogen. J Clin Endocrinol Metab. 2006;91:530–4. doi: 10.1210/jc.2005-1786. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Chen H, Tan J, Wang X, Liang H, Sun X, et al. Impaired release of tissue plasminogen activator from the endothelium in Graves' disease – Indicator of endothelial dysfunction and reduced fibrinolytic capacity. Eur J Clin Invest. 1998;28:1050–4. doi: 10.1046/j.1365-2362.1998.00381.x. [DOI] [PubMed] [Google Scholar]


